Abstract
AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase that plays an important role in maintaining cellular energy balance. The activity of AMPK is modulated both by the cellular AMP-to-ATP ratio and by upstream kinases. Recently, AMPK was shown to be phosphorylated and activated by LKB1, a protein kinase that plays a conserved role in epithelial polarity regulation in mammals and Drosophila. Here, we investigate the involvement of AMPK in the regulation of epithelial tight junction assembly and cell polarization in MDCK cells. We show that the level of AMPK phosphorylation increases during calcium-induced tight junction assembly and cell polarization and that this increase depends on the kinase activity of LKB1. Expression of a kinase-dead mutant of AMPK inhibits tight junction assembly as indicated by measurement of transepithelial resistance and analysis of ZO-1 localization to the tight junction after calcium switch. Conversely, 5-aminoimidizole-4-carboxamide riboside, an activator of AMPK, promotes transepithelial resistance development and tight junction assembly upon calcium switch. Furthermore, 5-aminoimidizole-4-carboxamide riboside partially protects the tight junctions from disassembly induced by calcium depletion. These results support an important role of AMPK in the regulation of epithelial tight junction assembly and disassembly and suggest an intriguing link between cellular energy status and tight junction function.
Keywords: 5-aminoimidizole-4-carboxamide riboside, LKB1, transepithelial resistance, Zo-1
AMP-activated protein kinase (AMPK) is an evolutionarily conserved energy sensor in eukaryotic cells (1–3). It is activated by allosteric binding of AMP and through phosphorylation of its Thr-172 residue in the activation loop by upstream kinases (1–3). In intact cells, the activation of AMPK can be triggered by an increased cellular AMP/ATP ratio under energy stress, such as hypoxia, ischemia, and glucose deprivation. AMPK can also be activated in response to physiological stimuli, such as exercise and contraction in skeletal muscle, and to the peptide hormones leptin and adiponectin (1–3). Upon activation, AMPK phosphorylates downstream regulatory proteins and enzymatic effectors to up-regulate ATP-producing catabolic pathways (i.e., fatty acid oxidation, glucose uptake, and glycolysis) and down-regulate ATP-consuming processes (i.e., fatty acid, cholesterol, glycogen, and protein synthesis).
Recently, our laboratory and others (4–6) have identified LKB1 as one of the upstream activating kinases of AMPK. LKB1 is a tumor suppressor that regulates cell growth, proliferation, metabolism, and polarity (7). PAR4 proteins, the orthologs of LKB1 in Caenorhabditis elegans and Drosophila, were found to be important for the establishment of polarity during embryogenesis (8, 9). Activation of mammalian LKB1 was shown to induce cell-autonomous polarization in intestinal epithelial cells (10). However, the molecular mechanism by which LKB1 regulates cell polarity is not clear. In addition to AMPK, LKB1 was shown to be responsible for the activation of 11 other AMPK-like kinases, including PAR1 kinases/microtubule affinity regulating kinases (MARKs) (11). Because PAR1 kinases have been implicated in cell polarity regulation, it has been suggested that they may mediate the effects of LKB1 on polarity (12). Whether other downstream kinases of LKB1 are also involved in cell polarity regulation is largely unknown. Here we report that AMPK plays a role in the regulation of epithelial tight junction assembly and disassembly in MDCK cells.
Results
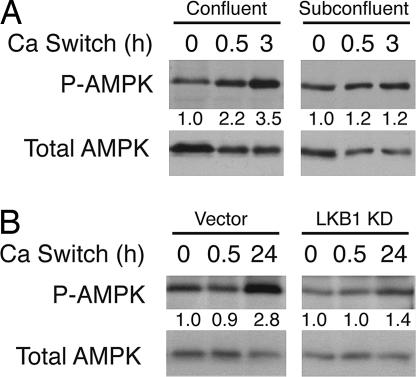
To investigate whether AMPK, as a downstream kinase of LKB1, also plays a role in cell polarity regulation, we first examined the phosphorylation status of AMPK Thr-172 during calcium switch in MDCK cell, a widely used model system to study mammalian tight junction assembly and epithelial polarity regulation (13). Calcium depletion from the medium in a confluent monolayer of MDCK cells causes loss of cell–cell junctions; readdition of calcium (calcium switch) triggers junction assembly and cell polarization (13). As shown in Fig. 1A, the level of AMPK Thr-172 phosphorylation increased ≈3.5-fold after readdition of calcium, suggesting that phosphorylation and activation of AMPK by upstream kinases occurs during tight junction assembly and cell polarization. In addition to LKB1, calmodulin-dependent protein kinase kinase was recently shown to be an upstream kinase for AMPK (14–16). To exclude the possibility that the increase of AMPK phosphorylation was due to the change of extracellular calcium level per se instead of the assembly of tight junctions after calcium switch, we performed similar calcium-switch experiments using subconfluent MDCK cells and found that the level of Thr-172 phosphorylation did not change significantly upon calcium switch (Fig. 1A). To further determine whether the up-regulation of AMPK Thr-172 phosphorylation depends on LKB1, we generated MDCK cells stably expressing a kinase-dead (KD) mutant (K78I) of LKB1 via retroviral transduction. When these cells were subjected to calcium switch, the increase of AMPK Thr-172 phosphorylation was greatly attenuated compared with that of control cells (Fig. 1B). These results together indicate that AMPK is phosphorylated at Thr-172 and activated by LKB1 during calcium-induced tight junction assembly and cell polarization.
Fig. 1.
The level of AMPK phosphorylation increases during calcium-induced tight junction assembly and cell polarization. (A) Confluent or subconfluent MDCK cells were subjected to calcium switch for the indicated times. Total cell lysates were used for Western blot analysis with the indicated antibodies. (B) MDCK cells stably expressing control vector or FLAG-tagged LKB1 KD were subjected to calcium switch for the indicated times. Total cell lysates were used for Western blotting with the indicated antibodies. Numbers indicate normalized phospho-AMPK/total AMPK ratios. The data represent three independent experiments.
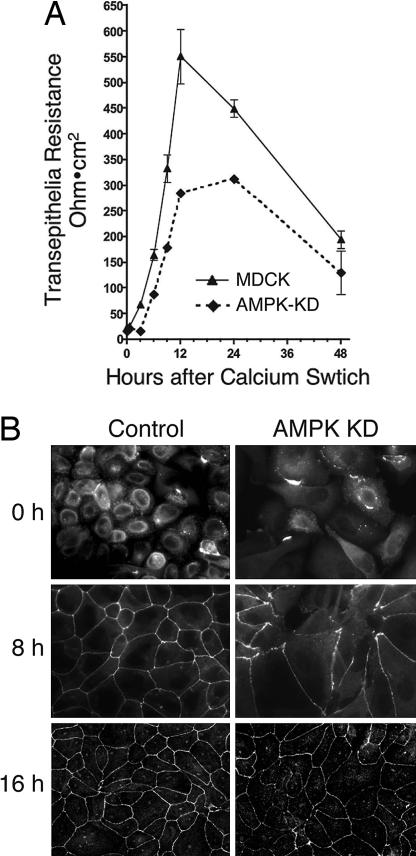
To directly assess whether AMPK may contribute to the assembly of tight junctions, we generated MDCK cell lines expressing the AMPKα1 KD mutant (D157A) and monitored the effect of calcium switch on transepithelial resistance (TER), a measurement for the paracellular barrier function and integrity of tight junctions (17). In control MDCK cells, the TER usually peaked at ≈12 h after calcium switch and returned to a lower steady-state level by 48 h (Fig. 2A). Expression of AMPK KD significantly reduced the peak level of TER, although the steady-state level did not change (Fig. 2A). These results indicate that the formation of functional tight junctions was suppressed by expression of AMPK KD mutant.
Fig. 2.
Expression of KD mutant of AMPK inhibits TER development and tight junction assembly. (A) MDCK cells stably expressing FLAG-tagged AMPK KD or control cells were grown on a Transwell filter for 3 days, incubated in low calcium medium for 16 h, and switched to normal calcium medium. TER was measured at the indicated time points after calcium switch. (B) MDCK stable lines grown on glass coverslips were incubated in low calcium medium for 16 h, switched to normal calcium medium for the indicated times, and stained for tight junction marker ZO-1.
Depletion of calcium in the medium causes the tight junction protein ZO-1 to translocate from the cell periphery to the cytoplasm. Upon readdition of calcium, ZO-1 moves back to the tight junctions as they assemble. Given the effect of the AMPK KD mutant on TER development after calcium switch, we examined the effect of this mutant on ZO-1 translocation. At 8 h after calcium switch, control MDCK cells showed recovery of ZO-1 labeling at most cell junctions, whereas cells expressing AMPK KD showed only partial ZO-1 translocation (Fig. 2B). At 16 h, ZO-1 translocation was complete in both cell lines. These results together with the TER data suggest that expression of AMPK KD significantly delayed the tight junction assembly and that the kinase activity of AMPK is required for the efficient development of functional tight junctions.
Although the expression of AMPK KD decreased the rate of ZO-1 assembly at the cell periphery after calcium switch, it did not appear to affect ZO-1 localization under standard cell culture conditions used for growth of MDCK cells (data not shown). These results suggest that AMPK activity accelerates assembly of tight junctions but that it may not be necessary for maintenance.
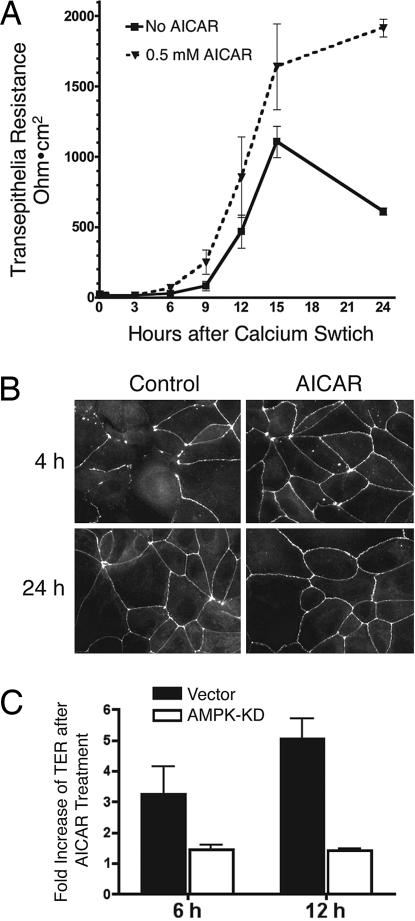
To further evaluate the role of AMPK in tight junction assembly, we treated MDCK cells with 5-aminoimidizole-4-carboxamide riboside (AICAR), a precursor of AMP that enters cells and causes activation of AMPK. It has been shown that AICAR activates AMPK but not other LKB1-dependent AMPK-like kinases, including MARKs/PAR1 kinases (11). We found that treatment of MDCK cells with AICAR enhances the peak TER levels after calcium switch (Fig. 3A). Consistent with this finding, AICAR also markedly promoted the translocation of ZO-1 to the cell periphery after calcium switch (Fig. 3B). These results are consistent with the results using AMPK KD and indicate that turning up AMPK activity accelerates tight junction assembly whereas turning down AMPK activity retards tight junction assembly. We found no changes in TER or in ZO-1 staining when confluent monolayers of MDCK cells were exposed to AICAR in normal growth medium (data not shown), suggesting that AICAR doesn't affect the structure of fully assembled tight junctions.
Fig. 3.
Activation of AMPK by AICAR promotes TER development and tight junction assembly. (A) MDCK cells grown on Transwell filters were incubated in low calcium medium for 16 h and switched to normal calcium medium containing 0.5 mM AICAR. TER was measured at the indicated time points. (B) MDCK cells grown on glass coverslips were incubated in low calcium medium for 16 h, switched to normal calcium medium containing 0.5 mM of AICAR for the indicated times, and stained for ZO-1. (C) MDCK cells stably expressing AMPK KD mutant or vector control were grown on a Transwell filter for 3 days, incubated in low calcium medium for 16 h, and switched to normal calcium medium with or without 0.5 mM AICAR. TER was measured at the indicated time points and normalized as the fold increase by comparing the values from cells with and without AICAR treatment.
To address whether the observed effects of AICAR on tight junction assembly were mediated by AMPK, we examined the effect of AICAR on tight junction formation in MDCK cells expressing AMPK KD. As shown in Fig. 3C, AICAR enhanced TER after calcium switch in MDCK cells infected with a control vector but failed to enhance TER in the cells expressing AMPK KD. These results indicate that the effect of AICAR on promoting TER development depends on the kinase activity of AMPK.
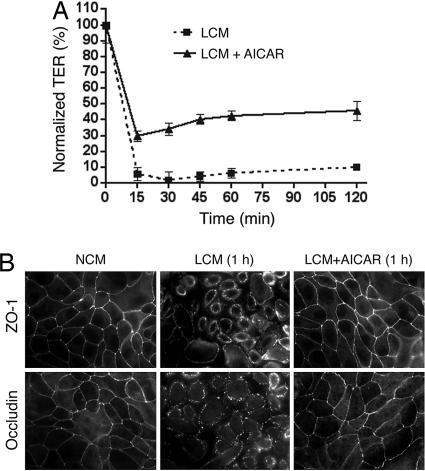
The stimulatory effects of AMPK on tight junction assembly raised the possibility that activation of AMPK could be beneficial to the maintenance of tight junction integrity under conditions that may disrupt tight junctions, such as extracellular calcium depletion. To test this hypothesis, we incubated confluent MDCK cells in low calcium medium in the presence or absence of 2 mM AICAR and examined the integrity of tight junctions through TER measurement and immunofluorescence analysis. As shown in Fig. 4A, the TER level of cells incubated in low calcium medium in the absence of AICAR quickly dropped to ≈5% of the baseline within 15 min, whereas the TER level of cells treated with AICAR dropped to and stayed at a much higher level (≈30% of the start point) for >2 h after calcium depletion (Fig. 4A). Immunofluorescence analysis revealed that, after incubation in the low calcium medium for 1 h, cells were rounded up and ZO-1 appeared to translocate from areas of cell–cell contact into the cytoplasm as tight junctions were disrupted (Fig. 4B). In contrast, there was no apparent change in cell shape in cells exposed to low calcium medium containing AICAR, and ZO-1 was maintained at areas of cell–cell contact (Fig. 4B). Another tight junction protein, occludin, also was retained at the areas of cell–cell contact in cells treated with AICAR (Fig. 4B). These results indicated that activation of AMPK by AICAR inhibits tight junction disassembly induced by calcium depletion.
Fig. 4.
AICAR protects calcium depletion-induced tight junction disassembly. MDCK cells were grown on Transwell filters (A) or glass coverslips (B) in normal growth medium for 3 days and changed to low calcium medium (LCM) in the presence or absence of 2 mM AICAR. (A) TER was measured at the indicated time points and normalized to the baseline values before switching to low calcium medium. (B) Cells were fixed at 1 h after being changed to low calcium medium and stained with the indicated antibodies.
Discussion
The results presented here indicate that, although AMPK may not be necessary for maintenance of tight junctions in confluent MDCK cells, it plays an important role in accelerating tight junction formation after calcium switch. The results also indicate that hyperactivation of AMPK can enhance the stability of tight junctions under conditions of calcium depletion. These results raise the interesting possibility that during conditions of mild energy stress leading to elevation of AMP, activation of AMPK stabilizes contacts between epithelial cells. These findings also raise the possibility that drugs that activate AMPK could provide a beneficial effect in diseases during which tight junctions are disrupted. Proinflammatory cytokines, such as TNF-α and IFN-γ (18), and certain virulence gene products from bacterial and viral pathogens, such as Helicobacter pylori CagA (19), Clostridium difficile toxin A (20), and rotavirus VP8 proteins (21), have been found to induce epithelial tight junction disassembly and thereby increase epithelial permeability. It would be interesting to test whether AICAR or other means of activating AMPK can also protect the structure and barrier function of tight junctions against these agents under pathophysiological conditions.
In addition to serving as paracellular diffusion barriers (22), mammalian tight junctions play critical roles in regulating epithelial proliferation and differentiation (23). In Drosophila, mutations in several epithelial junction proteins were found to disrupt epithelial polarity and induce overproliferation (24). Disruption of tight junctions and subsequent loss of cell polarity in epithelial have been linked to epithelial–mesenchymal transition and metastasis (23). On the other hand, tight junction assembly is often accompanied by the suppression of epithelial cell proliferation (23, 24). Thus, the observation here that activation of AMPK stabilizes tight junctions and the previous observations that activation of AMPK induces p53 activation and cell cycle arrest (25) and blocks the mTOR pathway (26, 27) are consistent with a general role for AMPK in repressing cell growth and division.
The function of LKB1 in cell polarization may help to explain its role as a tumor suppressor. However, the molecular mechanism underlying the regulation of cell polarization by LKB1 is not well understood. Several of the protein kinases activated by LKB1, such as Par1/MARKs (28), have been shown to be involved in regulating cell polarity. Our data presented here suggest that AMPK also plays a regulatory role in epithelial polarity and could mediate the effects of LKB1 in polarity regulation. An attractive model is that AMPK and the MARK family members have overlapping substrates explaining why AMPK is not essential for tight junction formation but contributes to the rate of formation and to the stability of the junctions. However, it is unlikely that AMPK can mediate all LKB1-dependent effects on cell polarity. For example, overexpression of MARK2 (also known as EMK1) was shown to promote reorganization of microtubule network and induce the formation of a hepatocyte-like lumen polarity in MDCK cells (28). However, we did not observe similar effects of AICAR in MDCK cells (data not shown).
Acetyl-CoA carboxylase (ACC) is a well established substrate of AMPK involved in fatty acid synthesis. Recent genetic analysis suggested that mutation in C. elegans pod-2, an ortholog of ACC, disrupted the anterior–posterior polarity at the one-cell stage (29). However, we did not detect a significant change in the level of phospho-ACC (Ser-79) upon calcium switch in MDCK cells (data not shown), suggesting that ACC may not play a critical role in this particular model for tight junction regulation. Future studies may reveal relevant substrates of AMPK involved in this process.
Materials and Methods
Antibodies and Reagents.
Phospho-Thr-172 AMPK and total AMPK antibodies were purchased from Cell Signaling Technology (Danvers, MA). ZO-1 and occludin antibodies were from Zymed (South San Francisco, CA). AICAR was obtained from Toronto Research Chemicals (Downsview, ON, Canada).
Cell Culture.
MDCK cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in DMEM containing 10% FBS. MDCK cells expressing AMPK KD and LKB1 KD mutants were generated by retroviral gene transfer. pBabe-puro retroviral constructs containing the FLAG-tagged AMPKα1 KD mutant (D157A) and LKB1 KD mutant (K78I) were described previously (5, 26). Amphotropic retrovirus were generated as described previously (5) and used to infect MDCK cells. Stable populations were obtained and maintained by selection with 2 μg/ml puromycin (Sigma, St. Louis, MO).
Calcium Switch and TER Measurements.
MDCK cells were plated at 1.5 × 105 cells per squared centimeter and allowed to establish confluent monolayers for 2–3 d before incubation for 16 h in low calcium medium (S-MEM containing 5% dialyzed FBS) and switched to normal calcium medium (low calcium medium supplemented with 1.8 mM CaCl2) for the indicated times. TER of MDCK cells grown on 12-mm polycarbonate Transwell filters (0.4 μm pore size; Corning Costar, Corning, NY) were measured by using an epithelial voltohmmeter (World Precision Instruments, Sarasota, FL) with three parallel filters for each group of cells at each time point. TER values were obtained by subtracting the blank values from the filters and the medium, and expressed in ohm·cm2.
Western Blot Analysis.
MDCK cells were lysed in boiling SDS-lysis buffer (10 mM Tris, pH 7.5/1% SDS/100 mM NaCl), and extracts were subjected to SDS/PAGE, immunoblotting, and enhanced chemiluminescence detection (Pierce, Rockford, IL) as described previously (5).
Immunofluorescence Analysis.
Cells grown on glass coverslips were fixed in 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 for 15 min, blocked with 5% FCS for 1 h, and incubated with primary antibodies overnight at 4°C, followed by incubation with Alexa Fluor 594-conjugated goat anti-mouse F(ab)2 and/or Alexa Fluor 488 goat anti-rabbit F(ab)2 (Molecular Probes, Carlsbad, CA) for 1 h. Specimens were analyzed with an Axiovert microscope with Axiocam Axiovision 4.2 software (Zeiss, Oberkochen, Germany) and the appropriate fluorescent filters (Chroma Technology, Rockingham, VT).
Acknowledgments
We thank Drs. Reuben Shaw (The Salk Institute for Biological Studies, La Jolla, CA) and Vivian Tang (Harvard Medical School) for reagents and helpful discussion. This work was supported by National Institutes of Health Grant GM56203 (to L.C.C.). B.Z. was supported by a Charles A. King Trust postdoctoral fellowship from the Bank of America, Co-Trustee.
Abbreviations
- AICAR
5-aminoimidizole-4-carboxamide riboside
- AMPK
AMP-activated protein kinase
- KD
kinase-dead
- MARK
microtubule affinity regulating kinase
- TER
transepithelial resistance.
Footnotes
The authors declare no conflict of interest.
References
- 1.Luo Z, Saha AK, Xiang X, Ruderman NB. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Carling D. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Alessi DR, Sakamoto K, Bayascas JR. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 8.Watts JL, Morton DG, Bestman J, Kemphues KJ. Development (Cambridge, UK) 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 9.Martin SG, St Johnston D. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 10.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 11.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spicer J, Ashworth A. Curr Biol. 2004;14:R383–R385. doi: 10.1016/j.cub.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Gumbiner B, Simons K. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 15.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Matter K, Balda MS. Methods. 2003;30:228–234. doi: 10.1016/s1046-2023(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 18.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ML, Pothoulakis C, LaMont JT. J Biol Chem. 2002;277:4247–4254. doi: 10.1074/jbc.M109254200. [DOI] [PubMed] [Google Scholar]
- 21.Nava P, Lopez S, Arias CF, Islas S, Gonzalez-Mariscal L. J Cell Sci. 2004;117:5509–5519. doi: 10.1242/jcs.01425. [DOI] [PubMed] [Google Scholar]
- 22.Farquhar MG, Palade GE. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matter K, Aijaz S, Tsapara A, Balda MS. Curr Opin Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Bilder D. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 25.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Zhu T, Guan KL. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 28.Cohen D, Brennwald PJ, Rodriguez-Boulan E, Musch A. J Cell Biol. 2004;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappleye CA, Tagawa A, Le Bot N, Ahringer J, Aroian RV. BMC Dev Biol. 2003;3:8. doi: 10.1186/1471-213X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]