Abstract
Developing Dictyostelium cells aggregate to form fruiting bodies containing typically 2 × 104 cells. To prevent the formation of an excessively large fruiting body, streams of aggregating cells break up into groups if there are too many cells. The breakup is regulated by a secreted complex of polypeptides called counting factor (CF). Countin and CF50 are two of the components of CF. Disrupting the expression of either of these proteins results in cells secreting very little detectable CF activity, and as a result, aggregation streams remain intact and form large fruiting bodies, which invariably collapse. We find that disrupting the gene encoding a third protein present in crude CF, CF45-1, also results in the formation of large groups when cells are grown with bacteria on agar plates and then starve. However, unlike countin− and cf50− cells, cf45-1− cells sometimes form smaller groups than wild-type cells when the cells are starved on filter pads. The predicted amino acid sequence of CF45-1 has some similarity to that of lysozyme, but recombinant CF45-1 has no detectable lysozyme activity. In the exudates from starved cells, CF45-1 is present in a ∼450-kDa fraction that also contains countin and CF50, suggesting that it is part of a complex. Recombinant CF45-1 decreases group size in colonies of cf45-1− cells with a 50% effective concentration (EC50) of ∼8 ng/ml and in colonies of wild-type and cf50− cells with an EC50 of ∼40 ng/ml. Like countin− and cf50− cells, cf45-1− cells have high levels of cytosolic glucose, high cell-cell adhesion, and low cell motility. Together, the data suggest that CF45-1 participates in group size regulation in Dictyostelium.
A remarkable aspect of development is the formation of multicellular structures of a predetermined size. For the entire population of fat or muscle cells in a body, there appear to be negative feedback loops using secreted factors to sense the amount of fat or muscle in the body (5, 19). However, little is known about the mechanisms that regulate the breakup of a primordium into substructures of a specific size, such as individual muscles. A simple example of the breakup of a primordium occurs during the formation of fruiting bodies by the simple eukaryote Dictyostelium discoideum.
Dictyostelium lives as isolated motile cells that eat bacteria on soil surfaces (for reviews, see references 6, 7, 17, 20, 21, and 28). As long as there are bacteria available, the cells keep dividing. As the population overgrows the food supply, the cells begin to starve. To get at least some of the cells to a different patch of soil, the starving cells cooperatively form multicellular structures called fruiting bodies, consisting of a mass of spores on top of a 1- to 2-mm-high column of stalk cells. The spores are dispersed by the wind, and a germinating spore can then start a new colony.
To form fruiting bodies, the cells aggregate by using relayed pulses of cAMP as a chemoattractant (6, 7). The aggregating cells form radial streams flowing toward a common center. Since there is a limit to the strength of the stalk, Dictyostelium has evolved a mechanism that senses the number of cells in a stream and causes the stream to break up into groups if there are too many cells in a stream (29).
To elucidate the mechanism causing the stream to break up into groups, we used shotgun antisense to mutagenize Dictyostelium cells and isolated smlAas, a transformant that formed large numbers of very small fruiting bodies due to excessive stream breakup (33). Disruption of smlA by homologous recombination gave rise to cells with the smlAas phenotype. The exudate from starving smlA− cells causes wild-type cells to form small fruiting bodies (1). The factor oversecreted by the smlA− cells (and secreted by wild-type cells, albeit at a lower level) is counting factor (CF), a 450-kDa complex of polypeptides (2). Disruption of countin, a gene encoding one of the components of CF, results in streams not breaking up. These streams then coalesce into one huge group that forms a huge fruiting body, which then collapses, spilling the spores on the ground. The same phenotype is seen in colonies of cells lacking CF50, a second component of CF (3). The effect of CF on group size suggests that CF is part of a negative feedback loop, with a high concentration of CF inducing stream breakup.
To predict possible parameters that could cause the morphogenesis of a stream of cells into a series of groups of cells, we used a computer to model the movement of cells in a stream (27). In the simulations, we found that if the cell-cell adhesion was low and/or the random component of cell motility was high, the stream would start to dissipate. If the adhesion then increased and/or the random motility decreased, the dissipated stream would coalesce into groups. Overexpressing an adhesion protein during Dictyostelium development causes the formation of unbroken streams and large aggregates, while blocking cell surface adhesion proteins with monoclonal antibodies causes the formation of broken streams and many small aggregates (16, 27, 32). In agreement with these observations and the simulations, it was found that CF inhibits cell-cell adhesion (27). The simulations also predicted that increasing cell motility would increase stream dissipation and hence stream breakup. Decreasing motility increases group size (34), and CF, which decreases group size, increases motility (34). Growing cells in the presence of glucose causes the formation of large groups (9), and we found that CF appears to regulate adhesion and motility, and thus group size, in part through a signal transduction pathway that involves glucose or a glucose metabolite (14).
After purification by ion-exchange and hydroxylapatite chromatography followed by native-gel electrophoresis, CF appears to contain prominent bands at approximately 30, 40, 45, 50, and 60 kDa. When added to starved wild-type cells, both recombinant countin (the 40-kDa band) and recombinant CF50 (the 50-kDa band) decrease group size, decrease adhesion, and increase motility (3, 8). It is thus possible that CF consists of countin and CF50 and that some of the other bands are contaminants and may have some other function. To begin to elucidate the function of these other secreted proteins, we have examined the effect of disrupting the gene encoding a protein present in the 45-kDa band.
MATERIALS AND METHODS
Sequence assembly.
A search of the Dictyostelium BLAST site (http://dicty.sdsc.edu/) was performed by using the tryptic sequences obtained from the 45-kDa band in the partially purified CF as a query. The mixed tryptic sequences were resolved by partially matching to cDNA fragments from this initial search. PCR was done by using primers to these cDNA fragments and a cDNA library as a template to obtain overlapping sequence. A consensus sequence was assembled by using the GCG package (Accelrys Inc., San Diego, Calif.) to combine the fragments. This consensus was then used to requery the database and obtain additional sequence fragments. The process was repeated until the complete cDNA sequence was obtained. Similarly, PCR with genomic DNA as a template was used to construct a second consensus incorporating genomic DNA fragments to give the cf45-1 genomic sequence.
Cell culture and Northern blots.
Cell culture was performed as described by Brock and Gomer (2) with the strains described in reference 3. Photography of aggregates was performed as described by Brock et al. (3). Streams of cells on agar plates were photographed with a ×2 lens on a Nikon Microphot FX using transmitted light; the SM/5 agar plates were spread with Klebsiella aerogenes bacteria and inoculated at one point with Dictyostelium cells. RNA isolation and Northern blots were performed following the procedures of Brock et al. (3). For a probe, a 583-bp fragment of cf45-1 corresponding to nucleotides 67 to 1069 (the DNA sequence is available as GenBank AY212268) was generated by PCR by using the primers GTGAATGCTGATTGTGCTATCG and CCACACTCATTTCCATTACCAGC with cDNA from vegetative cells as the template. Conditioned HL5 growth medium and conditioned starvation medium (CM) were prepared by following the procedures of Brock et al. (3) with the exception that the samples were not concentrated.
Disruption of the cf45-1 gene.
A construct for the disruption of cf45-1 by homologous recombination was assembled and purified exactly as described previously (3) with the exception that to generate a 1,234-bp fragment on the 5′ side of cf45-1, the primers 45KOSacIIF2 (CGATAATCATCCGCGGACAACAAACCTATGGAGG) and 45-1XbaIR (GCATGCTCTAGAGCACAATCAGCATTCAC) were used for PCR; a 1,339-bp fragment on the 3′ side was generated by using 45-1HindIII-F1 (CTCTCATTCAAGCTTTTATGAATTTGGTGGTTGGAC) and 45-1ApaIR3 (CGCATTGGGCCCGTCCCACATGTTTTGGGAAG). Dictyostelium Ax2 cells were transformed following the procedures of Shaulsky et al. (30). PCR was used to identify transformant clones with an insertion of the blasticidin resistance cassette in the cf45-1 gene. Two different cf45-1− clones were identified and were designated DB45-1-1 and DB45-1-5. They had the same phenotypes, and most of the experiments reported here were done with both clones. In all of the assays where both clones were examined, the two clones behaved identically. The data for cf45-1− shown in this report used the clone DB45-1-5.
Antibody production and sieving gel chromatography.
To make a recombinant fragment of CF45-1, PCR was performed by using cDNA as a template with the primers GGCAGCCATATGGATTGTGCTATCGATTTTGATAG and GCCGGATCCTCGAGTTAAGTTGATGCTGAACATG. This generated a PCR fragment with a NdeI site on the 5′ end and a XhoI site on the 3′ end, encoding the region starting with the putative first amino acid of the secreted protein and ending just before a serine-glycine-rich region near the C terminus of CF45-1 (Fig. 1, arrows). This serine-glycine-rich region has a high degree of similarity to a region near the C terminus of CF50 (3). The PCR product was digested with NdeI and XhoI. Ligation of the fragment into the pET15b expression vector (Novagen, Madison, Wis.), sequence verification, and expression of the His-tagged protein was performed as described by Brock et al. (3). Immunization of rabbits and production of affinity-purified antibodies was done at Bethyl Laboratories (Montgomery, Tex.). Western blots were performed following the procedures of Brock et al. (3) with the affinity-purified anti-CF45-1 antibodies at 0.4 μg/ml. Staining Western blots with anti-CF50 and anti-countin antibodies was performed as previously described (3). Recombinant CF50 was prepared following the procedures of Brock et al. (3). Sieving gel chromatography was done as previously described (2).
FIG. 1.
The predicted amino acid sequence of CF45-1. An underline indicates the sequence of the N terminus of one of the 45-kDa bands in the crude preparation of CF. Vertical arrows delineate the first and last amino acids encoded by the cf45-1 cDNA fragment that was expressed in bacteria and used to immunize rabbits for antibody production. Shading indicates a potential N-linked glycosylation site. The GenBank accession number is AY212268.
Production of recombinant CF45, group size assays, and lysozyme assays.
A recombinant protein corresponding to the full length of the polypeptide backbone of the secreted CF45-1 was produced as described above with the exception that the second primer was CGGATCCTCGAGTTATGATGAACCTGATCCCG. This generated a His-tagged fusion protein that contained the region from the first arrow in Fig. 1 to the stop codon. Group size assays with cells developing on filter pads were done as previously described (1). The recombinant CF45-1 proteins were assayed for lysozyme activity by following the procedures of Monchois et al. (25) with hen egg white lysozyme (Sigma) as a standard.
Adhesion, motility, cell type differentiation, and glucose levels.
Cell-cell adhesion and cell motility were assayed as described by Brock et al. (3). The assays for the cf45-1− cells were done in parallel at the same time as the assays done in that paper; hence, the values for the wild-type cells are the same in both studies. The percentage of cells expressing the cell type-specific markers CP2 or SP70 when starved at very low cell density was assayed as described by Wood et al. (36), and the effect of adding recombinant proteins to cells at low density was assayed by following the procedures of Brock et al. (3). Glucose levels were measured by following the procedures of Jang et al. (14).
RESULTS
cf45-1 is expressed in vegetative and early developing cells.
A crude purification of CF was previously obtained and found to contain several proteins (1). From a 45-kDa band in the preparation, a mixture of two different N-terminal sequences, namely, (D/A)-(N/C)-(A/S)-(V/I)-D-(F/Y)-(D/R)-(A/S)-(D/K)-(G/T)-(V/A)-(N/V), was obtained. The sequence of what appeared to be the more abundant amino acid at each step of the sequencing reaction was previously published as DNAVDFDADGVN (1). For initial sequence assembly, this polypeptide sequence was used to search databases. While this initial search produced no exact match, a partial match was found. Comparison of the mixed peptide sequence to the partial match from the database identified two amino acid sequences, namely, DCAIDFDSDTVN and ANSVDYRAKGAV, which when combined would produce the above mixed sequence.
One of the peptides (ANSVDYRAKGAV) was a close match to the predicted sequence of the CprF cysteine protease (the published sequence of CprF has a W instead of a Y at the sixth amino acid in the peptide) (26). The other peptide (Fig. 1, underlined) exactly matched part of the predicted amino acid sequence encoded by a Dictyostelium gene that we designated ctnC and that in the rest of this paper will be referred to as cf45-1. The cf45-1 gene has six introns, an open reading frame that has a 5′ ATG preceded by three A's, and an AATAAA polyadenylation signal 20 nucleotides after the stop codon.
The predicted molecular mass of the entire CF45-1 polypeptide backbone is 29.3 kDa, and the predicted pI is 3.6; for the region starting at the N terminus of the secreted protein and going to the predicted C terminus, the mass is 27.2 kDa and the pI is 3.4. Secondary structure prediction routines predict that the first 75% of the protein is a mixture of helices, turns, and sheets and that the last ∼25% of the protein is largely a random coil. In the open reading frame, there is a potential signal sequence immediately before the sequence of the N terminus of the protein we purified, suggesting that as with most secreted proteins, the N-terminal signal sequence is cleaved. For the secreted form of CF45-1, there are no large regions of hydrophobicity or charge. There is a predicted N-linked glycosylation site near the middle of the secreted portion of the protein. Many proteins secreted by Dictyostelium are glycosylated, and thus the molecular mass of the polypeptide backbone is ∼70% of that of the purified protein (2, 3, 13, 15, 35). In other cases, proteins can migrate on sodium dodecyl sulfate (SDS)-polyacrylamide gels with apparent molecular masses that are higher than their true masses (10, 22, 24). We thus hypothesize that the difference between the mass of the secreted CF45-1 polypeptide backbone (27 kDa) and 45 kDa is due to either glycosylation or anomalous migration on the SDS-polyacrylamide gels. There are no predicted O-linked glycosylation sites or other predicted motifs. From amino acids 49 to 219, there is a 29% identity and 43% similarity to Entamoeba histolytica lysozyme. The secreted portion of CF45-1 has a 67% identity to the secreted portion of CF50; both proteins have a serine- and glycine-rich domain at their C termini. Starting at amino acid 230 and ending at the C terminus, there is a sequence of 68 serines and glycines, with six instances of the motif SGSGSSS. The serine-glycine-rich tail of CF50 is 70 amino acids long and contains amino acids other than serine and glycine. The serine-glycine-rich tails of the two proteins have a 67% identity, and the secreted regions without the tails have a 68% identity.
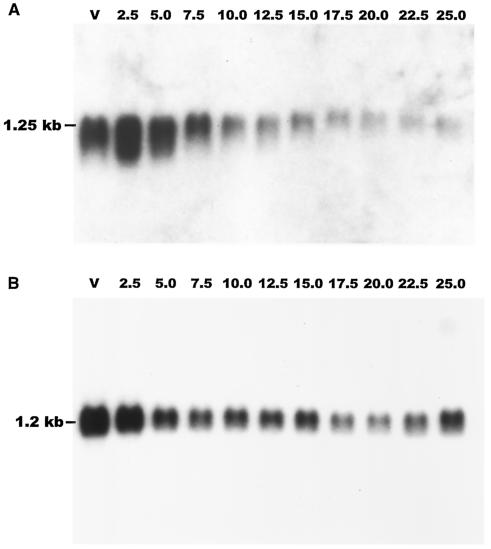
A Northern blot of vegetative cells and cells at different developmental times probed with a fragment of cf45-1 indicated that cf45-1 mRNA is present in vegetative cells and increases somewhat at 2.5 h of development, and then the message levels decline slightly. At 10 h, there is a strong decrease in message levels, and the cf45-1 mRNA levels then continue to decline until very little is seen at 25 h (Fig. 2A). A similar blot probed with a fragment of countin showed that countin mRNA is present in vegetative cells, and the levels then decline slightly through development (Fig. 2B). It was previously observed that cf50 mRNA shows an accumulation pattern roughly similar to that observed here for the cf45-1 mRNA (3), so together, the data indicate that cf50, cf45-1, and countin mRNAs are present during growth and early development.
FIG. 2.
cf45-1 and countin mRNAs are present in growing and developing cells. Ax2 wild-type cells were starved on filters, and samples were harvested at the indicated times (in hours) after starvation. (A) A Northern blot of RNA prepared from the samples was probed with a fragment of cf45-1. (B) A similar Northern blot was probed with a fragment of countin. V, vegetative cells.
CF45-1 is secreted, and cells lacking CF45-1 form huge groups.
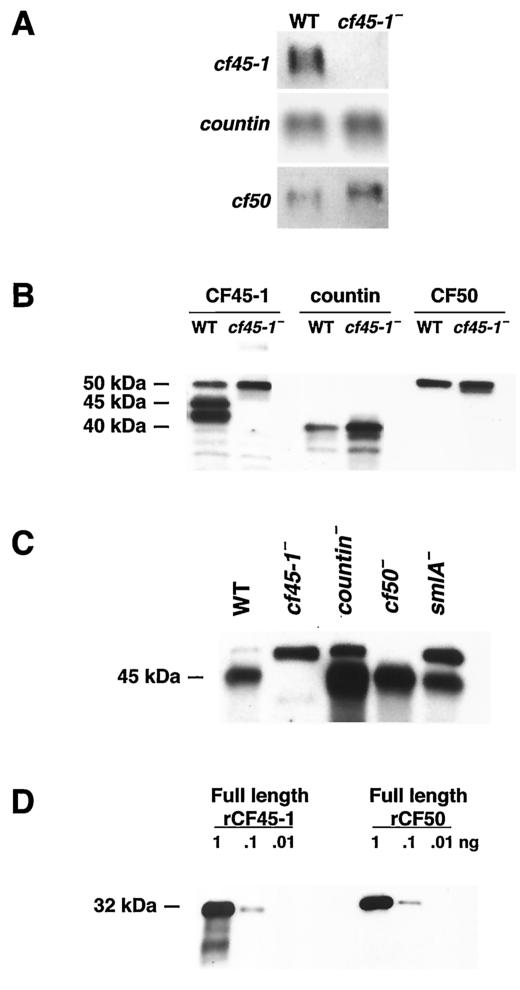
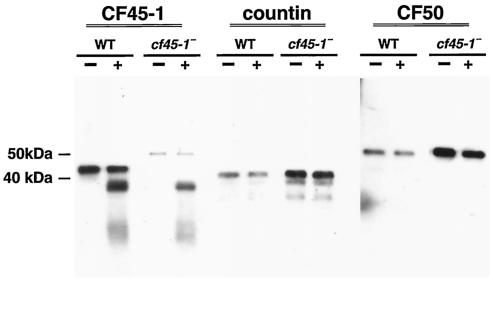
To examine the function of CF45-1, we used homologous recombination to disrupt its expression. The putative cf45-1− cells grew normally in liquid shaking culture. On agar plates spread with bacteria, the putative cf45-1− cells spread across the plate slightly faster than parental cells. A Northern blot of RNA from vegetative cells showed that the putative cf45-1− cells lack the cf45-1 mRNA and thus indeed have a disruption of cf45-1 expression (Fig. 3A). Compared to parental wild-type cells, cf45-1− cells have a higher level of cf50 mRNA and a slightly higher level of countin mRNA. A Western blot of vegetative cells stained with antibodies directed against a large portion of the polypeptide backbone of CF45-1 showed that cf45-1− cells lack the 45-kDa CF45-1 protein, although the antibodies appeared to also stain a band at 50 kDa and bands with both higher and lower molecular masses (Fig. 3B). Similar blots stained with anti-countin and anti-CF50 antibodies showed that compared to wild-type cells, cf45-1− cells had higher levels of countin and slightly higher levels of CF50 (Fig. 3B). Western blots of the exudate from cells developing for 20 h in shaking culture (CM) stained with anti-CF45-1 showed that the 45-kDa CF45-1 band was present in parental CM and absent from cf45-1− CM (Fig. 3C). There were higher levels of CF45-1 in countin− CM and cf50− CM and slightly higher levels in smlA− CM. However, the anti-CF45-1 antibodies often stained a band at approximately 50 kDa in the cf45-1− CM. This band was also present in parental, countin−, and smlA− CMs but was absent in cf50− CM. Since the region of CF45-1 used for antibody production has a 67% identity with CF50, we hypothesized that the 50-kDa band stained with the anti-CF45-1 antibodies is CF50. To test this directly, a Western blot of recombinant CF45-1 and recombinant CF50 was stained with anti-CF45-1 antibodies. As shown in Fig. 3D, the anti-CF45-1 antibodies indeed stain CF50. Together, the data indicate that cf45-1− cells lack the cf45-1 mRNA and the CF45-1 protein and that CF45-1 is secreted by developing cells.
FIG. 3.
Disruption of cf45-1 results in cells lacking the cf45-1 mRNA and the CF45-1 protein. (A) Northern blots of RNA from Ax2 parental and cf45-1− cells were probed with fragments of cf45-1 (top row), countin (middle row), or cf50 (bottom row). (B) Western blots of Ax2 parental (WT) or cf45-1− vegetative cells were stained with anti-CF45-1, anti-countin, or anti-CF50 antibodies; the position of molecular mass markers is shown at left. (C) A Western blot of 20-h CM (containing material secreted by developing cells) from the indicated cell lines was stained with anti-CF45-1 antibodies. (D) A Western blot of the indicated amounts of either recombinant CF45-1 or recombinant CF50 was stained with anti-CF45-1 antibodies; this indicates that the 50-kDa band that the anti-CF45-1 antibodies binds to is CF50. The molecular mass of the recombinant CF45-1 (including the His tag) is 31.5 kDa, and the mass of the recombinant CF50 is 33 kDa.
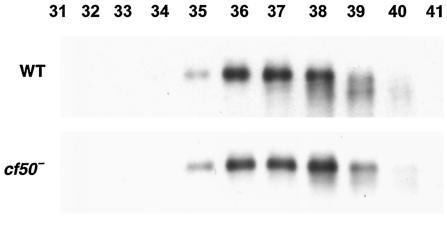
Sieving gel chromatography of CM indicated that countin and CF50 are both present in CM in fractions that elute in a peak with a maximum near 450 kDa (2, 3). When anti-CF45-1 antibodies were used to stain a Western blot of fractions from sieving gel chromatography of Ax2 CM, there was a broad peak with a maximum near 450 kDa (Fig. 4). Fractionation of CM from cf50− cells showed that the fractions containing countin had shifted to an apparently lower molecular mass (3). This was interpreted as indicating that countin and CF50 are in a complex and that removal of CF50 from the complex caused the complex to be apparently smaller (3). Similar fractionation of CM from cf50− cells indicated that CF45-1 was present in cf50− CM in a broad peak with a maximum lower than 450 kDa (Fig. 4). This indicates that removal of CF50 from CM affects the elution profile of CF45-1 in sieving gel chromatography. This in turn suggests that some of the CF45-1 may be in a complex with CF50.
FIG. 4.
CF45-1 appears to be part of a large complex in the CM from starving cells. CM was made from Ax2 parental or cf50− cells and then concentrated ∼10-fold. After size fractionation on a sieving gel column, the fractions were assayed for CF45-1 by Western blotting. Staining of the fractions from Ax2 CM was previously shown for countin and CF50; both proteins elute with a peak at fraction 37 (3). Molecular mass standards eluted with peaks at fractions 37 (443 kDa) and 41 (66 kDa).
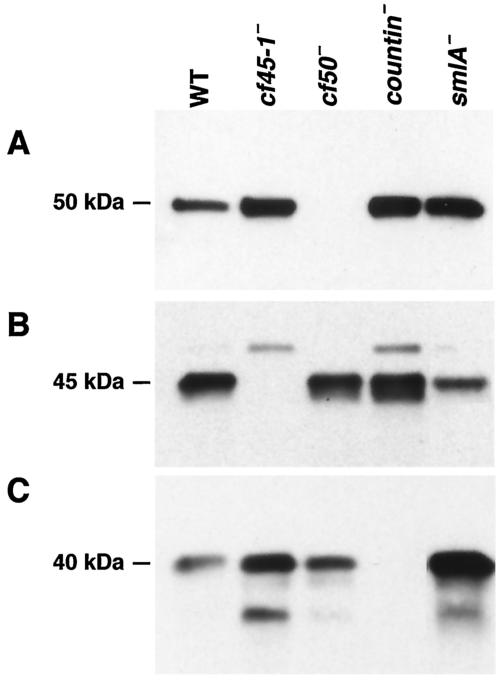
Both countin and CF50 are secreted into HL5 growth medium by growing cells (3). To determine if growing cells also secrete CF45-1, cells were grown in HL5 for 20 h, and the conditioned growth medium was clarified by centrifugation. A Western blot of the conditioned growth medium stained with anti-CF50 antibodies showed that cf45-1− cells accumulate more CF50 than parental cells (Fig. 5A). As previously observed, cf50− cells do not accumulate CF50, and countin− and smlA− cells accumulate more CF50 than parental cells. A blot of the same samples stained with anti CF45-1 antibodies showed staining in the exudate from wild-type cells of a band at 45 kDa (Fig. 5B). Conditioned HL5 from wild-type cells also often showed a weak staining of a band at 50 kDa. In the exudate from cf45-1− cells, the 45-kDa band was not stained, suggesting that it is CF45-1. There was weak staining of a 50-kDa band, while in the growth medium conditioned by cf50− cells, the 50-kDa band was absent and the 45-kDa band was present. This suggests that the 50-kDa band stained with anti-CF45-1 antibodies is an artifact due to the anti-CF45-1 antibodies cross-reacting with the similar (67% identity) CF50 protein. The growth medium from countin− and smlA− cells also contained CF45-1; interestingly, although smlA− cells appear to oversecrete CF activity, there was less CF45-1 in the smlA− growth medium than in the parental medium. Although there was approximately as much CF50 in the countin− and smlA− growth media, there was less staining of the 50-kDa band by the anti-CF45-1 antibodies in the smlA− media (Fig. 5B). This difference in the staining of the 50-kDa band was variable. Together with the observation that smlA− cells do not secrete significantly increased levels of CF45-1 into starvation medium, the relatively small amount of CF45-1 in the smlA− CM suggests that smlA− cells do not secrete CF45-1 as a stoichiometric complex with countin and CF50. A blot of the same growth medium samples stained with anti-countin antibodies showed that countin was present in all samples except the medium from the countin− cells (Fig. 5C). There appeared to be more countin in the growth medium conditioned by cf45-1− cells. It was previously observed that some countin protein appears to be degraded in the starvation medium from cf50− cells (3), and we observed here that there is a similar appearance of what appears to be a breakdown product of countin in the growth medium from cf45-1− cells.
FIG. 5.
Growing cells secrete CF45-1. The indicated cell types were inoculated into HL5 medium at 1 × 106 cells/ml and grown in shaking culture for 20 h. The conditioned growth medium was clarified by centrifugation. (A) A Western blot of the conditioned growth media from the indicated cell types was stained with anti-CF50 antibodies. Similar blots were stained with anti-CF45-1 (B) and anti-countin (C) antibodies.
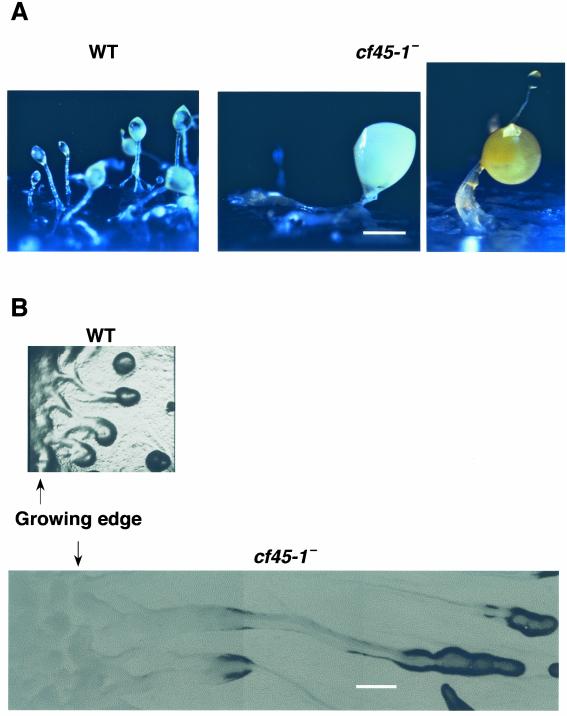
As shown in Fig. 6A, the cf45-1− cells formed huge misshapen fruiting bodies when the cells were grown on agar plates with bacteria and allowed to starve in situ. This appeared to be due in part to the cells forming huge streams (Fig. 6B). These large streams were observed from the time a colony of cf45-1− cells started forming streams to the time a colony covered an agar plate. However, when the cells were grown in liquid shaking culture and starved on filter pads in buffer or water, or on agar plates containing either phosphate buffer with magnesium (PBM), no buffer (water), or SM/5 medium, they occasionally formed fruiting bodies that were approximately half the size of wild-type fruiting bodies. The rest of the time when the cells grown in liquid were starved on filter pads at low cell densities, the cf45-1− cells formed larger groups than the parental cells. This behavior is in sharp contrast to countin− and cf50− cells, which under all the conditions described above form larger groups than parental cells.
FIG. 6.
cf45-1− cells form large groups and fruiting bodies. (A) Cells were grown on a bacterial lawn on an agar plate and allowed to overgrow the bacteria, starve, and form fruiting bodies. Bar, 0.5 mm. (B) The streams of cells formed at the edge of a colony of cells growing across a bacterial lawn were photographed. Bar, 1 mm.
Recombinant CF45-1 affects group size.
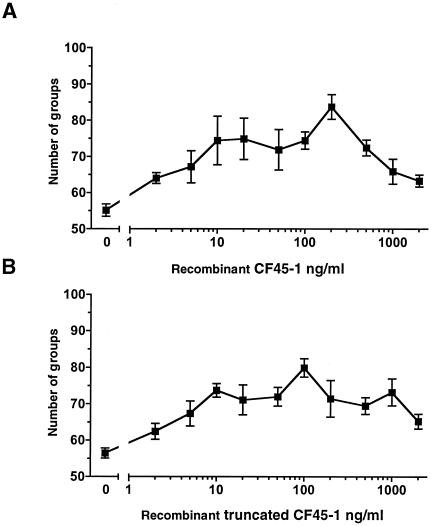
Addition of recombinant CF45-1 to the cf45-1− cells increased the number of groups that they formed (Fig. 7A). The 50% effective concentration (EC50) for recombinant CF45-1 was approximately 8 ng/ml. The truncated recombinant fragment of CF45-1 used for antibody production also increased the number of groups formed by cf45-1− cells (Fig. 7B). For both the full-length and truncated recombinant CF45-1s, there was a decrease in the effect on group size as the concentrations increased above ∼200 ng/ml. The number of groups formed by cf45-1− cells in the presence of 200 ng of recombinant CF45-1/ml was less than the number of groups formed by wild-type cells in the absence of exogenous CF45-1 (Fig. 8), suggesting that recombinant CF45-1 partially but not completely rescued the phenotype of cf45-1− cells.
FIG. 7.
Recombinant CF45-1 partially rescues the phenotype of cf45-1−cells. (A) cf45-1− cells were starved in the presence of different concentrations of full-length recombinant CF45-1. Values are means ± standard errors of the means from three independent experiments. The difference between buffer alone (0) and 200 ng/ml is significant (P < 0.005; t test). (B) cf45-1− cells were starved in the presence of recombinant truncated CF45-1; values are means ± standard errors of the means from three independent experiments. The difference between buffer alone (0) and 100 ng/ml of the truncated CF45-1 is significant (P < 0.005).
FIG. 8.
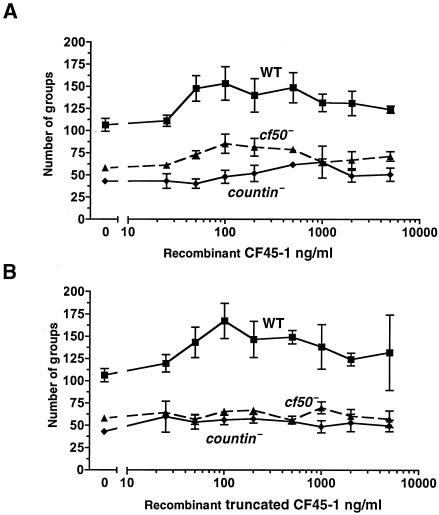
Recombinant CF45-1 affects group size in wild-type, countin−, and cf50− cells. (A) Cells were starved in the presence of different concentrations of full-length recombinant CF45-1. Values are means ± standard errors of the means from three independent experiments. For wild-type and cf50− cells, the differences between buffer alone (0) and 50 ng/ml are significant (P < 0.05; t test); for countin− cells, the difference between buffer (0) and 500 ng/ml is significant (P < 0.005). (B) Cells were starved in the presence of recombinant truncated CF45-1; values are means ± standard errors of the means from three independent experiments. The absence of error bars indicates that the standard error of the mean was smaller than the plot symbol. For wild-type and countin− cells, the differences between buffer alone (0) and 100 ng of the truncated CF45-1/ml were significant (P < 0.05); the difference between buffer (0) and 200 ng/ml was significant for cf50− cells (P < 0.05).
Addition of recombinant CF45-1 to wild-type cells increased the number of groups that they form (Fig. 8A). The maximal effect occurred at approximately 100 ng/ml; at higher concentrations, the effect of recombinant CF45-1 decreased somewhat. There was a slight effect on cf50− cells and a very slight effect of recombinant CF45 on countin− cells. This may be due to the fact that cf50− and countin− cells accumulate a large amount of extracellular CF45-1 (Fig. 3C). The EC50 for wild-type cells was approximately 35 ng/ml, while that for cf50− cells was ∼45 ng/ml. A much higher concentration of recombinant CF45-1 was needed to affect group size in countin− cells (Fig. 8A). The recombinant truncated CF45-1 protein used for antibody production also affected group size (Fig. 8B). The EC50 for wild-type cells was approximately 40 ng/ml. This protein also had a slight but detectable effect on countin− and cf50− cells. Together, the data indicate that CF45-1 can affect group size.
Since the predicted amino acid sequence of CF45-1 has similarity to that of lysozyme, and the recombinant CF45-1 and the truncated recombinant fragment of CF45-1 used for antibody production appeared to have bioactivity in group size assays, these proteins were tested for lysozyme activity. A hen egg white lysozyme standard had ∼100 units/μg in both PBM buffer and 100 mM potassium phosphate (pH 6.4). The full-length and truncated CF45-1 both had no significant activity in either buffer, with a detection limit of 0.007 units/μg.
Cells lacking CF45-1 appear to contain more countin and CF50 than parental cells (Fig. 3B) and secrete more of these proteins into growth medium (Fig. 5). We also observed that cf45-1− cells secrete more countin and CF50 during development (Fig. 9, middle and right panels). We also occasionally observed that the countin and CF50 proteins secreted by cf45-1− cells migrated on SDS-polyacrylamide gels as if their molecular masses were 38 and 48 kDa, respectively, but then a week later, the countin and CF50 secreted by the same cultures would have apparent molecular masses indistinguishable from those of the 40-kDa countin and 50-kDa CF50 secreted by wild-type cells, as seen in Fig. 9. To determine if extracellular CF45-1 can affect the amount of accumulated extracellular countin and CF50, recombinant CF45-1 was added to cells starving in shaking culture. As shown in the left panel of Fig. 9, wild-type cells secrete CF45-1, while cf45-1− cells do not, and the recombinant CF45-1 is detected by the anti-CF45-1 antibodies as a ∼37-kDa band on an SDS-polyacrylamide gel. The presence of the recombinant CF45-1 caused a very slight decrease in the amount of countin and CF50 secreted by both wild-type and cf45-1− cells (Fig. 9, middle and right panels).
FIG. 9.
Exogenous recombinant CF45-1 slightly decreases the overaccumulation of extracellular countin and CF50 by cf45-1− cells. Wild-type (WT) and cf45-1− cells were starved in shaking culture in the absence (−) or presence (+) of 100 ng of recombinant CF45-1/ml. After 6 h, the conditioned starvation buffer was collected, and Western blots of this material were stained for the presence of CF45-1 (left panel), countin (middle panel), or CF50 (right panel). Bars at left indicate the positions of molecular mass markers.
Although countin− and cf50− cells both form large fruiting bodies, examination of the expression of two cell type-specific markers indicated that countin− cells have a normal initial cell type differentiation, while that of cf50− cells is abnormal (2, 3) (Table 1). The marker CP2 is expressed in a subset of prestalk cells that gives rise to the first set of cells expressing other prestalk markers such as ecmA (4, 11), and the marker SP70 is a prespore protein that later appears on spore coats (11). As shown in Table 1, in the absence of added proteins, cf45-1− cells had a slightly altered initial cell type differentiation. The addition of recombinant CF50 partially rescued the abnormal initial cell type differentiation of the cf50− cells (3) (Table 1). Surprisingly, recombinant CF45-1 also caused a partial rescue of the differentiation. However, in cf45-1− cells, neither recombinant CF50 nor recombinant CF45-1 rescued the slight abnormality in initial differentiation of CP2-positive cells, although there was a slight decrease in the percentage of SP70-positive cells. Together, the data suggest that in terms of the initial cell type differentiation as assessed by the markers CP2 and SP70, cf45-1− cells have a phenotype intermediate between that of countin− and that of cf50− cells. Recombinant CF45-1 was also seen to have an effect on initial cell type differentiation when added to cf50− cells and a slight effect on cf45-1− cells.
TABLE 1.
Differentiation of cells into CP2-positive and SP70-positive cells at low cell densitya
| Cell line | Protein added | % CP2 positive | % SP70 positive |
|---|---|---|---|
| Ax2 | None | 11.8 ± 0.3 | 32.8 ± 0.7 |
| rCF45-1 | 10.5 ± 0.5 | 31.6 ± 1.2 | |
| rCF50 | 9.4 ± 0.2 | 28.9 ± 0.9 | |
| cf50− | None | 0.6 ± 0.2 | 49.2 ± 1.8 |
| rCF45-1 | 6.4 ± 1.4 | 44.6 ± 0.9 | |
| rCF50 | 8.5 ± 0.5 | 34.6 ± 0.6 | |
| cf45-1− | None | 8.3 ± 0.5 | 42.5 ± 0.8 |
| rCF45-1 | 8.3 ± 0.4 | 36.8 ± 0.7 | |
| rCF50 | 7.1 ± 0.2 | 37.3 ± 1.0 |
Approximately 2,000 cells of each cell type were starved in duplicate wells of an eight-well slide. When indicated, recombinant CF45-1 (rCF45-1) or recombinant CF50 (rCF50) was added to a final concentration of 100 ng/ml to the well. cAMP was added 6 h after starvation to induce accumulation of the SP70 and CP2 antigens. The cells were fixed 18 h after starvation, and the cells in one well were stained for CP2 while the cells in the other well were stained for SP70. Values are the means ± standard errors of the means of the percent positive cells from three separate experiments.
Like countin− and cf50− cells, cf45-1− cells have high glucose levels, high cell-cell adhesion, and low motility.
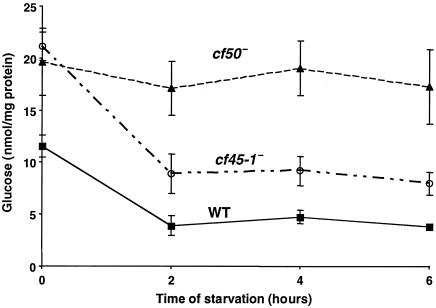
CF regulates group size in part by repressing levels of cytosolic glucose (14). It was previously observed that the Ax4 wild-type strain has approximately 13 nmol glucose/mg of protein, and the levels fall to approximately 9 nmol glucose/mg of protein at 6 h of development (14). Our laboratory strain of Ax2 wild-type cells form slightly smaller fruiting bodies than Ax4 cells. Interestingly, these cells have slightly lower glucose levels than Ax4 cells (Fig. 10). It was previously observed that countin− cells have higher levels of glucose than parental cells (14), and we found that cf50− cells also have higher levels of glucose than parental cells (Fig. 10). Vegetative cf45-1− cells have glucose levels that are as high as those of cf50− cells (Fig. 10). The glucose levels in cf45-1− cells then decrease during development, remaining, however, higher than the levels in parental cells (Fig. 10). Together, the data suggest that cf45-1− cells have higher levels of glucose than parental cells.
FIG. 10.
Both cf45-1− and cf50− cells have high levels of glucose. Cells were grown to 1 × 106 cells/ml in shaking culture in HL5, washed in PBM buffer, and starved in shaking culture for the indicated times. Cells were then collected by centrifugation, and the amount of glucose in the lysed cells was measured. WT indicates parental Ax2 cells. Values are means ± standard errors of the means from four independent experiments.
A key prediction from Monte Carlo simulations of cells in a stream was that a high cell-cell adhesion and/or a low random cell motility would keep a stream from breaking up, thus keeping the aggregation streams intact and leading to larger groups (27). Both countin− and cf50− cells have a higher cell-cell adhesion than their parental cells (3, 27). Compared to wild-type parental cells, cf45-1− cells had 8.9% ± 0.7% higher adhesion at 2 h of development, 7.5% ± 1.4% higher adhesion at 4 h, and 4.5% ± 1.1% higher adhesion at 6 h (means ± standard errors of the means from five separate experiments). The adhesion assays used cells that were forming streams on filter pads. The development and stream morphologies were similar from the time of starvation to 8 h of development, and the streams had not begun to coalesce. Thus, whereas during later development the cf45-1− cells, those cells in the interiors of huge groups might be starved for oxygen or be in the presence of high concentrations of waste products, in these assays, the cells were in structures of a similar size.
In addition to high cell-cell adhesion, both countin− and cf50− cells have low cell motility at 6 h after starvation, when wild-type streams are starting to break up (3, 34). cf45-1− cells also have significantly decreased cell motility at this time (Fig. 11). These assays were done with cells developing in submerged monolayer culture, so as above, these differences are not due to factors such as a lack of oxygen. Together, the data indicate that disruption of cf45-1 has qualitatively the same effect on cell-cell adhesion and cell motility as disruption of countin or cf50.
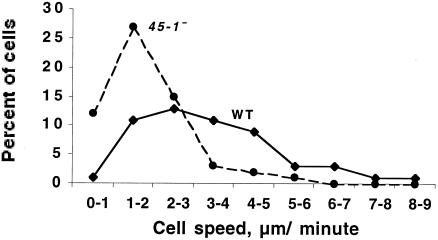
FIG. 11.
Developing cf45-1− cells move more slowly than parental Ax2 cells. Cells were starved in submerged culture for 6 h, and then fields of cells were observed by time-lapse videomicroscopy. For each cell, the approximate center of the cell was tracked for 5 min, and the cell speeds were then binned in 1 μm/min increments. The average speeds were 3.3 ± 0.2 μm/min for Ax2 cells and 1.9 ± 0.1 μm/min for cf45-1− cells (means ± standard errors of the means for 60 cells each, combining the observations from 20 cells in three separate experiments). A two-tailed Mann-Whitney test showed that the speed distributions were different, with a P value of <0.005.
DISCUSSION
CF, which plays a significant role in regulating the size of Dictyostelium fruiting bodies, appears to be a 450-kDa complex of proteins. It was previously found that countin and CF50, two proteins present in a crude purification of CF, are indeed components of CF (2, 3). In this report, we find that CF45-1, which was also found in the crude CF preparation, also appears to be involved in regulating group size. Like countin and CF50 (2, 3), CF45-1 is present in CM in fractions with a peak near 450 kDa. In the CM from cf50− cells, countin shows a sharp change in elution profile to an apparently lower molecular mass (3). In the cf50− CM, CF45-1 also shows a change in elution profile, although not as dramatic as that seen with countin. This suggests that at least some CF45-1 may be in a complex with CF50 and thus also with countin. Compared to wild-type parental cells, cells lacking countin or CF50 have higher glucose levels, higher cell-cell adhesion, and lower motilities and as a result form large groups and fruiting bodies (2, 3). Cells lacking CF45-1 also have high glucose levels, high adhesion, and low motility, and after overgrowing a bacterial lawn on an agar plate, they form large groups and fruiting bodies. Recombinant CF45-1 can affect group size when added to cells, suggesting that it has bioactivity. The ability of exogenous recombinant CF45-1 to reduce group size in cf45-1− cells suggests that the increased group size in the cf45-1− cells was due to disruption of the cf45-1− gene and not due to a second mutation.
Although the CF45-1 sequence has some similarity to that of lysozyme, recombinant CF45-1 has no detectable lysozyme activity. Recombinant CF50 also reduces group size when added to cells and has some similarity to lysozyme, but it has very little lysozyme activity (3). Countin has some similarity to amoebapores and has proteins that form holes in membranes, but countin has very little amoebapore activity at physiological pHs (8). Our working hypothesis is that like countin and CF50, CF45-1 may have evolved from digestive or defensive enzymes secreted by cells. Both CF50 and CF45-1 have large serine-glycine-rich tails. Other proteins with such regions are lustrin A, a matrix protein in abalone shell and pearls (31); loricrin, the major cornified envelope protein from human skin (12, 23); and keratin, a structural protein in skin (18). Thus, another possibility is that CF50 and CF45-1 may have evolved from structural proteins.
Adding different concentrations of recombinant CF45-1 to cf45-1− cells showed that the recombinant CF45-1 could decrease group size but that even at the optimal concentration, it could not decrease the group size of the cf45-1− cells to the size of the groups formed by parental cells. This suggests that the exogenous recombinant CF45-1 is not fully active, presumably due to misfolding and/or the lack of posttranslational glycosylations, and that this lack of activity cannot be compensated for by adding more of the protein. One possible explanation for this is that CF45-1 functions as part of a complex of a defined stoichiometry with some other protein(s), such as the other components of CF, and that above ∼200 ng of recombinant CF45-1/ml, some other protein becomes the limiting factor. This in turn suggests that CF45-1 is not the sole factor that determines group size.
Cells with a disruption of the gene encoding countin secrete CF50, and cf50− cells secrete countin (2, 3) (Table 2). cf45-1− cells accumulate abnormally high levels of extracellular CF50, and for unknown reasons, they secrete variable amounts of countin (Table 2). smlA− cells do not secrete CF45-1 as a stoichiometric complex with countin and CF50, suggesting that in this mutant, the secretion of CF45-1 is not coordinately regulated with the secretion of countin and CF50. When cf45-1− cells are starved on filter pads, they occasionally form smaller groups, and we have observed that this tends to occur when the cultures are secreting high levels of countin. Thus, our working hypothesis is that high extracellular levels of countin can force cf45-1− cells to form small groups. The observation that countin− and cf50− cells secrete more CF45-1 protein than wild-type cells, yet form larger groups, again suggests that the extracellular level of CF45-1 is not the sole determinant of group size. In addition, the observation that when cells lack either countin, CF45-1, or CF50, the secretion of the other two proteins is altered, often increasing, suggests that the three proteins affect each others' secretion or stability and that the proteins are not always secreted in equimolar amounts.
TABLE 2.
Summary of the observed levels of the three known Dictyostelium secreted group size-regulatory proteins in CM
| Protein | Level in the indicated cell typea
|
||||
|---|---|---|---|---|---|
| smlA− | WT | countin− | cf45-1− | cf50− | |
| CF50 | ++ | + | +++ | +++ | − |
| CF45-1 | ++ | + | +++ | − | +++ |
| Countin | +++ | + | − | Variable | Variable |
−, none observed; +, level seen in CM from wild type (WT) cells; ++, higher level than that seen with WT cells; +++, considerably higher level than that seen with WT cells. Variable indicates that for unknown reasons, cultures can have low levels along with degradation products, levels comparable to those seen in CM from WT cells, or even higher levels than those in CM from WT cells. Results are as indicated by Western blotting.
The EC50 for recombinant countin with respect to decreasing the size of groups formed by wild-type cells is ∼3 ng/ml (8). Similarly, the EC50 for semipurified CF is ∼100 ng/ml (2), and the EC50 for recombinant CF50 is ∼50 ng/ml (3). The EC50 for recombinant CF45-1 is ∼35 ng/ml when added to wild-type cells. Thus, the EC50 for recombinant CF45-1 is roughly comparable to that of recombinant CF50, and these are both somewhat higher than that of countin. Adding recombinant versions of either countin, CF45-1, or CF50 to wild-type cells causes the formation of smaller groups. These data suggest that there does not appear to be a single key component of CF. Instead, cells appear to respond to all three of the above proteins. Because of their different effects on the differentiation of CP2-positive and SP70-positive cells, it appears that they might have separate functions. Although one can envision a simple mechanism whereby one protein secreted at a fixed rate mediates group size regulation, for unknown reasons group size in Dictyostelium is regulated by at least three different proteins.
Acknowledgments
We thank Darrell Pilling for assistance with some of the graphs. We enthusiastically thank the members of the Japanese Dictyostelium cDNA sequencing consortium for the cDNA sequences and the members of the international Dictyostelium genomic DNA sequencing project for the genomic sequence. R.H.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Brock, D. A., F. Buczynski, T. P. Spann, S. A. Wood, J. Cardelli, and R. H. Gomer. 1996. A Dictyostelium mutant with defective aggregate size determination. Development 122:2569-2578. [DOI] [PubMed] [Google Scholar]
- 2.Brock, D. A., and R. H. Gomer. 1999. A cell-counting factor regulating structure size in Dictyostelium. Genes Dev. 13:1960-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock, D. A., R. D. Hatton, D.-V. Giurgiutiu, B. Scott, R. Ammann, and R. H. Gomer. 2002. The different components of a multisubunit cell number-counting factor have both unique and overlapping functions. Development 129:3657-3668. [DOI] [PubMed] [Google Scholar]
- 4.Clay, J. L., R. A. Ammann, and R. H. Gomer. 1995. Initial cell type choice in a simple eukaryote: cell-autonomous or morphogen-gradient dependent? Dev. Biol. 172:665-674. [DOI] [PubMed] [Google Scholar]
- 5.Clement, K., C. Vaisse, N. Lahlou, S. Cabrol, V. Pelloux, D. Cassuto, M. Gourmelen, C. Dina, J. Chambaz, J. Lacorte, A. Basdevant, P. Bougneres, Y. Lebouc, P. Froguel, and B. Guy-Grand. 1998. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398-401. [DOI] [PubMed] [Google Scholar]
- 6.Devreotes, P. 1989. Dictyostelium discoideum: a model system for cell-cell interactions in development. Science 245:1054-1058. [DOI] [PubMed] [Google Scholar]
- 7.Firtel, R. A. 1995. Integration of signaling information in controlling cell-fate decisions in Dictyostelium. Genes Dev. 9:1427-1444. [DOI] [PubMed] [Google Scholar]
- 8.Gao, T., K. Ehrenman, L. Tang, M. Leippe, D. A. Brock, and R. H. Gomer. 2002. Cells respond to and bind countin, a component of a multisubunit cell number counting factor. J. Biol. Chem. 277:32596-32605. [DOI] [PubMed] [Google Scholar]
- 9.Garrod, D. R., and J. M. Ashworth. 1972. Effect of growth conditions on development of the cellular slime mould Dictyostelium discoideum. J. Embryol. Exp. Morph. 28:463-479. [PubMed] [Google Scholar]
- 10.Gerst, J., L. Rodgers, M. Riggs, and M. Wigler. 1992. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc. Natl. Acad. Sci. USA 89:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomer, R. H., S. Datta, and R. A. Firtel. 1986. Cellular and subcellular distribution of a cAMP-regulated prestalk protein and prespore protein in Dictyostelium discoideum: a study on the ontogeny of prestalk and prespore cells. J. Cell Biol. 103:1999-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohl, D., T. Mehrel, U. Lichti, M. Turner, D. Roop, and P. Steinert. 1991. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J. Biol. Chem. 266:6626-6636. [PubMed] [Google Scholar]
- 13.Jain, R., and R. H. Gomer. 1994. A developmentally regulated cell surface receptor for a density-sensing factor in Dictyostelium. J. Biol. Chem. 269:9128-9136. [PubMed] [Google Scholar]
- 14.Jang, W., B. Chiem, and R. H. Gomer. 2002. A secreted cell-number counting factor represses intracellular glucose levels to regulate group size in Dictyostelium. J. Biol. Chem. 277:31972-31979. [DOI] [PubMed] [Google Scholar]
- 15.Jung, E., and K. Williams. 1997. The production of recombinant glycoproteins with special reference to simple eukaryotes including Dictyostelium discoideum. Biotechnol. Appl. Biochem. 25:3-8. [DOI] [PubMed] [Google Scholar]
- 16.Kamboj, R. K., T. Y. Lam, and C. H. Siu. 1990. Regulation of slug size by the cell adhesion molecule gp80 in Dictyostelium discoideum. Cell Regul. 1:715-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessin, R. H. 2001. Dictyostelium evolution, cell biology, and the development of multicellularity. Cambridge University Press, New York, N.Y.
- 18.Langbein, L., H. Heid, I. Moll, and W. Franke. 1993. Molecular characterization of the body site-specific human epidermal cytokeratin 9: cDNA cloning, amino acid sequence, and tissue specificity of gene expression. Differentiation 55:57-71. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S., and A. McPherron. 2001. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 98:9306-9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loomis, W. F. 1975. Dictyostelium discoideum: a developmental system. Academic Press, New York, N.Y.
- 21.Loomis, W. F. 1993. Lateral inhibition and pattern formation in Dictyostelium. Curr. Top. Dev. Biol. 28:1-46. [DOI] [PubMed] [Google Scholar]
- 22.McNew, J., F. Parlati, R. Fukuda, R. Johnston, K. Paz, F. Paumet, T. Sollner, and J. Rothman. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407:153-159. [DOI] [PubMed] [Google Scholar]
- 23.Mehrel, T., D. Hohl, J. Rothnagel, M. Longley, D. Bundman, C. Cheng, U. Lichti, M. Bisher, A. Steven, P. Steinert, S. Yuspa, and D. Roop. 1990. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 61:1103-1112. [DOI] [PubMed] [Google Scholar]
- 24.Meier, U., and G. Blobel. 1992. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell 70:127-138. [DOI] [PubMed] [Google Scholar]
- 25.Monchois, V., C. Abergel, J. Strugis, S. Jeudy, and J.-M. Claverie. 2001. Escherichia coli ykfE ORFan gene encodes a potent inhibitor of C-type lysozyme. J. Biol. Chem. 276:18437-18441. [DOI] [PubMed] [Google Scholar]
- 26.Ord, T., C. Adessi, L. Y. Wang, and H. H. Freeze. 1997. The cysteine proteinase gene cprG in Dictyostelium discoideum has a serine-rich domain that contains GlcNAc-1-P. Arch. Biochem. Biophys. 339:64-72. [DOI] [PubMed] [Google Scholar]
- 27.Roisin-Bouffay, C., W. Jang, and R. H. Gomer. 2000. A precise group size in Dictyostelium is generated by a cell-counting factor modulating cell-cell adhesion. Mol. Cell. 6:953-959. [PubMed] [Google Scholar]
- 28.Schaap, P. 1991. Intercellular interactions during Dictyostelium development., p. 147-178. In M. Dworkin (ed.), Microbial cell-cell interactions. American Society for Microbiology, Washington, D.C.
- 29.Shaffer, B. M. 1957. Variability of behavior of aggregating cellular slime moulds. Quart. J. Microsc. Sci. 98:393-405. [Google Scholar]
- 30.Shaulsky, G., R. Escalante, and W. F. Loomis. 1996. Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl. Acad. Sci. USA. 93:15260-15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen, X., A. Belcher, P. Hansma, G. Stucky, and D. Morse. 1997. Molecular cloning and characterization of Lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufesens. J. Biol. Chem. 272:32472-32481. [DOI] [PubMed] [Google Scholar]
- 32.Siu, C. H., and R. K. Kamboj. 1990. Cell-cell adhesion and morphogenesis in Dictyostelium discoideum. Dev. Genet. 11:377-387. [DOI] [PubMed] [Google Scholar]
- 33.Spann, T. P., D. A. Brock, D. F. Lindsey, S. A. Wood, and R. H. Gomer. 1996. Mutagenesis and gene identification in Dictyostelium by shotgun antisense. Proc. Natl. Acad. Sci. USA 93:5003-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang, L., T. Gao, C. McCollum, W. Jang, M. G. Vickers, R. Ammann, and R. H. Gomer. 2002. A cell number-counting factor regulates the cytoskeleton and cell motility in Dictyostelium. Proc. Natl. Acad. Sci. USA. 99:1371-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West, C. M., H. van Der Wel, and E. A. Gaucher. 2002. Complex glycosylation of Skp1 in Dictyostelium: implications for the modification of other eukaryotic cytoplasmic and nuclear proteins. Glycobiology 12:17R-27R. [DOI] [PubMed] [Google Scholar]
- 36.Wood, S. A., R. R. Ammann, D. A. Brock, L. Li, T. P. Spann, and R. H. Gomer. 1996. RtoA links initial cell type choice to the cell cycle in Dictyostelium. Development 122:3677-3685. [DOI] [PubMed] [Google Scholar]