Abstract
Autoimmune polyendocrine syndrome type 1 (APS1) is a rare autosomal recessive disorder caused by mutations in the autoimmune regulator (AIRE) gene. High titer autoantibodies (Aabs) toward intracellular enzymes are a hallmark for APS1 and serve as diagnostic markers and predictors for disease manifestations. In this study, we aimed to identify pituitary autoantigens in patients with APS1. A pituitary cDNA expression library was screened with APS1 sera and a tudor domain containing protein 6 (TDRD6) cDNA clone was isolated. Positive immunoreactivity against in vitro translated TDRD6 fragments was shown in 42/86 (49%) APS1 patients but not in patients with other autoimmune diseases or in healthy controls. By using immunohistochemistry, sera from 3/6 APS1 patients with growth hormone (GH) deficiency showed immunostaining of a small number of guinea pig anterior pituitary cells, and 40–50% of these cells were GH-positive. No such immunostaining was seen with sera from healthy controls. The APS1 Aab-positive, GH-negative cells may represent a novel subpopulation of anterior pituitary cells. In addition, 4/6 patient sera showed staining of a fiber-plexus in the pituitary intermediate lobe recognizing enzymes of monoamine and GABA synthesis. Thus, we have identified TDRD6 as a major autoantigen in APS1 patients and shown that several sera from GH-deficient patients stain specific cell populations and nerves in the pituitary gland.
Keywords: autoantigens, growth hormone deficiency, pituitary intermediate lobe, tudor domain containing protein 6
Autoimmune polyendocrine syndrome type 1 (APS1), also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (OMIM 240300), is a rare autosomal recessive disorder caused by mutations in the autoimmune regulator (AIRE) gene encoded on chromosome 21q22.3 (1, 2). The autoimmune regulator is an important mediator of central tolerance by promoting the expression of organ-specific antigens in the thymus (3–5).
The classical triad of APS1 include hypoparathyroidism, adrenal failure and chronic mucocutaneous candidiasis (6). In addition, other organ-specific autoimmune diseases such as gonadal failure, intestinal dysfunction, type 1 diabetes mellitus, hypothyroidism and pituitary insufficiency as well as ectodermal manifestations are found with variable penetrance.
High titer autoantibodies (Aabs) against intracellular key enzymes, primarily involved in the synthesis of steroids or neurotransmittors, is a hallmark of APS1 (7–10). These Aabs constitute important diagnostic markers and are sometimes predictive of disease manifestations (11, 12).
Hypopituitarism is a rare manifestation of APS1. Isolated growth hormone (GH)-deficiency has to the best of our knowledge only been reported in 10 APS1 patients (6, 13–17), isolated hypogonadotroph hypogonadism in one patient (13) and central diabetes insipidus in three (18–20). Three siblings with APS1 and partial adrenocorticotropic hormone deficiency have been described (21). One APS1 patient with multiple pituitary hormonal insufficiencies has also been reported (22).
Pituitary Aabs against prolactin secreting cells have been detected by conventional immunofluorescence in sera from a few APS1 patients (6, 23). Immunoreactivity against median eminence dopaminergic nerve terminals and pituitary gonadotrops have been shown in an APS1 patient with GH deficiency (24). By using an immunoblotting method with pituitary homogenate, Aabs to a 49-kDa antigen was detected in 39/67 (58%) APS1 patients (25). The 49-kDa pituitary protein has been identified as α-enolase (26), but the importance of enolase Aabs as markers for neuroendocrine autoimmunity has been debated (27). Moreover, no correlations between pituitary Aabs and pituitary dysfunction in APS1 have been described.
In this study, we aimed to identify novel pituitary autoantigens in patients with APS1 by immunohistochemistry and immunoscreening of a human pituitary cDNA expression library.
Results
Identification of Tudor Domain Containing Protein 6 (TDRD6) as an Autoantigen.
A pituitary cDNA expression library was constructed and screened with sera from two APS1 patients. Twenty-seven cDNA clones were isolated and partially sequenced. Four of the clones encoded tryptophan hydroxylase (TPH) isoform 1, a well known APS1 autoantigen (9). In vitro transcription and translation (ITT) of other cDNA clones resulted in 10 recombinant proteins that were used for immunoprecipitation with a test panel of sera from six APS1 patients and five healthy blood donors. Most of these recombinant products were recognized solely by the screening serum, by both APS1 sera and control sera or by none of the sera. A protein encoded by a tudor domain containing protein 6 (TDRD6) cDNA clone was, however, efficiently immunoprecipitated by two of the APS1 sera but not by any of the healthy blood donor sera and was therefore selected for further studies.
The TDRD6 gene which is located on chromosome 6p12.3 consists of four exons and spans over a region of 14 kb. The TDRD6 mRNA reference sequence reported in GenBank (accession no NM_001010870.1) is 6.8 kb long and encodes a 2,096-aa protein. Several different TDRD6 cDNA sequences with different putative transcriptional start sites and alternative splicing have been reported. The TDRD6 cDNA clone identified in this study spans from the middle of exon 1 to intron 3 and retains intron 1. Because of a termination codon, 6 bp into intron 1, the protein is truncated, and this cDNA is predicted to encode a 925-aa protein. Details on TDRD6 cDNA clones are in supporting information (SI) Fig. 5.
Immunoprecipitation of in Vitro Translated TDRD6 Fragment with Patient Sera.
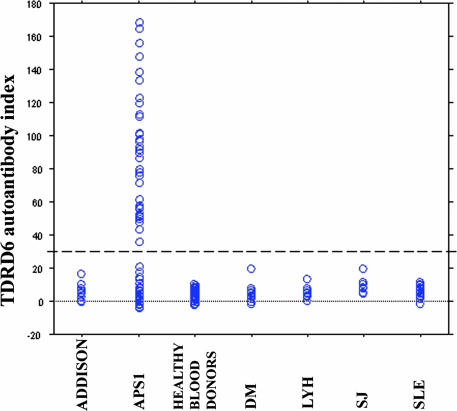
To determine whether immunoreactivity against TDRD6 was APS1 related, sera obtained from 86 APS1 patients, 93 patients with other autoimmune diseases and 90 healthy blood donors were tested for immunoreactivity against TDRD6. Forty-two of the 86 (49%) APS1 patients showed positive immunoreactivity against the in vitro translated TDRD6 fragment. No TDRD6 Aabs were found in sera from patients with isolated lymphocytic hypophysitis, Addison's disease, type 1 diabetes mellitus, Sjögren's syndrome, systemic lupus erythematosus, or healthy blood donors (Fig. 1). We found no associations between immunoreactivity against TDRD6 and clinical manifestations of APS1 (Table 1). Two of six APS1 patients with GH-deficiency showed positive immunoreactivity against TDRD6, but the limited number did not allow statistical interpretation.
Fig. 1.
Scattergram showing the immunoreactivity against TDRD6 fragment in sera from patients with APS1 (n = 86), lymphocytic hypophysitis (LyH) (n = 11), Addison's disease (n = 17), type 1 diabetes mellitus (DM) (n = 20), Sjögren's syndrome (SJ) (n = 20), systemic lupus erythematosus (SLE) (n = 25), and healthy blood donors (n = 90). The broken line indicates a cut-off value (30) of positive results.
Table 1.
Immunoreactivity against in vitro expressed TDRD6 correlated to clinical manifestations in 86 patients with APS1
| Clinical manifestation | Number with manifestation/total | Number with autoantibodies to TDRD6/total |
P* | |
|---|---|---|---|---|
| With manifestation | Without manifestation | |||
| Mucocutaneous candidiasis | 83/86 | 40/83 | 2/3 | 0.612 |
| Hypoparathyroidism | 71/86 | 37/71 | 5/15 | 0.258 |
| Adrenal insufficiency | 69/86 | 35/69 | 7/17 | 0.591 |
| Alopecia areata | 29/86 | 15/29 | 27/57 | 0.820 |
| Gonadal failure | 28/86 | 17/28 | 25/58 | 0.168 |
| Intestinal dysfunction | 22/86 | 12/22 | 30/64 | 0.624 |
| Vitiligo | 18/86 | 8/18 | 34/68 | 0.793 |
| Chronic active hepatitis | 16/86 | 10/16 | 32/70 | 0.274 |
| Pernicious anemia | 13/86 | 7/13 | 35/73 | 0.769 |
| Type 1 diabetes mellitus | 10/86 | 5/10 | 37/76 | 1.000 |
| Pituitary insufficiency | 6/86 | 2/6 | 40/80 | 0.677 |
*Calculated by use of two-tailed Fisher's exact test.
Immunohistochemical Findings.
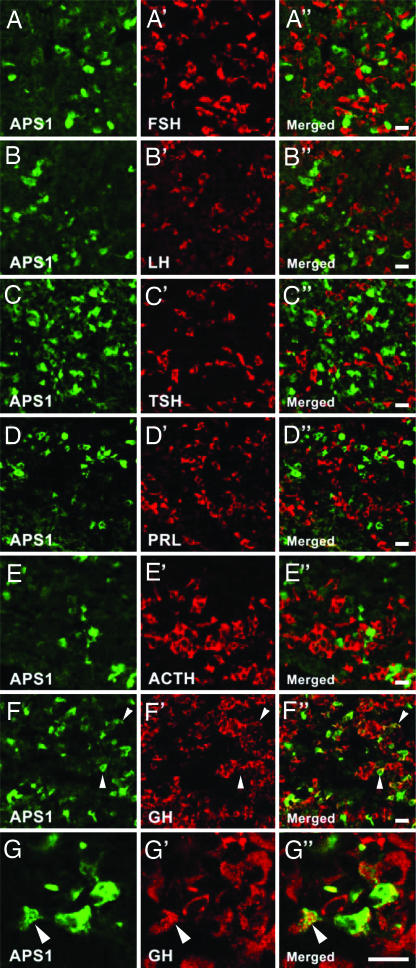
Three of the six (50%) APS1 patients with GH-deficiency (Table 2, patients 1, 3, and 6) showed immunoreactivity against a small cell population in the guinea pig anterior pituitary lobe. We selected the patient serum that showed the strongest immunoreactivity (Table 2, patient 3) and performed double-staining for the following pituitary hormones: follicle stimulating hormone, luteinizing hormone, thyroid stimulating hormone, prolactin, adrenocorticotropic hormone or GH. No coexistence was found (Fig. 2 A–E), the GH antiserum being an exception (Fig. 2 F and G). Thus, 40–50% of the APS1-positive cells were GH-positive with serum no. 3. Conversely, <1% of the GH cells stained with the APS1 serum. Subsequently, about the same percentage were observed for patients 1 and 6. The APS1 immunofluorescence pattern showed aggregates of staining, distinctly different from the small, singular granules containing GH, suggesting that the targeted autoantigen(s) is not colocalized or cosecreted with GH (Fig. 2G). None of the ten sera from healthy control subjects showed any staining.
Table 2.
Clinical characteristics and autoantibodies in six APS1 patients with GH deficiency
| Variables | Patient |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Characteristic | ||||||
| Sex | M | M | F | F | M | F |
| Age (years) at evaluation | 18 | 6 | 18 | 6 | 15 | 16 |
| Mucocutaneous candidiasis | + | + | + | +* | + | + |
| Adrenal insufficiency | + | − | + | +* | + | + |
| Hepatitis | + | + | − | − | + | + |
| Hypoparathyroidism | + | − | + | + | − | + |
| Intestinal dysfunction | + | + | − | − | − | + |
| Gonadal failure | − | − | + | − | − | + |
| Alopecia | − | +* | − | − | + | − |
| Vitiligo | + | +* | + | − | − | − |
| Iritis | + | + | − | − | − | − |
| Keratitis | − | − | − | − | + | − |
| Thyroiditis | − | − | − | − | − | + |
| Failure of exocrine pancreas | + | − | − | − | − | − |
| Autoantigen | ||||||
| Glutamic acid decarboxylase (GAD) | + | − | + | − | + | + |
| Aromatic-l-amino acid decarboxylase (AADC) | + | − | + | − | + | + |
| Tyrosine hydroxylase (TH) | − | + | + | − | + | − |
| Tryptophan hydroxylase (TPH) | + | + | + | − | + | + |
*Manifestation diagnosed after blood sampling for this study.
Fig. 2.
Double-staining of guinea pig anterior pituitary lobe with serum from an APS1 patient (green) and antisera against pituitary hormones (red). No colocalization of the APS1 serum immunoreactivity and reactivity against follicle stimulating hormone (A), luteinizing hormone (B), thyroid stimulating hormone (C), prolactin (D), or adrenocorticotropic hormone (E) was found. The APS1 serum and GH antiserum revealed colocalization (yellow) within some cells (F and G, arrowheads). The APS1 staining was distinctly different from the GH-positive granules (G, arrowheads). (Scale bars: 20 μm.)
The APS1 sera were preadsorbed with TDRD6 or enzymes (glutamic acid decarboxylase, GAD; aromatic-l-amino acid decarboxylase, AADC; tyrosine hydroxylase, TH; or TPH). Preadsorption with AADC, but with none of the other proteins, abolished the staining in one of three APS1 patients (Table 2, patient 1) (compare Fig. 3 B with A).
Fig. 3.
Preadsorption with AADC of an APS1 serum that stains cells in the guinea pig anterior pituitary lobe. Shown are before (A) and after (B) adsorption. (Scale bar: 50 μm.)
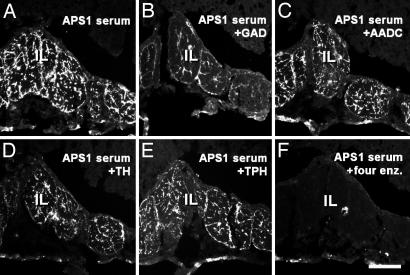
In the rat intermediate lobe, four APS1 sera (patients 1, 3, 5, and 6, Table 2) showed a distinctly stained fiber plexus (Fig. 4A). Adsorption of one APS1 serum (Table 2, patient 5) with GAD alone caused a strong reduction in the number of fluorescent terminals (compare Fig. 4 B with A), whereas the effect of AADC or TH was less pronounced (compare Fig. 4 C and D with A) and that of TPH negligible (compare Fig. 4 E with A). No fluorescent structures were seen after incubation with a mix of all four enzymes (compare Fig. 4 F with A).
Fig. 4.
Preadsorption with recombinant enzymes of an APS1 serum, which stained intermediate lobe (IL) of rat pituitary. Shown are before adsorption (A) and after adsorption with GAD (B), AADC (C), TH (D), TPH (E), and all four enzymes (F). (Scale bar: 100 μm.)
Table 2 shows presence of Aabs against GAD, AADC, TH, and TPH among the six APS1 patients with GH-deficiency as detected by immunoprecipitation. No sera from healthy controls showed immunoreactivity against any of the four enzymes.
Discussion
By immunoscreening of a human pituitary cDNA library, we have identified TDRD6 as a major autoantigen in APS1. TDRD6 is known to be mainly expressed in the testis and only at very low levels in other endocrine tissues like the pituitary, adrenal gland, and pancreas (http://expression.gnf.org) (28). The function of TDRD6 is unexplored, but the protein is known to contain seven so-called tudor domains, that is, ≈60-aa repeats initially found in developmentally important proteins and first ascribed a putative RNA-binding function (29). More recent studies have revealed, however, that tudor domains do not bind nucleic acids but instead proteins containing methylated arginines (30, 31). No other protein motifs were detected.
In the present study, TDRD6 Aabs were frequently found among APS1 patients (42/86; 49%) by using an immunoprecipitation assay. These Aabs seem to be highly APS1 specific, because no immunoreactivity against in vivo translated TDRD6 was detected in a large number of sera from patients with organ-specific and systemic autoimmune diseases or healthy blood donors. TDRD6 has previously been identified as an autoantigen in a single patient with colon cancer (32); however apparently not cancer-related, because sera from 29 patients with colorectal cancer, in addition to 16 normal blood donors, tested negative for TDRD6 Aabs (32).
We were unable to correlate TDRD6 immunoreactivity with any APS1 manifestation; perhaps not unexpected, because the number of APS1 patients with hypopituitarism is very small. Notably, the lowest P value was seen for correlation between TDRD6 Aabs and gonadal failure (P = 0.168). This observation is interesting considering the TDRD6 expression pattern. The APS1 patients in the present report are all well characterized, but there is always a possibility of unrecognized manifestations, presence of subclinical disease or appearance of Aabs before the onset of clinical disease. To fully elucidate the possible correlations between gonadal failure and TDRD6 Aabs, more studies on TDRD6 immunoreactivity in patients with isolated and combined gonadal failure are needed. Also, it remains to be verified whether or not TDRD6 Aabs are related to other so far unrecognized APS1 manifestations.
Pituitary insufficiency is a rare manifestiation of APS1 and diagnosed in only 6 of our 86 patients. All these patients had an isolated GH-deficiency, but only two of which tested positive for TDRD6 Aabs, not allowing statistical interpretation.
The prevalence of TDRD6 immunoreactivity among APS1 patients (49%) is comparable with the high rates of Aabs against side–chain cleavage enzyme (52%), AADC (51%), and TPH (45%) (11). However, TDRD6 is not structurally related to any of these APS1 autoantigens and sharing of antigen epitopes is not obvious.
In this study, Aabs against guinea pig anterior pituitary cells were detected in sera from 3/6 APS1 patients with GH-deficiency. Double-staining for pituitary hormones revealed a partial colocalization with GH-producing cells, somatotrophs. Recent publications suggest that Aabs against somatotrophs, when present in high titers, may be considered a good diagnostic marker for autoimmune forms of GH-deficiency (33, 34).
To what extent presence of APS1 autoantigen, apparently only in a small number of GH cells and apparently not in the GH storage granules, may contribute to the GH-deficiency in these patients is unclear. It also remains to be established whether the APS1 autoantigen-positive cells seemingly not expressing any of the the classical anterior pituitary hormones represent a novel cell subpopulation.
Several of the major APS1 autoantigens are key enzymes in neurotransmitter synthesis, such as GAD, AADC, TH, and TPH which are all present in the pituitary, and one of our screening sera did identify clones encoding TPH. Because of this finding, we performed preadsorption tests with GAD, AADC, TH, and TPH. In one APS1 patient preadsorption with AADC abolished the staining, whereas preadsorption with the other enzymes or TDRD6 did not alter the immunostaining seen with patient sera. These results indicate the presence of further target autoantigen(s) than TDRD6 and AADC in anterior pituitary cells.
The three sera also stained a nerve plexus in the intermediate pituitary lobe (compare ref. 24), which is known to receive a dopaminergic (35, 36) and a GABAergic (37) innervation, both of central origin. These terminals are at least in part identical, in agreement with coexistence of TH and GAD in arcuate neurons (38). In addition, there is a central serotonergic innervation of the intermediate lobe (39) which should contain TPH. Serotonin can also be present in dopamine terminals, but in this case after uptake by the dopamine transporter (40), that is these terminals do not contain TPH. Taken together, in the intermediate lobe, there are presumably nerve terminals containing TH plus AADC (dopamine), TH plus AADC plus GAD (mixed dopamine-GABA), GAD alone, and TPH alone. The preadsorption results seem to reflect that distribution, GAD enzyme being most efficient in reducing staining.
In conclusion, TDRD6 is a major autoantigen in APS1 patients, and 3/6 sera from GH-deficient patients stain specific cell populations and nerves in the guinea pig pituitary gland.
It may be speculated that presence of APS1 autoantigen in GH cells may contribute to the GH-deficiency. Alternatively, autoantibodies directed against the transmitter-synthesizing enzymes, acting at the level of the median eminence/arcuate nucleus, could alter brain control of the pituitary, contributing to this disorder.
Materials and Methods
Patients.
Serum samples from 86 APS1 patients, 42 men and 44 women, of Swedish (n = 10), Norwegian (n = 17), and Finnish (n = 59) origin were analyzed. We also included sera from 11 patients with lymphocytic hypophysitis (3 biopsy-proven, 8 suspected), 17 with autoimmune Addison's disease, 20 with type 1 diabetes mellitus, 20 with Sjögren's syndrome, and 25 with systemic lupus erythematosus. Ninety healthy Swedish blood donors served as controls. All subjects gave their informed consent to the study, which was approved by the local ethical committees at Uppsala University and Karolinska Institutet.
Immunohistochemistry.
Experiments were designed in accordance to guidelines on animal care. Male and female guinea pigs (weight 250–300 g) and male Sprague–Dawley rats (weight 250–350 g) (all animals from B & K, Stockholm, Sweden), housed under controlled environmental conditions, were deeply anesthetized and perfused via the ascending aorta with formalin/picric acid in phosphate buffer.§§ The pituitaries were immersed in the same fixative, rinsed with 10% sucrose in phosphate buffer, snap-frozen, cut at 14-μm thickness on a cryostat (Microm, Heidelberg, Germany) and thaw-mounted on chrome alum-gelatin-coated glass slides.
Sera from 6 APS1 patients diagnosed with GH-deficiency (2 Swedish and 4 Finnish, Table 2) and 10 healthy blood donors (diluted 1:2,000–1:10,000) were processed according to the tyramide signal amplification (TSA) immunohistochemical technique (41), that is, incubation overnight followed by horseradish peroxidase-conjugated rabbit or donkey anti-human IgG (1:200; Dako A/S, Copenhagen, Denmark; Jackson ImmunoResearch, West Grove, PA) by using the TSA-Plus Fluorescein System (PerkinElmer Life Science, Boston, MA).
For double labeling, the TSA technique was followed by conventional immunohistochemistry (42) with rabbit antisera against luteinizing hormone (1:1,600; Biogenesis, Poole, England), thyroid stimulating hormone (1:5,000; Chemicon International, Temecula, CA), or prolactin (1:400; a generous gift from N. M. György), mouse monoclonal antibodies against follicle stimulating hormone (1:500; Abcam, Cambridge, England), adrenocorticotropic hormone (1:1,000; Peninsula Laboratories, Belmont, CA), or a sheep antiserum against GH (1:5,000; Biogenesis). Appropriate secondary antibodies conjugated with FITC (Jackson ImmunoResearch) were used at (1:40–1:80) dilution.
The specificity of the binding was tested by preadsorption of human sera (diluted 1:2,000–1:4,000) with 60,000–120,000 cpm [35S]-radiolabeled GAD, AADC, TH, TPH, or TDRD6 expressed in vitro as described below.
Sections were mounted in a mixture of glycerol and PBS (3:1), containing 0.1% para-phenylenediamine as anti-fading agent (Sigma–Aldrich, Stockholm, Sweden). The sections were examined in a Zeiss confocal laser scanning system (Model 510) or a Bio-Rad Radiance Plus confocal laser scanning system (Bio-Rad, Hemel Hemstead, U.K.) installed on a Nikon Eclipse E600 fluorescence microscope (Tokyo, Japan) equipped with appropriate objectives and excitation and emission filters. Digital images were optimized for image resolution, and images with double labeling were merged in Adobe PhotoShop 9.02 (Adobe Systems, San Jose, CA).
In Vitro Transcription and Translation (ITT) of Enzymes and Immunoprecipitation.
cDNA clones corresponding to GAD, AADC, TH and TPH were subcloned into a pSP64 poly(A) vector as described (9, 10, 43, 44). Recombinant [35S]-radiolabeled enzymes were produced by ITT in a TnT SP6 Quick coupled reticulocyte lysate system (Promega, Madison, WI). The correct size of the radioactive product was verified by SDS/PAGE (Bio-Rad) and [35S]methionine incorporation was measured by trichloroacetic acid precipitation, followed by scintillation counting. The [35S]-radiolabeled enzymes were used in immunohistochemistry experiments described above. In addition, enzymes were used for immunoprecipitation with the 16 sera selected for immunohistochemistry experiments, essentially as described elsewhere (44). The results were expressed as an index [(cpm sample − cpm negative control) / (cpm positive control − cpm negative control) × 100]. Serum samples were run in duplicates. APS1 patients with known high titers of GAD, AADC, TH, or TPH Aabs, were used as positive controls; and 4% bovine albumin (Sigma, St. Louis, MO) as negative control. The upper normal limit of the Aab index was set to the mean value for blood donors plus 3 standard deviations.
Construction and Screening of a Human Pituitary cDNA Library.
A cDNA expression library was constructed from 5 μg of human pituitary gland Poly(A)+ RNA (Clontech, Alto, CA) by using the ZAP Express cDNA synthesis kit and ZAP Express cDNA Gigapack III Gold cloning kit (Stratagene Cloning Systems, La Jolla, CA). The library, containing 1.7 × 106 unique cDNA clones, was then amplified once. Sera from two APS1 patients (diluted 1:1,000 and 1:3,000) were used for immunoscreening of the library as described (8). In vitro excision of pBK-CMV phagemid vectors from the ZAP express vector were performed according to the manufacturer's protocol. Isolated cDNA clones were analyzed by 5′and 3′sequencing by using a dye-terminator-sequencing kit (Amersham Pharmacia Biotech, Uppsala, Sweden) and ABI 377 or 3700 sequencers (PerkinElmer Applied Biosystems, Foster City, CA). The sequence data were compared with available databases by using the Basic Local Alignment Search Tool (BLAST) (45). In addition, the cDNA clone encoding TDRD6 was completely sequenced by primer walking with internal primers (CyberGene, Huddinge, Sweden).
In Vitro Transcription and Translation (ITT) of TDRD6 and Immunoprecipitation.
Plasmids containing the cDNA fragment encoding TDRD6 were purified by using the Giagen midiprep kit (Qiagen, Hilden, Germany). Recombinant [35S]-radiolabeled TDRD6 was produced by ITT in a TnT reticulocyte lysate system (Promega) and used for immunoprecipitation with sera in a 96-well plate assay, essentially as described elsewhere (44). The results were expressed as an index [(cpm sample − cpm negative control)/(cpm positive control − cpm negative control) × 100] with serum samples run in duplicates. The APS1 serum identifying the TDRD6 clone in immunoscreening was used as positive control and 4% bovine albumin (Sigma chemicals) as negative control. An arbitrary upper normal limit of the Aabs index was set to 30 as this value separated the APS1 cohort into those with clearly elevated values and those with normal values. Recombinant [35S]-radiolabeled TDRD6 was also used in the immunohistochemistry experiments described above.
Statistical Analysis.
Statistical analysis was performed with Statistica version 7.1 (StatSoft, Tulsa, OK). Two-tailed Fisher's exact test was used to compare the frequencies of different disease manifestations in APS1 patients with and without TDRD6 Aabs. P < 0.05 was considered significant.
Acknowledgments
We thank Lars Berglund and Agneta Hilding for help with statistical analyses and Åsa Hallgren for technical assistance. The prolactin antibody was a generous gift from Dr. Nagy M. György, Semmelweis University, Budapest, Hungary. This work was supported by the European Union FP6 Program on Rare Diseases; the Karolinska Institutet; the Knut and Alice Wallenberg, Marianne and Marcus Wallenberg, Magnus Bergvall, and Torsten och Ragnar Söderberg Foundations; and the Swedish Research Council.
Abbreviations
- Aab
autoantibody
- AADC
aromatic-l-amino acid decarboxylase
- APS1
autoimmune polyendocrine syndrome type 1
- GAD
glutamic acid decarboxylase
- GH
growth hormone
- ITT
in vitro transcription and translation
- TDRD6
tudor domain containing protein 6
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase.
Footnotes
The authors declare no conflict of interest.
Data deposition: The TDRD6 mRNA reference sequence reported in this paper has been deposited in the GenBank database (accession no. EF 185284).
This article contains supporting information online at www.pnas.org/cgi/content/full/0610070104/DC1.
Zamboni L, De Martino C (1967) J Cell Biol 35:148A (abstr).
References
- 1.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 2.The Finnish-German APECED Consortium. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 4.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 5.Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betterle C, Greggio NA, Volpato M. J Clin Endocrinol Metab. 1998;83:1049–1055. doi: 10.1210/jcem.83.4.4682. [DOI] [PubMed] [Google Scholar]
- 7.Winqvist O, Karlsson FA, Kämpe O. Lancet. 1992;339:1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 8.Rorsman F, Husebye ES, Winqvist O, Björk E, Karlsson FA, Kämpe O. Proc Natl Acad Sci USA. 1995;92:8626–8629. doi: 10.1073/pnas.92.19.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekwall O, Hedstrand H, Grimelius L, Haavik J, Perheentupa J, Gustafsson J, Husebye E, Kämpe O, Rorsman F. Lancet. 1998;352:279–283. doi: 10.1016/S0140-6736(97)11050-9. [DOI] [PubMed] [Google Scholar]
- 10.Hedstrand H, Ekwall O, Haavik J, Landgren E, Betterle C, Perheentupa J, Gustafsson J, Husebye E, Rorsman F, Kämpe O. Biochem Biophys Res Commun. 2000;267:456–461. doi: 10.1006/bbrc.1999.1945. [DOI] [PubMed] [Google Scholar]
- 11.Söderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, et al. J Clin Endocrinol Metab. 2004;89:557–562. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 12.Coco G, Dal Pra C, Presotto F, Albergoni MP, Canova C, Pedini B, Zanchetta R, Chen S, Furmaniak J, Rees Smith B, et al. J Clin Endocrinol Metab. 2006;91:1637–1645. doi: 10.1210/jc.2005-0860. [DOI] [PubMed] [Google Scholar]
- 13.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 14.Franzese A, Valerio G, Di Maio S, Iannucci MP, Bloise A, Tenore A. J Endocrinol Invest. 1999;22:66–69. doi: 10.1007/BF03345481. [DOI] [PubMed] [Google Scholar]
- 15.Ward L, Paquette J, Seidman E, Huot C, Alvarez F, Crock P, Delvin E, Kämpe O, Deal C. J Clin Endocrinol Metab. 1999;84:844–852. doi: 10.1210/jcem.84.3.5580. [DOI] [PubMed] [Google Scholar]
- 16.Al-Herbish AS, Bailey JD, Kooh SW. Saudi Med J. 2000;21:765–768. [PubMed] [Google Scholar]
- 17.Perheentupa J. Endocrinol Metab Clin North Am. 2002;31:295–320. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- 18.Clifton-Bligh P, Lee C, Smith H, Posen S. Aust N Z J Med. 1980;10:548–551. doi: 10.1111/j.1445-5994.1980.tb04974.x. [DOI] [PubMed] [Google Scholar]
- 19.Hung SO, Patterson A. Br J Ophtalmol. 1984;68:367–369. [Google Scholar]
- 20.Scherbaum WA, Wass JA, Besser GM, Bottazzo GF, Doniach D. Clin Endocrinol. 1986;25:411–420. doi: 10.1111/j.1365-2265.1986.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 21.Castells S, Fikrig S, Inamdar S, Orti E. J Pediatr. 1971;79:72–79. doi: 10.1016/s0022-3476(71)80061-6. [DOI] [PubMed] [Google Scholar]
- 22.Arvanitakis C, Knouss RF. J Am Med Assoc. 1973;225:1492–1495. doi: 10.1001/jama.225.12.1492. [DOI] [PubMed] [Google Scholar]
- 23.Bottazzo GF, Pouplard A, Florin-Christensen A, Doniach D. Lancet. 1975;2:97–101. doi: 10.1016/s0140-6736(75)90004-5. [DOI] [PubMed] [Google Scholar]
- 24.Cocco C, Meloni A, Boi F, Pinna G, Possenti R, Mariotti S, Ferri GL. J Clin Endocrinol Metab. 2005;90:4108–4111. doi: 10.1210/jc.2004-2184. [DOI] [PubMed] [Google Scholar]
- 25.O'Dwyer DT, McElduff P, Peterson P, Perheentupa J, Crock PA. Acta Biomed Ateneo Parmense. 2007;78(Suppl 1) in press. [PubMed] [Google Scholar]
- 26.O'Dwyer DT, Smith AI, Matthew ML, Andronicos NM, Ranson M, Robinson PJ, Crock PA. J Clin Endocrinol Metab. 2002;87:752–757. doi: 10.1210/jcem.87.2.8205. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Tatsumi KI, Takano T, Murakami Y, Takao T, Yamakita N, Tahara S, Teramoto A, Hashimoto K, Kato Y, et al. Endocr J. 2003;50:697–702. doi: 10.1507/endocrj.50.697. [DOI] [PubMed] [Google Scholar]
- 28.Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, et al. Proc Natl Acad Sci USA. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponting CP. Trends Biochem Sci. 1997;22:51–52. doi: 10.1016/s0968-0004(96)30049-2. [DOI] [PubMed] [Google Scholar]
- 30.Selenko P, Spranglers R, Stier G, Buhler D, Fischer U, Sattler M. Nat Struct Biol. 2001;8:27–31. doi: 10.1038/83014. [DOI] [PubMed] [Google Scholar]
- 31.Cote J, Richard S. J Biol Chem. 2005;280:28476–28483. doi: 10.1074/jbc.M414328200. [DOI] [PubMed] [Google Scholar]
- 32.Scanlan MJ, Chen YT, Williamson B, Gure AO, Stockert E, Gordan JD, Tureci O, Sahin U, Pfreundschuh M, Old LJ. Int J Cancer. 1998;76:652–658. doi: 10.1002/(sici)1097-0215(19980529)76:5<652::aid-ijc7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.De Bellis A, Bizzarro A, Perrino S, Coronella C, Conte M, Pasquali D, Sinisi AA, Betterle C, Bellastella A. Clin Endocrinol. 2005;63:45–49. doi: 10.1111/j.1365-2265.2005.02296.x. [DOI] [PubMed] [Google Scholar]
- 34.De Bellis A, Salerno M, Conte M, Coronella C, Tirelli G, Battaglia M, Esposito V, Ruocco G, Bellastella G, Bizzarro A, et al. J Clin Endocrinol Metab. 2006;91:2484–2489. doi: 10.1210/jc.2006-0040. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarten HG, Björklund A, Holstein AF, Nobin A. Z Zellforsch Mikrosk Anat. 1972;126:483–517. doi: 10.1007/BF00306908. [DOI] [PubMed] [Google Scholar]
- 36.Björklund A, Moore RY, Nobin A, Stenevi U. Brain Res. 1973;51:171–191. doi: 10.1016/0006-8993(73)90371-5. [DOI] [PubMed] [Google Scholar]
- 37.Oertel WH, Mugnaini E, Tappaz ML, Weise VK, Dahl AL, Schmechel DE, Kopin IJ. Proc Natl Acad Sci USA. 1982;79:675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everitt BJ, Hökfelt T, Wu JY, Goldstein M. Neuroendocrinology. 1984;39:189–191. doi: 10.1159/000123977. [DOI] [PubMed] [Google Scholar]
- 39.Friedman E, Krieger DT, Mezey E, Leranth C, Brownstein MJ, Palkovits M. Endocrinology. 1983;112:1943–1947. doi: 10.1210/endo-112-6-1943. [DOI] [PubMed] [Google Scholar]
- 40.Vanhatalo S, Soinila S. Neurosci Res. 1994;21:143–149. doi: 10.1016/0168-0102(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 41.Adams JC. J Histochem Cytochem. 1992;40:1457–1463. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- 42.Coons AH. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- 43.Falorni A, Grubin CE, Takei I, Shimada A, Kasuga A, Maruyama T, Ozawa Y, Kasatani T, Saruta T, Li L, et al. Autoimmunity. 1994;19:113–125. doi: 10.3109/08916939409009539. [DOI] [PubMed] [Google Scholar]
- 44.Husebye ES, Gebre-Medhin G, Tuomi T, Perheentupa J, Landin-Olsson M, Gustafsson J, Rorsman F, Kämpe O. J Clin Endocrinol Metab. 1997;82:147–150. doi: 10.1210/jcem.82.1.3647. [DOI] [PubMed] [Google Scholar]
- 45.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]