Abstract
Using a differential display technique, the gene gtt1, which codes for a high-affinity glucose transporter, has been cloned from the mycoparasite fungus Trichoderma harzianum CECT 2413. The deduced protein sequence of the gtt1 gene shows the 12 transmembrane domains typical of sugar transporters, together with certain residues involved in glucose uptake, such as a conserved arginine between domains IV and V and an aromatic residue (Phe) in the sequence of domain X. The gtt1 gene is transcriptionally regulated, being repressed at high levels of glucose. When carbon sources other than glucose are utilized, gtt1 repression is partially alleviated. Full derepression of gtt1 is obtained when the fungus is grown in the presence of low carbon source concentrations. This regulation pattern correlates with the role of this gene in glucose uptake during carbon starvation. Gene expression is also controlled by pH, so that the gtt1 gene is repressed at pH 6 but not at pH 3, a fact which represents a novel aspect of the influence of pH on the gene expression of transporters. pH also affects glucose transport, since a strongly acidic pH provokes a 40% decrease in glucose transport velocity. Biochemical characterization of the transport shows a very low Km value for glucose (12 μM). A transformant strain that overexpresses the gtt1 gene shows a threefold increase in glucose but not galactose or xylose uptake, a finding which confirms the role of the gtt1 gene in glucose transport. The cloning of the first filamentous ascomycete glucose transporter is the first step in elucidating the mechanisms of glucose uptake and carbon repression in aerobic fungi.
Filamentous fungi are ubiquitous organisms able to obtain energy from very different substrates. Consequently, some of them are important pathogens for other fungi, insects, plants, and animals, and some are quite relevant in biotechnology. The wide use of strains of the genus Trichoderma is based on their ability to degrade plant polymers, such as cellulose (20), and to antagonize other fungi (18). For this reason, strains of Trichoderma harzianum have been commonly used as agents for the biocontrol of plant pathogenic fungi. Several mechanisms have been considered to be key factors in antagonistic interactions: lysis of host cell walls, antibiosis, competition for nutrients, induced resistance in plants, and inactivation of host enzymes (15). Both the lysis of fungal cell walls and the efficient use of available nutrients are based on the ability of Trichoderma strains to obtain ATP from the metabolism of different sugars, such as those obtained from polymers widespread in vegetal and fungal sources: cellulose, hemicellulose, xylan, mannan, arabinan, and chitin, among others.
The majority of the enzymes involved in the degradation of these polymers are repressed by glucose (2, 20, 24). The mechanism(s) by which glucose repression is triggered remains undiscovered, despite the large amount of information obtained from Saccharomyces cerevisiae (13). Unlike that of the unicellular ascomycete S. cerevisiae, the metabolism of mycelial fungi is preferentially respiratory in the presence of oxygen. S. cerevisiae and other fermentative yeasts usually grow on glucose-enriched substrates, such as grapes. Fermentative metabolism correlates perfectly with the availability of high glucose concentrations. Most filamentous fungi, however, have evolved a preference for respiratory metabolism and a low-rate but high-ATP-yield process (35). The impact of this difference on general carbon repression mechanisms is not yet known due to the scarcity of data available on glucose metabolism in easily manageable organisms with respiratory metabolism. Taking advantage of expressed sequence tag analysis and microarrays, Chambergo et al. (5) have reported general changes in the regulation of critical genes that control the metabolic flux to fermentative or respiratory behavior in Trichoderma reesei. However, whether it is glucose transport into the cell or sugar phosphorylation that provokes carbon repression (13) is still a question that remains to be answered, and the role of the glucose transport system that provides enough glucose to support growth when low-rate respiratory metabolism is utilized also remains to be discovered.
Sugar transport in S. cerevisiae has been extensively studied (4, 22, 28, 32). The hexose transporters belong to a transporter superfamily termed the major facilitators superfamily (28). Seventeen genes have been cloned and characterized as being involved in glucose and fructose transport. The activities of all of these transporters generate two glucose uptake systems: a constitutive low-affinity system (Km, 15 to 20 mM) and a glucose-repressed high-affinity system (Km, 1 to 2 mM) (22, 32). A simpler situation is observed in Kluyveromyces lactis, which has only one low-affinity (Km, 20 to 50 mM) inducible glucose transporter (56) and only one high-affinity (Km, 1 mM) constitutively expressed glucose transporter (3).
For Neurospora crassa, two glucose uptake systems have been described: a constitutive low-affinity system (Km, 8 mM) and an inducible high-affinity system (Km, 10 μM) (44-47). Fructose and galactose transport systems have also been identified (36, 37). The differences among the values for fermentative yeasts and fungi with respiratory metabolism may reflect the different availabilities of glucose in the environments of a very diverse group of ascomycetes. DNA sequences from a putative transporter in filamentous ascomycetes have not been reported, but a putative glucose sensor gene, rco3, has been cloned (27). Only two genes encoding glucose transporters in filamentous fungi have been cloned and characterized to date; both are from basidiomycete species: hxt1 from Uromyces fabae (53) and AmMst1 from Amanita muscaria (30).
In this work, we report the isolation of a gene, gtt1, which codes for a glucose transporter in T. harzianum CECT 2413. When overexpressed, gtt1 provokes a threefold enhancement of glucose transport velocity, a fact which makes gtt1 the first glucose transporter identified in an ascomycete-related species. We show that the gtt1 gene is repressed in the presence of high glucose concentrations and strongly induced in the presence of carbon starvation. We discuss a possible role of gtt1 during glucose assimilation in mycoparasitism.
MATERIALS AND METHODS
Strains, media, and growth conditions.
T. harzianum CECT 2413 was obtained from the Colección Española de Cultivos Tipo (Burjassot, Valencia, Spain). T. harzianum was maintained on potato dextrose agar (2% [wt/vol] commercial mashed potatoes [dehydrated potato flakes], 2% [wt/vol] dextrose, 2% [wt/vol] agar). For liquid cultures, mycelia were grown in 250-ml flasks containing 100 ml of minimal salt medium (MM) (34) supplemented with various carbon sources and 0.5% (wt/vol) ammonium sulfate as a nitrogen source and incubated at 22°C on a rotary shaker (200 rpm). When needed, media were buffered with either 0.2 M sodium citrate (pH 3) or 0.2 M 2-(N-morpholino)ethanesulfonic acid-KOH (pH 6). All media used for transformation were as described previously (34).
Mycelia for both Northern experiments and the differential display technique were obtained in two steps as follows. MM with 2% glucose was inoculated with a spore suspension (final concentration of 106 spores per ml) and incubated for 36 h as described above. Mycelia were collected, washed extensively with 2% MgCl2 and distilled water, and used to reinoculate medium containing the carbon source described for each experiment. After 8 h of incubation, mycelia were collected and frozen at −80°C until used for RNA extraction.
Cloning of the gtt1 gene, differential display technique, and cDNA isolation.
The differential display technique was performed as follows. Mycelia were precultured, collected, and washed as described above and used to reinoculate flasks containing MM with 2 or 0.2% glucose and buffered at either pH 3 or pH 6. The four resultant cultures were incubated for 8 h. One hundred micrograms of RNA from each of the four cultures was isolated and treated with 10 U of RNase-free DNase I. Finally, the RNA was treated with phenol, precipitated, and stored at 1 μg/μl at −80°C.
Three different reverse transcription reactions were performed, each with a different oligo(dT) (AAGCT11M, 2 μM, where M is G, A, or C; provided by GenHunter, Nashville, Tenn.). Two hundred nanograms of each RNA preparation and 20 μM deoxynucleoside triphosphates were also used in a 20-μl reaction with Moloney murine leukemia virus Superscript II (Gibco BRL, Paisley, United Kingdom) in accordance with the manufacturer's instructions. Two microliters of each reaction mixture (12 different reaction mixtures) was amplified by PCR carried out with 0.2 μM concentrations of both the arbitrary primers (H-AP primers, set 10; GenHunter) and the oligo(dT) primers (GenHunter), 2 μM deoxynucleoside triphosphates, and 0.2 μl of [α-33P]dATP. Forty cycles (94°C for 30 s, 40°C for 2 min, and 72°C for 30 s) were completed by using an Ampli-Taq polymerase PCR kit (Roche Molecular Systems, Branchburg, N.J.). This protocol yielded 96 different reactions, since eight different arbitrary primers were used with the 12 different reaction mixtures.
Four microliters of each reaction mixture was separated by 6% polyacrylamide gel electrophoresis in Tris-borate-EDTA buffer. Electrophoresis was performed with Genomyx LR equipment (Beckman Coulter Inc., Fullerton, Calif.) in accordance with the manufacturer's instructions. The dried gel was exposed to Kodak Biomax MR film (Amersham Biosciences, Barcelona, Spain). Gel bands showing differential expression were excised, rehydrated, reamplified with the corresponding pair of primers, and cloned by using pGEM-T-easy (Promega, Madison, Wis.). Bands were sequenced commercially. One of them, which matched yeast transporters, was used to screen a λ-ZAP-II library (Stratagene, La Jolla, Calif.) constructed with mRNA from a culture containing fungal cell walls as the sole carbon source (B. Suárez, unpublished data) in accordance with the manufacturer's instructions. The complete cDNA was isolated, sequenced, and named gtt1.
Sequence analysis.
Protein sequences were aligned by using the CLUSTAL W algorithm (17). Alignment was treated as described previously (39). Briefly, sequences without homology to any of the other aligned sequences were deleted from the alignment, and a single base was left to cause a gap. All gaps exceeding a single amino acid were considered missing information and were replaced by question marks. This edited alignment was analyzed with the PHYLIP package, version 3.5.c (http://evolution.genetics.washington.edu/phylip). To obtain statistical support for the branches, 100 data sets were created by using SEQBOOT. Distance matrices were calculated with the PROTDIST program, branch lengths were evaluated by the neighbor-joining method (42), and the tree consensus sequence was generated with CONSENSE. To obtain unrooted parsimony analysis, the PROTPARS program was used (jumble option with n = 25), and the tree consensus sequence also was generated with CONSENSE. The GTR1 sequence from humans was used as an outgroup.
DNA procedures and Southern analysis.
Standard molecular techniques were performed throughout these studies (43). Genomic DNA isolation and analysis were carried out as described previously (8, 23). Southern blot analysis of genomic DNA was performed with the complete cDNA for gtt1 as a probe, and a high-stringency hybridization solution (50% formamide) and a washing solution were used as described previously (43).
RNA extraction and Northern analysis.
Mycelia were lysed by using a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.) with 2.3-mm-diameter steel beads. RNA was isolated by the acid phenol extraction procedure (6). Ten micrograms of total RNA from each sample was separated on 1.2% agarose- formaldehyde gels, blotted onto nylon membranes, and hybridized (43). Blots were probed with the complete gtt1 cDNA. Probes were labeled with [α-32P]dCTP by using an oligolabeling kit (Amersham Pharmacia Biotech). The loading control of Northern blots was checked by using radish 18S rRNA as a probe.
Transformation procedure.
Protoplast preparation and transformation were carried out as described by Penttilä et al. (34). T. harzianum was cotransformed with plasmids pLMRS3::gtt1 and p3SR2 (34); the latter carries the A. nidulans amdS gene as a selection marker, allowing growth in media with acetamide as the sole nitrogen source. Plasmid pLMRS3::gtt1 was constructed by fusing gtt1 cDNA (1.9 kb) to the pki (pyruvate kinase) promoter and to the cbh2 (cellobiohydrolase II) terminator, both from T. reesei (26). The cDNA was obtained with primers Gtt1U (5′-ATGGTCAAGGTCCTCTAGACAAAGCATCAA-3′) and Gtt1L (5′-AGCCTACCGCCATGCATAAGATTATCATCG-3′), which introduced XbaI and NsiI restriction enzymes sites. PCR was carried out for 35 cycles of 30 s at 94°C, 30 s at 55°C, and 90 s at 72°C with Expand high-fidelity polymerase (Roche Molecular Systems). The DNA was treated with XbaI and NsiI, and the fragment was cloned into plasmid pLMRS3 (26) that had been treated with the same enzymes. Cotransformation was conducted with a 1:10 (amdS::gtt1) plasmid ratio. Colonies obtained after transformation and selection were allowed to grow on potato dextrose agar in order to produce spores, which were collected and spread onto selective medium plates. This process was repeated twice. Transformants were then checked for mitotic stability, and those that showed 100% of spores growing on selective medium were chosen for further study.
[14C]glucose uptake studies.
T. harzianum was used to inoculate MM with 2% glycerol as a carbon source at a final concentration of 107 conidia per ml. Cultures were incubated for 19 h at 22°C. Spores were swollen, but less than 1% showed a germ tube protrusion. At this point, cells were collected by centrifugation at 4°C and washed four times with MM without a carbon source. Finally, cells were twofold concentrated in MM. When needed, MM was buffered with 0.1 M phosphate buffer at pH 3, 4, 5, or 6. The assay was performed with 250-μl aliquots. Equal volumes of 2 mM, 1 mM, 200 μM, 100 μM, 20 μM, 10 μM, and 2 μM glucose solutions (each containing 2 μM [14C]glucose [31 Ci/mmol]) were added to prewarmed cells, and transport reactions were carried out for 5, 30, 60, and 90 s at 30°C. Reactions were stopped by the addition of 1.5 ml of ice-cold 200 mM nonradioactive glucose. Cells were filtered through HAWP 02500 nitrocellulose filters (Millipore, Bedford, Mass.) and washed twice with the same glucose solution. Radioactivity was measured by using a Wallac 1409 scintillation counter. Cell viability was obtained by plating serial dilutions of 250-μl aliquots on potato dextrose agar plus 0.1% (vol/vol) Triton X-100. Colonies were counted after 3 days of incubation. Transport experiments were performed at least three times in duplicate, with similar results. The double-reciprocal plot was plotted with data relative to wild-type minimal values in order to minimize variability among experiments.
Nucleotide sequence accession number.
The gtt1 nucleotide sequence was assigned GenBank accession number AJ269534.
RESULTS
The sequence of the gtt1 gene, isolated by differential display, shows features of sugar transporter genes.
Antagonistic interactions between Trichoderma and fungal hosts take place in poor environments with a low availability of nutrients, mainly carbon sources. Therefore, we were interested in isolating genes which were differentially expressed in cultures grown in the presence of high or low glucose concentrations. We used a differential display technique with RNA isolated from mycelia obtained by cultivating T. harzianum CECT 2413 in media containing either 2 or 0.2% glucose and buffered at either pH 3 or pH 6 as described in Materials and Methods. Media were buffered because a number of genes involved in the antagonism of T. harzianum, such as genes for proteases (9) and glucanases and chitinases (M. A. Moreno-Mateos and T. Benítez, unpublished data), are pH controlled. Gene expression controlled by pH has also been described for fungal pathogens for insects and mammals, such as Metarhizium anisopliae and Candida albicans (7, 48). One of the clones expressed only in medium containing a low glucose concentration was chosen for further characterization in this study. The DNA fragment obtained was used as a probe to screen a λ−ZAP-II cDNA library.
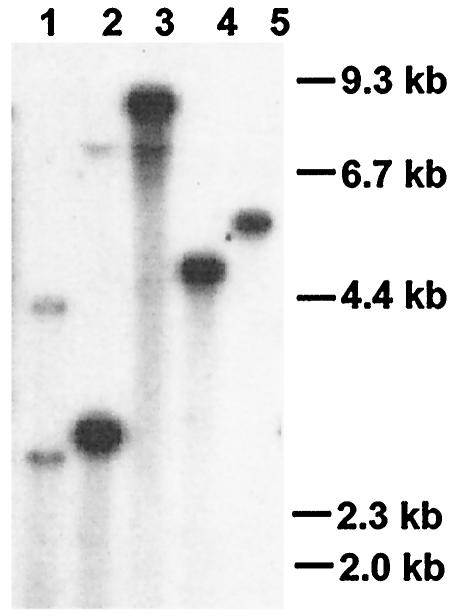
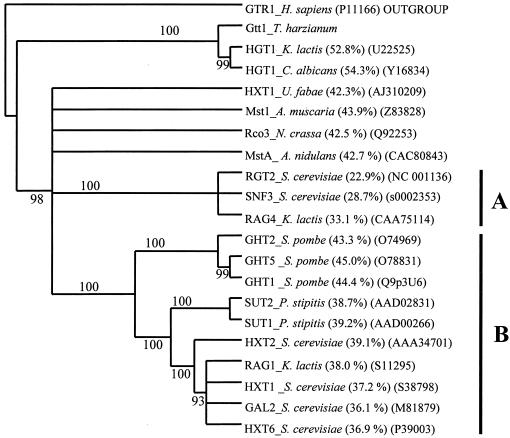
The 1,972-bp cDNA isolated was named gtt1 and had a deduced product of 561 amino acids with a calculated molecular mass of 62.1 kDa. The gtt1 gene is present in the genome of T. harzianum as a single copy (Fig. 1), and no other hybridization signals were detected under low-stringency conditions (data not shown). A high degree of similarity to fungal glucose transporters was observed by means of the BLASTX algorithm (1). Some of these proteins were aligned by using the CLUSTAL W algorithm (17), and the corresponding distance and parsimony phylogenetic trees were obtained. Only the distance phylogenetic tree is displayed (Fig. 2), since parsimony methods gave very similar results. The Gtt1 protein grouped with Hgt1 from K. lactis (3) and Hgt1 from C. albicans (52); these results seem to indicate that these proteins constitute a different subfamily of transporters (4). The rest of the proteins grouped with yeast sensors (Rgt, Snf3, and Rag4; group A) or yeast transporters (group B), whereas the filamentous fungal proteins represented separate branches without statistical support for being grouped together. Rco3, a putative sensor protein from N. crassa (27), did not group with the other sensors. The Gtt1 protein showed 53 and 54% identities, respectively, with high-affinity glucose transporters, such as Hgt1 from K. lactis (3) and Hgt1 from C. albicans (52). The Gtt1 protein deduced sequence was also very similar (44%) to that of sugar transporter MstA from the basidiomycete A. muscaria (30) and Hxt1 from U. fabae (53), glucose transporters from Schizosaccharomyces pombe (ca. 44%) (16), and the putative glucose sensor Rco3 from N. crassa (42.5%) (27). The Gtt1 protein lacks the long C-terminal tail that functions as a signal transducer in the yeast proteins and that is also present in Rco3 (31) (Fig. 3). The amino- and carboxyl-terminal regions predicted to be on the cytosolic side did not show a high degree of homology in the sequences analyzed (Fig. 3).
FIG. 1.
Southern blot analysis. Genomic DNA was digested with the following restriction enzymes: SalI (lane 1), XhoI (lane 2), XbaI (lane 3), ClaI (lane 4), and BamHI (lane 5). Complete cDNA of gtt1 was used as a probe. Single bands were detected, unless enzymes which cut the gtt1 sequence were used, such as SalI and XhoI.
FIG. 2.
Homology of the Gtt1 protein to other fungal transporters. A distance phylogenetic tree was obtained as described in Materials and Methods (parsimony methods gave very similar results). Sequences were aligned by using the CLUSTAL W algorithm (17), and a distance tree of 100 bootstrapped data sets was generated by using the PROTDIST program and the neighbor-joining method (42). Each number on the tree represents the number of times the group located to the right occurred in 100 different trees (12). Branches not supported (<90) were collapsed. Designations in parentheses are GenBank accession numbers. Percentages in parentheses indicate the percent similarity to the Gtt1 protein. A and B indicate groups.
FIG. 3.
Alignment of various fungal transporters and transporter-like proteins. (A) Alignment of deduced protein sequences, from top to bottom, for Gtt1 from T. harzianum, Hgt1 from K. lactis, HXT1 from U. fabae, Snf3, Gal2, and Hxt2 from S. cerevisiae, and Rco3 from N. crassa. Sequences were aligned by using the CLUSTAL W algorithm (17). Horizontal black bars indicate the transmembrane domains of Gtt1 obtained by using the TopPred algorithm (54). Black shading indicates highly conserved residues, and grey shading indicates lesser degrees of similarity. The black arrow indicates Arg151, the striped arrow indicates Phe412, and the grey arrow indicates Trp425 (positions relative to those of the Gtt1 sequence).
Twelve putative transmembrane domains were detected by using the TopPred algorithm (54) and the hydropathy profile algorithm of Kyte and Doolite (21) (data not shown). These domains are distributed with a long extracellular loop present between domains I and II, an intracellular loop located between segments VI and VII, and another intracellular loop located between domains IX and X (Fig. 3). This model has also been described for mammalian glucose transporter GHT1 (14, 29). Some specific amino acids, such as several leucines and isoleucines between domains I and II, which are probably involved in the oligomerization of transporter proteins, are well conserved (32). The trimeric domain GRR is present in the loops in the cytoplasm that connect domains II and III and domains VIII and IX, although the second one is disrupted in the Gtt1 protein by the dimer FT. Arg151 is conserved in all of the glucose transporters studied (Fig. 3). The sequence of Gtt1 also shows a Phe412 residue well conserved in other glucose transporters, such as Hxt2 from S. cerevisiae, where its relevance in sugar specificity has been proved (19). The same role has been attributed to Tyr440 in the Hxt2 sequence. The sequence of Gal2, a yeast galactose transporter, shows substitutions of these Phe and Tyr residues by Tyr446 and Trp455, respectively. The Gtt1 sequence also shows a tryptophan residue (Trp425) instead of the tyrosine residue of Hxt2 (Fig. 3). The presence of tryptophan is also observed in Hgt1 from K. lactis and Hxt1 from U. fabae, as well as other glucose transporters.
Northern blot analysis of gtt1.
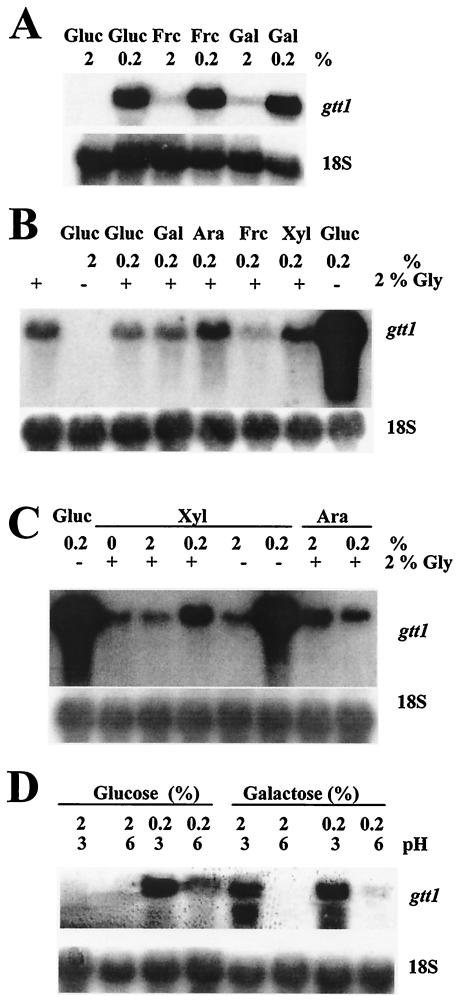
Northern blot experiments were carried out in order to investigate the relationship between the availability of a carbon source and the level of expression of gtt1. First, different hexoses, such as glucose, fructose, and galactose, were used at high (2%) and low (0.2%) concentrations (Fig. 4A). When high sugar concentrations were used, weak signals were detected with fructose and galactose but not glucose. gtt1 mRNA accumulation was strong in the presence of all of the sugars at low concentrations. Thus, gtt1 transcription was fully derepressed when sugars were present at low concentrations. Specifically, the lack of glucose, even when another carbon source was present at a high concentration, provoked a slight derepression.
FIG. 4.
Northern blot analysis. RNA was extracted from mycelia grown under the conditions indicated in Materials and Methods. Various carbon sources were used at 2 or 0.2% (wt/vol): glucose (Gluc), fructose (Frc), galactose (Gal), arabinose (Ara), xylose (Xyl), and glycerol (Gly). In panels B and C, the presence and absence of 2% glycerol are indicated by plus and minus signs, respectively. In panel D, media were buffered at the indicated pHs. Radish 18S rRNA was used as a loading control.
A second set of experiments was designed to determine the role of other carbon sources, such as glycerol, on gtt1 mRNA accumulation (Fig. 4B). The use of glycerol as the sole carbon source gave rise to a basal level of gtt1 mRNA compared to what was seen under derepression conditions (0.2% glucose). The addition of small amounts of sugar(s) to glycerol-containing media did not induce or repress the expression of gtt1, except for pentoses. When 0.2% xylose or arabinose was used in combination with glycerol as carbon sources, a small increase (threefold with xylose) in the level of expression of gtt1 was observed. However, this increase was almost negligible compared to the expression observed in cultures with glucose at a low concentration. The use of xylose at a high concentration in combination with glycerol did not increase the expression of gtt1 compared to that seen in glycerol-containing media (Fig. 4C). Therefore, gtt1 was slightly derepressed when glycerol was used as a sole carbon source, and this basal level was increased when pentoses, such as xylose or (to a lesser extent) arabinose, but not hexoses were present at low concentrations. The derepression of gtt1 was maximal when glucose (or other sugars) was used at a very low concentration as the sole carbon source, that is, under carbon starvation conditions.
Another set of Northern blot experiments was carried out in order to investigate the hierarchy between pH and carbon source regulation of gtt1 expression (Fig. 4D). Cultures containing glucose or galactose at high (2%) and low (0.2%) concentrations and at pH 6 or 3 were used. As expected, gtt1 was repressed at high glucose levels and induced at low levels. However, the gtt1 mRNA level was lower when the pH of the low-glucose culture was near neutrality (pH 6) than in the presence of an acidic pH (pH 3). This pH effect on gene expression was more noticeable when galactose was used as the carbon source, since expression was observed at pH 3 even with a high galactose concentration. Buffering cultures at pH 6 seems to repress the expression of gtt1, even with a low galactose concentration. In conclusion, acidic pH conditions provoked gtt1 mRNA accumulation with high galactose but not high glucose concentrations.
The Gtt1 protein is a glucose transporter.
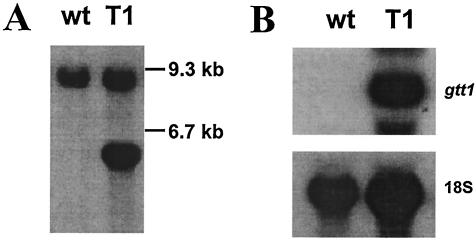
Due to similarities between gtt1 and other sequences found in sequence databases and to its transcription pattern, a potential role in the transport of hexoses could be assigned to the Gtt1 protein. To examine this hypothesis, we first tried to complement yeast mutants, such as strains RE700 and EBY.W4000, which are unable to grow in the presence of glucose due to the lack of glucose or hexose transporters (38, 57). Vector pAJ401, which contains the yeast pgk promoter, was used for gtt1 expression in yeasts. The transformants obtained were tested for their ability to grow in the presence of glucose or fructose. Plasmid pAJ401::gtt1 did not appear to complement the hexose uptake defects in either of the two strains, in spite of the fact that gtt1 mRNA was being properly detected (data not shown). For this reason, we decided that the function of Gtt1 should rather be tested with filamentous fungi. We have tried extensive gene disruption when analyzing the gtt1 gene and other genes (T. Benítez, personal communication). However, we have obtained no null mutants of T. harzianum CECT 2413 to date. These results led us to design a strategy other than deletion to determine which sugar(s) was transported by Gtt1. A construct in which the gtt1 gene was placed under the control of the constitutive pyruvate kinase gene promoter from T. reesei was made. This promoter allows a high level of expression when glycerol is used as the sole carbon source (8). T. harzianum CECT 2413 was transformed, and a strain, designated T1, that carried one extra copy of the gtt1 gene (Fig. 5A) was isolated and characterized. Strain T1 showed a higher gtt1 expression level than the wild-type strain when either 2% glucose (Fig. 5B) or 2% glycerol (data not shown) was used. Hence, the use of glycerol as a sole carbon source allowed us to analyze two isogenic strains, one of which showed enhanced expression of gtt1. Strain T1 did not show either growth or antagonistic defects compared to the wild-type strain (data not shown).
FIG. 5.
Characterization of strain T1. (A) Southern blot analysis. Genomic DNA was digested with the XbaI enzyme. wt, wild-type strain. (B) Northern blot analysis. RNA was extracted from mycelia grown with 2% glucose as a carbon source as described in Materials and Methods. For both panels A and B, the complete gtt1 gene was used as a probe, and radish 18S rRNA was used as a loading control.
To measure transport capacity, spores of both strains were germinated in MM containing 2% glycerol as a carbon source for 19 h. Glycerol was used instead of glucose because of the possibility that posttranscriptional mechanisms could affect the stability and/or activity of the protein. Since the gtt1 gene was expressed when glycerol was used, it was assumed that those mechanisms would be active under these conditions. Low glucose concentrations were not used because the high gtt1 mRNA expression levels in the wild-type strain were very similar to those in strain T1 under strong derepression conditions (data not shown). Almost none of the spores showed protrusion of the germ tube, but all were swollen. These cells were used to measure the transport of glucose, xylose, and galactose. Xylose was chosen because of the possibility that enhanced expression in media containing glycerol plus xylose could reflect a role in pentose transport. We also used galactose because Gtt1 shares a conserved amino acid (Trp425) involved in transport specificity with Gal2, and we tried to assess whether Gtt1 plays a role in the uptake of galactose.
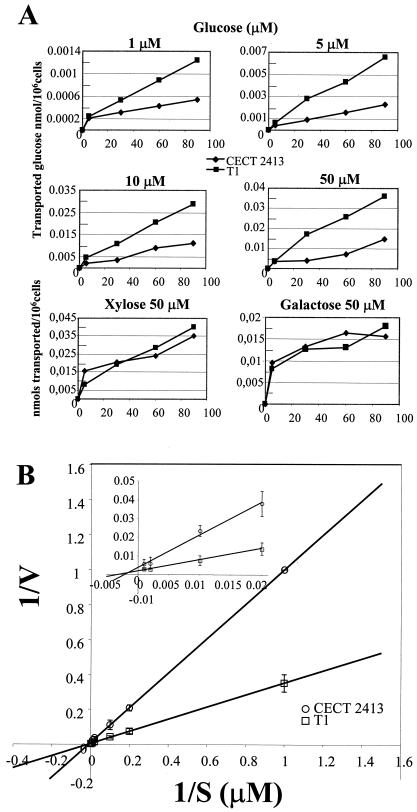
Strain T1 was able to transport glucose two to three times faster than the wild-type strain when low concentrations of glucose were used in measurements (1 μM to 1 mM) (Fig. 6A). When the data were plotted as double-reciprocal plots (Lineweaver-Burk transformation), a Km of about 12 μM could be calculated for the high-affinity glucose transport component of T. harzianum (Fig. 6). The calculated Vmax (obtained with the double-reciprocal plot) for strain T1 was about 1.9 × 10−3 nmol/106 cel/s, whereas the value for the wild-type strain was about two to threefold lower: 0.9 × 10−4 nmol/106 cel/s. No enhancement of transport capability for either xylose or galactose was observed for strain T1 under the conditions tested (Fig. 6A). These results led us to conclude that gtt1 codes for a high-affinity glucose transporter.
FIG. 6.
Kinetics of uptake of d-glucose. (A) Uptake of d-glucose, d-xylose, and d-galactose (nanomoles of glucose transported per million living cells) for Trichoderma strains CECT 2413 (wild-type strain) and T1 (strain that overexpresses the gtt1 gene). The amount of sugar used in each transport assay is indicated above each graph. (B) Double-reciprocal plots used to obtain the Km and Vmax values for high-affinity d-glucose transport. The inset is an amplification of the 1/S values from 0 to 0.02 μM. V, velocity. Error bars indicate standard deviations.
Glucose transport is disturbed at an acidic pH.
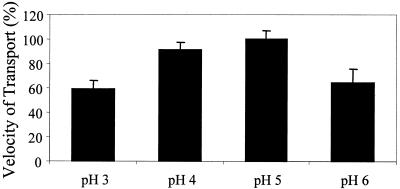
One of the most interesting aspects of gtt1 was the pH control of gene expression (Fig. 4C). Acidic media, with a high availability of protons, could give rise to the induction (or derepression) of gtt1 (through PacC or a similar transcription factor) (11) in order to utilize this gradient of protons to transport glucose. Alternatively, Gtt1 activity might be disturbed at a strongly acidic pH. Then, the lack of glucose uptake might trigger the derepression or induction of the gtt1 gene by a CreA-like mechanism (10, 41). To investigate these hypotheses, we measured the transport velocity of strain T1 grown for 19 h in glycerol-containing medium buffered at different pHs (pH 3, 4, 5, or 6) by using a transport assay (Fig. 7). The uptake of glucose was diminished about 20% at pH 4 with respect to pH 5 and about 40% at pH 3. Since cells were grown under the same conditions, this effect can be explained only by a slowing down of the glucose transporter function at an acidic pH.
FIG. 7.
Decreased uptake of d-glucose at acidic pH. The uptake of d-glucose was measured by using 10 μM d-glucose and different phosphate buffers at pH 3, 4, 5, or 6. Transport velocity was obtained by using slopes of the linear regression of d-glucose uptake at 0, 5, 30, 60, and 90 s. Error bars indicate standard deviations.
DISCUSSION
We report the isolation and characterization of the gtt1 gene, the first glucose transporter gene described for an ascomycete-related filamentous fungus. Although we have highly detailed information about the transport of hexoses in yeasts such as S. cerevisiae and K. lactis, only two transporter proteins have been described for filamentous fungi, both in basidiomycetes: AmMstA from A. muscaria (30) and Hxt1 from U. fabae (53).
Twenty HXT genes involved in hexose transport have been isolated from S. cerevisiae, all of them with significant similarities in their sequences. However, gtt1 seems to be present in a single copy in the T. harzianum genome. S. cerevisiae has to carry out fermentative metabolism when glucose is available at a high concentration (grape must). Fermentation is a process that yields low amounts of energy (2 mol of ATP/mol of glucose), so a high flux of glucose, transported by a number of permeases, is necessary to ensure the generation of enough energy during processes such as enological fermentation, when sugar availability changes constantly (25). Environments inhabited by Trichoderma strains are nutrient poor, and their resources are exploited by their extracellular hydrolases. Trichoderma has respiratory metabolism (5), which produces higher ATP yields (36 mol of ATP/mol of glucose). For these reasons, two transporters with different Km values may be sufficient to sustain the respiratory metabolism and growth of T. harzianum under field conditions, without large oscillations in sugar concentrations. The second transporter probably is not related to Gtt1, since low-stringency hybridization did not show additional signals. This situation resembles that in K. lactis, in which only two transporters have been identified: one with a high affinity and one with a low affinity.
Distance phylogenetic tree analysis, which places the Gtt1 protein near other high-affinity hexose transporters from ascomycetes, such as K. lactis and C. albicans, and sequence features indicated a role for Gtt1 in glucose transport in T. harzianum. Moreover, the pattern of gtt1 gene transcription is very similar to that described for high-affinity hexose transporter genes. A high expression level is observed only when glucose is present at a very low concentration (0.2%). The same result has been observed with hxt2 from S. cerevisiae (55). The gtt1 gene is also repressed when T. harzianum is cultivated in media with 2% glucose, a feature typical of the genes for transporters involved in the high-affinity uptake of hexoses, such as HXT2, HXT4, HXT6, and HXT7 (32). These two characteristics provide a useful mechanism for ensuring that a gene will be transcribed when needed (4). In K. lactis, hgt1 gene regulation is different: this high-affinity transporter gene is transcribed constitutively, whereas a low-affinity transporter gene is induced by large amounts of glucose. The same pattern has been described for Aspergillus niger (51). These strategies have a common element: one of the genes is constitutively transcribed, whereas the other one is regulated. These findings allow us to suggest that, on the basis of the model of K. lactis, T. harzianum very likely has another gene coding for a hexose transporter; this gene will be a low-affinity transporter gene and probably will be constitutively expressed.
gtt1 is fully repressed only when high levels of glucose are present. Partial derepression is observed when the sugar used is other than glucose (glycerol, fructose, galactose, xylose, and arabinose). Derepression is fully obtained when sugars are available only at low levels (0.2%). That is, gtt1 seems to be only repressible and derepressible but not inducible, since small amounts of glucose combined with glycerol did not raise gtt1 mRNA levels. Glycerol in T. harzianum is probably metabolized to pyruvate via glycolysis or to glucose via gluconeogenesis (13). T. harzianum requires abundant sugar phosphates and depends on gluconeogenesis for providing carbohydrates mostly for cell wall biosynthesis and hyphal growth (51). If glycerol is rapidly metabolized, then catabolic repression could be exerted by glucose synthesized from glycerol through gluconeogenesis. The rate of growth of T. harzianum in glycerol is similar to that in glucose (data not shown), so that the efficiency of glycerol catabolism might influence both the growth rate and the glucose concentration inside the cell; carbon catabolite regulation might be mediated by the repressor Cre1p in a manner similar to that of Mig1p (10, 13, 40, 41). Alternatively, the results might indicate a mechanism of carbon repression different from that in yeasts and filamentous fungi, which is related to the participation of the repressor proteins Mig1 and CreA, as has been suggested by other authors (11). gtt1 regulation differs substantially from that shown for the most similar transporter genes. While gtt1 expression seems to be repressible, HGT1 from K. lactis (3) and AmMstA from A. muscaria (30) were highly expressed in media containing high glucose concentrations. The expression of the HXT1 gene from U. fabae was restricted to haustoria (53), although studies with shake flask cultures to analyze carbon regulation of gene expression have not been done to date, as have been done for the HGT1 gene from C. albicans (52).
The use of galactose allowed us to identify a novel aspect of transporter regulation in T. harzianum: the role of pH. gtt1 seems to be fully repressed in the presence of 2% glucose at neutral or acidic pH, although the derepression observed at low glucose levels was higher at acidic pH than at neutral pH. This effect was observed in a more extreme manner when galactose was used, since gtt1 expression was obtained even when galactose was used at high concentrations at acidic pH but not at neutral pH. Transport experiments showed a decrease in glucose transport at acidic pH, as has been described for A. niger (51). Gtt1 activity seems to be disturbed at a strongly acidic pH compared to the transport obtained at pH 5. This decrease provokes a slow reduction in intracellular glucose levels and may be the reason why acidic pH represses gtt1 expression in cultures with low glucose concentrations. The decrease in glucose uptake observed at pH 6 could be due to the reduction in gtt1 expression (Fig. 4D).
In the present study, the deduced Km for high-affinity transport in T. harzianum grown in glycerol was about 12 μM. gtt1 is present in a single copy, and there are no closely related genes in the genome of T. harzianum CECT 2413. These facts, together with the enhancement of glucose uptake in strain T1—which carries one additional copy—indicate a significant role for Gtt1 in the transport of glucose when T. harzianum is grown in the presence of low levels of sugars. Unfortunately, the low Km value obtained prevented us from measuring possible differences in growth rates between strain T1 and the wild-type strain at micromolar concentrations of glucose. No differences in growth rates between strain T1 and the wild-type strain were detected at 0.5, 5, and 25 mM glucose (data not shown). However, at these concentrations, a second, low-affinity glucose transporter might be active. The Km value is lower than those measured for S. cerevisiae (32) and K. lactis (3), both ca. 1 to 2 mM, and S. pombe (16), A. muscaria (30), and U. fabae (53) (all ca. 0.4 mM). However, most of these data were obtained with heterologous systems, by taking advantage of strain RE700 of S. cerevisiae (38, 57) and/or growing microorganisms at high glucose concentrations, which may involve the transcriptional induction of only low-affinity transporters. A very low Km (10 μM) has been estimated for the transport of glucose in N. crassa grown in media with low concentrations of this sugar (44, 45). Similar kinetic data (Km, 15 μM) have also been obtained for Candida utilis grown in the presence of glycerol (33). Both reactions are mediated by active transport, probably also the case for T. harzianum. U. fabae—a biotrophic basidiomycete—possesses an H+ ATPase and a proton-coupled glucose transport system located at the haustorial interface during the process of infection of Vicia faba (49, 50, 53, 58). This information suggests a very attractive idea: antagonism combined with a very-low-Km transporter could allow T. harzianum not only to obtain energy from hydrolyzed polymers but also to rapidly take sugar molecules into the cells, processes which could represent very useful mechanisms for competing for nutrients during mycoparasitic interactions. We have determined that gtt1 mRNA levels are increased when T. harzianum is confronted with the fungus Rhizoctonia solani (data not shown). However, it is difficult to determine specific expression due to the strong signal observed during the carbon starvation conditions needed to provoke an antagonistic interaction.
The isolation of gtt1, the first high-affinity glucose transporter gene isolated from an ascomycete-related filamentous fungus, provides a new tool to help learn whether mechanisms of glucose repression similar to those described for fermentative yeasts are present in aerobic fungi and whether these represent key metabolic differences between fermentative yeasts and fungi with respiratory metabolism.
Acknowledgments
We are grateful to Daniel Ramón and Andrew MacCabe (Instituto de Agroquímica y Tecnología de Alimentos, Valencia, Spain) for help with initial sugar uptake measurements; to Rafael C. Jiménez Domenech for help with sequence analysis; to Belén Suárez for providing us with the λ-ZAP-II library; and to Ana M. Rincón, Hamza El-Dorry, and Rosario Lagunas (Instituto de Investigaciones Biomédicas, Madrid, Spain) for critical reading of the manuscript.
M.A.M.-M. is the recipient of a grant from the Ministerio de Ciencia y Tecnología. This work was supported by grants PAI CVI-107 and CICYT IFD97-0668, IFD97-0820, and AGL2000-0524.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Benítez, T., M. C. Limón, J. Delgado-Jarana, and M. Rey. 1998. Glucanolytic and other enzymes and their genes, p. 101-127. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Taylor & Francis, Ltd., London, England.
- 3.Billard, P., S. Menart, J. Blaisonneau, M. Bolotin-Fukuhara, H. Fukuhara, and M. Wesolowski-Louvel. 1996. Glucose uptake in Kluyveromyces lactis: role of the HGT1 gene in glucose transport. J. Bacteriol. 178:5860-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boles, E., and C. P. Hollenberg. 1997. The molecular genetics of hexose transport in yeasts. FEMS Microbiol. Rev. 21:85-111. [DOI] [PubMed] [Google Scholar]
- 5.Chambergo, F. S., E. D. Bonaccorsi, A. J. Ferreira, A. S. Ramos, J. R. J. J. R. Ferreira, J. Abrahao-Neto, J. P. Farah, and H. El-Dorry. 2002. Elucidation of the metabolic fate of glucose in the filamentous fungus Trichoderma reesei using expressed sequence tag (EST) analysis and cDNA microarrays. J. Biol. Chem. 277:13983-13988. [DOI] [PubMed] [Google Scholar]
- 6.Chomzynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 7.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Jarana, J., J. A. Pintor-Toro, and T. Benítez. 2000. Overproduction of β-1,6-glucanase in Trichoderma harzianum is controlled by extracellular acidic proteases and pH. Biochim. Biophys. Acta 1481:289-296. [DOI] [PubMed] [Google Scholar]
- 9.Delgado-Jarana, J., A. M. Rincón, and T. Benítez. 2002. Aspartyl protease from Trichoderma harzianum CECT 2413: cloning and characterization. Microbiology 148:1305-1315. [DOI] [PubMed] [Google Scholar]
- 10.Ebbole, D. J. 1998. Carbon catabolite repression of gene expression and conidiation in Neurospora crassa. Fungal Genet. Biol. 25:15-21. [DOI] [PubMed] [Google Scholar]
- 11.Espeso, E. A., J. Tilburn, H. N. Arst, Jr., and M. A. Peñalva. 1993. pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J. 12:3947-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1985. Confidence limits on phylogeneties: an approach using bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 13.Gancedo, J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould, G. W., and M. J. Seater. 1997. Introduction to the facilitative glucose transporter family, p. 1-38. In G. W. Gould (ed.), Facilitative glucose transporter. Chapman & Hall, New York, N.Y.
- 15.Harman, G. E. 2000. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 84:377-393. [DOI] [PubMed] [Google Scholar]
- 16.Heiland, S., N. Radovanovic, M. Hofer, J. Winderickx, and H. Lichtenberg. 2000. Multiple hexose transporters of Schizosaccharomyces pombe. J. Bacteriol. 182:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins, D., J. Thompson, T. Gibson, J. D. Thompson, D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressivemultiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjeljord, L., and A. Tronsmo. 1998. Trichoderma and Gliocladium in biological control: an overview, p. 131-151. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Taylor & Francis, Ltd., London, England.
- 19.Kasahara, M., E. Shimoda, and M. Maeda. 1997. Amino acid residues responsible for galactose recognition in yeast Gal2 transporter. J. Biol. Chem. 272:16721-16724. [DOI] [PubMed] [Google Scholar]
- 20.Kubicek, C. P., and M. Penttilä. 1998. Regulation of production of plant polysacchride degrading enzymes by Trichoderma, p. 49-71. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Taylor & Francis, Ltd., London, England.
- 21.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 22.Lagunas, R. 1993. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 10:229-242. [DOI] [PubMed] [Google Scholar]
- 23.Limón, M. C., J. M. Lora, I. García, J. de la Cruz, A. Llobell, T. Benítez, and J. A. Pintor-Toro. 1995. Primary structure and expression pattern of the 33-kDa chitinase gene from the mycoparasitic fungus Trichoderma harzianum. Curr. Genet. 28:478-483. [DOI] [PubMed] [Google Scholar]
- 24.Lorito, M. 1998. Chitinolytic enzymes and their genes, p. 73-99. In G. E. Harman and C. P. Kubicek (ed.), Trichoderma and Gliocladium, vol. 2. Taylor & Francis, Ltd., London, England.
- 25.Luyten, K., C. Riou, and B. Blondin. 2002. The hexose transporters of Saccharomyces cerevisiae play different roles during enological fermentation. Yeast 19:713-726. [DOI] [PubMed] [Google Scholar]
- 26.Mach, R. L., M. Schindler, and C. P. Kubicek. 1994. Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr. Genet. 25:567-570. [DOI] [PubMed] [Google Scholar]
- 27.Madi, L., S. A. McBride, L. A. Bailey, and D. J. Ebbole. 1997. rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 146:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marger, M. D., and M. H. Saier. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 29.Mueckler, M. 1994. Facilitative glucose transporters. Eur. J. Biochem. 219:713-725. [DOI] [PubMed] [Google Scholar]
- 30.Nehls, U., J. Wiese, M. Guttenberger, and R. Hampp. 1998. Carbon allocation in ectomycorrhizas: identification and expression analysis of an Amanita muscaria monosaccharide transporter. Mol. Plant-Microbe Interact. 11:167-176. [DOI] [PubMed] [Google Scholar]
- 31.Özcan, S., J. Dover, and M. Johnston. 1998. Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J. 17:2566-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Özcan, S., and M. Johnston. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peinado, J. M., P. J. Cameira-dos-Santos, and M. C. Loureiro-Dias. 1989. Regulation of glucose transport in Candida utilis. J. Gen. Microbiol. 135:195-201. [DOI] [PubMed] [Google Scholar]
- 34.Penttilä, M., H. Nevalainen, M. Ratto, E. Salminen, and J. Knowles. 1987. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155-164. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer, T., S. Schuster, and S. Bonhoeffer. 2001. Cooperation and competition in the evolution of ATP-producing pathways. Science 292:504-507. [DOI] [PubMed] [Google Scholar]
- 36.Rand, J. B., and E. L. Tatum. 1980. Characterization and regulation of galactose transport in Neurospora crassa. J. Bacteriol. 141:707-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand, J. B., and E. L. Tatum. 1980. Fructose transport in Neurospora crassa. J. Bacteriol. 142:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reifenberger, E., E. Boles, and M. Ciriacy. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245:324-333. [DOI] [PubMed] [Google Scholar]
- 39.Retief, J. D. 2000. Phylogenetic analysis using PHYLIP, p. 243-258. In S. A. Krawetz (ed.), Bioinformatics: methods and protocols, vol. 132. Humana Press Inc., Totowa, N.J. [DOI] [PubMed]
- 40.Ronne, H. 1995. Glucose repression in fungi. Trends Genet. 11:12-17. [DOI] [PubMed] [Google Scholar]
- 41.Ruijter, G., and J. Visser. 1997. Carbon repression in aspergilli. FEMS Microbiol. Lett. 151:103-114. [DOI] [PubMed] [Google Scholar]
- 42.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Scarborough, G. A. 1970. Sugar transport in Neurospora crassa. J. Biol. Chem. 245:1694-1698. [PubMed] [Google Scholar]
- 45.Scarborough, G. A. 1970. Sugar transport in Neurospora crassa. II. A second glucose transport system. J. Biol. Chem. 245:3985-3987. [PubMed] [Google Scholar]
- 46.Schneider, R. P., and W. R. Wiley. 1971. Kinetic characteristics of the two glucose transport systems in Neurospora crassa. J. Bacteriol. 106:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider, R. P., and W. R. Wiley. 1971. Regulation of sugar transport in Neurospora crassa. J. Bacteriol. 106:487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St. Leger, R. J., J. O. Nelson, and S. E. Screen. 1999. The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 145:2691-2699. [DOI] [PubMed] [Google Scholar]
- 49.Struck, C., M. Hahn, and K. Mendgen. 1996. Plasma membrane H+-ATPase activity in spores, germ tubes, and haustoria of the rust fungus Uromyces viciae-fabae. Fungal Genet. Biol. 20:30-35. [DOI] [PubMed] [Google Scholar]
- 50.Szabo, L. J., and W. R. Bushnell. 2001. Hidden robbers: the role of fungal haustoria in parasitism of plants. Proc. Natl. Acad. Sci. USA 98:7654-7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres, N. V., J. M. Riol-Climas, M. Wolschek, and C. P. Kubicek. 1996. Glucose transport by Aspergillus niger: the low-affinity carrier is only formed during growth on high glucose concentrations. Appl. Microbiol. Biotechnol. 44:490-494. [Google Scholar]
- 52.Varma, A., B. B. Singh, N. Karnani, H. Lichtenberg-Frate, M. Hofer, B. B. Magee, and R. Prasad. 2000. Molecular cloning and functional characterisation of a glucose transporter, CaHGT1, of Candida albicans. FEMS Microbiol. Lett. 182:15-21. [DOI] [PubMed] [Google Scholar]
- 53.Voegele, R. T., C. Struck, M. Hahn, and K. Mendgen. 2001. The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc. Natl. Acad. Sci. USA 98:8133-8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Heijne, G. 1992. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 55.Wendell, D. L., and L. F. Bisson. 1994. Expression of high-affinity glucose transport protein Hxt2p of Saccharomyces cerevisiae is both repressed and induced by glucose and appears to be regulated posttranslationally. J. Bacteriol. 176:3730-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wesolowski-Louvel, M., P. Goffrini, I. Ferrero, and H. Fukuhara. 1992. Glucose transport in the yeast Kluyveromyces lactis. I. Properties of an inducible low-affinity glucose transporter gene. Mol. Gen. Genet. 233:89-96. [DOI] [PubMed] [Google Scholar]
- 57.Wieczorke, R., S. Krampe, T. Weierstall, K. Freidel, C. P. Hollenberg, and E. Boles. 1999. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464:123-128. [DOI] [PubMed] [Google Scholar]
- 58.Wirsel, S. G., R. T. Voegele, and K. W. Mendgen. 2001. Differential regulation of gene expression in the obligate biotrophic interaction of Uromyces fabae with its host Vicia faba. Mol. Plant-Microbe Interact. 14:1319-1326. [DOI] [PubMed] [Google Scholar]