Abstract
Ullrich congenital muscular dystrophy is a severe genetically and clinically heterogeneous muscle disorder linked to collagen VI deficiency. The pathogenesis of the disease is unknown. To assess the potential role of mitochondrial dysfunction in the onset of muscle fiber death in this form of dystrophy, we studied biopsies and myoblast cultures obtained from patients with different genetic defects of collagen VI and variable clinical presentations of the disease. We identified a latent mitochondrial dysfunction in myoblasts from patients with Ullrich congenital muscular dystrophy that matched an increased occurrence of spontaneous apoptosis. Unlike those in myoblasts from healthy donors, mitochondria in cells from patients depolarized upon addition of oligomycin and displayed ultrastructural alterations that were worsened by treatment with oligomycin. The increased apoptosis, the ultrastructural defects, and the anomalous response to oligomycin could be normalized by Ca2+ chelators, by plating cells on collagen VI, and by treatment with cyclosporin A or with the specific cyclophilin inhibitor methylAla3ethylVal4-cyclosporin, which does not affect calcineurin activity. Here we demonstrate that mitochondrial dysfunction plays an important role in muscle cell wasting in Ullrich congenital muscular dystrophy. This study represents an essential step toward a pharmacological therapy of Ullrich congenital muscular dystrophy with cyclosporin A and methylAla3ethylVal4 cyclosporin.
Keywords: collagen VI, mitochondria, permeability transition
Collagen VI is an extracellular matrix protein forming a microfibrillar network that is particularly abundant in the endomysium of skeletal muscle. The protein is composed of three different α-chains encoded by separate genes named COL6A1, COL6A2, and COL6A3 in humans (1). Mutations of collagen VI genes cause two skeletal muscle diseases, Bethlem myopathy (Mendelian Inheritance in Man no. 158810; www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=158810) and Ullrich congenital muscular dystrophy (UCMD) (Mendelian Inheritance in Man no. 254090;www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=254090).
Bethlem myopathy is a disorder characterized by slowly progressive axial and proximal muscle weakness with flexion finger contractures (2, 3). The disease presents intrafamilial variability with different onset (from prenatal onset to onset in midadulthood) and is usually mild but slowly progressive, with some affected individuals >50 years of age needing aids for outdoor mobility (4, 5). The mode of inheritance is autosomal dominant, and mutations can affect any of the three COL6 genes. Expression of collagen VI appears normal or mildly reduced in the endomysium of most patients (1).
UCMD is a severe wasting disease of axial muscle with contractures and coexisting distal joint hyperlaxity, usually present at birth (6, 7). Involvement of the diaphragm is prominent, with early and severe respiratory failure. Muscle biopsies from UCMD patients usually show a marked decrease or complete absence of collagen VI (1). UCMD is classically regarded as an autosomal recessive disease, but cases have been described (1) with de novo dominant mutations, which were confirmed in two patients described here. Homozygous or compound heterozygous COL6 mutations typically have a severe UCMD phenotype (8, 9) although they may occasionally present a milder Bethlem myopathy-like disease (10). Mutations in the coding region of COL6 genes were excluded in some patients with the clinical UCMD phenotype, suggesting a possible genetic heterogeneity for the disease (8).
Mice with targeted disruption of the Col6a1 gene display an early-onset myopathic syndrome that resembles Bethlem myopathy despite their total lack of collagen VI (11). The murine phenotype affects diaphragm and other skeletal muscles and is characterized by increased apoptosis and ultrastructural defects of mitochondria and sarcoplasmic reticulum (11, 12). We have shown that skeletal muscle fibers derived from the Col6a1−/− mice have a latent mitochondrial defect caused by inappropriate opening of the permeability transition pore (PTP) (12), an inner membrane channel that plays a role in several forms of cell death and can be desensitized by cyclosporin (Cs) A (13). Short-term treatment of the Col6a1−/− mice with CsA led to a dramatic recovery from the muscle lesions, suggesting that the disease caused by collagen VI deficiency in mice can be cured downstream of the genetic lesion by desensitizing the mitochondrial PTP (12). Establishing whether mitochondria are equally involved in the pathogenesis of the genetically and clinically heterogeneous UCMD represents a major challenge, the main hurdle to the potential application in humans of the regimen defined in the mouse model. To fill this gap and to assess whether increased incidence of apoptosis and latent mitochondrial dysfunction are present in UCMD, we have studied biopsies and myoblasts from patients affected by UCMD.
Results
The five patients we studied are representative of the spectrum of severity of UCMD (Table 1). All patients had a congenital onset. Three (patients 2, 3, and 5) never achieved the ability to stand and walk, one (patient 4) was able to stand only with support, and one (patient 1) achieved the ability to walk. The expression of collagen VI ranged from mild (patients 1 and 4) to marked reduction (patients 2 and 3) to complete absence (patient 5). Patients 1 and 5 had genetically proven UCMD (10, 14), whereas the mutation of patients 2 and 4 is identified here (see Materials and Methods). Genetic analysis was not available for patient 3, who presented the typical clinical and immunohistochemical features of UCMD. The mutation affected the COL6A1 gene in three cases (patients 2, 4, and 5) and the COL6A3 gene in one case (patient 1) and was undefined in one case (patient 3).
Table 1.
Clinical and genetic characteristics of patients
| Patient | Age | Phenotype | Collagen VI | Mutation |
|---|---|---|---|---|
| 1 | 23 | Floppy at birth. First steps at 13 months. Limb girdle wasting and weakness, multiple joint contractures, and distal laxity. | Mild reduction in muscle and fibroblasts | COL6A3 homozygous nonsense Arg465Stop mutation (ref. 10) |
| 2 | 6 | Floppy at birth with talus of the feet. Never able to stand or walk. Diffuse muscle wasting and weakness, contractures, and distal laxity. | Marked reduction in muscle | COL6A1 de novo heterozygous del15 921–935 mutation |
| 3 | 3 | Floppy at birth. Congenital hip dislocation. Never able to walk. Diffuse muscle wasting and weakness, contractures, and distal laxity. | Marked reduction in muscle | Not known |
| 4 | 9 | Floppy at birth. Able to stand with support. Diffuse muscle wasting and weakness. Spine thoracic kyphosis, keloid formation, proximal contractures, and distal laxity. | Mild reduction in muscle and fibroblasts | COL6A1 de novo heterozygous Gly284Arg mutation |
| 5 | 11 | Floppy at birth. Congenital hip dislocation; only seated. Diffuse muscle weakness, scoliosis, diffuse contractures, and distal laxity. | Absent in muscle fibres and fibroblasts | COL6A1 homozygous delG1456 mutation (ref. 14) |
All patients listed experienced onset of muscle weakness at birth. Patient 5 was the only patient listed who required mechanical ventilation, at 11 years of age, and became the only patient to have died, also at 11 years of age.
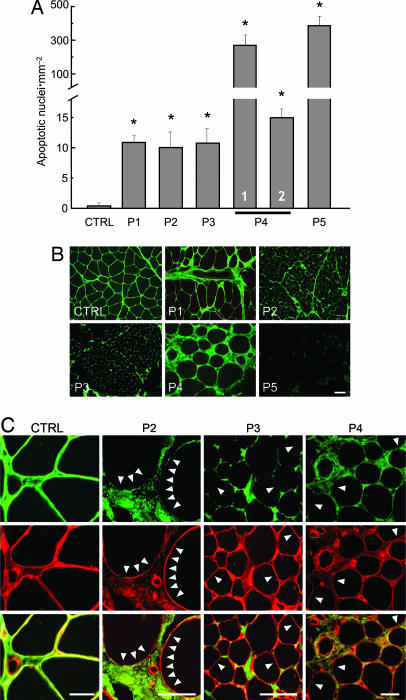
We studied the occurrence of apoptosis in biopsies from quadriceps muscle. When compared with that of a healthy donor, the frequency of apoptotic nuclei was much higher in all of the UCMD samples, with values ranging between an ≈10-fold increase for patients 1, 2, and 3 to a >300-fold increase for patient 5 (Fig. 1A). Two biopsies were available for patient 4, taken at 4 and 9 years of age. Interestingly, the incidence of apoptosis was always much higher than that observed in cultures from the healthy donor, yet the frequency decreased by 1 order of magnitude between the two measurements. This important finding indicates that the occurrence of apoptosis is variable in the course of the disease and probably explains why there is no quantitative match between increased apoptosis and reduced collagen VI expression in the muscle biopsies of UCMD patients, as revealed by a selective antibody against collagen VI (Fig. 1B). Reduced labeling of collagen VI was always prominent at the level of basal lamina, as documented by the absence of colocalization with perlecan, whereas the protein was still detectable in the extracellular matrix in perivascular fibrotic regions between muscle fibers (Fig. 1C). These findings suggest that the critical factor for development of muscular dystrophy is the selective deficiency of collagen VI in the endomysium.
Fig. 1.
Incidence of apoptosis and expression of collagen VI in muscle biopsies from a healthy donor and UCMD patients. (A) Biopsies from a healthy donor (CTRL) and five UCMD patients (P1–P5) were scored for the presence of apoptotic nuclei with the TUNEL reaction. For patient 4, columns 1 and 2 refer to biopsies taken at 4 and 9 years of age, respectively. ∗, P < 0.01 for each patient vs. healthy donor. (B) Muscle biopsies from a healthy donor (CTRL) and five UCMD patients (P1–P5) were studied by immunofluorescence for collagen VI. (Scale bar, 20 μm.) (C) Muscle biopsies from a healthy donor (CTRL) and three UCMD patients (P2–P4) were studied by immunofluorescence for collagen VI (COLVI, green fluorescence) (Top) and perlecan (red fluorescence) (Middle), and a merge is shown in Bottom. Arrowheads mark examples of a lack of collagen VI staining at the basal lamina. (Scale bars, 20 μm.)
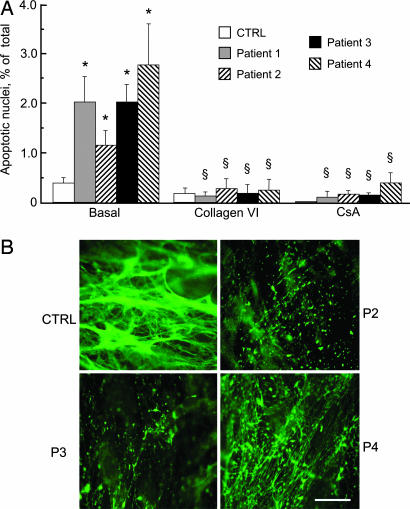
To experimentally test the existence of a causal link between the lack of collagen VI and apoptosis, we established muscle cell cultures from the biopsies and studied the occurrence of apoptosis after plating them on plastic or collagen VI. We found that the cultures from all patients displayed a higher incidence of apoptosis compared with those from healthy donors and that plating on collagen VI or treatment with CsA fully normalized the occurrence of apoptosis in the patient samples (Fig. 2A). Cultures from patients 2, 3, and 4 displayed low levels of collagen VI (Fig. 2B) as expected and as already had been shown for patient 1 (10), whereas collagen VI was completely absent in cell cultures obtained from patient 5 (14).
Fig. 2.
Incidence of apoptosis and expression of collagen VI in muscle cell cultures from a healthy donor and UCMD patients. (A) Primary myoblast cultures from a healthy donor (CTRL) and four UCMD patients were plated on plastic dishes or on collagen VI and scored for the presence of apoptotic nuclei by the TUNEL reaction. Where indicated, cells plated on plastic dishes were incubated with 1.6 μM CsA for 2 h. Data are the mean of three independent experiments ± SD. *, P < 0.01 for each patient vs. healthy donor; §, not significant for each patient vs. healthy donor. (B) Collagen VI was detected by immunofluorescence in cultured myoblasts from a healthy donor (CTRL) and three UCMD patients (P2–P4). Note that in the healthy donor collagen VI is secreted and organized in a dense fibrillar network, whereas in the UCMD patients collagen VI staining is severely reduced, with residual labeling in the form of small dots. (Scale bar, 20 μm.)
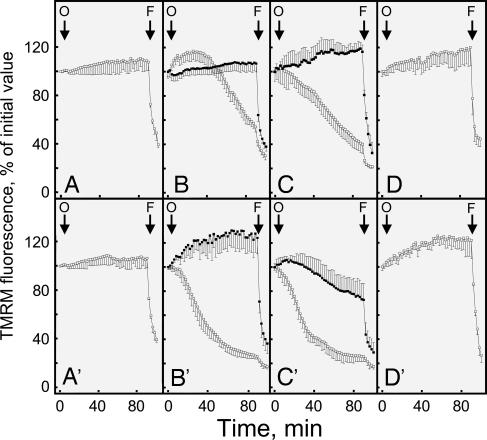
We next studied mitochondrial function in living muscle cell cultures to assess whether the antiapoptotic effects of collagen VI and CsA could be traced to mitochondria. As expected, addition of the F1FO ATP synthase inhibitor oligomycin to cultures established from the healthy donors did not cause mitochondrial depolarization, which was instead promptly elicited by the addition of the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (Fig. 3 A and A′). The addition of oligomycin was instead followed by mitochondrial depolarization in the cells from all UCMD patients, although only the results from patients 1 and 2 are shown for clarity (open symbols in Fig. 3 B, B′, C, and C′). Remarkably, the response to oligomycin was fully normalized by treatment with CsA (filled symbols in Fig. 3 B and B′) or with the intracellular Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (filled symbols in Fig. 3 C and C′) or by plating cells on collagen VI (Fig. 3 D and D′), suggesting an involvement of the PTP in the pathogenesis of UCMD. These findings suggest that cells from UCMD patients have a latent mitochondrial dysfunction that can be selectively amplified by oligomycin, as has been shown previously for the mouse model of collagen VI deficiency (12).
Fig. 3.
Changes of mitochondrial TMRM fluorescence induced by oligomycin in muscle cell cultures from a healthy donor and UCMD patients. Myoblasts from two healthy donors (A and A′) and from UCMD patients 1 (B–D) and 2 (B′–D′) were loaded with TMRM and studied as described in Materials and Methods. Cells were seeded on glass (A–C and A′–C′) or on collagen VI (D and D′). Where indicated by arrows, 6 μM oligomycin (O) and 4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (F) were added in the absence of further treatments (open symbols) or after treatment for 30 min with 1.6 μM CsA or 5 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester (filled symbols in B and B′ and C and C′, respectively). Similar results were obtained with cells from patients 3 and 4, and the results shown are representative of at least five repeats per patient.
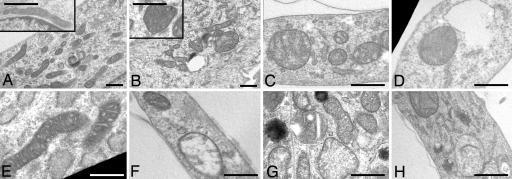
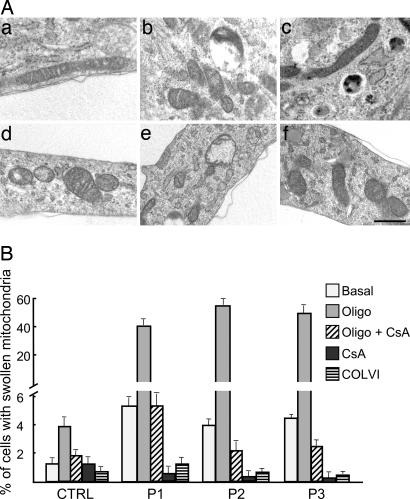
The muscle cell cultures established from healthy donors and from patients 1–3 were further studied by using electron microscopy (Fig. 4). In the UCMD samples, the mitochondrial area/perimeter ratio was significantly increased to attain values 62.5%, 75%, and 50% higher than those of healthy donors for patients 1, 2, and 3, respectively (P < 0.05). This finding indicates that, in UCMD muscle cells, mitochondria are significantly less elongated than in normal samples. We also found that 4–8% of mitochondria of patients had an increased short axis value (>400 nm) compared with control (<300 nm). Taken together, these findings suggest the presence of a fraction of mitochondria with increased size in UCMD cells. A small percentage of cells from UCMD patients (between 4% and 5%, compared with 1% for cells from healthy donors) also displayed swollen mitochondria, with hypodense matrix and absence of cristae (Fig. 4). Remarkably, when the UCMD cells were plated on collagen VI, the mitochondrial area/perimeter ratio and short axis values became similar to those of healthy donors (Fig. 4E), and plating on collagen VI or treatment with CsA decreased the numbers of cells with swollen mitochondria to those observed in cultures from healthy donors (Fig. 5B). Treatment of cultures with oligomycin increased the percentage of cells with swollen mitochondria to 4% in control and to >40% in UCMD patients (Fig. 5). The effect of oligomycin could be prevented by treatment with CsA, and the percentages of cells bearing swollen mitochondria were restored to values similar to basal in all cell cultures (Fig. 5B).
Fig. 4.
Mitochondrial ultrastructure in muscle cell cultures from healthy donors and UCMD patients. (A–E) Electron micrographs of myoblasts from a healthy donor (A) and UCMD patients 1, 2, and 3 (B, C, and D, respectively) plated on plastic or on collagen VI (patient 2, E). (A and B Insets) Higher magnifications from the same samples. (F–H) Representative examples of swollen mitochondria found in the myoblasts of patients 1 (F), 2 (G), and 3 (H). For further details and for a morphometric analysis see Results. (Scale bars, 300 nm.)
Fig. 5.
Effects of oligomycin, CsA, and collagen VI on mitochondrial ultrastructure in healthy donor and UCMD patient cell cultures. (A) Electron micrographs of myoblasts from a healthy donor (a–c) and UCMD patient 2 (d–f) plated on plastic in the absence (a and d) or presence of 6 μM oligomycin (b and e) or 6 μM oligomycin plus 1.6 μM CsA (c and f). (Scale bar, 400 nm.) (B) Percentage of cells with swollen mitochondria in the myoblasts of a healthy donor (CTRL) or UCMD patients 1, 2, and 3 (P1–P3) plated on plastic in the absence (Basal) or presence of 6 μM oligomycin (Oligo), 1.6 μM CsA, or both or of the same cells plated on collagen VI (COLVI). Data are the mean of three independent experiments ± SD.
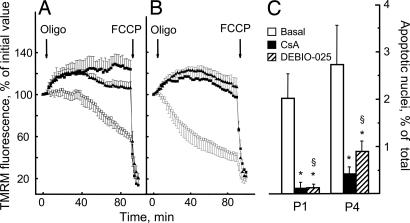
To sort the inhibitory effects of CsA on the PTP from those on calcineurin (15), we studied the effects of methylAla3ethylVal4-cyclosporin (also called Debio 025), a nonimmunosuppressive cyclosporin where sarcosine in position 3 and methyl leucine in position 4 of CsA are replaced by d-methyl alanine and ethyl valine, respectively. This specific cyclophilin inhibitor maintains the inhibitory properties displayed by CsA on the PTP in isolated mitochondria (16) and is more potent than CsA at inhibiting the permeability transition in mice in vivo while displaying a total lack of inhibition of calcineurin (L. Nicolosi, E. Palma, M. E. Soriano, A. Rasola, F. Chiara, F. Finetti, G. Vuaginiaux, J.-M. Dumont, C. T. Baldari, and P. Bernardi, unpublished observations). Debio 025 was as effective as CsA at preventing oligomycin-dependent mitochondrial depolarization in cells from UCMD patients (Fig. 6 A and B) and restored the occurrence of apoptosis to the level displayed by cells from a healthy donor (Fig. 6C).
Fig. 6.
Effects of CsA and Debio 025 on mitochondrial TMRM fluorescence and incidence of apoptosis in myoblast cultures from UCMD patients. (A and B) Myoblasts from UCMD patients 1 (A) and 4 (B) were loaded with TMRM and studied as described in Materials and Methods. Cells were seeded on glass, and, where indicated by arrows, 6 μM oligomycin (Oligo) and 4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) were added in the absence of further treatments (open symbols) or after treatment for 30 min with 1.6 μM CsA (filled squares) or 1.6 μM Debio 025 (filled triangles). (C) Myoblasts from patients 1 (P1) and 4 (P4) were cultured on plastic dishes and scored for the presence of TUNEL-positive nuclei either in the absence of further treatments (Basal) or after incubation for 2 h with 1.6 μM CsA or 1.6 μM Debio 025. Data are the mean of at least four independent experiments ± SD. *, P < 0.01 compared with basal condition; §, not significant for Debio 025 vs. CsA.
Discussion
Here we have established that patients affected by UCMD show an increased rate of apoptosis in skeletal muscle in vivo and in myoblast cultures derived from biopsies. The muscle cell cultures also display a measurable fraction of altered mitochondria, with morphological alterations that range from shape changes to overt swelling. These changes are matched by a latent mitochondrial dysfunction that can be revealed by the addition of the F1FO ATPase inhibitor oligomycin, which caused mitochondrial depolarization only in the cultures from UCMD patients. Oligomycin also dramatically increased the number of swollen mitochondria, this effect being particularly prominent in the cultures from UCMD patients.
Oligomycin is expected to cause hyperpolarization in healthy respiring cells, where the mitochondrial membrane potential is maintained by proton pumping by the respiratory chain and the proton electrochemical gradient is used to drive ATP synthesis (17). The mitochondrial depolarization induced by oligomycin in muscle fibers from Col6a1−/− mice (12) and in myoblasts from UCMD patients (this work) is therefore an anomalous response, which suggests that the membrane potential is not maintained by respiration but rather by the mitochondrial ATP synthase working “in reverse” to pump protons from the matrix to the intermembrane space, thereby consuming glycolytic ATP. This respiratory deficiency could be due to depletion of pyridine nucleotides and cytochrome c, which can be lost via the PTP even in the absence of overt swelling (18–20). Treatment of cultures from UCMD patients with CsA prevented mitochondrial dysfunction and normalized the incidence of apoptosis, indicating that inappropriate opening of the PTP is the basis for both events.
It is remarkable that the mitochondrial defect can be revealed in primary cultures from patients with clinical signs of UCMD, irrespective of whether the primary genetic defect is in the COL6A1 or COL6A3 gene. Furthermore, the mitochondrial defect is present both in homozygous (patient 1) and in heterozygous (patients 2 and 4) mutations, indicating that the genotypic profile of collagen VI genes alone may not be predictive of the disease phenotype. These findings suggest that mitochondria are involved in the pathogenesis of all cases of UCMD and that additional genetic and/or environmental factors play a role in the individual susceptibility to muscle fiber demise and regeneration. Consistent with this, the mouse model produced by targeted disruption of the Col6a1 gene displays a complete absence of collagen VI, yet the phenotype resembles more the milder Bethlem myopathy than UCMD (11). This observation indicates that in the mouse model compensatory mechanisms may limit the net loss of muscle fibers and that similar mechanisms may be present to a variable degree in patients with collagen VI disorders. It should be noted that, together with the decreased levels of endomysial collagen VI, the anomalous response to oligomycin could represent a useful diagnostic tool for patients whose genetic lesion is undefined, as shown in this work for patient 3.
The trophic signals relayed from collagen VI to mitochondria remain elusive, although a prominent role for intracellular Ca2+ can be inferred both from studies in the mouse model (12) and from the effects of the intracellular Ca2+ chelator in the myoblasts from the UCMD patients. Moreover, lack of collagen VI caused a marked decrease of the BCL-2/BAX ratio attributable to decreased BCL-2 expression (data not shown). This decreased expression may synergize with Ca2+ in increasing the PTP open time (21), causing the release of cytochrome c and activation of caspase 3. Irrespective of the details of the mechanism, however, it is reassuring that the mitochondrial defects and ensuing apoptosis are normalized by cyclophilin inhibitors. Thus, the pathogenic chain of events downstream of the collagen VI genetic lesion can be interrupted by proper drugs, at least in the early stages, an observation that has tremendous potential for therapy. Indeed, disease progression depends on the balance between cell death and regeneration, and decreasing the rate of cell death by even a small factor may lead to a substantial improvement in the clinical course of the disease. We are also confident that the identification of the signaling pathways engaged by collagen VI upstream of mitochondria will offer additional targets and increase the chances of an effective therapy.
Besides its mitochondrial effects, CsA is well known to cause immunosuppression through the inhibition of calcineurin (22), which could be a major drawback in long-term treatment of patients. A key observation made here is that Debio 025, a specific cyclophilin inhibitor that desensitizes the PTP through mitochondrial cyclophilin D but has no inhibitory effects on calcineurin (16), is as effective as CsA at normalizing mitochondrial function and apoptotic rates in UCMD cells. This finding demonstrates that calcineurin is not involved in the cytoprotective effects of CsA and leads to perspectives on the pharmacological treatment of patients affected by collagen VI disorders.
Materials and Methods
Participants.
UCMD was diagnosed according to the criteria of the European Neuromuscular Center (23). All probands were examined and all underwent a muscle biopsy. Genetic data for patients 1 and 5 were published previously (10, 14), whereas the molecular defect and the associated reduction of collagen VI for patients 2 and 4 were determined in the course of the present work (see below and Figs. 1B and 2B). All participants provided written informed consent, and approval was obtained from the ethics committee of the Rizzoli Orthopedic Institute (Bologna, Italy). The basic features of the patients analyzed in this study are summarized in Table 1.
Sequence Analysis.
For sequence analysis of collagen VI genes, DNA was extracted by standard methods from whole blood, after informed consent was obtained from patients. Exons 9, 14, and 15 of the COL6A1 gene were PCR-amplified, including 3′ and 5′ intronic boundaries (≈100 bp), and sequenced. The sequences of the oligonucleotides we used are available upon request. Amplification reactions were performed in a final volume of 25 μl containing 100 ng of genomic DNA, 200 μM dNTPs, 0.5 μM each primer, 1.5 mM MgCl2, and 1 unit of Taq polymerase (Invitrogen, Carlsbad, CA). Thermal PCR conditions were as follows: 94°C for 5 min for 1 cycle; 94°C for 30 sec, 67°C for 30 sec, and 72°C for 30 sec for 2 cycles; 94°C for 45 sec, 66°C for 30 sec, and 72°C for 1 min for 5 cycles; and 94°C for 45 sec, 66°C for 30 sec, and 72°C for 1 min for 23 cycles. All PCR products were column-purified (Microcon; Millipore, Billerica, MA) and sequenced on an ABI 3100 automated sequencer (Applied Biosystems, Foster City, CA). In patient 2, sequence analysis of COL6A1 exon 9 revealed the presence of a 15-nucleotide deletion [spanning positions 35,374–35,388 of genomic sequence (GenBank no. AJ011932) and 921–935 of mRNA sequence (GenBank no. NM_001848)] occurring in heterozygosity. The identified deletion represents a COL6A1 mutation and is predicted to cause the loss of six normal amino acid residues (GlyLeuProGlyGluLys) and the insertion of a Glu missense residue (fusion codon GAG) in the triple helical domain of an α1 (VI)-chain. The del15 921–935 mutation occurred de novo in this patient, as revealed by sequence analysis of the genomic DNA of both parents, therefore suggesting an autosomal dominant mode of inheritance. In patient 4, sequence analysis of COL6A1 exon 9 revealed the presence of a G→A variation occurring in heterozygosity [nucleotide 35,400 (GenBank no. AJ011932) and nucleotide 850 (GenBank no. NM_001848)]. This variation is predicted to cause a Gly284Arg substitution in the triple helical domain of α1 (VI)-chain and corresponds to a known COL6A1 mutation previously reported as a heterozygous change in three UCMD patients (1, 14). The Gly284Arg missense mutations occurred de novo in patient 4, as revealed by sequence analysis of the genomic DNA of both parents, which is again compatible with an autosomal dominant mode of inheritance.
Myoblast Cultures.
We obtained muscle biopsies from healthy donors and UCMD patients after obtaining the informed consent of patients and approval of the ethics committee of the Rizzoli Orthopedic Institute (Bologna, Italy). Myoblast cultures were established from muscle biopsies of two unaffected controls and from patients 1–4. Myoblasts were prepared by enzymatic and mechanical treatment of muscle biopsies and by plating in DMEM supplemented with 20% FCS, penicillin, streptomycin, and amphotericin B (Sigma, St. Louis, MO) as previously described (24) and then stored in liquid nitrogen. Unless otherwise stated, cells were grown to confluence in tissue culture dishes and incubated with DMEM for 90 min at 37°C with no additions, in the presence of 6 μM oligomycin (Sigma) or in the presence of 6 μM oligomycin and 1.6 μM CsA (Sigma) or 1.6 μM Debio 025 (Debiopharm, Lausanne, Switzerland). When needed, dishes were coated with whole tetrameric collagen VI molecules (5 μg × cm2), previously purified from adult murine tissues as described (25).
Electron Microscopy.
Cultures were washed with PBS, fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) and postfixed with 1% osmium tetroxide in veronal buffer. All samples were detached from the plastic dish with propylene oxide, centrifuged, and embedded in Epon E812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a Philips (Eindhoven, the Netherlands) EM400 electron microscope at 100 kV. For morphometric analysis, micrographs were taken at ×17,000 magnification, and the area, perimeter, and short axis of mitochondria were determined by using the AnalySIS program (Soft Imaging System, Muenster, Germany). Three hundred cells were examined for each sample. For determination of area, perimeter, and short axis, cells with swollen mitochondria and necrotic aspects were not considered.
Detection of Proteins by Fluorescence Microscopy.
Unfixed frozen sections of muscle biopsies from patients and from controls were double-labeled with mouse anti-collagen type VI (MAB 1944, 1:200 dilution; Chemicon, Temecula, CA) and rat anti-perlecan antibodies (1:100 dilution; Chemicon) for 1 h at room temperature in PBS containing 2% BSA, followed by fluorescein isothiocyanate-conjugated anti-mouse (Dako, Glostrup, Denmark), and tetramethylrhodamine isothiocyanate-conjugated anti-rat antibodies (Sigma). All samples were mounted with ProLong Antifade reagent (Molecular Probes, Carlsbad, CA) and observed with a Nikon (Tokyo, Japan) E600 fluorescence microscope. Cultured myoblasts from patients and controls were grown to confluence on coverslips and were treated with 0.25 M ascorbic acid for 5 days, to allow collagen molecules to be hydroxylated and secreted. Samples were fixed with cold methanol at −20°C for 7 min and incubated with anti-collagen VI (MAB 1944, 1:100 dilution; Chemicon) overnight at 4°C, followed by incubation with fluorescein isothiocyanate-conjugated anti-mouse antibodies (Dako).
Detection of Apoptosis.
We measured the rate of apoptosis in muscle biopsies and in myoblast cultures using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) method. Frozen sections (7 μm thick) were prepared from muscle biopsies of an unaffected control and five UCMD patients and fixed in 50% acetone/50% methanol. TUNEL was performed by using the ApopTag in Situ Apoptosis Detection kit (Chemicon). Samples were stained with peroxidase/diaminobenzidine to reveal TUNEL-positive nuclei. Myoblast cultures were seeded onto Lab-Tek plastic chamber slides (Nunc, Roskilde, Denmark) and grown to confluence in DMEM supplemented with 20% FCS. Cells were fixed in 50% acetone/50% methanol and processed for TUNEL analysis by using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI). Visualization of all nuclei was performed by staining with Hoechst 33258 (Sigma). The number of total and TUNEL-positive nuclei was determined in randomly selected fields by using a Zeiss (Oberkochen, Germany) Axioplan microscope (×40 magnification) equipped with a digital camera. Data are expressed as mean ± SD. Data were analyzed with the unpaired Student's t test, and values of P < 0.01 were considered significant.
Mitochondrial Membrane Potential.
This was measured based on the accumulation of tetramethylrhodamine methyl ester (TMRM). Myoblasts were seeded onto 24-mm-diameter round glass coverslips and grown for 2 days in DMEM supplemented with 20% FCS. The extent of cell and, hence, mitochondrial loading with potentiometric probes is affected by the activity of the plasma membrane multidrug resistance pump, which is inhibited by CsA. Treatment with this drug may therefore cause an increased mitochondrial fluorescence that can be erroneously interpreted as an increase of the mitochondrial membrane potential (26). To prevent this artifact and to normalize the loading conditions, in all experiments with TMRM the medium was supplemented with 1.6 μM CsH, which inhibits the multidrug resistance pump but not the PTP (27). Cells were rinsed once and then incubated in serum-free DMEM supplemented with 1.6 μM CsH and loaded with 10 nM TMRM for 30 min. At the end of each experiment, mitochondria were fully depolarized by the addition of 4 μM of the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP). Cellular fluorescence images were acquired with an Olympus (Center Valley, PA) IX71/IX51 inverted microscope equipped with a xenon light source (75 W) for epifluorescence illumination and with a 12-bit digital cooled charge-coupled device (CCD) camera (Micromax, Princeton Instruments, Trenton, NJ). For detection of fluorescence, 568 ± 25-nm bandpass excitation and 585-nm longpass emission filter settings were used. Images were collected with an exposure time of 100 msec by using a ×40, 1.3 N.A. oil immersion objective (Nikon). Data were acquired and analyzed by using CellR software (Olympus). Clusters of several mitochondria (10–30) were identified as regions of interest, and fields not containing cells were taken as the background. Sequential digital images were acquired every 2 min, and the average fluorescence intensity of all relevant regions was recorded and stored for subsequent analysis.
Acknowledgments
We thank all individuals from the families who participated in this study and Debiopharm (Lausanne, Switzerland) for providing Debio 025. This study was supported by Telethon Italy Grant GGP04113, the Fondazione Carisbo, Association Française contre les Myopathies Grant 9398, and the Progetti di Ricerca di Interesse Nazionale of the Ministero dell′Università e della Ricerca (MIUR–PRIN).
Abbreviations
- UCMD
Ullrich congenital muscular dystrophy
- PTP
permeability transition pore
- Cs
cyclosporin
- TMRM
tetramethylrhodamine methyl ester.
Footnotes
The authors declare no conflict of interest.
References
- 1.Lampe AK, Bushby KM. J Med Genet. 2005;42:673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethlem J, Wijngaarden GK. Brain. 1976;99:91–100. doi: 10.1093/brain/99.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Merlini L, Morandi L, Granata C, Ballestrazzi A. Neuromuscul Disord. 1994;4:503–511. doi: 10.1016/0960-8966(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 4.Pepe G, Giusti B, Bertini E, Brunelli T, Saitta B, Comeglio P, Bolognese A, Merlini L, Federici G, Abbate R, et al. Biochem Biophys Res Commun. 1999;258:802–807. doi: 10.1006/bbrc.1999.0680. [DOI] [PubMed] [Google Scholar]
- 5.de Visser M, de Voogt WG, la Riviere GV. Muscle Nerve. 1992;15:591–596. doi: 10.1002/mus.880150510. [DOI] [PubMed] [Google Scholar]
- 6.Ullrich O. Z Ges Neurol Psychiatr. 1930;126:171–201. [Google Scholar]
- 7.Camacho Vanegas O, Bertini E, Zhang RZ, Petrini S, Minosse C, Sabatelli P, Giusti B, Chu ML, Pepe G. Proc Natl Acad Sci USA. 2001;98:7516–7521. doi: 10.1073/pnas.121027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan TC, Zhang RZ, Sudano DG, Marie SK, Bonnemann CG, Chu ML. Am J Hum Genet. 2003;73:355–369. doi: 10.1086/377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker NL, Morgelin M, Peat R, Goemans N, North KN, Bateman JF, Lamande SR. Hum Mol Genet. 2005;14:279–293. doi: 10.1093/hmg/ddi025. [DOI] [PubMed] [Google Scholar]
- 10.Demir E, Sabatelli P, Allamand V, Ferreiro A, Moghadaszadeh B, Makrelouf M, Topaloglu H, Echenne B, Merlini L, Guicheney P. Am J Hum Genet. 2002;70:1446–1458. doi: 10.1086/340608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Hum Mol Genet. 1998;7:2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- 12.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, et al. Nat Genet. 2003;35:267–271. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 13.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 14.Giusti B, Lucarini L, Pietroni V, Lucioli S, Bandinelli B, Sabatelli P, Squarzoni S, Petrini S, Gartioux C, Talim B, et al. Ann Neurol. 2005;58:400–410. doi: 10.1002/ana.20586. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Farmer JDJ, Lane WS, Friedman J, Weissman I, Schreiber SL. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 16.Hansson MJ, Mattiasson G, Mansson R, Karlsson J, Keep MF, Waldmeier P, Ruegg UT, Dumont JM, Besseghir K, Elmer E. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell P. Science. 1979;206:1148–1159. doi: 10.1126/science.388618. [DOI] [PubMed] [Google Scholar]
- 18.Vinogradov A, Scarpa A, Chance B. Arch Biochem Biophys. 1972;152:646–654. doi: 10.1016/0003-9861(72)90261-5. [DOI] [PubMed] [Google Scholar]
- 19.Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 20.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 21.Milanesi E, Costantini P, Gambalunga A, Colonna R, Petronilli V, Cabrelle A, Semenzato G, Cesura AM, Pinard E, Bernardi P. J Biol Chem. 2006;281:10066–10072. doi: 10.1074/jbc.M513708200. [DOI] [PubMed] [Google Scholar]
- 22.Clipstone NA, Crabtree GR. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 23.Pepe G, Bertini E, Bonaldo P, Bushby K, Giusti B, de Visser M, Guicheney P, Lattanzi G, Merlini L, Muntoni F, et al. Neuromuscul Disord. 2002;12:984–993. doi: 10.1016/s0960-8966(02)00139-6. [DOI] [PubMed] [Google Scholar]
- 24.Cenni V, Sabatelli P, Mattioli E, Marmiroli S, Capanni C, Ognibene A, Squarzoni S, Maraldi NM, Bonne G, Columbaro M, et al. J Med Genet. 2005;42:214–220. doi: 10.1136/jmg.2004.026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatelli P, Bonaldo P, Lattanzi G, Braghetta P, Bergamin N, Capanni C, Mattioli E, Columbaro M, Ognibene A, Pepe G, et al. Matrix Biol. 2001;20:475–486. doi: 10.1016/s0945-053x(01)00160-3. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 27.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]