Abstract
i.v. infusion of native autoantigen or its altered peptide variants is an important therapeutic option for the treatment of autoimmune diseases, because it selectively targets the disease-inducing T cells. To learn more about the mechanisms and kinetics of this approach, we visualized the crucial initial effects of i.v. infusion of peptides or intact protein on GFP-tagged autoaggressive CD4+ effector T cells using live-video and two-photon in situ imaging of spleens in living animals. We found that the time interval between i.v. injection of intact protein to first changes in T cell behavior was extremely short; within 10 min after protein application, the motility of the T cells changed drastically. They slowed down and became tethered to local sessile stromal cells. A part of the cells aggregated to form clusters. Within the following 20 min, IFN-γ mRNA was massively (>100-fold) up-regulated; surface IL-2 receptor and OX-40 (CD 134) increased 1.5 h later. These processes depleted autoimmune T cells in the blood circulation, trapping the cells in the peripheral lymphoid organs and thus preventing them from invading the CNS. This specific blockage almost completely abrogated CNS inflammation and clinical disease. These findings highlight the speed and efficiency of antigen recognition in vivo and add to our understanding of T cell-mediated autoimmunity.
Keywords: autoimmunity, live imaging
One of the most efficient and specific ways to treat organ-specific autoimmune disease is based on the systemic administration of the known autoantigen in soluble form. Pioneering studies by Levine et al. (1) showed that infusion of water-soluble myelin basic protein (MBP) efficiently prevents experimental autoimmune encephalomyelitis (EAE), if given before or briefly after transfer of pathogenic MBP-specific CD4+ T cells. The mechanism underlying this therapeutic success remained unclear for many years, until it was shown that high doses of protein antigen drive specific autoreactive T cells into activation-induced cell death (2).
We infused high doses of MBP to terminate an unfolding EAE at defined time points to determine the distinct phases of the autoimmune response. We found that the mechanisms that halt the autoimmune response after MBP infusion act stunningly fast, indeed too fast to detect their onset by conventional methods. We therefore combined several cellular, molecular, and imaging technologies to study in detail the cellular events surrounding the effect of the soluble autoantigen MBP on MBP-specific encephalitogenic T cells in vivo. In particular, GFP-transduced MBP-specific effector T cells (TMBP-GFP cells) were used, a system that allows the tracing of effector cells in the recipient organism from cell transfer to onset of clinical disease and to recovery from the disease (3). Our data show that the effect of soluble antigen treatment in vivo is extremely fast. Two-photon microscopy and video recording document that the presence of soluble antigen led to a profound change in the behavior of (auto)antigen-specific T cells in peripheral immune organs within 10 min. The cells were arrested to form clusters, presumably around antigen-presenting cells (APCs). They became strongly activated and were trapped within the organs. After a given period, during which the cells were nonresponsive to the specific antigen, they seemed to undergo antigen-related cell death.
Results
Clinical Effects of i.v. Antigen Injection.
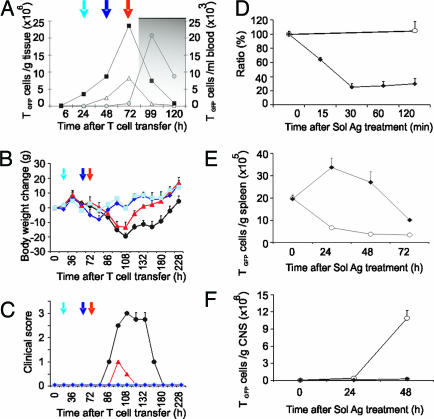
The effect of soluble MBP on preclinical EAE induced in Lewis rats was examined by transfers of 5 × 106 freshly activated TMBP-GFP cells. In the absence of MBP infusion, the animals lost weight and developed paralytic disease 78 h after T cell transfer [maximal clinical score 2.9 ± 0.4; supporting information (SI) Table 1]. i.v. infusion of single doses of 500 μg of MBP 24, 48, or 72 h after T cell transfer [posttransfer (p.t.)] essentially suppressed the development of clinical disease (clinical scores: 0, 0, and 0.8 ± 0.2, respectively; Fig. 1 A–C; SI Table 1). In contrast, i.v. ovalbumin (OVA) injection did not influence the course or severity of clinical EAE (SI Table 1). The treatment effects on clinical EAE were mirrored by changed CNS inflammation: the numbers of TMBP-GFP cells and recruited immune cells were drastically reduced (SI Fig. 6 A and B). Also 48 h after i.v. MBP infusion, there was a significant reduction of IL-2, IFN-γ, TNFα, and IL-2 receptor mRNA, as shown by quantitative PCR analysis of snap-frozen spinal cords (SI Fig. 6 C).
Fig. 1.
Clinical effect of i.v. MBP infusion in different phases of EAE and kinetics of TMBP-GFP cells. (A) Homing of TMBP-GFP cells into blood (black squares), spleen (white triangles), and CNS (gray circles) during adoptive transfer EAE. Arrows indicate the time points of i.v. MBP infusion. Gray background indicates onset of clinical disease. (B and C) Weight loss (B) and clinical score (C) after transfer of TMBP-GFP cells. i.v. MBP infusion was performed 24 (light blue, square), 48 (dark blue, diamonds), or 72 (red, triangles) h p.t. Not-treated control, black circles. Representative data of three independent experiments. Means ± SD of three animals/group. (D) TMBP-GFP cells in the blood 0, 15, 30, 60, and 120 min after i.v. MBP (diamonds) or OVA (circles). Ratio (%): TMBP-GFP/leukocytes at the indicated time points/TMBP-GFP/leukocytes at time point 0. Representative data of three independent experiments. Means ± SD of three measurements. (E) TMBP-GFP cells in the spleen of i.v. OVA- (circles) or i.v. MBP- (diamonds) treated animals. Cytofluorometric counts of TMBP-GFP cells 0, 24, 48, or 72 h after i.v. antigen. Means ± SD of three rats per time point. Representative data of three independent experiments. (F) TMBP-GFP cells in the CNS of OVA- (circles) or MBP- (black diamonds) treated animals 0, 24, and 48 h after i.v. antigen. Means ± SD of three rats per time point. Representative data of four independent experiments.
Kinetics of Effector T Cells After i.v. Antigen.
Infusion of i.v. MBP 48 h p.t., i.e., during the prodromal EAE when the TMBP-GFP cells accumulated in blood and spleen (Fig. 1A, ref. 4), led to a rapid and profound depletion of TMBP-GFP cell numbers in the circulating blood (Fig. 1D). At the same time, TMBP-GFP cells accumulated within the spleen, where they reached peak levels 24 h later (Fig. 1E). MBP treatment arrested the cells in the spleen milieu, thus preventing them from exiting the organ and invading the CNS (Fig. 1F). The arrested T cells lost their reactivity to specific antigen. TMBP-GFP cells sorted from spleens 24 h after i.v. MBP infusion did not proliferate in vitro upon presentation of MBP by professional APCs. In contrast, TMBP-GFP cells from OVA-injected control animals proliferated vigorously, as did TMBP-GFP cells propagated in culture (SI Fig. 7).
Motility of Effector T Cells in the Spleen After i.v. Antigen.
Substantial proportions of freshly transferred activated TMBP-GFP cells settle in peripheral lymphoid organs, where they profoundly remodel their gene expression profiles, presumably as a consequence of interaction with the local milieu (4, 5). Intravital video recording and two-photon imaging were used to directly document their behavior within the spleen (4, 5).
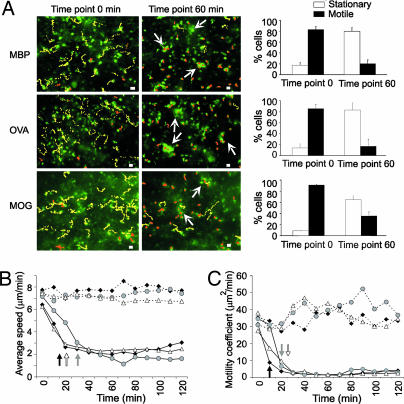
The TMBP-GFP cells were distributed throughout the red pulp and the T cell areas 60 h after T cell transfer (SI Fig. 8). The vast majority of TMBP-GFP cells (>80%) was in permanent motion, with only a few scattered T cells attached to anchoring points (Fig. 2A; SI Movies 1 and 2). The motile TMBP-GFP cells moved around apparently at random, as indicated by the low vector sum of their trajectories (SI Fig. 9 A) and the proportional increase of their displacements over time (refs. 6 and 7; SI Fig. 9 B). Their mean velocity was 7–8 μm/min with peaks of >25 μm/min (Fig. 2B; SI Fig. 10). Moreover, their locomotion was not constant but followed a “stop-and-go” mode, i.e., phases of rapid progression alternated with phases of respite (SI Fig. 11).
Fig. 2.
Effect of soluble antigen on effector T cell motility in the spleen. (A) Migratory paths of TMBP-GFP (Top), TOVA-GFP (Middle), and TMOG-GFP cells (Bottom) before (Left) and 60 min after (Right) i.v. antigen infusion. Videomicroscopy, 10-min videos, 30-sec intervals, 60 h p.t. The dotted lines indicate trajectories of motile (yellow) or stationary (orange) cells. Representative cells of three videos per T cell line and time point. Arrows indicate cell clusters. (Scale bars, 10 μm.) Right bar histograms, proportion of stationary (white) vs. motile (black) TGFP cells before and 60 min after i.v. antigen. Cells were defined as stationary if they migrated <10 μm per 10 min and as motile cells if they moved >10 μm per 10 min. Means ± SD from three independent videos/antigen/T cell line. (B) Average velocity and (C) motility coefficient of TMBP-GFP (black diamonds), TOVA-GFP (gray circles), and TMOG-GFP (white triangles) cells in spleens after specific (filled lines) or control antigen (dotted lines) infusion. Video microscopy, 120-min videos, 30-sec intervals, 60 h p.t. Values represent means of three different videos per antigen and T cell lines comprising 312 MBP, 326 OVA, and 308 MOG T cells. A total of 163,800 time points were analyzed. Arrows indicate the time points, from which the values significantly decreased (P < 0.001).
i.v. injection of MBP had an almost instantaneous effect on the behavior of the TMBP-GFP cells (SI Movies 3 and 4). It took no more than 10–15 min for the cells to lose speed (Fig. 2 B and C). After 30 min, this process was completed and remained stable for the entire observation period of 2 h (Fig. 2 B and C; SI). OVA- and myelin oligodendrocyte glycoprotein (MOG)-specific T cells (TOVA-GFP and TMOG-GFP cells, respectively) showed the same behavior after infusion of OVA or MOG, respectively (Fig. 2 B and C; SI; SI Movie 5).
Antigen infusion arrested most of the TMBP-GFP cells (Fig. 2A) but did not immobilize them completely. Their average velocity was reduced to ≈2 μm/min (SI Fig. 9), and the cells seemed to be tethered at particular points (SI Movies 3–5). Many TMBP-GFP cells aggregated to form clusters (Fig. 2A; SI Movie 6). The effect of MBP infusion was specific, because treatment with control antigen (OVA) did not influence the migratory behavior of TMBP-GFP cells (Fig. 2 B and C; SI Movies 1 and 2; SI Fig. 10) but affected TOVA-GFP cells (Fig. 2; SI Fig. 10; SI Movie 5). TMOG-GFP cells behaved in all respects like TMBP-GFP cells and TOVA-GFP cells after i.v. injection of MOG (Fig. 2; SI).
To visualize the interaction of TMBP-GFP cells with potential stromal APCs, i.v. Texas red-conjugated dextran was infused, which is rapidly taken up by splenic phagocytes. In the absence of specific antigen and while proceeding through the spleen milieu, TMBP-GFP cells seemed to form brief contacts with red-fluorescent phagocytes (SI Movie 7). Once MBP was introduced, however, these contacts became durable and stable (SI Movie 8). If T cell/APC pairs could be clearly identified, the contacts remained constant (SI Movie 8). However, the possibility of a more promiscuous behavior in areas with dense networks of labeled APCs cannot be excluded.
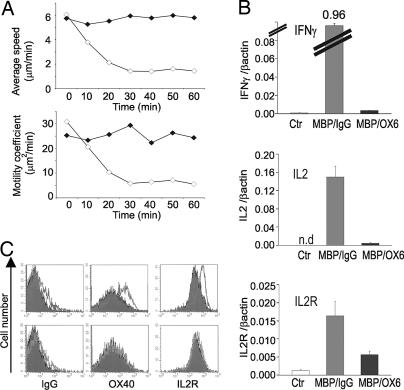
The TMBP-GFP effector cell response to MBP depended on MHC class II molecules (Fig. 3). i.v. injection of blocking anti-class II antibodies 1 h before soluble MBP almost completely prevented tethering and cluster formation of the cells (Fig. 3A) and consequently reduced up-regulation of cytokine mRNA and membrane activation markers (Fig. 3 B and C).
Fig. 3.
MHC class II dependence of T cell activation in the spleen upon i.v. antigen infusion. (A) Average velocity (Upper) and motility coefficient (Lower) of TMBP-GFP cells after i.v. infusion of MBP in the presence of neutralizing anti-MHC class II (black) or isotype control (white) antibodies. Video recordings of spleens 60 h p.t. Means of five independent video recordings comprising 384 cells and 25,200 movement steps, 60-min recording, 30-sec intervals. (B) Quantitative PCR of TMBP-GFP cells sorted from spleens before antigen injection (white bars, Ctr) or 4 h after i.v. MBP in the presence of neutralizing anti-MHC class II (black, MBP/OX6) or isotype control antibodies (gray, MBP/IgG). Ex vivo TMBP-GFP cells were obtained from four animals and measured in two independent quantitative PCRs. (C) Cytofluorometric analysis of OX-40 antigen and IL-2 receptor on TMBP-GFP cells 4 h after i.v. MBP (overlays) in the presence of isotype control (Upper) or neutralizing anti-MHC class II antibodies (Lower). Filled histograms, no i.v. antigen treatment.
Antigen Uptake and Presentation by Splenic APCs.
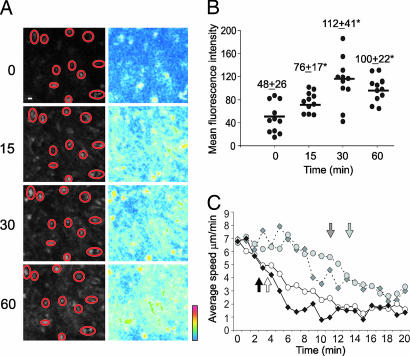
If the extremely rapid response of TMBP-GFP cells to soluble MBP involves specific recognition of the autoantigen, then the uptake and processing of MBP must have occurred even faster. To examine these processes in real time by intravital microscopy, DQ Ovalbumin (DQ OVA, Invitrogen, Karlsruhe, Germany) was used as a model antigen. This fluorochrome-labeled autoquenched substitute of OVA becomes fluorescent only upon proteolytic digestion but keeps its antigenicity for T cells (not shown). DQ OVA tracer uptake and processing by substantial numbers of spleen cells were observed as early as 10–15 min after injection (Fig. 4A and B; SI Movie 9). Labeled cells were isolated and identified by cytofluometry. The majority of fluorescent cells (>80%) was an MHC class II-positive population composed of B cells, monocytes/macrophages, dendritic cells, and endothelial cells (SI Fig. 12 A and SI Table 2). To test their capacity to present antigen to effector T cells, splenocytes were isolated ex vivo 30 min and 2 h after i.v. antigen administration and immediately fixed with glutaraldehyde, which efficiently stops antigen processing, leaving intact the presentation capacity of APCs (8). The splenocytes after i.v. MBP-, but not i.v. OVA-, infusion induced strong IFN-γ and IL-2 receptor expression of TMBP-GFP cells added to the fixed cells (SI Fig. 12 B and C).
Fig. 4.
Antigen proteolysis and presentation within the spleen after i.v. antigen. (A) Kinetics of DQ OVA proteolysis in the spleen. Intravital confocal recording of the spleen before (0) and 15, 30, and 60 min after i.v. infusion of DQ OVA. (Left) Original fluorescence. (Right) Pseudocolor-coded pictures. Red circles indicate the selected regions of interest (ROIs) (magnification, 10 μm). (B) Quantification of mean fluorescence intensities of selected ROIs. Means ± SD; ∗, P < 0.001. (C) Average velocity of MBP peptide-specific T cells (diamonds) or OVA peptide-specific T cells (circles) after i.v. infusion of intact proteins (MBP dark gray or OVA light gray, respectively; dotted lines) or after peptides (MBP or OVA peptides, black and white, respectively). Video recordings (20-min, 30-sec intervals) of spleens 60 h p.t. Arrows indicate the time points at which the velocity became significantly reduced (P < 0.05). Means of two independent videos per antigen, including 512 cells and 16,800 analyzed time intervals.
Intracellular antigen processing might be bypassed by direct binding of peptide degradation products on surface MHC class II molecules, a pathway that would considerably accelerate antigen presentation and the recognition process by effector T cells. This appears unlikely, because our protein preparations were extensively dialyzed to exclude peptide contamination and were controlled by gel electrophoresis (not shown). Further, the motility changes of TMBP-GFP and TOVA-GFP effector cells occurred significantly earlier after i.v. injection of specific peptides compared with intact protein (3 vs. 10 min, respectively; Fig. 4C). This difference was even more pronounced in TOVA-GFP cells (3 vs. 13 min, respectively; Fig. 4C).
Early Transcription and Protein Changes.
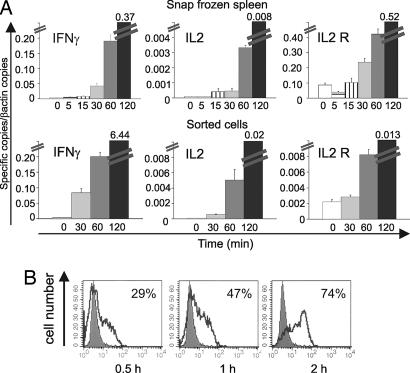
To correlate the arrest of TMBP-GFP cells and cluster formation with T cell activation, spleens were isolated 5, 15, 30, 60, and 120 min after i.v. MBP infusion; the tissues were snap-frozen in liquid nitrogen, and mRNA was isolated. Real-time PCR analysis showed strongly elevated transcription of IFN-γ mRNA (10- to 100-fold) beginning 30 min after antigen infusion. This up-regulation was followed by increases of IL-2, IL-2 receptor, TNF-α, STAT-1, and T-bet mRNAs (Fig. 5A; SI Table 3). In contrast, IL-4, CD3, CCR-2, CXCR-3, and CXCR-4 remained constant (SI Table 3). These changes most likely took place in splenic TMBP-GFP cells; similar kinetics were seen in TMBP-GFP cells sorted from spleens (Fig. 5A; SI Table 3). The elevation of IFN-γ production was confirmed on the protein level. Intracellular staining and FACS analysis of TMBP-GFP cells recovered from the spleen 0.5 h after i.v. antigen transfer showed that ≈30% of the T cells were positive for intracellular IFN-γ, increasing to ≈75% 1.5 h later (Fig. 5B). Changes in membrane activation markers on effector T cells were noted beginning 2 h after i.v. antigen; there was a partial down-modulation of CD3/αβTCR complex and the levels of OX-40 antigen and IL-2 receptor increased (SI Fig. 13 A). Similar kinetics in mRNA regulations, IFN-γ production, and membrane protein changes were observed in TOVA-GFP cells after i.v. injection of OVA (SI Fig. 13 B–D).
Fig. 5.
Kinetics of T cell activation after i.v. antigen infusion. (A) Quantitative PCR of snap-frozen spleens (Upper) or of ex vivo-sorted TMBP-GFP cells (Lower). Numbers indicate time points of sampling after i.v. MBP. Ex vivo TMBP-GFP cells were obtained from four animals and measured in two independent quantitative PCRs. Representative data of two independent experiments. (B) Intracellular IFN-γ staining in TMBP-GFP from spleens 0.5, 1, and 2 h after i.v. MBP. Filled histogram, control (no antigen); overlay histogram, after i.v. antigen. Representative data of three independent experiments.
Discussion
EAE is caused by activated CNS-specific T cells, which reach their target by way of the bloodstream. However, instead of directly invading the CNS after activation, they undergo a radical tuning process, which enables them to penetrate the blood–brain barrier. This tuning lasts 3–4 days and takes place within the peripheral lymphoid organs, in particular the spleen. This scenario is of clinical interest. At least in theory, the effector cells are especially accessible and vulnerable to therapeutic modification or depletion during their stay in the periphery and might be intercepted before invading their target organ.
We have now studied the earliest events occurring in the prodromal spleen after infusion of high-dosed soluble autoantigens, a time-honored therapeutic approach to prevent EAE. In untreated rats, activated effector T cells reach the spleen and settle in “T cell areas” and also in the red pulp, milieus controlled by special sets of stromal cells (9). Within a short period, the T cells reduce expression of their activation markers, while up-regulating a large number of other genes, including chemokine receptors (4). This reprogramming is most likely the result of interactions with the local lymphatic microenvironment, in particular local stromal cells. The imaging approaches showed that in the bona fide absence of autoantigen, the effector T cells unceasingly crisscrossed the splenic milieus. Their motion was apparently nondirected and proceeded in a stop-and-go manner. In this respect, the behavior of our autoimmune effector T cells recalls findings of previous studies, which traced naïve T cells introduced into lymph nodes containing dendritic APCs (10). Although the present work did not analyze in detail the nature of the cell-to-cell interactions at this stage, our imaging data suggest that the effector T cells make multiple short-lasting contacts with local stroma, which remain mostly sessile, in contrast to dendritic cells contacting naïve CD4+ T cells (11). Because antigen-independent contact formation followed by calcium activation in T cells has been described elsewhere (12), we are tempted to conclude that the putatively stochastic contacts observed here could play a role in the reprogramming of the effector T cells.
Infusion of soluble antigen almost instantaneously led to radical changes of T cell behavior. Within no more than 10 min, we noted a significant deceleration of T cell migration, and after another 30 min, most of the effector T cells were stopped, aggregating to form cell clusters mostly around antigen-containing phagocytes. These findings strongly contrast with antigen encounters by naïve mouse CD4+ T cells, where stable cluster formation is preceded by a period of multiple transient T cell–APC contacts (10, 13, 14).
A T cell response that can be demonstrated as soon as 10 min after introduction of antigen is unexpectedly rapid. The reaction involves antigen recognition by the T cells, because it depends on infusion of the relevant antigen and can be blocked by anti-MHC class II antibodies. A complex chain of reactions must have occurred within this short period. These reactions include the uptake of antigenic proteins by local APCs and the processing and presentation in the proper MHC class II context. These processes are then followed by antigen recognition by TCRs and the proper response by the T cells.
Transport of i.v. antigen into the splenic white pulp is very fast, making use of special conduits, which accommodate proteins smaller than 70 kDa (15). The antigens used in our present study were below this threshold: MBP (18 kDa), OVA (45 kDa), and MOG (12 kDa). Once they arrive, their uptake and processing start promptly. According to current concepts, protein antigens processed for presentation to CD4+ T cells are taken up by APCs by endo-, pino-, or phagocytosis (16). Previous studies estimated the time required for antigen cleavage and embedding in nascent MHC class II proteins to range between 3 h (17) and ≈20 min (18). Our studies using DQ OVA, a fluorochrome-substituted OVA useful for antigen processing (19, 20), indicate that the kinetics are faster. Within 10–15 min, proteolytic digestion of DQ OVA could be clearly demonstrated in potential splenic APCs, including B cells, macrophages, dendritic cells, and endothelial cells (SI Movie 9 and SI Table 2).
Direct binding of suitable peptides by membrane-bound class II proteins circumvents the intracellular processing cascade and significantly accelerates the specific T cell presentation/recognition process. Indeed, in accord with other reports (21), effector T cells in our study were promptly arrested after i.v. infusion of peptide (2–4 min; Fig. 4C).
It is known that T cell signaling and activation occur within a few minutes after antigen recognition and are accompanied by profound changes in T cell calcium (22) and cell motility (23, 24). The dynamics of T cellular effector functions, e.g., the release of proinflammatory cytokines, are less clear. Although substantial up-regulation of IL-2 mRNA was observed in Jurkat cells 2 h after stimulation with Con A and phorbol myristate acetate (25), the lymph nodes and spleens of mice carrying antigen-primed transgenic T cells showed a cytokine response 30 min after exposure to specific peptide (26). This is similar to our own observation that proinflammatory cytokine expression is initiated immediately after T cell arrest and accompanied by a 100-fold increase in IFN-γ mRNA synthesis 30 min after i.v. antigen infusion (Fig. 5 and SI Fig. 13).
Besides documenting a fast (auto-)immune response in vivo, our results contribute to a better understanding of EAE. According to a widely accepted view, the autoimmune infiltrate in EAE is built up by two waves of T cell invasion (27, 28). The first wave occurs within the first few hours after T cell transfer and involves a limited number of activated T cells (“pioneer cells”). After an interval of 3–4 days, a second wave enters the CNS. This time it is composed of millions of postactivated effector T cells (4, 29). Soluble antigen infusion at 48 h p.t., as in our current studies, shuts off the recirculation of autoimmune T cells immediately and completely and later causes depletion of the cells by apoptosis. Because such treatment totally prevents the formation of histological infiltrations and of clinical EAE, the numerical and functional contribution of the early intra-CNS pioneer cells to the florid infiltrations arising with onset of disease must be negligible. Consecutively, the autoaggressive effector T cells of the second inflammatory phase are the culprits which control autoimmune inflammation and disease.
We have confirmed that i.v. infusion of soluble neural autoantigen is a most efficient and specific treatment of EAE. However, can this strategy be translated into a practicable therapy for human autoimmune disease? There are several problems to be overcome. i.v. infusion of high protein doses may induce allergic shock responses, which occurred in some patients who received s.c. injections of altered peptide ligands (APL; refs. 30 and 31), and which were impressively demonstrated in murine EAE after i.p. injections of myelin protein peptide (32). Furthermore, in the presence of autoantibodies, the infusion of autoantigen could cause immune complex disease. We should also recall that early attempts to infuse MBP into multiple sclerosis (MS) patients yielded disappointing results (33–35), and similar outcomes were noted in trials of other human autoimmune disease (36). In retrospect, this finding is not too surprising, considering that an infused autoantigen acts exclusively on specific T cells. Thus, knowledge of the pathogenic target autoantigen would be required. However, in MS, as in most other human autoimmune diseases, the autoantigen is not known. In fact, more than one autoantigen may be the target of T cell-mediated immune attacks, and each patient may have distinct profiles of autoantigens. To add another degree of complexity, these autoantigenic profiles may not be static. Because of “determinant spreading,” they may drift into new patterns over time (37, 38). Several groups have designed chimeric proteins combining more than one myelin autoantigen for therapy (39, 40). It will be difficult to cover the entirety of encephalitogenic proteins for therapy. It is important to note that high-dose treatment with a particular autoantigen is a negative therapy, one aimed at deleting specific T cells from the immune repertoire. Thus, so long as the identification of individual autoantigens remains an unsolved problem, therapeutic concepts that promote bystander suppression of undefined autoimmune effector T cells, like treatment with APLs, promise a clear advantage (41).
Materials and Methods
Animals and Antigens.
Lewis rats (6–8 weeks old) were obtained from the animal facility of the Max Planck Institute for Biochemistry (Martinsried, Germany). MBP was purified from guinea pig brains as reported (42). Hen egg OVA was obtained from Sigma (St. Louis, MO). DQ OVA and Texas red dextran (70 kDa) were bought from Invitrogen (Carlsbad, CA). Recombinant MOG (amino acids 1–120) was produced as described (43). Synthetic MBP (amino acids 68–86) and OVA (amino acids 323–339) peptides were obtained from the Laboratory of Bioorganic Chemistry (Max Planck Institute for Biochemistry). All animal experiments were performed according to Bavarian state regulations for animal experimentation and were approved by the responsible authorities (Animal license: nos. 209.1/211-2531-56/99 and 209.1/211-2531-36/04).
TGFP Cells and EAE Induction.
CD4+ TGFP cells were generated and tested for phenotype, cytokine profile, and antigen specificity, as described (3). T cells encoding the gene for red fluorescent protein (RFP) were established as described (3). The vector pMSCVneo-IRES2-RFP was constructed as follows: the EcoRI/HpaI fragment of pIRES2-Dsred2 (Becton Dickinson, Heidelberg, Germany) was inserted into the murine stem cell retrovirus pMSCVneo (Becton Dickinson). Packaging lines were established as described (3). EAE was induced by i.v. injection of 5 × 106 T cell blasts. Animals were monitored daily by measuring weight and examining disease scores as follows: 0, no disease; 1, flaccid tail; 2, gait disturbance; 3, complete hind limb paralysis; 4, tetraparesis; and 5, death.
Intravital Imaging.
Surgical procedure and intravital imaging settings.
Animals were anesthetized by i.p. injection of xylazine/ketamine (10 and 50 mg/kg, respectively). Adequate oxygenation was ensured by an oxygen (100% O2) mask. Body temperature was controlled by a heating pad. Temperature- and moistness-controlled chambers were constructed for upright and inverted microscope (SI Fig. 14). Surgically exposed spleens with intact vasculature were placed in chambers superfused with carbogenated HBSS (95% O2, 5% CO2).
Live imaging.
Two-photon and confocal system.
Time-lapse two-photon laser-scanning microscopy analyzing the movements of TGFP cells in all three spatial dimensions was performed with a LeicaSP2 confocal system (Leica Microsystems, Wetzlar, Germany) combined with a 10-W Millenia-Tsunami laser system (Spectra Physics, Darmstadt, Germany). For spleen imaging, a 40× 1.25-N.A. oil immersion objective (Leica) was used. The power of the excitation light was controlled by an electrooptic modulator (LINOS Photonics, Göttingen, Germany). Fluorescence was detected by an external photomultiplier tube (Leica) with suitable filter sets. Image acquisition and online analysis were performed by the Leica confocal software (Leica). The image resolution was 586 nm/pixel in x-y, with a field of view of 300 × 300 μm2. Penetration into the spleen reached 80–100 μm. Images were taken by 3-μm z interval over a total depth of 20–40 μm. Images were Kalman-averaged over two to four frames. One stack was acquired every 30–60 sec.
Fluorescence videomicroscopy.
Time-lapse recordings were performed by using an inverted microscope [Zeiss (Oberkochen, Germany) Axiovert 200M] equipped with a 20× 0.4-N.A. objective (Zeiss). Images were acquired by using a Coolsnap-HQ camera (Photometrics, Roper Scientific, Tuscon, AZ) in 30-sec intervals and processed by MetaMorph (Visitron Systems, Puchheim, Germany).
Image analysis.
Four-dimensional (x, y, z, t) image stacks taken by two-photon laser-scanning microscopy were processed with Leica confocal software. Individual stacks were subjected to a Gaussian spatial filter and rescaled. To facilitate an overview, the 3D stacks were volume-rendered as 2D images. All image analysis was done by visual inspection of the individual image sections. Image J software (freeware, provided by Wayne Rasband, National Institutes of Health, Bethesda, MD) was used to evaluate cell trajectories and velocity. To calculate the motility coefficient (MC), the following formula was used: MC = x2/4t, where x means displacement of individual T cells in 10-min time intervals (t) (7). Both two-photon imaging and video microscopy recording were used for analyzing T cell motility in spleen. As reported earlier (5, 7), these techniques are complementary and give comparable results as shown in SI Table 4.
Cell Isolation, Cytofluorometry, and FACS.
Single-cell suspensions of organs were prepared as described (4). The organs after explanation were immediately put on ice. Homogenization and cell sorting were performed at 4°C. TGFP cells were sorted in PBS containing 2% glucose using FACS Vantage (Becton Dickinson) or MoFlo sorter (Cytomation Bioinstruments, Freiburg, Germany). Cytofluorometric analysis was performed with FACS-Calibur operated by Cell Quest software (Becton Dickinson). The following monoclonal antibodies were used: W3/25 (anti-CD4), OX33 (CD45RA, B cells), RECA-1 (endothelial cells) (all Serotec, Düsseldorf, Germany), R73 (αβTCR), OX-6 (rat MHC class II), OX-40 antigen (CD134), OX-39 (CD25, IL-2 receptor α chain), CD8α, CD8β, CD11a (LFA-1α), CD11b (integrin αM chain), CD11b/c (OX42), CD11c (integrin αX chain) (all Becton Dickinson), and IgG isotype controls (Sigma). Allophycocyanin-labeled anti-mouse antibody (Invitrogen, Karlsruhe, Germany) was used as secondary antibody. Staining of anti-MHC class II-pretreated splenocytes was performed by using biotinylated primary OX-39 and OX-40 antibodies (Becton Dickinson) and R-phycoerythrin-Cy5-conjugated streptavidin as detection reagent (Dako, Glostrup, Denmark).
Ex Vivo Reactivity Assay.
TMBP-GFP cells isolated from spleens after i.v. MBP were cocultured with professional thymic APCs in 96-well plates (in DMEM 1% rat serum) in the presence of specific or control antigen (10 μg/ml MBP or OVA, respectively). Amplification of ex vivo-isolated TMBP-GFP cells was measured by cytofluorometry, as described (44). Their numbers were determined in relation to a known absolute number of added phycoerythrin-labeled plastic beads (Becton Dickinson). The amplification rate was calculated in relation to the TGFP cell numbers at day 0.
Quantitative PCR and Intracellular IFN-γ Staining.
mRNA was extracted by using TRIzol and reversed to cDNA. Taqman analysis was performed as reported (4) by using ABI Prism 5700 Sequence Detector Taqman (PE Applied Biosystems, Darmstadt, Germany). The expression of a housekeeping gene (β-actin) was set into relation to the specific mRNA. Data were obtained by independent duplicate measurements. The threshold cycle value of the individual measurements did not exceed 0.5 amplification cycles.
Intracellular IFN-γ staining was performed as described by using anti-mouse/rat IFN-γ antibody (clone DB-1; Becton Dickinson) (45). Control IgG (mouse IgG MOPC31) was obtained from Sigma.
Supplementary Material
Acknowledgments
We thank Ingeborg Haarmann, Sabine Kosin, and Karin Nispel for excellent technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB455-A8 and Hertie Foundation Grant 1.01.1/04/010.
Abbreviations
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- OVA
ovalbumin
- p.t.
posttransfer
- EAE
experimental autoimmune encephalomyelitis
- APC
antigen-presenting cell
- TMBP-GFP cells,
GFP-transduced MBP-specific effector T cells
- TMOG-GFP
GFP-transduced MOG-specific effector T cells
- TOVA-GFP cells
GFP-transduced OVA-specific effector T cells.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608383104/DC1.
References
- 1.Levine S, Hoenig EM, Kies MW. Science. 1968;161:1155–1157. doi: 10.1126/science.161.3846.1155. [DOI] [PubMed] [Google Scholar]
- 2.Critchfield JM, Racke MK, Zuñiga-Pflücker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 3.Flügel A, Willem M, Berkowicz T, Wekerle H. Nat Med. 1999;5:843–847. doi: 10.1038/10567. [DOI] [PubMed] [Google Scholar]
- 4.Flügel A, Berkowicz T, Ritter T, Labeur M, Jenne D, Li Z, Ellwart J, Willem M, Lassmann H, Wekerle H. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami N, Nägerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MJ, Wei SH, Cahalan MD, Parker I. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MJ, Wei SH, Parker I, Cahalan MD. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 8.Shimonkevitz R, Kappler JW, Marrack P, Grey H. J Exp Med. 1983;158:303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mebius RE, Kraal G. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 10.Miller MJ, Safrina O, Parker I, Cahalan MD. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VLJ, Amigorena S. Science. 2005;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 12.Revy P, Sospedra M, Barbour B, Trautmann A. Nat Immunol. 2001;2:925–931. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 13.Catron DM, Itano AA, Pape KA, Mueller DL, Jenkins MK. Immunity. 2004;21:341–347. doi: 10.1016/j.immuni.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Mempel TR, Henrickson SE, von Andrian UH. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 15.Nolte MA, Beliën JAM, Schadee-Eestermans IL, Jansen W, Unger WWJ, Van Rooijen N, Kraal G, Mebius RE. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trombetta ES, Mellman I. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 17.Manickasingham S, Reis e Sousa C. J Immunol. 2000;165:5027–5034. doi: 10.4049/jimmunol.165.9.5027. [DOI] [PubMed] [Google Scholar]
- 18.Harding CV, Geuze HJ. J Cell Biol. 1992;119:531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez GM, Diment S. Eur J Immunol. 1995;35:1823–1827. doi: 10.1002/eji.1830250705. [DOI] [PubMed] [Google Scholar]
- 20.Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin LM. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Zell T, Khoruts A, Ingulli E, Bonnevier JL, Mueller DL, Jenkins MK. Proc Natl Acad Sci USA. 2001;98:10805–10810. doi: 10.1073/pnas.191567898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnadieu E, Cefal D, Tan YP, Paresys G, Bismuth G, Trautmann A. J Immunol. 1992;148:2643–2653. [PubMed] [Google Scholar]
- 23.Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VLJ, Bismuth G, Trautmann A, Germain RN, Delon J. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 24.Negelescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 25.Weiss A, Shields R, Newton M, Manger B, Imboden J. J Immunol. 1987;1138:2169–2176. [PubMed] [Google Scholar]
- 26.Hayashi N, Liu D, Min B, Ben-Sasson S, Paul WE. Proc Natl Acad Sci USA. 2002;99:6187–6191. doi: 10.1073/pnas.092129899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 28.Hickey WF, Hsu BL, Kimura H. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 29.Ransohoff RM, Kivisäkk P, Kidd G. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 30.Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, et al. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 31.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L. Nat Med. 2000:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 32.Pedotti R, Mitchell D, Wedemeyer J, Karpuj M, Chabas D, Hattab EM, Tsai M, Galli SJ, Steinman L. Nat Med. 2001;2:216–222. doi: 10.1038/85266. [DOI] [PubMed] [Google Scholar]
- 33.Campbell B, Vogel PJ, Fisher E. Arch Neurol. 1973;29:10–15. doi: 10.1001/archneur.1973.00490250028003. [DOI] [PubMed] [Google Scholar]
- 34.Gonsette RE, Delmotte P, Demonty L. J Neurol. 1977;216:27–31. doi: 10.1007/BF00312812. [DOI] [PubMed] [Google Scholar]
- 35.Romine JS, Salk J. In: Multiple Sclerosis. Hallpike J, Adams CWM, Tourtellotte WW, editors. Cambridge, UK: Cambridge Univ Press; 1983. pp. 621–630. [Google Scholar]
- 36.Tian J, Olcott A, Hanssen L, Zekzer D, Kaufman DL. Immunol Today. 1999;20:190–194. doi: 10.1016/s0167-5699(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 37.Vanderlugt CL, Miller SD. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 38.Robinson WH, Fontoura P, Lee BJ, De Vegvar HEN, Tom J, Pedotti R., DiGennaro CD, Mitchell DJ, Fong D, Ho PPK, et al. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 39.McFarland HI, Lobito AA, Johnson MM, Palardy GR, Yee CSK, Jordan EK, Frank JA, Tresser N, Genain CP, Mueller JP, et al. J Immunol. 2001;166:2116–2121. doi: 10.4049/jimmunol.166.3.2116. [DOI] [PubMed] [Google Scholar]
- 40.Zhong M.-C., Kerlero de Rosbo N, Ben-Nun A. J Clin Invest. 2002;110:81–90. doi: 10.1172/JCI15692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hohlfeld R, Wekerle H. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eylar EH, Kniskern PJ, Jackson JJ. Methods Enzymol. 1979;32B:323–341. [PubMed] [Google Scholar]
- 43.Adelmann M, Wood J, Benzel I, Fiori P, Lassmann H, Matthieu J-M, Gardinier MV, Dornmair K, Linington C. J Neuroimmunol. 1995;63:17–27. doi: 10.1016/0165-5728(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 44.Kawakami N, Odoardi F, Ziemssen T, Bradl M, Ritter T, Neuhaus O, Lassmann H, Wekerle H, Flügel A. J Immunol. 2005;175:69–81. doi: 10.4049/jimmunol.175.1.69. [DOI] [PubMed] [Google Scholar]
- 45.Kawakami N, Lassmann S, Li Z, Odoardi F, Ritter T, Ziemssen T, Klinkert WEF, Ellwart J, Bradl M, Krivacic K, et al. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.