Abstract
Erythrocytes infected with malaria parasites exhibit marked increases in permeability to organic and inorganic solutes. The plasmodial surface anion channel (PSAC), an unusual voltage-dependent ion channel induced on the host membrane after infection, may play a central role in these permeability changes. Here, we identified a functional PSAC mutant through in vitro selection with blasticidin S. Resistance to blasticidin S was generated during culture and correlated with significant reductions in permeability to multiple solutes, consistent with uptake via a common pathway. Single channel recordings revealed marked changes in PSAC gating with the addition of a subconductance state not present in wild-type channels. The channel's selectivity profile and pharmacology also were significantly altered. Eventual loss of the mutant phenotype upon removal of selective pressure and slower growth of mutant parasites suggest that PSAC serves an important role in intracellular parasite survival. These findings provide solid evidence for the uptake of diverse solutes via PSAC and implicate one or more parasite genes in expression of this channel.
Keywords: drug resistance, malaria, nutrient acquisition, fitness cost
The malaria parasite, Plasmodium falciparum, is a highly successful intracellular pathogen. In the human host, replication in erythrocytes leads to an exponentially increasing number of parasites and accounts for much of the clinical syndrome of malaria. To facilitate its rapid intracellular growth, the parasite remodels the erythrocyte cytoplasm (1), adds virulence factors to the surface of infected cells (2), and increases erythrocyte permeability to many small solutes. Increased uptake of solutes may be mediated by the plasmodial surface anion channel (PSAC) (3). This unusual ion channel has a single-channel conductance of 20 pS in molar Cl− solutions and fast-flickering voltage-dependent gating. PSAC also has an atypical selectivity profile for anions (SCN− > I− > Br− > Cl−) and effectively excludes Na+ despite permeability to bulky cations (4). Various antagonists have quantitatively identical effects on PSAC's open probability (5–7) and uptake of organic solutes including sugars (8), amino acids (9), purines (10), organic cations (11), and some vitamins (12), suggesting that PSAC functions as a shared route for the increased uptake of these diverse solutes after infection.
Nevertheless, PSAC's role in solute transport is debated because other ion channels have been observed by some workers (13–16) and because a single ion channel with broad permeability to diverse solutes but stringent exclusion of Na+ is unprecedented. It is also debated whether PSAC and other putative channels are parasite-encoded proteins or modified human proteins (5). Addressing these issues is an important hurdle for transport studies in malaria and for drug development programs that seek to target the parasite-induced permeability changes.
Blasticidin S is a fungal toxin (molecular weight 422.2) that kills most prokaryotic and eukaryotic cells by inhibiting mRNA-directed translation on ribosomes (17). This agent has been extensively used in molecular transfections of various cell types because it can select for the expression of detoxifying enzymes. Here, we identify and characterize a P. falciparum mutant that achieves blasticidin S resistance without the expression of heterologous resistance genes. This mutant was refractory to synchronization with sorbitol, suggesting defects in the parasite-induced permeability changes. Our studies reveal markedly altered functional properties of PSAC resulting from changes in the parasite genome. The altered properties of this mutant channel are essential for blasticidin S resistance.
This PSAC mutant directly addresses several important questions regarding how malaria parasites transform the erythrocyte's permeability. Our findings also have broad implications for molecular biology studies that use blasticidin S in selective transfections.
Results
Identification and Characterization of a Blasticidin S-Resistant Mutant.
Blasticidin S has been successfully used in P. falciparum genetic transfection experiments (18–20), including the production of a mutant with effective silencing of all members of the large var gene family (21). In these transfections, resistant parasites were selected through expression of the Aspergillus blasticidin S deaminase (BSD), which converts blasticidin S to a nontoxic deaminohydroxy derivative. We therefore were puzzled when an attempted genetic disruption in the FCB parasite isolate yielded blasticidin S-resistant parasites that lacked the bsd sequence from the transfection plasmid, as evaluated by PCR and Southern blotting (data not shown). To definitively establish resistance via parasite-specific mechanisms, we challenged several commonly available parasite isolates (FCB, HB3, W2, and 7G8) with blasticidin S under normal in vitro culture conditions. Microscopic examination showed no detectable parasites within 4 days of drug pressure for all of these lines; however, resistant parasites subsequently were recovered from FCB in each of four independent attempts, but never from the other three isolates. We then used limiting dilution to obtain a clonal population of blasticidin S-resistant parasites, hereafter referred to as FCB-br1.
FCB-br1 Exhibits Reduced Permeabilities to Diverse Organic Solutes.
During routine in vitro culture, we found that the mutant FCB-br1 parasites were refractory to synchronization in isotonic sorbitol solutions (22). Sorbitol synchronization is based on the increased permeability of infected erythrocytes to this sugar alcohol. In an isotonic sorbitol solution, mature trophozoite-infected erythrocytes import sorbitol via PSAC and/or other parasite-induced channels; continued uptake produces an osmotic imbalance and leads to cell lysis. Immature ring stage forms survive this treatment because they do not exhibit increased sorbitol permeability. Therefore, failure to synchronize FCB-br1 cultures suggests defects in the parasite-induced sorbitol permeability.
We evaluated and compared the induced permeabilities in FCB-br1 and its wild-type parent with a quantitative light-scattering assay for cell lysis (23). These experiments revealed statistically significant decreases in the mutant's permeability not only to sorbitol, but also to phenyltrimethylammonium chloride (PhTMA-Cl) and l-proline (Fig. 1A), structurally unrelated solutes that also exhibit increased permeability after infection. Importantly, the ratios of permeability coefficients for these solutes indicated that the decreases are not uniform in magnitude, with sorbitol exhibiting a 16-fold reduction, whereas the decrease in proline permeability was only 5.0-fold (Student's t test, P < 0.002; Fig. 1B). These findings implicate transport of these structurally dissimilar solutes via a shared ion channel; they also suggest that functional changes in this channel alter its selectivity for permeating solutes in the FCB-br1 parasite. Our observations are not compatible with simple down-regulation of an ion channel, which would produce quantitatively similar reductions for each solute.
Fig. 1.
The blasticidin S-resistant mutant, FCB-br1, exhibits reduced permeability to diverse solutes. (A) Osmotic lysis kinetics at 37°C in isotonic solutions of sorbitol, PhTMA-Cl, or proline, as indicated. In each graph, upper and lower traces represent lysis kinetics for FCB and FCB-br1, respectively. Lysis of FCB-br1 is slower in each solute, indicating reduced permeability across the host erythrocyte membrane. (B) Ratios of permeability coefficients (PFCB/PFCB-br1) for the solutes in A, shown as the mean ± SEM of four to five measurements. Permeability coefficients were calculated as the reciprocal of the time to 50% lysis, as formally justified for passive diffusion via PSAC (23). (C) Immunoblot showing reduced labeling of hemoglobin by sulfosuccinimidyl-6-(biotinamido)hexanoate in FCB-br1 parasites (lane 2) when compared with FCB parasites (lane 1), as detected with a monoclonal antibody against biotin. The blot was stripped and reprobed with an anti-hemoglobin antibody to confirm equal protein loading. Because the extent of hemoglobin labeling correlates with reagent permeation via PSAC (4), the blot reflects reduced reagent permeability in FCB-br1.
We also found that sulfosuccinimidyl-6-(biotinamido)hexanoate, a bulky water-soluble reagent with known PSAC permeability (4), had markedly reduced entry into FCB-br1 when compared with FCB. We quantified the uptake of this reagent by measuring the extent of hemoglobin labeling (Fig. 1C). Reduced uptake in the FCB-br1 isolate suggests that this reagent enters infected erythrocytes via the same ion channel as sorbitol, PhTMA-Cl, and proline.
Direct inhibition of channels by blasticidin S is unlikely because its addition to osmotic lysis solutions at concentrations up to 100 μg/ml does not reduce solute permeability for either FCB-br1 or FCB (data not shown). Washout of blasticidin S by culturing the FCB-br1 parasite for 2 weeks without this toxin revealed sustained reductions in sorbitol permeability, definitively excluding direct effects of blasticidin S or its metabolites. Our studies implicate changes in the parasite genome.
Associated Defects in PSAC.
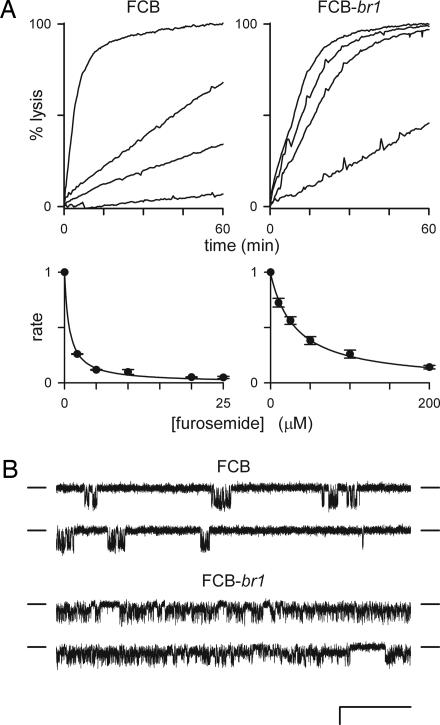
We used the whole-cell voltage-clamp method to examine changes in conductive Cl− currents induced by the intracellular parasite. These experiments revealed a dramatic reduction in the magnitude of whole-cell currents in the FCB-br1 isolate, but no significant change in their characteristic inward-rectifying voltage dependence (Fig. 2A). To explore the molecular basis of these defects, we obtained low-noise recordings in the cell-attached configuration (Fig. 2B). Under our experimental conditions, only one channel type was seen on erythrocytes infected with FCB; single channel conductance, gating, and voltage dependence matched those previously described for PSAC on other parasite isolates (3, 5). Identical experiments with FCB-br1 revealed PSAC activity with obvious changes in gating and conductance. Most conspicuously, single channel recordings exhibited frequent transitions to a level between the open and closed states, known as a subconductance state (24). This level was apparent in all points histograms from extended recordings (Fig. 2C), indicating that these mutated PSAC channels spend a significant fraction of time in this state. The correlation between defects in PSAC gating and reductions in both whole-cell currents and organic solute permeability strongly support a central role for PSAC in the transport of Cl− and the various solutes examined in Fig. 1.
Fig. 2.
Electrophysiological differences between FCB and FCB-br1. (A) Whole-cell voltage-clamp recordings from erythrocytes infected with FCB or FCB-br1 trophozoites with solution A in both bath and pipette compartments. Each group of traces represents superimposed current responses to 50-ms pulses from a holding potential of 0 mV to values between −100 and +100 mV in 10-mV increments. The FCB-br1 isolate induces markedly smaller Cl− currents. (B) Single channel recordings in solution A at imposed membrane potential (Vm) of −100 mV. Whereas PSAC activity on FCB is indistinguishable from that on other wild-type isolates, activity on FCB-br1 is visibly altered. Black dashes on the sides of each trace represent the closed channel level; the subconductance level seen on FCB-br1 is demarcated with a red line behind each of those traces. (Scale bars: A, 25 ms and 1.0 nA; B, 6.7 ms and 0.83 pA.) (C) All points histograms calculated from single channel recordings as in B for FCB and FCB-br1 isolates (black and red traces, respectively). Each histogram appears as a smooth curve because of narrow bin widths (0.02 pA) and calculation from ≈2 × 106 samples (20 s of single channel recording). The mean closed (c), open (o), and subconductance (s) levels are marked with one-letter abbreviations.
We further explored this link with furosemide, a well studied PSAC antagonist that interacts with an extracellular site distinct from the channel pore (5, 25). We found that furosemide was significantly less effective at inhibiting proline uptake by erythrocytes infected with FCB-br1 (Fig. 3A). Inhibitory dose responses revealed an ≈40-fold reduction in furosemide affinity when compared with the wild-type channel in FCB parasites. Single channel recordings confirmed that less effective inhibition of the mutant PSAC accounts for this change (Fig. 3B). Reduced permeability of organic solutes, altered selectivity and gating, and reduced affinity for furosemide suggest that the FCB-br1 parasite expresses a PSAC with a globally altered structure.
Fig. 3.
Markedly reduced sensitivity to furosemide of PSAC expressed on FCB-br1. (A) (Upper) Osmotic lysis kinetics in proline at 20°C in the presence of 0, 10, 25, and 200 μM furosemide (top to bottom traces in each graph, respectively). Proline uptake by FCB-br1 is less well inhibited by furosemide. (Lower) The corresponding dose responses for inhibition. Symbols represent the mean ± SEM lysis rates normalized to 1.0 without furosemide, calculated as described in ref. 23 from up to five measurements at each concentration. Solid lines represent a least-squares best fit to y = K0.5/(K0.5 + x) with K0.5 estimates of 0.8 and 32 μM for FCB and FCB-br1, respectively. (B) Single PSAC recordings in solution A with 10 μM furosemide in bath and pipette. Vm = −100 mV. (Scale bars: 100 ms and 2 pA.) Closed channel currents are indicated by dashes to the sides of each trace. Furosemide significantly reduces channel openings (downward deflections from the closed channel level) for PSAC on the FCB isolate but has a negligible effect on FCB-br1 channels when compared with their activities without inhibitor (Fig. 2).
Reversion of Blasticidin S Resistance in Culture Leads to Loss of Altered PSAC Activity.
To investigate whether blasticidin S resistance results from irreversible changes in the parasite genome, we propagated FCB-br1 in the absence of blasticidin S and found that the defective sorbitol lysis phenotype was lost after ≈4 weeks in culture (FCB-br1-rev in Fig. 4). Restored rapid lysis in sorbitol was associated with wild-type sensitivity to growth inhibition by blasticidin S. It also correlated with restored PSAC-mediated whole-cell conductances and with loss of the subconductance state in single channel recordings. We then rechallenged FCB-br1-rev with blasticidin S and grew out a new mutant, which we named FCB-br2. Although FCB-br2 may represent a mixed population of parasites because limiting dilution was not used to generate individual clones, functional studies indicate it also has defective sorbitol uptake, reduced whole-cell Cl− conductance, and defective PSAC gating (Fig. 4). Thus, blasticidin S resistance is tightly linked to the identified defects in PSAC activity. The relatively rapid reversion we observed may reflect changes in the expression of genes, one or more mutation hotspots, or transcriptional switches among paralogous genes.
Fig. 4.
Loss of resistance to blasticidin S is linked to the reappearance of wild-type PSAC activity. (A) Osmotic lysis kinetics in sorbitol for FCB (black trace), FCB-br1 (red), FCB-br1-rev (green), and FCB-br2 (blue). Wild-type sorbitol permeability is restored in the blasticidin S-sensitive revertant; subsequent selection of blasticidin S resistance yields reduced sorbitol permeability, similar to that seen with FCB-br1. (B) Correlation between in vitro growth inhibition by blasticidin S (red bar, normalized to 1 for complete microscopic clearance of parasites after 4 days in culture with blasticidin S), whole-cell chord conductance in solution A (blue bar, mean ± SEM of n = 10–29 cells for each isolate), and apparent sorbitol permeability coefficient (green bars, mean ± SEM of n = 3–6 trials for each isolate, calculated as the reciprocal of the lysis halftime). (C) Single PSAC recordings with the same conditions as in Fig. 2B. (Scale bars: 50 ms and 2 pA.) Whereas PSAC on FCB-br1-rev exhibits wild-type gating, FCB-br2 channel gating resembles that of FCB-br1.
Slower Growth of Parasites Carrying Functional PSAC Mutants.
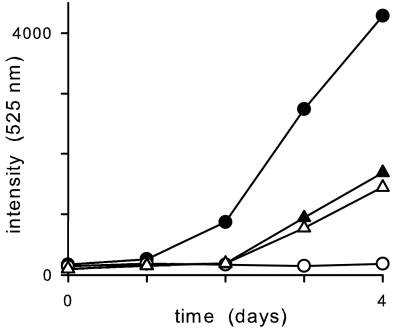
Nutrient acquisition by the intraerythrocytic parasite may critically depend on PSAC-mediated increases in organic solute permeability (3). If this hypothesis is correct, the altered PSAC activity expressed on FCB-br1 mutants may affect adversely parasite growth and survival. We therefore quantified in vitro growth rates for FCB and FCB-br1 with and without blasticidin S selective pressure. When compared in the absence of blasticidin S, we found that FCB-br1 parasites grow significantly slower than wild-type parasites (Fig. 5), suggesting that defects in PSAC adversely affect expansion of in vitro cultures. Because FCB-br1's growth rate was not affected detectably by the addition of blasticidin S, the selected genomic changes appear to confer complete resistance to blasticidin S under our conditions.
Fig. 5.
In vitro growth rates for FCB (circles) and FCB-br1 (triangles) with and without blasticidin S (open and filled symbols, respectively) quantified by using SYBR Green I-based fluorescent detection of parasite DNA in arbitrary units. Experiments evaluating growth rates by microscopic determination of parasitemias produced similar results (data not shown).
Discussion
P. falciparum mutants that carry heritable changes in host erythrocyte permeability have not been described previously. The mutant described here can be recognized easily because its cultures cannot be synchronized by incubation in isotonic sorbitol, as commonly used in malaria research laboratories to study parasite developmental stages. This method is based on sorbitol's high permeability through wild-type PSAC; it spares immature parasite stages and achieves synchronization of cultures because PSAC activity is not induced until some 18 h after erythrocyte invasion. FCB-br1 parasites resist sorbitol synchronization because they express PSAC activity with markedly lower sorbitol permeability. These mutant channels also exhibit altered selectivity for various solutes, defective gating, and reduced affinity for the inhibitor furosemide. The identification of a PSAC mutant has several important implications for intraerythrocytic malaria parasite biology.
Evidence for a Single Ion Channel Shared by Diverse Solutes.
Other reports from electrophysiological studies have suggested that there may be multiple distinct ion channels on erythrocytes with or without infection by malaria parasites (13–16). A persistent question therefore has been whether different ion channels mediate the uptake of each solute whose permeability increases after infection. Although inhibition by multiple inhibitors produces quantitatively parallel effects on PSAC single channel recordings and the uptake of each organic solute (5, 6, 26), some workers remain skeptical of this finding because available inhibitors may be nonspecific and because electrophysiological methods cannot directly study the transport of uncharged solutes. The identified mutant represents independent evidence for a single ion channel shared by the various solutes we tested (Fig. 1). Because this mutant also exhibits corresponding reductions in PSAC single channel and whole-cell currents, the permeability of organic solutes now can be confidently linked to PSAC.
Parasite Genes Encode PSAC.
Our findings require the involvement of parasite genes in the expression of PSAC activity. Human erythrocytes lack mutable genetic material; they therefore cannot accrue changes to account for functional mutants. PSAC gating polymorphisms identified through surveys of geographically divergent parasite isolates also insinuate parasite genetic elements (5). PSAC's unusual selectivity profile (4) and various other functional properties (3, 25–27) also distinguish it from known human anion channels and suggest one or more parasite genes.
PSAC Serves an Important Function for the Intracellular Parasite.
Stringent conservation of PSAC's biophysical properties on phylogenetically divergent malaria parasites suggests that the channel serves an important biological role for the intraerythrocytic parasite (28). One proposal is that it provides the parasite with access to serum nutrients by increasing their permeability across the host erythrocyte membrane (3); removal of metabolic waste products also may be facilitated by PSAC. Other possible roles include volume regulation of infected erythrocytes and changing the ionic composition of erythrocyte cytosol to support other parasite activities. Although each of these proposed functions is plausible, experimental limitations have thwarted attempts to determine PSAC's exact biological role and have raised questions regarding whether it serves any function for the intracellular parasite. The identified mutant supports an important biological role because the observed defects in PSAC compromise in vitro parasite survival. Both the slower growth rate of FCB-br1 and its reversion to the wild-type phenotype upon removal of blasticidin S are consistent with a fitness cost associated with blasticidin S resistance (29).
How Does Blasticidin S Select for a PSAC Mutant?
Blasticidin S has been used as a selective agent in P. falciparum transfections with plasmids carrying the bsd gene. Our demonstration that resistance to blasticidin S can be selected without plasmids reveals a potential limitation to its use in transfections. However, we note that resistance could be generated only from the FCB parasite. This isolate presumably carries a permissive genetic background for the selection of required changes in PSAC. It is possible that this unique background results from polymorphisms in more than one parasite gene; in this scenario, acquisition of blasticidin S resistance may require multiple changes at the level of the parasite genome.
We propose that the altered PSAC activity in FCB-br1 contributes to blasticidin S resistance by reducing host membrane permeability to this toxin. This proposal is consistent with the structural similarity of blasticidin S to nucleosides with known permeability through PSAC (10). It is also consistent with the observation that blasticidin S has limited membrane permeability in some cell lines; in these cells, toxicity resulting from inhibition of translation occurs only when blasticidin S permeability is increased (30, 31). Finally, blasticidin S-resistant yeast, identified after UV treatment to increase mutation rates, appear to use reduced permeability as the primary mechanism of resistance (32).
We envision that the selected changes in PSAC serve to alter its selectivity profile and prohibit blasticidin S access to its intracellular target. Importantly, changes in the channel's selectivity profile permit maintenance of essential transport functions such as adequate levels of nutrient uptake. More drastic changes such as production of nonfunctional channels presumably would be lethal. Although PSAC is an acknowledged antimalarial target (7), these findings suggest it also may be an important route for delivery of other hydrophilic antimalarials to their intracellular targets. Such approaches will need to consider resistance via selection of parasites with reduced permeability.
Materials and Methods
In Vitro Selection of Mutant Parasites.
Resistant P. falciparum parasites were selected by culturing under standard conditions in the presence of 2.5 μg/ml blasticidin S·HCl (Invitrogen, Carlsbad, CA). At this concentration, microscopic examination revealed essentially complete sterilization of cultures within 4 days after addition of blasticidin S. In experiments with the FCB isolate, rare parasites were detected in smears as soon as 4 weeks later. Transient removal of blasticidin S pressure appeared to speed the selection of fully resistant parasites. FCB-br1 was cloned from cultures of blasticidin S-resistant parasites by the limiting dilution technique (33).
Osmotic Lysis Kinetics.
Osmotic lysis experiments were performed as described in ref. 23. Briefly, trophozoite-infected RBCs were enriched to 95–99% by percoll/sorbitol separation (34) with modifications that improve recovery of infected erythrocytes with reduced sorbitol permeability (35). Enriched cells were washed in PBS (150 mM NaCl/20 mM NaH2PO4, pH 7.5), and resuspended at 0.5% hematocrit in buffered osmotic lysis solution containing 280 mM sorbitol, 280 mM proline, or 145 mM PhTMA-Cl supplemented with 20 mM Na-Hepes/0.1 mg/ml BSA, pH 7.4. Osmotic swelling and lysis in these solutions then were continuously followed by recording transmittance of 700-nm light through the cell suspension (DU640 spectrophotometer with Peltier temperature control, Beckman Coulter, Fullerton, CA). This method produces estimates of permeation rates through PSAC that quantitatively match those obtained by using either radioisotope flux or patch-clamp recordings (23).
Sulfo-NHS Ester Labeling and Immunoblot.
Intact enriched trophozoite-stage RBCs were labeled with 0.4 mM sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (Pierce Endogen, Rockford, IL) at a hematocrit of 0.5% in PBS for 15 min at room temperature. After terminating the labeling with 100 mM Tris (pH 7.4), the cells were washed, hypotonically lysed in 20 mM Na2HPO4/1 mM EDTA/1 mM EGTA, pH 8.6, and ultracentrifuged (100,000 × g, 1 h) to separate soluble intracellular proteins from membrane-associated proteins. Soluble fractions corresponding to 7.5 × 105 cells were separated in a reducing SDS 4–12% polyacrylamide gel, transferred to nitrocellulose membrane, blocked and probed with horseradish peroxidase-conjugated anti-biotin IgG (Jackson ImmunoResearch, West Grove, PA), and visualized with SuperSignal ECL Substrate (Pierce Endogen). The blot then was stripped and reprobed with horseradish peroxidase-conjugated anti-hemoglobin IgG (Bethyl Laboratories, Montgomery, TX).
Electrophysiology.
Cell-attached recordings on trophozoite stage-infected RBCs were obtained as described in ref. 5 in symmetric bath and pipette solutions of 1,000 mM choline-Cl/115 mM NaCl/10 mM MgCl2/5 mM CaCl2/20 mM Na-Hepes, pH 7.4 (solution A). This hypertonic solution increases the signal-to-noise ratio for single PSAC detection by permitting higher rates of Cl− flux through open channels and by reducing pipette RC noise. When used in single channel recordings, furosemide was present at identical concentrations in both pipette and bath compartments.
The whole-cell patch-clamp configuration was obtained with brief electrical pulses to disrupt the membrane patch. Whole-cell chord conductances (γ) were calculated between −100 mV and 0 mV, where PSAC-mediated currents are largest and have approximately linear current-voltage profiles in both the wild-type and mutant channels.
Recordings in both configurations used quartz pipettes pulled to tip diameters <0.5 μm and resistances of 1–3 MΩ in solution A. All recordings were low-pass filtered at 5 kHz (8-pole Bessel) and digitized at 100 kHz. All-points histograms and all other analyses were carried out with home-written code.
Parasite Growth Rates.
Growth rates for blasticidin S-sensitive and -resistant parasites were determined by using matched cultures seeded at 0.5% hematocrit and a parasitemia of 0.2% with and without 2.5 μg/ml blasticidin S. On each subsequent day, identical aliquots were harvested, washed, and subjected to freeze-thaw before dilution to 0.5% hematocrit with SYBR Green I nucleic acid stain (used at twice the recommended concentration; Invitrogen). Parasite DNA then was quantified as a marker of growth by measuring fluorescence (485 nm excitation, 525 nm emission). Standard curves using serial dilutions of parasite cultures revealed a linear relationship between fluorescence and parasitemia over the range used in our study (data not shown).
Acknowledgments
We thank Michelle Doll for help with cell culture and Thomas E. Wellems for comments on this work. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and by the Medicines for Malaria Venture.
Abbreviations
- PhTMA
phenyltrimethylammonium
- PSAC
plasmodial surface anion channel.
Footnotes
The authors declare no conflict of interest.
References
- 1.Trager W, Rudzinska MA, Bradbury PC. Bull W H O. 1966;35:883–885. [PMC free article] [PubMed] [Google Scholar]
- 2.Leech JH, Barnwell JW, Miller LH, Howard RJ. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai SA, Bezrukov SM, Zimmerberg J. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA. Mol Biochem Parasitol. 2003;132:27–34. doi: 10.1016/j.molbiopara.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA. Blood. 2004;104:4279–4286. doi: 10.1182/blood-2004-05-2047. [DOI] [PubMed] [Google Scholar]
- 6.Desai SA, Alkhalil A, Kang M, Ashfaq U, Nguyen ML. J Biol Chem. 2005;280:16861–16867. doi: 10.1074/jbc.M414629200. [DOI] [PubMed] [Google Scholar]
- 7.Kang M, Lisk G, Hollingworth S, Baylor SM, Desai SA. Mol Pharmacol. 2005;68:34–40. doi: 10.1124/mol.104.010553. [DOI] [PubMed] [Google Scholar]
- 8.Homewood CA, Neame KD. Nature. 1974;252:718–719. doi: 10.1038/252718a0. [DOI] [PubMed] [Google Scholar]
- 9.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Mol Biochem Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 10.Upston JM, Gero AM. Biochim Biophys Acta. 1995;1236:249–258. doi: 10.1016/0005-2736(95)00055-8. [DOI] [PubMed] [Google Scholar]
- 11.Staines HM, Rae C, Kirk K. Biochim Biophys Acta. 2000;1463:88–98. doi: 10.1016/s0005-2736(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 12.Saliba KJ, Horner HA, Kirk K. J Biol Chem. 1998;273:10190–10195. doi: 10.1074/jbc.273.17.10190. [DOI] [PubMed] [Google Scholar]
- 13.Staines HM, Powell T, Ellory JC, Egee S, Lapaix F, Decherf G, Thomas SL, Duranton C, Lang F, Huber SM. J Physiol. 2003;552:177–183. doi: 10.1113/jphysiol.2003.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duranton C, Huber SM, Tanneur V, Brand VB, Akkaya C, Shumilina EV, Sandu CD, Lang F. J Gen Physiol. 2004;123:417–426. doi: 10.1085/jgp.200308919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verloo P, Kocken CH, van der WA, Tilly BC, Hogema BM, Sinaasappel M, Thomas AW, De Jonge HR. J Biol Chem. 2004;279:10316–10322. doi: 10.1074/jbc.M311540200. [DOI] [PubMed] [Google Scholar]
- 16.Bouyer G, Egee S, Thomas SL. Blood Cells Mol Dis. 2006;36:248–254. doi: 10.1016/j.bcmd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi H, Yamamoto C, Tanaka N. J Biochem (Tokyo) 1965;57:667–677. [PubMed] [Google Scholar]
- 18.Mamoun CB, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. Proc Natl Acad Sci USA. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Wang Q, Sims PF, Hyde JE. Mol Biochem Parasitol. 2002;123:1–10. doi: 10.1016/s0166-6851(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 20.Sidhu AB, Verdier-Pinard D, Fidock DA. Science. 2002;298:210–213. doi: 10.1126/science.1074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzikowski R, Frank M, Deitsch K. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambros C, Vanderberg JP. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 23.Wagner MA, Andemariam B, Desai SA. Biophys J. 2003;84:116–123. doi: 10.1016/S0006-3495(03)74836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamill OP, Sakmann B. Nature. 1981;294:462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- 25.Desai SA. Nanomed Nanotech Biol Med. 2005;1:58–66. [Google Scholar]
- 26.Lisk G, Kang M, Cohn JV, Desai SA. Eukaryot Cell. 2006;5:1882–1893. doi: 10.1128/EC.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumeister S, Winterberg M, Duranton C, Huber SM, Lang F, Kirk K, Lingelbach K. Mol Microbiol. 2006;60:493–504. doi: 10.1111/j.1365-2958.2006.05112.x. [DOI] [PubMed] [Google Scholar]
- 28.Lisk G, Desai SA. Eukaryot Cell. 2005;4:2153–2159. doi: 10.1128/EC.4.12.2153-2159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson DI, Levin BR. Curr Opin Microbiol. 1999;2:489–493. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 30.Contreras A, Carrasco L. J Virol. 1979;29:114–122. doi: 10.1128/jvi.29.1.114-122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiguro J, Miyazaki M. Curr Genet. 1985;9:179–181. doi: 10.1007/BF00436968. [DOI] [PubMed] [Google Scholar]
- 33.Rosario V. Science. 1981;212:1037–1038. doi: 10.1126/science.7015505. [DOI] [PubMed] [Google Scholar]
- 34.Aley SB, Sherwood JA, Howard RJ. J Exp Med. 1984;160:1585–1590. doi: 10.1084/jem.160.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alkhalil A, Hill DA, Desai SA. Cell Microbiol. 2006 Nov 3; doi: 10.1111/j.1462-5822.2006.00834.x. doi: [DOI] [PubMed] [Google Scholar]