Abstract
1,N6-ethanoadenine (EA) forms through the reaction of adenine in DNA with the antitumor agent 1,3-bis(2-chloroethyl)-1-nitrosourea, a chemotherapeutic used to combat various brain, head, and neck tumors. Previous studies of the toxic and mutagenic properties of the DNA adduct EA have been limited to in vitro experiments using mammalian polymerases and have revealed the lesion to be both miscoding and genotoxic. This work explores lesion bypass and mutagenicity of EA replicated in vivo and demonstrates that EA is neither toxic nor mutagenic in wild-type Escherichia coli. Although the base excision repair glycosylase enzymes of both humans and E. coli possess a weak ability to act on the lesion in vitro, an in vivo repair pathway has not yet been demonstrated. Here we show that an enzyme mechanistically unrelated to DNA glycosylases, the adaptive response protein AlkB, is capable of acting on EA via its canonical mechanism of oxidative dealkylation. The reaction alleviates the unrepaired adduct's potent toxicity through metabolism at the C8 position (attached to N1 of adenine), producing a nontoxic and weakly mutagenic N6 adduct. AlkB is shown here to be a geno-protective agent that reduces the toxicity of DNA damage by converting the primary adduct to a less toxic secondary product.
Keywords: 1,3-bis(2-chloroethyl)-1-nitrosourea; DNA repair
In the DNA base adduct 1,N6-ethanoadenine (EA), the exocyclic nitrogen of adenine is connected to the N1 ring nitrogen by a saturated two-carbon bridge, creating a five-membered ring (Fig. 1A). This adduct is formed by the reaction of DNA with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) (1–3) and is possibly responsible for the mutagenic property of this anticancer agent. EA is repaired to some extent by the human alkyladenine DNA glycosylase MPG (also called AAG, APNG, or ANPG) (4), although its excision is 65-fold less efficient than that of the structurally related 1,N6-ethenoadenine (εA) (Fig. 1A). Similarly, the Escherichia coli repair protein 3-methyladenine DNA glycosylase (AlkA) recognizes and excises the EA adduct, but with 20-fold lower activity than toward εA (5). Because EA lacks the structural features needed to form instructional hydrogen bonds with thymine and diverges from adenine in both size and shape, it is likely to be both a toxic and a mutagenic lesion.
Fig. 1.
Experimental outline. (A) Structures of adenine (A), EA, and εA. (B) Overview of experimental procedure for mutation detection.
In the absence of repair, EA blocks polymerase bypass and miscodes during attempted replication by mammalian DNA polymerases in vitro (6). The analogous εA adduct, which is missing the identical hydrogen-bonding capabilities, is both toxic and mutagenic in E. coli when unrepaired (7). Because the EA adduct is likely to be mutagenic and genotoxic when unrepaired and is excised only inefficiently by base excision repair glycosylases, another method of repair beyond the action of AlkA would greatly aid the cell in preserving genomic stability and viability. Given that the structurally similar εA lesion is repaired by AlkB, a protein up-regulated in response to alkylating agents, it was hypothesized that EA may also be a substrate.
The E. coli AlkB protein is the product of one of four genes up-regulated in response to methylating agents in a biochemical network termed the adaptive response (8). Ada is a bifunctional methyltransferase that serves not only to reverse DNA damage directly by irreversibly accepting methyl groups from O6-methylguanine and one isomer of backbone methylphosphotriesters, but also as a transcriptional regulator, inducing expression at three promoters after methylation of Cys-38. AlkA, as mentioned above, is a 3-methyladenine DNA glycosylase responsible for excision of damaged bases as the first step in the base excision repair pathway. AidB is homologous to human isovaleryl CoA dehydrogenase, a central player in the leucine metabolism pathway, but its functional role in the adaptive response has not yet been elucidated beyond its ability to bind double-stranded DNA (9).
AlkB is an α-ketoglutarate dioxygenase that uses non-heme iron(II) as a cofactor and molecular oxygen and α-ketoglutarate as cosubstrates to reverse directly, through oxidative demethylation, 1-methyladenine (m1A) and 3-methylcytosine, lesions formed predominantly in single-stranded DNA under conditions of alkylative stress (10, 11). Hydroxylation of the DNA-bound methyl group is coupled with decarboxylation of α-ketoglutarate, releasing succinate and carbon dioxide while producing an unstable hydroxymethyl intermediate that decomposes, releasing formaldehyde and the restored adenine or cytosine. Although homologs of AlkB have been found in many other bacterial species, as well as in Saccharomyces pombe, Caenorhabditis elegans, Drosophila, mice, humans, and plant RNA viruses (12–14), proteins similar in sequence are apparently lacking in many other species, both bacterial and eukaryotic (13). Interestingly, however, expression of several Saccharomyces cerevisiae genes that possess no amino acid homology to AlkB in AlkB-deficient E. coli has been shown to complement the methyl methanesulfonate-sensitive phenotype imparted by the mutation (15). The mouse AlkB homologs, mABH2 and mABH3 (16), and two of the eight identified human homologs, hABH2 and hABH3 (17–20), have been shown to possess the same ability to reverse methylated DNA base damage, although substrate preferences vary among enzymes (21, 22). Recently, mABH2 has been demonstrated to remove endogenously formed m1A adducts in mice (23).
Since the initial discovery of AlkB's mechanism of action in the repair of m1A and 3-methylcytosine, the substrate range of the protein has expanded to include 3-ethylcytosine (24), 1-methylguanine (17, 24), 3-methylthymine (17, 18, 24), and 1-ethyladenine (20) lesions in DNA, demonstrating the enzyme's biochemical versatility. The recently solved x-ray crystal structure (25) shows a particularly malleable region within the nucleotide-recognition lid that may account for the accommodating nature of the protein.
In addition to these methylated and ethylated substrates, AlkB has been recently shown to directly repair εA and 1,N6-ethenocytosine. Here, the protein employs a second, distinct chemical mechanism of direct reversal: epoxidation of the double bond of etheno lesions resulting in the release of glyoxal and the undamaged base (7). The human homolog hABH3 has also been shown to reverse εA directly, albeit inefficiently, in vitro (26). In addition to the broad pool of DNA substrates that AlkB repairs, the protein and its human homolog hABH3 also are able to remove methyl groups from RNA, allowing reactivation of methylated RNA phage (27) and restoring the function of methylated mRNA and tRNA molecules in vitro (28).
Given the structural similarity of EA to known substrates for AlkB (εA and 1-alkyladenines), we hypothesized that it could itself be a substrate for this versatile enzyme. To investigate this possibility, a 16-mer oligonucleotide containing a site-specific EA was inserted into a single-stranded M13 phage genome, and the ability of DNA polymerase to replicate past the lesion was measured in wild-type and alkB E. coli (Fig. 1B). Although replication of the adduct-containing genome was at least as efficient as that of an unmodified control genome in AlkB-containing cells, replication was severely impaired in an AlkB-deficient strain, indicating that the protein does indeed play a role in alleviating the toxicity of the damaged base. In vitro reconstitution of the repair reaction followed by mass spectral analysis revealed that AlkB is able to hydroxylate EA oxidatively, resulting in a product that rapidly converts to a reactive ring-opened aldehydic isomer that can form secondary adducts with primary amines. Despite the demonstrated ability of AlkB to metabolize EA, the primary role of AlkB in vivo was to counter lesion toxicity rather than lesion mutagenesis, because EA mutagenicity was quantifiable but low in cells either proficient or deficient in AlkB.
Results
Bypass of EA in Vivo.
EA is a nontoxic adduct when replicated in wild-type (AlkB+) cells, because it shows a level of bypass comparable with that seen for the control T (Fig. 2). In striking contrast, when a viral genome containing a single EA lesion is replicated in cells deficient in AlkB-mediated repair, the adduct reveals itself to be extremely toxic, given that it is bypassed only 14% as well as the control. This vast difference in lesion tolerance between wild-type and alkB E. coli is suggestive of a role for AlkB in mitigating lesion toxicity through repair of this adduct. The bypass characteristics of εA were investigated again in this work as an established benchmark against which to compare EA. The data obtained here are in agreement with those previously observed for εA (7), because bypass was comparable with controls in wild-type cells but dropped to only 9% in AlkB-deficient cells.
Fig. 2.
Lesion bypass of EA and εA in wild-type and alkB E. coli as compared with a lesion-free control containing thymine (T) at the relevant site.
In Vitro Repair/Metabolism of EA in DNA.
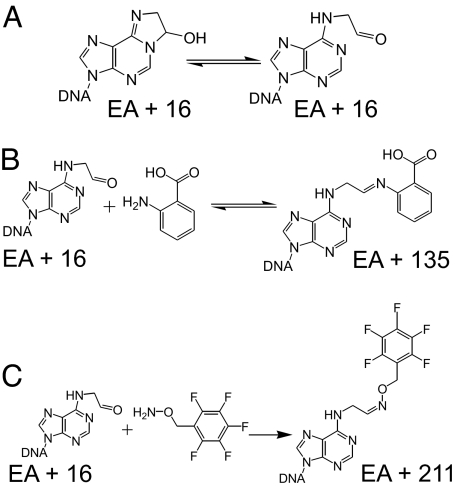
To determine the cause of this large change in bypass, the repair reaction was reconstituted in vitro. Purified AlkBΔN11 protein was incubated with the EA-containing 16-mer oligonucleotide used for genome construction in Hepes buffer containing the requisite cofactors and cosubstrates to achieve enzymatic activity. The products of the reaction were monitored by using MALDI-TOF mass spectrometry. Incubation of the oligonucleotide and two molecular weight markers in buffer lacking protein produced three peaks, the flanking two corresponding to the 15- and 17-mer controls, and the central peak representing the 16-mer substrate containing the unaltered lesion (Fig. 3A). After incubation with protein for 30 min at 37°C, the substrate DNA was converted entirely to two products, one with a mass of +16 relative to EA and the other with a mass of +135 (Fig. 3B). The +16 peak is consistent with the hydroxylation of EA (Fig. 4A). The regiochemistry shown (hydroxylation at the C8 position) mimics the product of the oxidative hydroxylation reaction mechanism that AlkB has been shown to employ in the repair of simple methylated lesions, such as m1A (10, 11, 29). Additionally, this C8-hydroxylated product could rearrange to form the ring-opened aldehydic isomer (Fig. 4A). The +135 peak is consistent with the Schiff base product of this ring-opened aldehyde with the anthranilic acid (molecular weight, 137.1) component of the matrix used for ionization (Fig. 4B). Indeed, the addition of a 100-fold molar excess of the trapping reagent O-(2,3,4,5,6-pentafluorobenzyl)hyroxylamine (PFBHA) (molecular weight, 213.1) before matrix exposure produced quantitative conversion to a +211 product, consistent with the oxime product of the ring-opened EA aldehyde and PFBHA (Fig. 4C).
Fig. 3.
MALDI-TOF mass spectra of in vitro repair reaction of AlkB with a 16-mer oligonucleotide containing a single EA residue. (A) Control incubation of oligonucleotide and molecular weight standards without AlkB. (B) Complete conversion of EA to +16 and +135 products after a 30-min incubation at 37°C. (C) Complete conversion to a single +211 product by the addition of a 100-fold excess of PFBHA after incubation with AlkB.
Fig. 4.
Putative structures of repair reaction products as seen by mass spectrometry. (A) Hydroxylated product (EA + 16) of AlkB metabolism of EA and its ring-opened aldehydic isomer. (B) Product (EA + 135) of ring-opened isomer reaction with anthranilic acid. (C) Product (EA + 211) of ring-opened isomer reaction with PFBHA.
Mutational Frequency and Specificity of EA.
Analysis of the base composition of progeny phage at the site that originally contained the lesion reveals that EA is only weakly mutagenic in wild-type cells. DNA polymerase is able to faithfully replicate a transfected genome containing the EA adduct >98% of the time (Fig. 5). Similarly, εA is also replicated correctly, because >97% adenine is seen at the site of interest in this work and >99% adenine has been seen previously (7). The 2% mutation frequency of EA in wild-type cells is composed of ≈1% EA→C and ≈0.5% each EA→G and EA→T. Perhaps surprisingly, however, given the dramatic alteration in toxicity, the mutational signature of EA does not change substantially when the adduct is replicated in alkB cells, in which case the observed base composition at the lesion site is ≈96% adenine, 2% cytosine, and 1% each guanine and thymine, yielding only ≈4% total mutagenesis. In contrast, εA is seen to be ≈37% mutagenic in a repair-deficient strain, producing 22% εA→T, 8% εA→C, and 7% εA→G. The results generated by the εA control are in agreement with previous studies of εA mutagenicity (7).
Fig. 5.
Mutational frequency and specificity of EA and εA replicated in wild-type and alkB E. coli.
Discussion
The anticancer chemotherapeutic agent BCNU is a bifunctional electrophile that reacts with DNA to form a range of adducts, including chloroethyl- and hydroxyethyl- monosubstituted bases, saturated ethano adducts, and inter- and intrastrand cross-links (30–33). The compound has been shown to exhibit both genotoxic and mutagenic properties and, although the potent toxicity of the drug is attributed to quantitatively minor 1-(3-cytosinyl)-2-(1-guanyl)ethane interstrand cross-links (32), the mutagenic characteristics are thought to be due to other damaged bases (33). It has been hypothesized that EA is to some extent responsible for the mutagenicity of BCNU, and this investigation into the mutagenic signature of EA when replicated within cells was intended to shed light on that question.
The EA adduct is an addition to the continuously expanding list of substrates of the promiscuous direct reversal repair enzyme AlkB. Although the unrepaired adduct is exceedingly toxic, AlkB is able to mitigate that toxicity completely, rendering the lesion as easily bypassed as a normal base in E. coli that possess functional protein. Such lesion bypass evidence has been exploited in the past to establish an adduct as a substrate for AlkB. For example, the structurally related adduct 1,N6-ethenoadenine was seen to exhibit the same characteristic nontoxic properties in wild-type cells (85% bypass) while allowing replication only 5% of the time in alkB cells (7). Through further studies, this alleviation in toxicity was demonstrated to be attributable to direct reversal of the εA adduct by AlkB.
Indeed, mass spectral analysis of an in vitro reconstitution of the repair reaction revealed that AlkB is capable of metabolizing the EA adduct, most likely through the protein's well established mechanism of oxidative hydroxylation at the carbon connected to the N1 of adenine. Although the data do not rule out hydroxylation of EA at the C7 position (that attached to the exocyclic nitrogen; see Fig. 1A), this scenario is unlikely as AlkB did not demonstrate repair activity of 6-methyladenine (data not shown), and an adduct attached at the N1 position of adenine would likely block replication [as m1A (24)], making its presence inconsistent with the lack of toxicity observed here.
Application of the canonical repair mechanism of AlkB in this situation diverges from the usual pathway in that the oxidized carbon that is normally released as formaldehyde is in this case covalently tethered to the base as an acetaldehyde residue (Fig. 6), providing opportunity for further reaction. Indeed, the hydroxylated adduct seems to isomerize to yield a ring-opened aldehydic form, which, at least in vitro, can then readily react with primary amines in the system, as demonstrated by the observed MALDI peaks possessing molecular weights consistent with the Schiff base product of the aldehydic ring-opened EA metabolite and either anthranilic acid from the matrix or PFBHA added exogenously as a trapping reagent. Reaction with anthranilic acid speaks to the high reactivity of the EA product, as the DNA is mixed with the matrix immediately before analysis, and yet the metabolite reacts extensively with an aromatically substituted amine in that brief amount of time. If this rapid reactivity holds in vivo, the action of AlkB on EA could lead to the formation of a range of secondary adducts, possibly including various small-molecule lesions, intrastrand or interstrand DNA cross-links, and protein–DNA cross-links.
Fig. 6.
Proposed reaction pathway through which AlkB metabolizes EA. Restoration of hydrogen bonding ability might favor formation of the reactive ring-opened aldehydic species in double-stranded DNA.
The mutational spectrum produced by replication of EA in wild-type E. coli is similar to that observed for the structurally related εA adduct. Neither is substantially miscoding, as >98% and >97% adenine is present at the lesion site after replication of EA and εA, respectively. Given the analogous structures of the two adducts, however, it was expected that their mutational signatures would resemble each other, because they are impaired in hydrogen bonding capability in the same manner at identical sites. As has been demonstrated to be the case for εA, it was suspected that the low mutation frequency of EA could be due to repair of the adduct before it is encountered by a replicative polymerase. Consequently, the coding characteristics of EA also were examined in an AlkB-deficient strain of the bacteria. Despite displaying potent toxicity when unrepaired, EA is poorly mutagenic, even in this cellular context. The coding specificity of EA in this repair-deficient background was observed to be 96% adenine, 2% cytosine, and 1% each guanine and thymine, in contrast to ≈64% adenine, 22% thymine, 8% cytosine, and 7% guanine for εA. The large differences in mutagenicity are most likely attributable to differential recognition by replicative polymerase of the EA lesion as compared with εA.
Although neither εA nor EA can participate in hydrogen bonding at the N6 position, there are other characteristics of the two adducts that could account for their differential mutagenic signatures. Although εA is planar, the saturated portion of the imidazole ring of EA may cause the adduct to take on an altered conformation within the active site of DNA polymerase. Additionally, the aromatic nature of εA could stabilize stacking interactions with the amino acid side-chains of the polymerase, possibly resulting in differential positioning of the lesion within the active site relative to EA and leading to variation in what is incorporated opposite the adduct. Indeed, observed differences in the abilities of the base excision repair glycosylases of both humans and E. coli to recognize εA and EA have been attributed to these characteristics (4, 5).
Although AlkB is able to act on EA, we show here that direct reversal of a damaged base is in this case incomplete (Fig. 6), resulting in the formation of a secondary adduct that may react further to create a variety of DNA lesions. Interestingly, this conversion to an N6 adduct completely alleviates the toxicity of EA, indicating that neither it nor any downstream product blocks the progress of polymerase during replication. Additionally, replication past an EA adduct in the presence of AlkB results in incorporation of thymine >98% of the time, suggesting that the N6 adduct and any derivatives it may form are able to code mainly as adenine, perhaps in part due to the restoration of hydrogen bonding ability (Fig. 6). Also surprising is the finding that in the absence of AlkB unaltered EA, which lacks an instructional hydrogen bond necessary to stabilize pairing with an incoming thymine nucleotide, is still able to code as adenine 96% of the time, indicating that EA possesses unique characteristics that enable it to be read correctly, either by replicative or bypass polymerases. A similar phenomenon has been seen in other contexts (34, 35), but the relative contributions of size, shape, and hydrogen bonding ability to faithful replication are not yet clear. Although the AlkA pathway is active in these cells, it is not likely responsible for the observed weakly mutagenic phenotype given its poor affinity for EA and the single-stranded context in which the adduct exists within cells in this system (5, 32, 33). The link between BCNU and EA has been established firmly only in vitro; however, the failure to detect EA in DNA isolated from treated cells or tissues is not entirely surprising. The rapidity of metabolism by AlkB (and perhaps the protein's human homologs) seen here would hinder a search for intact adduct by altering the structure of EA before analysis could be performed. Even a small quantity of the unmodified adduct, however, would carry major biological significance because of its extreme toxicity.
The poor mutagenesis induced by replication of EA seen here is in stark contrast to the miscoding nature of the adduct when copied by mammalian polymerases in vitro (6), suggesting either that further studies into alternative repair pathways that could be active on EA in E. coli are warranted or that fundamental dissimilarities in the replicative polymerases of mammals and bacteria cause the adduct to be read differently in the two systems. Although this study does not speak to the mutagenicity of EA, an investigation into the ability of hABH3 to metabolize EA would be useful to determine whether the adduct is in fact responsible for the mutagenic property of BCNU in humans. It may also be possible that AlkB and its mammalian homologs employ this pathway of metabolism to a secondary lesion to alleviate the toxicity and/or mutagenicity of additional cyclic lesions formed by this or other compounds.
Materials and Methods
Bacterial Strains.
The E. coli strains used for transfection and replication of lesion-containing viral genomes were HK81 (as AB1157, but nalA; wild type) and HK82 (as AB1157, but nalA alkB22; AlkB-deficient). AlkB status was confirmed by PCR amplification and sequencing analysis of the alkB gene of both strains. NR9050 E. coli were used for quantification of transfection efficiency, whereas the bacterial strain used for regrowth of progeny phage was SCS110 (Stratagene, La Jolla, CA).
Oligonucleotides.
Oligonucleotides were synthesized by using phenoxyacetal-protected phosphoramidites and capping reagents from Glen Research (Sterling, VA) (24). Approximately 50 mg of EA phosphoramidite (Chemgenes, Wilmington, MA), which has previously been site-specifically incorporated into an oligonucleotide (36), were used during a 30 min coupling with the solid support. After synthesis, the resin was incubated with concentrated ammonium hydroxide for 1 h before lyophilization. The 16-mer insert oligonucleotide 5′-GAAGACCTXGGCGTCC-3′ (X = EA) was purified by anion exchange and reversed-phase HPLC, and the molecular weight was verified by MALDI-TOF mass spectrometry (4,916.21 observed and 4,916.22 calculated). Sixteen-mer oligonucleotides of the same sequence, but where X = εA or thymine, were synthesized and purified as described in ref. 7. Scaffold oligonucleotides (of 5′-GGTCTTCCACTGAATCATGGTCATAGC-3′ and 5′-AAAACGACGGCCAGTGAATTGGACGC-3′) used in genome construction were obtained from Integrated DNA Technologies (Coralville, IA).
Genome Construction.
Single-stranded M13 phage genomes containing a single site-specific lesion were constructed as described in refs. 24 and 37. Briefly, 20 pmol of M13mp7L2 single-stranded bacteriophage genomic DNA were linearized by digestion with EcoRI, which incises the hairpin region at a unique restriction site. Two oligonucleotide scaffolds, one complementary to the 5′ end of the linearized phage genome and the 3′ end of the 16-mer insert and the other complementary to the 3′ end of the genome and the 5′ end of the insert, were annealed to the M13 DNA. Sixteen-mer oligonucleotide inserts containing EA, εA, or thymine at the lesion site were phosphorylated by T4 polynucleotide kinase. Phosphorylated inserts were added to the genome/scaffold mixture, and genomes were recircularized by ligation with T4 DNA ligase. After removal of the scaffolds by T4 DNA polymerase, genomes were extracted with phenol/chloroform/isoamyl alcohol (25:24:1), desalted in four washes on Centricon-100 spin-dialysis columns (Millipore, Billerica, MA), and stored at −20°C.
Genome Concentration Normalization.
Genome concentrations were normalized by annealing the scaffolds used for construction to the recircularized genomes, digesting the partially double-stranded product with HinFI, and dephosphorylating the resulting fragment with shrimp alkaline phosphatase (Roche, Basel, Switzerland). Genomes were then 5′-radiolabeled with [γ-32P]ATP and T4 polynucleotide kinase (USB, Cleveland, Ohio), and digested with HaeIII, and the products were separated by denaturing PAGE. Band intensities were quantified by using phosphorimagery (Molecular Dynamics, which is now part of GE Healthcare, Piscataway, NJ), and the concentration of each genome was normalized to that of the most dilute by the addition of water.
Preparation of Electrocompetent Cells.
Three baffled flasks each containing 150 ml of LB medium were inoculated with 1.5 ml of a saturated overnight culture of the strain to be transformed (wild-type HK81 or AlkB-deficient HK82) and grown with shaking at 37°C to an OD600 of ≈0.5 (≈2.5 h). The three cultures were then pelleted, and the cells were pooled, followed by three resuspension/repelleting cycles at 4°C using 175 ml of water. The final resuspension of cells was in 6 ml of 10% glycerol, after which the cells were transformed within 2 h.
Examination of Lesion Bypass Efficiency.
Lesion-containing genomes were mixed to a ratio of ≈10:1 with an internal standard competitor genome containing a thymine nucleobase instead of a lesion in an insert that is 3 nt longer than that bearing the lesion. We mixed 100 μl of electrocompetent cells with 4.8 μl of genome mixture, and transferred them to a prechilled, 2-mm-gap electroporation cuvette, which we exposed to ≈2.5 kV and ≈125 Ω. After electroporation, cells were immediately transferred to 10 ml of fresh LB medium and grown for 6 h at 37°C. Cells were then pelleted, and the supernatant containing progeny phage was removed and stored at 4°C. The number of successful transformation events was monitored by immediately plating 10 μl of the 10-ml culture onto a lawn of NR9050 indicator bacteria. Each independent event resulted in a plaque, which enabled the quantification of initial events and ensured a robust progeny population for analysis. All electroporation procedures reported here produced >104 events, with the vast majority resulting in >105.
Because not all copies of the lesion-bearing genome were incorporated into cells during the electroporation process, the isolated supernatant contained some unreplicated genomes in addition to the postreplication progeny phage. To eliminate analysis of these residual genomes, an amplification of the viable progeny phage was conducted. We regrew 100 μl of progeny phage (≈109) for 6 h in 10 ml of LB medium inoculated with 10 μl of an overnight culture of SCS110 E. coli (Stratagene). Only viable progeny phage, i.e., those previously replicated within a cell, were amplified, whereas the unincorporated genomes were not affected, effectively increasing the signal-to-noise ratio by several orders of magnitude. Cells were again pelleted, and the progeny-containing supernatant was isolated and stored at 4°C.
Before PCR amplification, single-stranded DNA was isolated from the progeny phage with a QIAprep Spin M13 kit (Qiagen, Valencia, CA). Amplification of the region of interest was performed by using PCR primers (5′-YCAGCTATGACCATGATTCAGTGGAAGAC-3′ and 5′-YCAGGGTTTTCCCAGTCACGACGTTGTAA-3′, where Y signifies amino modification of the 5′ nucleotide) as described in refs. 7, 24, 35, and 37. After phenol/chloroform/isoamyl alcohol extraction and removal of salt and organic residue with Sephadex G50 fine resin (GE Healthcare), the PCR product was digested with BbsI and dephosphorylated with shrimp alkaline phosphatase. T4 polynucleotide kinase was used to 5′-radiolabel the resulting fragment, which was further digested by HaeIII. Radiolabeled products were then separated by PAGE, and the intensity of each band was quantified by phosphorimagery. Comparison of the intensity of the 18-mer band (corresponding to the lesion-containing genome) to that of the 21-mer band (representing the internal standard) gave a measure of lesion bypass.
Construction and Purification of AlkBΔN11.
The AlkB ORF, excluding the first 11 aa, was PCR-amplified with the oligonucleotides 5′-GGAATTCCATATGCAAGAGCCACTGGCGG (NdeI restriction site) and 5′- CCGCTCGAGGCCTTGAAAATATAGGTTTTCTTTTTTACCTGCCTG (XhoI site). The PCR product was purified, digested with NdeI and XhoI (NEB, Ipswich, MA), and ligated into a C-terminal-histidine-tag-containing pET24a vector (Novagen, San Diego, CA) that had been digested by NdeI and XhoI. The ligation mixture was transformed into DH5α cells for verification by sequencing. pET24a-AlkBΔN11 was transformed into BL21(DE3) (Novagen, which is now part of EMD Biosciences, San Diego, CA) cells for expression.
pET24a-AlkBΔN11 cells were grown at 37°C to an A600 of 0.4, at which time isopropyl-β-d-thiogalactoside was added at 1 mM and the temperature was lowered to 30°C. Cells were harvested after 4 h by centrifugation, resuspended in 10 mM Tris (pH 7.3)/300 mM NaCl/2 mM CaCl2/10 mM MgCl2/5% glycerol/1 mM 2-mercaptoethanol (lysis buffer), and homogenized by sonication. Lysate was recovered by centrifugation. The supernatant was loaded onto an Ni-NTA column (Qiagen), washed at 20 mM, and eluted at 70 and 250 mM imidazole in lysis buffer. The eluent was dialyzed against 50 mM N-[tris(hydroxymethyl) methyl]-2-aminoethanesulfonic acid and loaded onto an SP Sepharose cation exchange column (Amersham Pharmacia, which is now part of GE Healthcare). AlkBΔN11 was eluted by a linear gradient of 0–1 M NaCl over 75 ml. The fractions containing AlkBΔN11 were pooled, and purity was established by SDS/PAGE. As previously reported (25), AlkBΔN11 has similar activity to the full-length protein in standard AlkB assays (data not shown).
Examination of Reaction Products by MALDI-TOF Mass Spectrometry.
Purified AlkBΔN11 protein (10.5 pmol) was incubated with 25 pmol of EA 16-mer in 10 μl of buffer (70 μM (NH4)2Fe(SO4)2·6H2O/0.9 mM α-ketoglutarate/1.8 mM l-ascorbate/46.5 mM Hepes, pH 8.0) and molecular weight standards (15-mer and 17-mer) for 30 min at 37°C. Reactions were quenched by application of the sample to a ZipTipC18 pipette tip (Millipore) followed by 0.1 M triethylamine washes to remove cofactors. In one reaction, ≈2 nmol of the trapping reagent PFBHA was added before cofactor removal. Quenched samples were then analyzed by MALDI-TOF mass spectrometry.
Analysis of EA Mutational Spectrum.
The mutational specificity and frequency of the EA adduct was determined exactly as in the lesion bypass assay described above up to the PCR step. A different set of primers (5′-YCAGCTATGACCATGATTCAGTGGAAGAC-3′ and 5′YTGTAAAACGACGGCCAGTGAATTGGACG-3′) was used to obtain specific amplification of progeny from genomes that originally contained the lesion. The second primer was mismatched when annealed to the internal standard, which was therefore not amplified. Samples were processed as in the lesion bypass assay through the PAGE step, at which point the 18-mer bands were excised from the gel and the DNA fragments were extracted by crushing and soaking. Importantly, the restriction enzyme used in this assay incised the DNA to expose the base that corresponds to the original site of the lesion at the 5′ end, which was then radiolabeled. This site-specific labeling allows for precise inquiry into the base present at the lesion site after replication. The extracted DNA was desalted on Sephadex G50 fine resin (GE Healthcare), lyophilized, and digested to free nucleotide monophosphates by nuclease P1 (USB). The digested nucleotides were separated on a polyethyleneimine thin layer chromatography plate, and the resulting spots, each representing a normal base, were quantified by phosphorimagery. Because only the 5′ base was radiolabeled, the thin layer chromatography represents the base composition at the lesion site after replication by E. coli, enabling calculation of both mutation frequency and specificity.
Acknowledgments
This work was supported by National Institutes of Health Grants T32-ES07020, R01-CA/ES80024, R01-GM69857, and P30-ES002109.
Abbreviations
- EA,
1,N6-ethanoadenine
- εA,
1,N6-ethenoadenine
- BCNU
1,3-bis(2-chloroethyl)-1-nitrosourea
- m1A
1-methyladenine
- PFBHA,
O-(2,3,4,5,6-pentafluorobenzyl)hyroxylamine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Ludlum DB. IARC Sci Publ 1986. 1986;78:71–81. [PubMed] [Google Scholar]
- 2.Ludlum DB. IARC Sci Publ 1986. 1986;70:137–146. [PubMed] [Google Scholar]
- 3.Ludlum DB. Mutat Res. 1990;233:117–126. doi: 10.1016/0027-5107(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 4.Guliaev AB, Hang B, Singer B. Nucleic Acids Res. 2002;30:3778–3787. doi: 10.1093/nar/gkf494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guliaev AB, Singer B, Hang B. DNA Repair. 2004;3:1311–1321. doi: 10.1016/j.dnarep.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Hang B, Chenna A, Guliaev AB, Singer B. Mutat Res. 2003;531:191–203. doi: 10.1016/j.mrfmmm.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Delaney JC, Smeester L, Wong C, Frick LE, Taghizadeh K, Wishnok JS, Drennan CL, Samson LD, Essigmann JM. Nat Struct Mol Biol. 2005;12:855–860. doi: 10.1038/nsmb996. [DOI] [PubMed] [Google Scholar]
- 8.Sedgwick B, Lindahl T. Oncogene. 2002;21:8886–8894. doi: 10.1038/sj.onc.1205998. [DOI] [PubMed] [Google Scholar]
- 9.Rohankhedkar MS, Mulrooney SB, Wedemeyer WJ, Hausinger RP. J Bacteriol. 2006;188:223–230. doi: 10.1128/JB.188.1.223-230.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Nature. 2002;419:174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 11.Falnes PO, Johansen RF, Seeberg E. Nature. 2002;419:178–182. doi: 10.1038/nature01048. [DOI] [PubMed] [Google Scholar]
- 12.Aravind L, Koonin EV. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-research0007. RESEARCH0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falnes PO, Rognes T. Res Microbiol. 2003;154:531–538. doi: 10.1016/S0923-2508(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 14.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE. DNA Repair. 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Wei YF, Chen BJ, Samson L. J Bacteriol. 1995;177:5009–5015. doi: 10.1128/jb.177.17.5009-5015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DH, Jin SG, Cai S, Chen Y, Pfeifer GP, O'Connor TR. J Biol Chem. 2005;280:39448–39459. doi: 10.1074/jbc.M509881200. [DOI] [PubMed] [Google Scholar]
- 17.Falnes PO. Nucleic Acids Res. 2004;32:6260–6267. doi: 10.1093/nar/gkh964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivisto P, Robins P, Lindahl T, Sedgwick B. J Biol Chem. 2004;279:40470–40474. doi: 10.1074/jbc.M407960200. [DOI] [PubMed] [Google Scholar]
- 19.Sedgwick B. Nat Rev Mol Cell Biol. 2004;5:148–157. doi: 10.1038/nrm1312. [DOI] [PubMed] [Google Scholar]
- 20.Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Proc Natl Acad Sci USA. 2002;99:16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falnes PO, Bjoras M, Aas PA, Sundheim O, Seeberg E. Nucleic Acids Res. 2004;32:3456–3461. doi: 10.1093/nar/gkh655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koivisto P, Duncan T, Lindahl T, Sedgwick B. J Biol Chem. 2003;278:44348–44354. doi: 10.1074/jbc.M307361200. [DOI] [PubMed] [Google Scholar]
- 23.Ringvoll J, Nordstrand LM, Vagbo CB, Talstad V, Reite K, Aas PA, Lauritzen KH, Liabakk NB, Bjork A, Doughty RW, et al. EMBO J. 2006;25:2189–2198. doi: 10.1038/sj.emboj.7601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaney JC, Essigmann JM. Proc Natl Acad Sci USA. 2004;101:14051–14056. doi: 10.1073/pnas.0403489101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Edstrom WC, Benach J, Hamuro Y, Weber PC, Gibney BR, Hunt JF. Nature. 2006;439:879–884. doi: 10.1038/nature04561. [DOI] [PubMed] [Google Scholar]
- 26.Mishina Y, Yang CG, He C. J Am Chem Soc. 2005;127:14594–14595. doi: 10.1021/ja055957m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aas PA, Otterlei M, Falnes PO, Vagbo CB, Skorpen F, Akbari M, Sundheim O, Bjoras M, Slupphaug G, Seeberg E, et al. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 28.Ougland R, Zhang CM, Liiv A, Johansen RF, Seeberg E, Hou YM, Remme J, Falnes PO. Mol Cell. 2004;16:107–116. doi: 10.1016/j.molcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Begley TJ, Samson LD. Trends Biochem Sci. 2003;28:2–5. doi: 10.1016/s0968-0004(02)00010-5. [DOI] [PubMed] [Google Scholar]
- 30.Lemoine A, Lucas C, Ings RM. Xenobiotica. 1991;21:775–791. doi: 10.3109/00498259109039517. [DOI] [PubMed] [Google Scholar]
- 31.Eisenbrand G, Muller N, Denkel E, Sterzel W. J Cancer Res Clin Oncol. 1986;112:196–204. doi: 10.1007/BF00395912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong WP, Kirk MC, Ludlum DB. Cancer Res. 1982;42:3102–3105. [PubMed] [Google Scholar]
- 33.Wiencke JK, Wiemels J. Mutat Res. 1995;339:91–119. doi: 10.1016/0165-1110(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 34.Potapova O, Chan C, DeLucia AM, Helquist SA, Kool ET, Grindley ND, Joyce CM. Biochemistry. 2006;45:890–898. doi: 10.1021/bi051792i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim TW, Delaney JC, Essigmann JM, Kool ET. Proc Natl Acad Sci USA. 2005;102:15803–15808. doi: 10.1073/pnas.0505113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chenna A, Maruenda H, Singer B. IARC Sci Publ 1999. 1999;150:89–101. [PubMed] [Google Scholar]
- 37.Delaney JC, Essigmann JM. Methods Enzymol. 2006;408:1–15. doi: 10.1016/S0076-6879(06)08001-3. [DOI] [PubMed] [Google Scholar]