The incretins are gastrointestinal hormones that promote the release of insulin. They thus represent promising therapeutic tools to tackle the current type 2 diabetes epidemic. Exploitation of the incretin glucagon-like peptide 1 (GLP-1) has resulted in effective pharmacological agents for the treatment of diabetes. Current GLP-1-based therapies are limited by the nausea they engender at relatively high doses and by the need to administer them parenterally. In this issue of PNAS, Chen et al. (1) and Knudsen et al. (2) describe nonpeptidic molecules that act as specific GLP-1 receptor agonists. These molecules therefore may provide valuable clues to the development of orally active GLP-1 receptor agonists for therapeutic use.
GLP-1 has a number of propitious effects on glucose control. It directly stimulates insulin release from the pancreatic β cell and suppresses the release of glucagon from the α cell. Gastric emptying is slowed by GLP-1 administration, thus slowing digestion and absorption and moderating blood glucose excursions. Acute central or peripheral GLP-1 administration suppresses appetite in animals and humans and chronically reduces body weight. Perhaps most excitingly, GLP-1 has been reported to increase β cell mass in rodents, reduce cell apoptosis and increase the glucose responsiveness of rodent and human islets in vitro, and stimulate the differentiation of rodent and human islet precursor cells into β cells (3–5).
Unfortunately, its short half-life in conjunction with the required i.v. or s.c. administration route makes it impractical to use GLP-1 itself as a therapy. In addition, high doses of GLP-1 are associated with nausea, making it impossible to administer larger doses to compensate for the short half-life. Molecules that can activate the same pathways as GLP-1 but have improved pharmacological characteristics are therefore of great interest.
Exendin-4 or exenatide is a peptide analogue of GLP-1 isolated from the venom of the Gila monster lizard Heloderma suspectum. Exenatide, marketed under the name Byetta, is licensed in the United States and Europe as a treatment for type 2 diabetes. It has a longer biological half-life than GLP-1, and twice-daily s.c. injections of exenatide have clinically significant effects. Thirty-week clinical trials in type 2 diabetics have shown that exenatide reduces hemoglobin A1c (HbA1c) and fasting plasma glucose levels, causes weight loss, and is generally well tolerated. As might be predicted, mild or moderate nausea was the most frequent adverse event, but it appeared to lessen with time and did not seem to be responsible for the weight loss (6–8). Liraglutide, a GLP-1 agonist with a longer half-life than exenatide, and an exenatide long-acting release preparation are being developed to reduce the required frequency of administration. However, the need for injection still limits the clinical utility of these drugs.
Glucagon-like peptide 1 has been reported to increase the glucose responsiveness of islets in vitro.
The exact mechanisms by which GLP-1 is broken down in vivo have not been comprehensively identified, but it appears that dipeptidyl peptidase-4 (DPP-4) plays a significant role. DPP-4 inhibitors therefore are thought to potentiate endogenous GLP-1 signaling and, accordingly, have been shown to increase insulin release and promote β cell growth and survival. A number of small-molecule orally active DPP-4 inhibitors have been developed, including vildagliptin. Clinically, vildagliptin appears to have similar effects on HbA1c to exenatide. However, it has not been found to effect gastric emptying or promote weight loss. This might be because treatment with DPP-4 inhibitors only modestly increases postprandial GLP-1 levels. In addition, because DPP-4 is believed to be involved in the breakdown of a number of other biologically active factors, blocking its actions may have less specific effects than exenatide (5).
Research thus has turned to orally active GLP-1 mimetics. However, protein and peptide hormones and neurotransmitters, by their very nature, can be difficult to mimic with the small molecules most favorable to oral activity. Unlike classical neurotransmitters that are small molecules themselves, peptides often have large receptor interaction sites, and the residues important to receptor binding and activation can be dispersed across their secondary structure. The GLP-1 receptor belongs to the G protein-coupled receptor (GPCR) family B. Although there are a number of examples of GPCRs being activated by small-molecule mimetics (including the opioid receptors, which are activated by morphine and a range of related compounds), these receptors all belong to GPCR family A. No small-molecule agonists have been discovered for any member of family B, although small-molecule antagonists have been described for the corticotrophin-releasing hormone receptor 1 (9), the calcitonin gene-related peptide receptor (10), and the glucagon receptor (11). The secondary structure of GLP-1 has not been well characterized, but it is a typical family B ligand, and studies suggest that several pairs of spatially separate residues are involved in its binding to the GLP-1 receptor. The GLP-1 receptor therefore might be expected to require a relatively large ligand that, in turn, would make it less likely to be orally active. Thus, the development of an orally active GLP-1 agonist is a challenging goal.
Indeed, Knudsen et al. (2) found that none of the 500,000 small molecules they tested were specific agonists as assessed by competitive binding to the GLP-1 receptor. However, using a functional assay, they managed to discover that substituted quinoxalines specifically activated the GLP-1 receptor, although they did not displace GLP-1 binding from these receptors. By synthesizing and testing further compounds, they discovered more potent GLP-1 agonists. These agonists often had bell-shaped dose–response curves, although Knudsen et al. (2) report identifying similar compounds that do not inhibit intracellular cyclic adenosine monophosphate production at high concentrations. The chemical compound analyzed in more detail, referred to as “compound 2,” not only agonizes the GLP-1 receptor, but also increases its binding affinity for GLP-1. The mechanism is unknown, although it appears that binding is allosteric, and Knudsen et al. (2) suggest that it may stimulate receptor dimerization. Compound 2 significantly increases glucose-stimulated insulin release from wild-type mouse pancreatic islets and from perfused rat pancreas, although not from GLP-1 receptor knockout mouse islets. It is not particularly potent, and its oral bioavailability is not reported (2). However, these findings suggest that this class of compound may be a useful starting point for the design of further drugs based on the GLP-1 signaling system. They also suggest the importance of searching for allosteric modulators in addition to classic agonists when screening small-molecule libraries.
In contrast, Chen et al. (1) report their discovery of orthosteric GLP-1 agonists that are orally active in rodents. They initially screened nearly 50,000 compounds, and subsequent investigation revealed that larger molecules, substituted cyclobutanes, could act as GLP-1 agonists. The compounds S4P and Boc5 then were synthesized for further study). Boc5 appears to be a full orthosteric GLP-1 receptor agonist, the effects of which can be blocked by exendin(9–39) and which amplifies glucose-stimulated insulin secretion from isolated rat pancreatic islets.
Boc5 also appears to agonize the GLP-1 receptor in vivo. Acute i.p. administration of Boc5 dose-dependently reduced food intake in mice. This effect appeared to be specific, because it was abolished by pretreatment with exendin(9–39). Chronic administration of Boc5 in db/db mice normalized HbA1c, blood glucose levels, and reduced body weight gain. Boc5-treated mice also had greater glucose tolerance and lower insulin levels, suggesting improved insulin sensitivity. Excitingly, Boc5 appears to be orally active, acutely and specifically reducing food intake after oral administration. However, the chronic effects of oral Boc5 administration were not investigated (1).
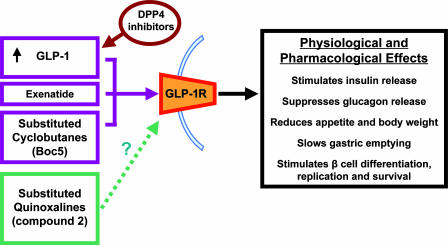
Both of these papers (1, 2) demonstrate that it is possible to synthesize nonpeptidic molecule GLP-1 receptor agonists (Fig. 1). Further work now is required to build on these findings. It may be possible to design more potent substituted quinoxalines to be tested in vivo. Although the db/db mouse is a convenient model of diabetes, it does not represent the normal physiology or pathophysiological of human type 2 diabetes, and it therefore will be important to investigate the effects of these molecules in other models. It also will be interesting to see whether these allosteric and orthosteric agonists possess the other activities of GLP-1, for example, whether the substituted quinoxalines inhibit food intake and whether they and Boc5 influence gastric emptying. The antidiabetic effects of Boc5 appear to extend beyond the treatment period, which the authors suggest may reflect an effect on β cell neogenesis and differentiation. If orally available GLP-1 receptor agonists can reproduce in a clinical setting the effects of GLP-1 on β cell differentiation, replication, and survival observed in animal models and in vitro, they may prove to be the magic bullet the current diabetes epidemic requires.
Fig. 1.
GLP-1 signaling as a target for antidiabetic compounds. Exenatide and Boc5 are orthosteric agonists, and compound 2 is an allosteric agonist, of the GLP-1 receptor (GLP-1R), although the exact mechanism by which it works is unknown. Dipeptidyl peptidase-4 (DPP4) inhibitors potentiate the effects of GLP-1.
Footnotes
References
- 1.Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, Zhang R, Zhang S, Lin L, Chen K, et al. Proc Natl Acad Sci USA. 2007;104:943–948. doi: 10.1073/pnas.0610173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, Holst JJ, Jeppesen CB, Johnson MD, de Jong JC, et al. Proc Natl Acad Sci USA. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen PJ, Holst JJ. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Weir GC. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 5.Drucker DJ, Nauck MA. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 7.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Dagnino R, Jr, De Souza EB, Grigoriadis DE, Huang CQ, Kim KI, Liu Z, Moran T, Webb TR, Whitten JP, et al. J Med Chem. 1996;39:4358–4360. doi: 10.1021/jm960149e. [DOI] [PubMed] [Google Scholar]
- 10.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling A, Hong Y, Gonzalez J, Gregor V, Polinsky A, Kuki A, Shi S, Teston K, Murphy D, Porter J, et al. J Med Chem. 2001;44:3141–3149. doi: 10.1021/jm000547o. [DOI] [PubMed] [Google Scholar]