Abstract
Transcriptional activation of cytochrome P450 (CYP) genes and various drug metabolizing enzymes by the prototypical inducer phenobarbital (PB) and many other drugs and chemicals is an adaptive response of the organism to exposure to xenobiotics. The response to PB is mediated by the nuclear receptor constitutive androstane receptor (CAR), whereas the chicken xenobiotic receptor (CXR) has been characterized as the PB mediator in chicken hepatocytes. Our previous results suggested an involvement of AMP-activated protein kinase (AMPK) in the molecular mechanism of PB induction. Here, we show that the mechanism of AMPK activation is related to an effect of PB-type inducers on mitochondrial function with consequent formation of reactive oxygen species (ROS) and phosphorylation of AMPK by the upstream kinase LKB1. Gain- and loss-of-function experiments demonstrate that LKB1-activated AMPK is necessary in the mechanism of drug induction and that this is an evolutionary conserved pathway for detoxification of exogenous and endogenous chemicals. The activation of LKB1 adds a proximal target to the so far elusive sequence of events by which PB and other drugs induce the transcription of multiple genes.
Keywords: drug metabolism, induction, mitochondria, reactive oxygen species
Evolution has provided organisms with an elaborate defense system against foreign compounds (xenobiotics). The liver of vertebrates contains numerous enzymes that can transform potentially toxic xenobiotics (e.g., nutrients or drugs) or endobiotics (e.g., bile acids) to inactive and excretable metabolites. The expression of these enzymes can be adapted to the needs for detoxification by a process called induction. Phenobarbital (PB) is the prototype of a number of drugs that induce their own and the metabolism of other xenobiotics. Induction of drug metabolism is part of a pleiotropic response of the liver to xenobiotic exposure, which includes liver hypertrophy, tumor promotion, and induction of numerous genes in addition to those encoding for drug-metabolizing enzymes and drug transporters (1). PB also was shown to decrease the transcription of gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase 1 (PEPCK1) and glucose-6-phosphatase (G6P), and of several hepatic genes responsible for fatty acid metabolism (2). Moreover, PB increases the transcription of δ-aminolevulinic acid synthase 1 (ALAS1), the rate-limiting enzyme in the synthesis of heme, the prosthetic group of cytochromes P450 (CYPs) (3, 4). The molecular details of the mechanisms by which PB causes these effects are incompletely understood.
The transcriptional activation by PB of genes encoding drug-metabolizing enzymes, such as Cyp2b10 in mouse and CYP2B6 in human, is mediated by the nuclear receptor constitutive androstane receptor (CAR) (5). The interaction of PB with CAR is complex. PB apparently does not bind directly to CAR, but rather triggers its translocation from cytoplasm to the nucleus by as yet unknown mechanisms. In addition, phosphorylation and dephosphorylation events strongly affect PB induction of CYPs (for a review see ref. 6).
Interestingly, some of the effects of PB on energy metabolism in the liver were found to be CAR-mediated. Cyp2b10 is up-regulated during fasting and in diabetes (7, 8) and insulin has a repressive effect on induction of CYPs (9). These and other observations point to an interaction between the energy state of liver cells and expression of CYPs and to a physiological role of CAR in the responses to metabolic and nutritional stress.
An important energy sensor is AMP-activated protein kinase (AMPK). AMPK responds to any cellular stress that threatens to lower ATP levels by arresting nonessential ATP-using functions and stimulating ATP-generating pathways (10). Among the several genes regulated by AMPK is PEPCK1 (11) an effect also exerted by PB. Because the effect of PB on CAR is influenced by phosphorylation and dephosphorylation events and the regulation of some CYPs can be affected by metabolic and nutritional stress, we investigated the role of AMPK in the induction response. AMPK indeed was shown to be activated during PB-mediated induction of CYP2B6 in human hepatoma-derived cells (12) and in primary cultures of human and mouse hepatocytes (13). However, the mechanism by which these drugs increase AMPK activity was unknown.
The phenomenon of PB induction appears conserved in evolution. We have recently shown that in chicken hepatoma cells, the chicken X receptor (CXR) confers PB-type induction by functionally identical or exchangeable signaling pathways triggered by the nuclear receptors CAR and pregnane X receptor (PXR) in mammals (14, 15). In contrast to mammalian hepatoma cells, the chicken leghorn male hepatoma (LMH) cell line maintains a large spectrum of CYP gene induction by PB as well as by other drugs providing an accessible model for induction research.
In the present study, we have explored the mechanism by which AMPK is involved in the induction of three drug-inducible genes in chicken liver, namely CYP2H1, CYP3A37, and ALAS1. Our data confirm dose-dependent increase of AMPK activity after exposure of LMH cells to PB and extend this effect to metyrapone. The role of AMPK is further established through gain- and loss-of-function experiments. Most importantly, we observed that PB and metyrapone increase mitochondrial ROS generation and trigger the interaction of AMPK with one of its upstream kinases, LKB1. Our experiments confirm and extend the involvement of AMPK signaling in liver drug responses as an evolutionary conserved system from birds to mammals and suggest a mechanism by which inducer drugs activate AMPK.
Results
AMPK Activation by PB and Metyrapone in LMH Cells.
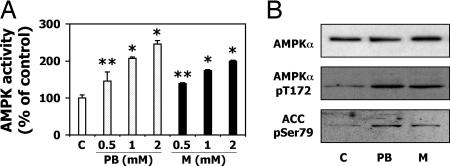
Because AMPK signaling pathways have not been characterized previously in LMH cells, AMPKα1, and AMPKα2 protein expression, localization and activation by the classical mammalian AMPK activator AICAR were analyzed in these cells [supporting information (SI) Fig. 6]. Recent studies have shown that PB activates AMPK in a human hepatoma-derived cell line (12). To examine whether this activation is also the case in LMH cells, two classical inducers of CYP2H1, CYP3A37, and ALAS1 (16), PB and metyrapone, were tested for their capacity to activate AMPK in LMH cells. Both compounds increased the AMPK activity after 1h treatment in a dose-dependent manner (Fig. 1A). Because the activity of AMPK correlates with phosphorylation of Thr-172 on its α catalytic subunit (17), Western blots were used to confirm that the increased activity was due to higher AMPK-Thr-172 phosphorylation (Fig. 1B). Phosphorylation of acetyl CoA carboxylase (ACC), a well known target of AMPK and indicator of its activation (18), also was increased. In this experiment, we thus demonstrate that two different inducers of CYPs trigger dose-dependent AMPK activation.
Fig. 1.
Chicken AMPKα subunits are activated by PB-type inducers. (A) LMH cells were treated with increasing doses of PB or metyrapone for 1h. AMPK activity is shown as percentage of the control. ∗, P < 0.01; ∗∗, P < 0.05. (B) Phosphorylation of AMPK-Thr-172 and ACC-Ser-79 is shown by Western blot after 1 h treatment with 500 μM PB or metyrapone (M).
Activation or Overexpression of AMPKα Subunits Affect CYP2H1, CYP3A37, and ALAS1 Gene Expression.
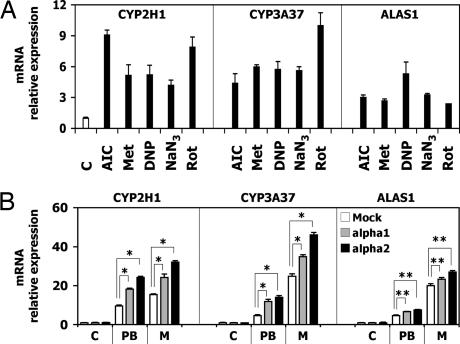
To test whether modulation of AMPK activity alone affects CYP2H1, CYP3A37, and ALAS1 gene expression, compounds known to activate AMPK by different mechanisms were tested. CYP2H1, CYP3A37 and ALAS1 mRNA were increased by AICAR, metformin, sodium azide (NaN3), dinitrophenol (DNP), and rotenone, but to a lower extent than by PB and metyrapone (Fig. 2A).
Fig. 2.
Activation or overexpression of AMPKα subunits affect CYP2H1, CYP3A37, and ALAS1 gene expression. (A) LMH cells were treated with 1 mM AICAR (AIC) or metformin (Met), 0.2 mM DNP, 1 mM NaN3, or 1 μM rotenone (Rot) for 16 h. Gene expression was analyzed by RT-PCR. (B) LMH cells transiently transfected with AMPKα subunits were treated with 500 μM PB or metyrapone (M) for 16 h. Gene expression was analyzed by RT-PCR. ∗, P < 0.01; ∗∗, P < 0.05.
When LMH cells were transiently transfected with rat AMPKα1 and AMPKα2 subunits, an increase of CYP2H1, CYP3A37, and ALAS1 induction by PB and metyrapone was observed (Fig. 2B). The latter showed a more pronounced effect most likely because of difference in expression of the two proteins, as documented in Western blots (data not shown). The basal expression level of the three genes was not altered. The increase in CYP induction obtained by AMPKα transfection was moderate probably as a result of the limited availability of AMPKβ and AMPKγ subunits, which were not cotransfected, but are known to be necessary for AMPK activation (19). These data show that induction of CYP2H1, CYP3A37, and ALAS1 is enhanced by increasing the expression of AMPKα.
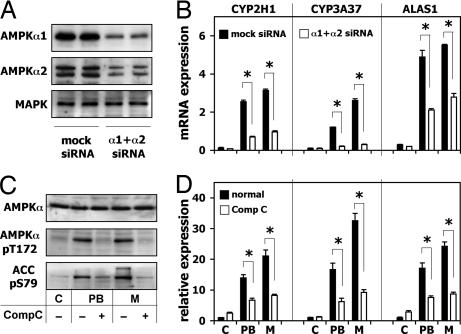
Down-Regulation of AMPKα Activity by siRNA or Compound C Decreases PB- and Metyrapone-Mediated Induction of CYP2H1, CYP3A37, and ALAS1.
To establish whether AMPK expression is necessary for drug-mediated induction of CYP2H1, CYP3A37 and ALAS1, AMPKα1 and AMPKα2 subunits were down-regulated by gene-specific siRNA duplexes. mRNA levels of AMPKα1 and AMPKα2 were reduced to ≈40% of the corresponding mRNAs of the control cells, whereas the AMPK activity was decreased to ≈50% of the control (SI Fig. 7A). The protein expression level also was clearly reduced as shown in a Western blot (Fig. 3A) with MAPK protein expression used as negative control for the siRNA specificity. Even if the down-regulation of the two AMPKα subunits was not complete, it drastically reduced the effect of both PB and metyrapone on CYP2H1 and CYP3A37 mRNA (Fig. 3B). In the case of ALAS1, the effect of siRNA was weaker, suggesting that ALAS1 is subject to other regulatory mechanisms.
Fig. 3.
Down-regulation of AMPKα activity by diced siRNA or Compound C decreases PB- and metyrapone-mediated induction of CYP2H1, CYP3A37, and ALAS1. (A) LMH cells were transiently transfected with AMPKα-specific siRNA. AMPKα protein expression was detected by Western blot. (B) LMH cells transiently transfected with siRNA were treated with 500 μM PB or metyrapone for 16 h. mRNA expression was measured by RT-PCR. ∗, P < 0.01. (C) Activation of AMPK by 500 μM PB or metyrapone with or without 30-min pretreatment with 20 μM Compound C is shown by Western blot evidencing the phosphorylation of AMPK-Thr-172 and ACC-Ser-79. (D) LMH cells were incubated 16 h with 500 μM PB or metyrapone with or without pretreatment for 30 min by 20 μM Compound C. mRNA levels were measured by RT-PCR. ∗, P < 0.01. M, metyrapone.
Another way to modulate the AMPK activity is Compound C, which is a specific and well-studied inhibitor of the kinase (20). Preincubation of LMH cells with Compound C abolished the AMPK activation by DNP completely (SI Fig. 7B), confirming the potency of this inhibitor. Preincubation of LMH cells with Compound C prevented the increased phosphorylation of AMPK-Thr-172 and ACC-Ser-79 observed when LMH cells were treated with PB or metyrapone (Fig. 3C) and it drastically reduced the increase in mRNA expression of CYP2H1, CYP3A37, and ALAS1 genes without changing their basal activity (Fig. 3D).
These experiments firmly establish that an activation of AMPK is necessary for the effect of PB and metyrapone on the transcriptional activation of CYPs and also influences the regulation of ALAS1. Knowing that PB and metyrapone activate AMPK and that this kinase is essential to mediate their drug effects on CYPs gene expression, we now focused on how these drugs can switch on AMPK activity.
The AMPK Upstream Kinase LKB1 Interacts with AMPKα upon PB and Metyrapone Treatment.
Several mechanisms of AMPK activation have been described, all involving the activation of AMPK by upstream kinases. Because it is known that LKB1 is the upstream kinase of AMPK in the liver (21), we tested its function on the effects of PB and metyrapone on CYPs and ALAS1 mRNA.
LKB1 and a LKB1 dominant negative mutant were transiently overexpressed in LMH cells. After treatment with PB or metyrapone, no statistically significant change in the CYP2H1, CYP3A37, and ALAS1 mRNA expression was detected with either the WT or the dominant negative mutant of LKB1 (SI Fig. 8). However, an LKB1 involvement cannot be excluded by this experiment, because it is known that this kinase forms an active heterotrimeric complex with two accessory proteins, Ste20-related adaptor protein (STRAD) and the mouse protein 25 (MO25) (22), which may thus be the limiting factors in LMH cells preventing the activation of transfected LKB1.
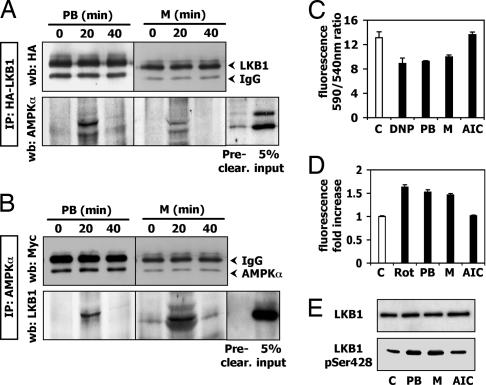
Therefore, a direct interaction between AMPK and LKB1 was considered. Coimmunoprecipitation experiments were performed to find out whether PB and metyrapone trigger an LKB1-AMPKα interaction. Western blots of LKB1-immunoprecipitated lysates revealed the appearance of a protein corresponding to AMPKα after 20 min of PB and metyrapone treatment, which disappeared at 40 min of exposure (Fig. 4A). Because AMPKα showed weak binding affinity to the protein G agarose beads in the preclearing step, we performed the vice versa experiment by immunoprecipitating AMPKα and assaying LKB1 by Western blot. As a result, after 20 min, a band appeared that corresponds to LKB1 (Fig. 4B). These experiments demonstrate that PB and metyrapone cause an interaction between LKB1 and AMPKα and establish LKB1 as a target of these drugs.
Fig. 4.
PB and metyrapone trigger the interaction of AMPKα with the upstream kinase LKB1 and affect mitochondrial membrane potential, ROS production, and LKB1 phosphorylation. (A) Immunoprecipitation of overexpressed HA-LKB1 by anti-HA antibody upon 500 μM PB or metyrapone. (B) Immunoprecipitation of overexpressed Myc-AMPKα upon 500 μM PB or metyrapone treatment. (C) JC-1 fluorescence was detected after 1 h treatment with 500 μM PB or metyrapone, 0.4 mM DNP, or 1 mM AICAR. Results are expressed as the 590 nm/540 nm fluorescence ratio in comparison with the control sample ratio. P < 0.05. (D) DCF fluorescence was detected after 1-h treatment with 500 μM PB or metyrapone, 5 μM rotenone, or 1 mM AICAR for 1 h. The result is shown as fold increase in comparison with the control sample. P < 0.01. (E) Phosphorylation of LKB1-Ser-428 upon PB and metyrapone is proven in a Western blot. M, metyrapone; Rot, rotenone; AIC, AICAR.
PB and Metyrapone Affect Mitochondrial Membrane Potential, ROS Production, and Phosphorylation of LKB1-Ser-428.
The involvement of LKB1 in the drug effects on CYPs led us to investigate by which mechanism PB and metyrapone prompt LKB1-AMPKα interaction and hence activate the latter by phosphorylation at Thr-172. It was reported that some stimuli like AICAR provoke changes in the AMP/ATP ratio leading to AMPK activation by LKB1 (23). Another recently described mechanism is the AMPK activation by metformin (24), which triggers mitochondrial ROS formation resulting in AMPK phosphorylation by LKB1. Troglitazone, an anti-diabetic drug, activates AMPK by a mechanism involving mitochondrial membrane depolarization (25).
In our experiments, we could not detect changes in the AMP/ATP ratio caused by treatment with PB and metyrapone (data not shown), suggesting an AMP/ATP ratio-independent mechanism in this system. We then tested for effects of PB and metyrapone on the mitochondrial membrane potential. DNP, an uncoupler that leads to a membrane potential drop, was used as positive control. Exposure of LMH cells to 500 μM PB or metyrapone, or 0.4 mM DNP caused changes in mitochondrial membrane potential (Fig. 4C), whereas AICAR as expected had no effect.
PB and metyrapone were then tested for their capacity to enhance intracellular ROS production. Both PB and metyrapone increased ROS production to a similar extent, as did rotenone (positive control) (Fig. 4D). As expected, AICAR did not affect ROS levels, because of its different AMPK activating mechanism. ROS were recently shown to activate AMPK by promoting the phosphorylation of LKB1 at Ser-428 (26). PB and metyrapone also caused an increase in LKB1-Ser-428 phosphorylation (Fig. 4E). These data clearly show that PB and metyrapone affect mitochondrial functions.
Interference with ROS Production Affects PB- and Metyrapone-Mediated CYP2H1, CYP3A37, and ALAS1 Gene Expression and AMPK Activation.
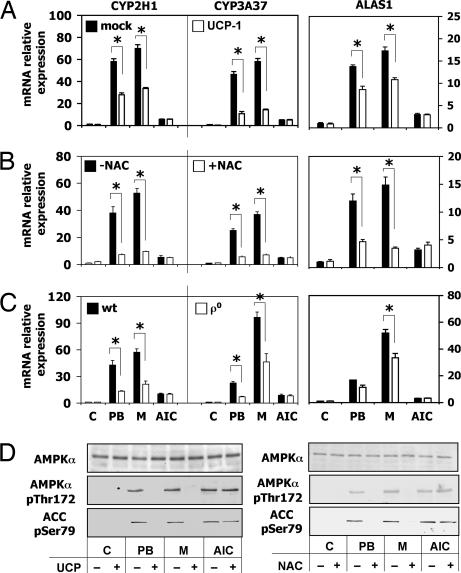
To assess whether PB- and metyrapone-stimulated ROS increase plays an important role in CYP induction, we decided to modulate the cellular ROS production by either overexpression of uncoupling protein 1 (UCP-1) (27) or ROS scavenging by N-acetyl l-cysteine (NAC) (28). Transfection of UCP-1 in LMH cells provoked a considerable decrease of the effect of PB and metyrapone on mRNA levels of both CYP2H1 and CYP3A37 (Fig. 5A), whereas the effect on ALAS1 was smaller. As expected, the AICAR effect on CYP induction was not altered. To establish whether ROS production is indeed critical for AMPK activation by PB and metyrapone, the phosphorylation of AMPK-Thr-172 and ACC-Ser-79 was assessed in LMH cells overexpressing UCP-1. The AMPKα and ACC phosphorylation normally triggered by PB and metyrapone was diminished by UCP-1, indicating that the increase of ROS produced by these drugs is linked to their ability to activate AMPK (Fig. 5D Left). In addition, ROS scavenging by NAC strongly diminished the PB and metyrapone induction of CYP2H1, CYP3A37, and ALAS1 (Fig. 5B) without altering the AICAR effect on these genes. Cotreatment of PB- or metyrapone-induced cells with NAC decreased AMPK-Thr-172 and ACC-Ser-79 phosphorylation (Fig. 5D Right). These experiments show that ROS generation is necessary for activation of AMPK by PB and metyrapone. Because the mitochondrial respiration chain is the major site of ROS production, we investigated the role of mitochondria in drug induction.
Fig. 5.
Decrease in intracellular ROS production by UCP-1 overexpression or by NAC-mediated scavenging attenuates drug-mediated increase of CYP2H1, CYP3A37 and ALAS1 gene expression and AMPK activity. (A) LMH cells transiently transfected with UCP-1 were incubated with 500 μM PB or metyrapone, or 1 mM AICAR for 16 h. Gene expression was detected by RT-PCR. ∗, P < 0.01. (B) LMH cells were incubated with 500 μM PB or metyrapone, or 1 mM AICAR, with or without 10 mM NAC for 16 h. mRNA levels were measured by RT-PCR. ∗, P < 0.01. (C) LMH WT or LMHρ0 cells were incubated with 500 μM PB or metyrapone for 16 h. mRNA levels were detected by RT-PCR. ∗, P < 0.01. (D) Activation of AMPK after 1 h treatment with 500 μM PB or metyrapone, or 1 mM AICAR is shown by Western blot evidencing the phosphorylation of AMPK-Thr-172 and ACC-Ser-79. M, metyrapone, AIC, AICAR.
PB and Metyrapone-Mediated Induction of CYP2H1, CYP3A37, and ALAS1 Is Mediated by Effects on Mitochondria.
To provide evidence that mitochondria act as mediators of PB and metyrapone in the cascade which leads to increased ROS production and subsequently to AMPK activation, mitochondrial DNA (mtDNA) was destroyed by ethidium bromide, which is an inhibitor of DNA/RNA synthesis (29), generating LMH cells with a decreased number of functional mitochondria (LMHρ°). After PB or metyrapone treatment, LMHρ° cells showed a drastically decreased induction of CYP2H1, CYP3A37, and ALAS1 gene expression (Fig. 5C) without a change of the basal level of the three genes. A complete inhibition of drug induction was not observed presumably because of still functional mitochondria present in the cells. As expected, the AICAR induction of CYP2H1, CYP3A37, and ALAS1 was not affected in the LMHρ° cells indicating that the AICAR effect is not mediated by mitochondria. These data imply an important role for mitochondria in the mechanism of drug induction and identify these organelles as targets of inducer drugs.
Discussion
In the present study, we demonstrate that PB induces drug-metabolizing enzymes such as CYPs by a cascade of events that involves an initial interaction with mitochondrial function and leads to phosphorylation of LKB1, the upstream kinase of AMPK. LKB1 then phosphorylates the AMPKα subunit at Thr-172 and activates AMPK. This activation of AMPK is a necessary step in the induction of these genes by PB, as recently shown in human hepatoma cells (12), primary mouse and human hepatocytes (13) and here in LMH cells. The direct interaction of the two kinases LKB1 and AMPK was evidenced by coimmunoprecipitation experiments and was associated with the drug-induced phosphorylation of LKB1 at Ser-428. The activation of LKB1 adds a proximal target to the so-far elusive sequence of events by which PB induces the transcription of multiple genes.
Our findings also raise numerous new questions. For instance, do all PB-type inducers work by affecting LKB1 and thereby AMPK? Is activation of AMPK a necessary step of all inducers of CYPs? Is activation of AMPK sufficient to explain the pleiotropic effect of PB on gene transcription? Which kinase activates LKB1 and how are these mechanisms related to mitochondrial functions?
The fact that metyrapone, another inducer drug, in all our experiments mimicked the dose-dependent response of PB, suggests an identical mechanism for this and possibly other inducers.
An interesting drug is metformin, used in the treatment of diabetes, which increases AMPK activity by a similar or identical mechanism. Recently, some studies proposed that this drug increases mitochondrial ROS production leading to AMPK activation (24). Clearly, if a compound like PB activates AMPK in a similar way than metformin, the question arises if PB can be used to treat diabetes. Indeed, PB has been used beneficially in patients who did not respond well to metformin treatment (30). However, even if these two drugs activate AMPK by ROS increase, they do not share all their effects. In our experiments in cell culture metformin activated expression of CYPs as did PB and metyrapone, supporting the role of AMPK activation in the induction process. However, metformin apparently is not an inducer in animal or human liver in vivo. This lack of induction is most probably due to its rapid renal excretion, which prevents sustained accumulation in the liver required for induction.
LKB1-AMPK Activation Is Necessary for Induction of CYP Genes.
The experiments reported here confirm and extend previous studies, which suggest that induction of CYPs by PB requires increased AMPK activity. This interpretation is derived from the following results: (i) AMPK activity is dose-dependently increased upon PB or metyrapone treatment; (ii) AMPKα overexpression enhances induction of CYPs by PB and metyrapone; (iii) down-regulation of AMPKα expression by siRNA drastically reduced induction. These experiments in avian cells establish that AMPK is necessary for the PB induction of CYPs and reveal evolutionary conservation of the mechanism of drug-mediated induction of CYPs.
PB Interacts with Mitochondria.
An important observation in explaining the effect of PB and metyrapone on LKB1 was that these drugs lead to increased production of ROS. This finding is supported by the observation that overexpression of UCP-1 inhibits drug induction of CYPs and that ROS scavenging by NAC decreases this effect on drug induction. In addition, we prepared LMH cells with a decreased number of functional mitochondria and the PB and metyrapone effect on expression of CYPs was strongly decreased, indicating the role of these organelles in the drug-elicited effect. The fact that some inducers can cause the formation of ROS by decoupling the electron flow in the CYP reaction cycle and that this phenomenon may relate in some way to the induction process has been proposed many years ago (for a review, see ref. 31). ROS are commonly thought to be toxic, but evidence is now accumulating that ROS might play a role as signaling molecules if tightly regulated. Our experiments strongly suggest a role for ROS in drug induction but the precise mechanism remains unknown and further studies are needed to unravel the direct downstream targets of these molecules. Preliminary experiments suggest that PB has the same effect also in primary cultures of human hepatocytes.
Cell lines are tumor-derived cells and for this reason they may be less dependent than primary cells on the respiratory chain to generate ATP. It is well known that cell lines usually have predominant anaerobic metabolism and low mitochondrial respiration. Our results show that mitochondrial functions are required by PB and metyrapone to exert their inducing effects on CYPs. Because in most cell lines the phenomenon of drug induction is not maintained, we speculate that reduced mitochondrial function may be responsible. If this is the case, LMH cells, which are highly inducible, should have higher aerobic metabolism in comparison to other cell lines. This hypothesis will require further investigation. A correlation between mitochondrial dysfunctions/oxidative stress and diabetes has been repeatedly reported and knowing that transcriptional regulation of CYPs is affected in diabetes suggests that these two effects may be somehow related. Moreover, the mechanism of PB and metyrapone-mediated induction of CYPs by means of effects of these drugs on mitochondria may also be considered in regard to the recent findings that mitochondrial dysfunction is involved in aging (32) and to the observation that in elderly there is a decline in drug metabolism capacity (33).
How is AMPK Activated?
AMPK is activated by several stimuli, which are sensed as stress for the cells/organism. Previous studies in a hepatoma cell line (12) and experiments done in our laboratory in primary cultures of human hepatocytes (13) detected AMP/ATP ratio changes in response to PB treatment. In LMH cells, we could not detect changes in AMP/ATP ratio in response to PB and metyrapone probably because of high AMP levels masking an effect on AMP/ATP ratio.
In this study, we demonstrated changes in mitochondrial membrane potential and ROS generation caused by PB and metyrapone. These effects as well as AMP/ATP ratio changes are not mutually exclusive, suggesting that, if inducer drugs target the mitochondria, several changes may occur at the same time. In fact, an inhibition of the mitochondrial respiration chain could explain all of these effects.
Our results propose mitochondria as a target for inducer drugs. Obviously, further studies are required to understand how exactly drugs affect these organelles.
AMPK Targets in the Mechanism of Drug Induction.
Our results implicate LKB1/AMPK in the drug induction mechanism and also raise new questions about the target/s of this cascade. Which proteins does AMPK phosphorylate and how does phosphorylation lead to drug-mediated increased expression of CYPs? Major efforts should be directed to answering this question. Dephosphorylation of CAR by the protein phosphatase 2A was recently shown to be necessary for nuclear translocation triggered by PB (34). Is this dephosphorylation related to the AMPK activation caused by drugs? If CAR has to be dephosphorylated to translocate, which kinase does phosphorylate CAR? Preliminary experiments suggest that CAR is not a phosphorylation target of AMPK (M. Matis and U.A.M., unpublished data).
AMPK activation by inducers could play a role in different ways, such as by affecting transcriptional coactivators or corepressors of nuclear receptors, as well as CAR translocation. Does AMPK phosphorylate cofactors interacting with CAR? In fact, AMPK was already shown to affect p300 (35) and peroxisome proliferators-activated receptor γ coactivator 1α (PGC-1α) (36), two transcriptional coactivators.
If we consider that AMPK is usually activated in stress situations, it is not unreasonable that this kinase is activated by compounds which up-regulate CYPs. Drugs are probably sensed by organisms as a stress, because switch to drug metabolism for detoxification purposes is an energy consuming process. For this reason, rapid initial AMPK activation by drugs could switch off unnecessary pathways allowing the organism to concentrate on the disposal of these compounds.
Transcriptional Regulation of ALAS1.
ALAS1 is the rate-limiting enzyme in heme synthesis and is up-regulated by drugs when the demand for reconstitution of CYPs increases. ALAS1 is transcriptionally regulated by the same nuclear receptors that drive drug-mediated induction of CYPs (3). Thus, it was reasonable to test whether AMPK is also involved in ALAS1 regulation. In our experiments, both by overexpression of AMPKα subunits or by siRNA down-regulation or by ROS scavenging, the effect on ALAS1 was present but not as strong as that on CYPs. These observations suggest that other mechanisms act on ALAS1 transcriptional regulation. Indeed, the PB effect on ALAS1 was detected also in CAR−/− mice, indicating a CAR-independent mechanism. Because ALAS1 has a central role in heme production, it is reasonable to assume that this enzyme is tightly regulated by several pathways. In support of these data, we recently observed that the transcription coactivator PGC-1α, which is a target of AMPK, is responsible for the nutritional regulation of ALAS1 (37).
In conclusion, we demonstrate that PB- and metyrapone-mediated transcriptional regulation of three chicken hepatic drug-inducible genes, CYP2H1, CYP3A37, and ALAS1, is achieved by a signaling cascade involving mitochondrial functions leading to ROS generation, LKB1 phosphorylation, and consequent interaction with AMPKα, which is in turn phosphorylated and activated. These findings add knowledge regarding the mechanism by which PB and metyrapone lead to transcriptional regulation of CYPs via AMPK. Our results reveal that inducers of CYPs affect the AMPK upstream kinase LKB1.
Our future studies are directed at understanding how ROS production leads to activation of AMPK, which is/are the downstream target/s of AMPK and which is the LKB1 upstream kinase in this pathway. In addition, future experiments will address whether AMPK activation affects directly or indirectly CAR activation or cytosolic-nuclear transfer. The availability of CAR−/− mice and mice with liver-specific deficiency of AMPKα1 or AMPKα2 subunits provides interesting models for these questions. Understanding the molecular mechanism of drug-mediated induction of CYPs is of importance for the molecular links between expression of CYPs and the metabolic state of the liver, for the problems caused by drug–drug interactions and adverse drug reactions, and for the crosstalk between disposal of endogenous and exogenous molecules.
Materials and Methods
Reagents.
Metyrapone, N-acetyl l-cysteine, metformin, and protein G agarose beads were obtained from Sigma (Buchs, Switzerland) and AICAR from Toronto Research (North York, ON, Canada). Phenobarbital, dinitrophenol, rotenone, and sodium azide were purchased from Merck (Dietikon, Switzerland) and Compound C from Calbiochem (Laufelfingen, Switzerland). Antibodies against AMPKα1 and AMPKα2 were purchased from Upstate Biotechnology (Lutern, Switzerland), and antibodies against ACC-pSer79, AMPK-pThr172, HA-tag, MAPK, AMPKα, LKB1, and LKB1-pSer428 were purchased from Cell Signaling Technology (Allschwil, Switzerland).
Culture and Transient Transfection of LMH Cells.
LMH cells were cultivated and treated as described (15). For transfection, cells were seeded for 3 days, and, when they reached ≈80% surface density, they were transfected for 48 h by using the Nucleofector Kit T (AMAXA Biosystems, Cologne, Germany) with Solution T according to the AMAXA protocol. Alternatively, cells were transfected for 48 h by Lipofectamine 2000 (Invitrogen, Nivelles, Belgium) as described in the manufacturer's protocol.
RNA Isolation and RT-PCR Analysis.
RNA from LMH cells was isolated by TRIzol Reagent. After reverse transcription, mRNA levels were quantified by real-time PCR. GAPDH mRNA levels were used for normalization of the results. The data are shown as mRNA relative expression to control sample. The primers used for real-time PCR measurements have been published (16).
AMPK Activity Measurement.
Cells treated for 1 h at 37°C with different compounds were harvested in 400 μl of lysis buffer containing 50 mM Tris, pH 7.5/50 mM NaF/1 mM EDTA/1 mM EGTA/1 mM sodium pyrophosphate/250 mM mannitol/1% Triton X-100/protease inhibitors (Roche Molecular Biochemicals, Rotkreuz, Switzerland)/5 μg/ml soybean trypsin inhibitors/0.2 mM sodium orthovanadate/1 mM DTT. After PEG precipitation, 15 μg of total proteins were used in a 40-μl reaction in the presence of 75 mM MgCl2/0.5 mM ATP/0.3 mM AMP/0.2 mM SAMS (Upstate Biotechnology)/0.4 mM DTT/1mCi/100 μl (1 Ci = 37 GBq) [γ-32P]ATP for 10 min at 30°C. At the end of the incubation, 35 μl of supernatant from the reaction mixture were spotted on Whatman filter papers, which were then washed three times with 0.75% phosphoric acid, washed once with acetone, and then allowed to dry before scintillation counting.
Western Blots.
LMH lysates were prepared as described above, but lysis was performed in 200 μl of buffer. The lysates were centrifuged at 20,800 × g and 4°C for 10 min, and the supernatant was transferred to a fresh tube, snap-frozen in liquid nitrogen, and stored at −80°C. Thirty micrograms of total proteins were separated on 10% SDS/PAGE and blotted onto a nitrocellulose membrane. Proteins were visualized according to the enhanced chemiluminescence protocol (Amersham Pharmacia Biotech, Zurich, Switzerland).
AMPKα1 and AMPKα2 Down-Regulation by Diced siRNA Duplexes.
RNAi analysis was performed by BLOCK-iT Dicer RNAi Kit (Invitrogen). Primers used for the sense and antisense DNA templates amplification are summarized in SI Table 1. The generation of diced siRNA duplexes and their transfection in LMH cells were done as described in the manufacturer's protocol.
Immunoprecipitation.
LMH cells transfected with HA-LKB1 or Myc-AMPKα1/2 by Lipofectamine 2000 for 48 h were treated with PB or metyrapone for 20 or 40 min. The cells were scraped in 150 mM NaCl/50 mM Tris, pH 8/1% Triton X-100/protease inhibitors (Complete Mini EDTA-free), left 15 min on ice, sonicated two times for 5 s, and centrifuged for 10 min at 4°C and 20,800 × g. A BCA assay was used to determine the protein content of the supernatant, which was then incubated for 30 min at 4°C under rotation with protein G agarose beads for preclearing. After removal of the beads, antibody was added to the lysates, which were incubated under rotation at 4°C for 2 h. Finally, protein G agarose beads were rotated with lysates under the same conditions. The beads were washed three times with lysis buffer and two times with PBS before being resuspended in protein loading buffer.
Mitochondrial Membrane Potential Measurement.
LMH cells cultivated in serum-free medium for 24 h were detached by trypsin and resuspended in 10 ml of serum-free Williams E medium. Approximately 4 × 105 cells were transferred to an Eppendorf tube and incubated at 37°C for 20 min with 1 μl of a 5 mg/ml JC-1 (Invitrogen) stock in DMSO, and either with 500 μM PB or metyrapone, 1 mM AICAR, or 0.2 mM DNP. After two washing steps with 1 ml of PBS and a 5-min centrifugation at 400 × g, the cells were resuspended in 300 μl of PBS, and the fluorescence was measured in triplicates with 100 μl of cell suspension. The ratio of the fluorescence at 590 nm and at 540 nm was calculated and depicted on a graph.
ROS Measurement.
LMH cells were detached with trypsin, washed with PBS, and incubated at 37°C with carboxydichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Molecular Probes, Nivelles, Belgium) for 30 min. After treatment with 500 μM PB, metyrapone, 1 mM AICAR, or 5 μM rotenone for 1 h, cells were washed once with PBS and the fluorescence was measured at 535 nm.
Preparation of LMHρ0 Cells.
LMH cells were grown for 12 weeks in medium containing 50 ng/ml ethidium bromide, 50 mg/ml uridine, and 1 mM pyruvate. The ρ0 status of the cells was confirmed by the decrease of mitochondrial marker genes (data not shown).
Immunofluorescence.
For details, see SI Materials and Methods.
Statistics.
Significant differences between means were determined by the two-tailed Student t test for paired samples. Error bars represent standard deviation of at least three experiments.
Supplementary Material
Acknowledgments
We thank Dr. Joohun Ha (University of Ulsan College of Medicine, Seoul, South Korea) for providing AMPKα1 and AMPKα2 expression plasmids, Dr. Daniel Ricquier (CNRS, Paris, France) for the UCP-1 expression plasmid, Dr. Tomi Mäkelä (Helsinki, Finland) for the LKB1 and LKB1 SL26 mutant plasmids, and Marianne Sutter (ETH, Zürich, Switzerland) for AMP/ATP ratio measurements. This work was supported by the Swiss National Science Foundation.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AICAR
5′-phosphoribosyl-5-aminoimidazol-4-carboxamide
- ALAS1
δ-aminolevulinic acid synthase 1
- AMPK
AMP-activated protein kinase
- CAR
constitutive androstane receptor
- CYPs
cytochromes P450
- LMH
leghorn male hepatoma
- PB
phenobarbital
- ROS
reactive oxygen species.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610216104/DC1.
References
- 1.Handschin C, Meyer UA. Pharmacol Rev. 2003;55:649–673. doi: 10.1124/pr.55.4.2. [DOI] [PubMed] [Google Scholar]
- 2.Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Podvinec M, Handschin C, Looser R, Meyer UA. Proc Natl Acad Sci USA. 2004;101:9127–9132. doi: 10.1073/pnas.0401845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakizaki S, Yamamoto Y, Ueda A, Moore R, Sueyoshi T, Negishi M. Biochim Biophys Acta. 2003;1619:239–242. doi: 10.1016/s0304-4165(02)00482-8. [DOI] [PubMed] [Google Scholar]
- 5.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 6.Swales K, Negishi M. Mol Endocrinol. 2004;18:1589–1598. doi: 10.1210/me.2003-0397. [DOI] [PubMed] [Google Scholar]
- 7.Sakuma T, Honma R, Maguchi S, Tamaki H, Nemoto N. Xenobiotica. 2001;31:223–237. doi: 10.1080/00498250110046451. [DOI] [PubMed] [Google Scholar]
- 8.Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. J Biol Chem. 2004;279:19832–19838. doi: 10.1074/jbc.M313601200. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida Y, Kimura N, Oda H, Kakinuma A. Biochem Biophys Res Commun. 1996;229:182–188. doi: 10.1006/bbrc.1996.1777. [DOI] [PubMed] [Google Scholar]
- 10.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 12.Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- 13.Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, da Silva Xavier G, Rutter GA, Viollet B, Meyer UA. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- 14.Handschin C, Podvinec M, Meyer UA. Proc Natl Acad Sci USA. 2000;97:10769–10774. doi: 10.1073/pnas.97.20.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handschin C, Podvinec M, Stockli J, Hoffmann K, Meyer UA. Mol Endocrinol. 2001;15:1571–1585. doi: 10.1210/mend.15.9.0701. [DOI] [PubMed] [Google Scholar]
- 16.Handschin C, Meyer UA. J Biol Chem. 2000;275:13362–13369. doi: 10.1074/jbc.275.18.13362. [DOI] [PubMed] [Google Scholar]
- 17.Stein SC, Woods A, Jones NA, Davison MD, Carling D. Biochem J. 2000;345:437–443. [PMC free article] [PubMed] [Google Scholar]
- 18.Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- 19.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 20.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 21.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boudeau J, Scott JW, Resta N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR. J Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 23.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou M-H, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, IV, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 25.Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, Klip A. Diabetologia. 2005;48:954–966. doi: 10.1007/s00125-005-1713-7. [DOI] [PubMed] [Google Scholar]
- 26.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 28.Oeda T, Henkel R, Ohmori H, Schill WB. Andrologia. 1997;29:125–131. doi: 10.1111/j.1439-0272.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 29.King MP, Attardi G. Science. 1989;246:500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 30.Lahtela JT, Arranto AJ, Sotaniemi EA. Diabetes. 1985;34:911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- 31.Waxman DJ, Azaroff L. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chomyn A, Attardi G. Biochem Biophys Res Commun. 2003;304:519–529. doi: 10.1016/s0006-291x(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 33.Woodhouse K, Wynne HA. Drugs Aging. 1992;2:243–255. doi: 10.2165/00002512-199202030-00007. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinpour F, Moore R, Negishi M, Sueyoshi T. Mol Pharmacol. 2006;69:1095–1102. doi: 10.1124/mol.105.019505. [DOI] [PubMed] [Google Scholar]
- 35.Leff T. Biochem Soc Trans. 2003;31:224–227. doi: 10.1042/bst0310224. [DOI] [PubMed] [Google Scholar]
- 36.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. Proc Natl Acad Sci USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.