Abstract
A significant number of viral and cellular mRNAs utilize cap-independent translation, employing mechanisms distinct from those of canonical translation initiation. Cap-independent translation requires noncanonical, cellular RNA-binding proteins; however, the roles of such proteins in ribosome recruitment and translation initiation are not fully understood. This work demonstrates that a nucleo-cytoplasmic SR protein, SRp20, functions in internal ribosome entry site (IRES)-mediated translation of a viral RNA. We found that SRp20 interacts with the cellular RNA-binding protein, PCBP2, a protein that binds to IRES sequences within the genomic RNAs of certain picornaviruses and is required for viral translation. We utilized in vitro translation in HeLa cell extracts depleted of SRp20 to demonstrate that SRp20 is required for poliovirus translation initiation. Targeting SRp20 in HeLa cells with short interfering RNAs resulted in inhibition of SRp20 protein expression and a corresponding decrease in poliovirus translation. Our data have identified a previously unknown function of an SR protein (i.e., the stimulation of IRES-mediated translation), further documenting the multifunctional nature of this important class of cellular RNA-binding proteins.
Keywords: cap-independent translation, poliovirus, SR protein, IRES, poly(rC) binding protein
Introduction
Since the discovery of internal ribosome entry site (IRES)-mediated translation initiation in 1988 (Jang et al, 1988; Pelletier and Sonenberg, 1988), the study of eukaryotic translation mechanisms has led to the discovery of processes distinct from the widely accepted view of initiation via a scanning ribosome traversing from the 5′ terminal cap structure of mRNAs to a methionine start codon 50–100 nucleotides downstream. These processes require formation of protein–RNA complexes at sites hundreds of nucleotides distal to the 5′ terminus of the mRNA. The exact determinants required for such complexes are not completely understood, but involve RNA secondary/tertiary structures, canonical components of the eukaryotic translation initiation apparatus and noncanonical RNA-binding proteins (for a review, see Belsham and Sonenberg, 2000; Jackson, 2005). These RNA-binding proteins may facilitate ribosome recruitment directly via interaction with IRES sequences and ribosomal proteins or initiation factors. Alternatively, their mode of action may be exerted indirectly via structural changes in the mRNA that then allow binding of initiation factors and/or ribosomal subunits.
It has been well documented that the cellular RNA-binding protein poly(rC)-binding protein 2 (PCBP2) binds to the 5′ noncoding region (NCR) of several picornavirus mRNAs and is required for IRES-mediated translation initiation specified by these positive-strand RNA viruses, including poliovirus (Blyn et al, 1996, 1997; Gamarnik and Andino, 1997; Graff et al, 1998; Walter et al, 1999). However, it is not understood how PCBP2 functions to facilitate the recruitment of ribosomes to the poliovirus IRES element. One possibility is that the PCBP2 complex recruits ribosomal proteins through protein–protein interactions with additional cellular factors. To address this possibility, we carried out a yeast two-hybrid screen of a HeLa cell cDNA library to identify other cellular proteins that interact with PCBP2 and may function in poliovirus translation. Among the proteins we identified as interacting with PCBP2 was the cellular RNA-binding protein, SRp20. SRp20 belongs to the SR protein family, members of which have been shown to function in cellular mRNA splicing and nucleo-cytoplasmic RNA export (Zahler et al, 1992; Huang and Steitz, 2001; for a review, see Graveley, 2000; Hertel and Graveley, 2005; Huang and Steitz, 2005). Several SR proteins including SRp20 and two highly homologous proteins, ASF/SF2 and 9G8, have been shown to shuttle between the nucleus and cytoplasm (Caceres et al, 1998). SRp20 contains two functional domains, an RNA recognition motif (RRM) at the N-terminus of the protein and an RS domain within the C-terminal half of the protein which contains arginine–serine repeats (Caceres et al, 1997). The RS domain of SR proteins mediates several previously defined protein–protein interactions (Wu and Maniatis, 1993; Graveley, 2000) and has been shown to contact a splice site branch point when bound to an exonic splicing enhancer (Shen et al, 2004).
SR proteins are essential factors for constitutive splicing and the regulation of alternative splice site utilization for many cellular mRNAs (Tacke and Manley, 1999; Sanford et al, 2005a). They have also been implicated in alternative functions in cellular gene expression, possibly providing a link between mRNA processing, export and translation (for a review, see Graveley, 2005; Huang and Steitz, 2005). SRp20 and 9G8 were shown to bind polyadenylated mRNAs in the nucleus and cytoplasm of HeLa cells, and this binding was shown to be important in promoting nucleo-cytoplasmic export of intronless mRNAs (Huang and Steitz, 2001). Based upon these adaptor functions of SR proteins connecting mRNA processing pathways to nuclear export pathways, it is possible that SR proteins bound to specific mRNAs could target these RNP complexes to sites of protein synthesis in the cytoplasm. Indeed, there is recent evidence that certain SR proteins function in cap-dependent translation (Sanford et al, 2004; Blaustein et al, 2005). ASF/SF2 and 9G8 were shown to co-sediment with 80S ribosomes and polysomes (Sanford et al, 2004). Overexpression of ASF/SF2 in cells caused a stimulation in cap-dependent translation of reporter constructs containing SR protein-binding sites. The phosphorylation state of ASF/SF2 was shown to influence its binding to cellular mRNAs, as well as its function in translation (Sanford et al, 2005b). SRp20 was also detected in ribosomal subunit fractions, but overexpression of SRp20 did not have a stimulatory effect on cap-dependent translation (Sanford et al, 2004). Studies carried out in yeast have also shown that SR protein homologues are important for translation initiation (Windgassen et al, 2004). In this manuscript, we demonstrate that SRp20 interacts with the cellular RNA-binding protein, PCBP2. We provide evidence that a functional complex between PCBP2 and one member of the SR family of cellular proteins (SRp20) is required for efficient IRES-mediated translation of poliovirus RNA. Thus, our data suggest a novel role for SR proteins in mediating cap-independent translation initiation.
Results
Genetic evidence for SRp20 interaction with PCBP2
To elucidate the mechanism of IRES-mediated translation using poliovirus as a model system, we examined the putative interactions of cellular proteins that may function in IRES-mediated translation. PCBP2 was used as a bait protein to screen a HeLa cDNA library. One cellular factor identified in the yeast two-hybrid assay was SRp20. The interaction between SRp20 and PCBP2 resulted in yeast growth on selective media and 100-fold β-gal activation over background in a quantitative liquid assay, suggesting that the interaction is significant (data not shown). To define the domains required for this interaction, we tested several truncated forms of PCBP2 for their ability to interact with SRp20 in the yeast two-hybrid assay. When the C-terminal portion of PCBP2 (the KH3 domain) was deleted, PCBP2 could no longer interact with SRp20 in the yeast two-hybrid assay, suggesting that this domain is required for the interaction (Figure 1). However, truncations in the N-terminal portion of PCBP2 did not affect the interaction with SRp20. Previously published studies showed that amino-acid substitutions in the KH3 domain of PCBP2 caused a decrease in the ability of PCBP2 to function in cap-independent translation (Walter et al, 2002). Taken together, these data provide the initial genetic evidence that the interaction between the KH3 domain of PCBP2 and SRp20 may be involved in IRES-mediated translation.
Figure 1.
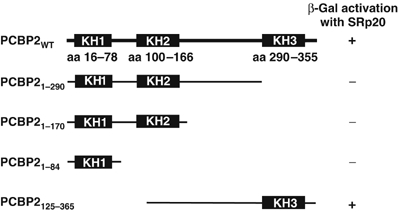
Interaction of SRp20 and truncated forms of PCBP2 in the yeast-two hybrid assay. The schematic shows the K-homologous RNA-binding domains (KH1, KH2 and KH3) in the full-length PCBP2, and the amino-acid positions of these domains are cited from reference Makeyev and Liebhaber (2002). Positive β-gal activation was determined using the colorimetric assay.
Interaction of SRp20 with PCBP2 in HeLa cells
We examined the interaction of endogenous PCBP2 and SRp20 in mock-infected and poliovirus-infected HeLa cells by carrying out co-immunoprecipitation assays (Brunner et al, 2005) with antibodies specific for PCBP or SRp20 (Figure 2A). Lysates from either infected or mock-infected HeLa cells were immunoprecipitated with a monoclonal antibody to SRp20, and interacting proteins were detected by Western blot analysis with a polyclonal PCBP antibody. When compared to assays performed with a nonspecific antibody (mouse IgG) serving as a negative control (lane 7), PCBP and SRp20 were shown to interact in cytoplasmic extracts from mock-infected and poliovirus-infected HeLa cells at 0, 2 or 5 h post infection (Figure 2A, top panel and lanes 1–6). A similar experiment was carried out by immunoprecipitating protein complexes with polyclonal PCBP antibody (bottom panel of Figure 2A, lanes 1–6) or with pre-immune serum, as a negative control (lane 7), and detecting protein complexes by Western blot analysis with an SRp20 monoclonal antibody. As shown for the approach utilized in the top panel of Figure 2A, PCBP and SRp20 can be co-immunoprecipitated in mock- and poliovirus-infected HeLa cell lysates (Figure 2A, bottom panel and lanes 1–6). These data demonstrate that PCBP and SRp20 can interact during a poliovirus infection of human cells.
Figure 2.

Biochemical evidence for SRp20 interaction with PCBP2. (A) Co-immunoprecipitation assays of PCBP2 and SRp20 using lysates from mock-infected (lanes 1, 3 and 5) or poliovirus-infected (2, 4, 6 and 7) HeLa cells. Protein complexes were immunoprecipitated with a monoclonal SRp20 antibody (Zymed Laboratories) and interacting proteins detected by Western blot analysis with a polyclonal PCBP antibody (top panel). Equal amounts of total protein were loaded in each lane. The negative control (lane 7) used nonimmune mouse IgG to detect nonspecific protein complexes. The bottom panel displays a similar co-immunoprecipitation assay, using a polyclonal PCBP antibody to immunoprecipitate complexes and a monoclonal SRp20 antibody to detect interacting proteins by Western blot analysis. The negative control (lane 7) using pre-immune serum from rabbits used to generate the PCBP antibody. (B) GST pull-down assays with GST-PCBP2 and wild-type or truncated SRp20. GST pull-down assays utilized in vitro translated, 35S-methionine-labeled SRp20, or a truncated form of SRp20 (SRp20-ΔRS, containing a deletion of the RS domain), and a full-length GST-PCBP2 fusion protein. Input levels of wild-type and SRp20-ΔRS are shown in lanes 1 and 2, respectively. Proteins that interacted with GST-PCBP2 were isolated on glutathione-Sepharose-4B, resolved by SDS–PAGE and visualized by autoradiography. GST pull-down assays were carried out with increasing amounts of translation mixtures of wild-type SRp20 (lanes 4 and 5) or SRp20-ΔRS (lanes 8 and 9). Nonspecific protein–protein interactions were detected using GST alone (lanes 3 and 7). Pull-down assays with wild-type SRp20 and GST-PCBP2 were also carried out in the presence of RNase A and T1 to eliminate any potential RNA tethering between the two proteins (lane 6). The symbols on the left-hand side of the figure designate wild-type SRp20 (with both the RRM and SR domains) and SRp20-ΔRS (missing the SR domain). The data displayed in panel B are representative of three independent GST pull-down assays.
Evidence for in vitro interaction between SRp20 and PCBP2
To further examine the interaction between SRp20 and PCBP2, we performed GST pull-down assays with a GST-PCBP2 fusion protein and in vitro translated, 35S-methionine-labeled SRp20. Wild-type SRp20 and ΔRS-SRp20 (SRp20 containing a deletion of the RS domain; Sciabica et al, 2003) were tested for their interaction with full-length GST-PCBP2 (Figure 2B). Wild-type SRp20 interacts with GST-PCBP2 (lanes 4 and 5), but not with a GST-negative control (lane 3). This interaction is not dependent on RNA, as treatment with RNase A and T1 had no inhibitory effect on the interaction (lane 6). Interestingly, the treatment with RNase increased the interaction of PCBP2 and SRp20, suggesting that RNA within the reactions may mask or sequester the protein domains required for the protein–protein interaction and subsequently inhibit the interaction. The SRp20 protein that contained a deletion of the RS domain did not display any significant interaction with GST-PCBP2 above background levels with GST alone (compare lanes 8 and 9 with lane 7 in Figure 2B). These results suggest that the RS domain is required for the protein–protein interaction with PCBP2.
SRp20 is required for efficient poliovirus IRES-mediated translation in HeLa cell extracts
Previous studies demonstrating the requirement for PCBP2 in poliovirus translation used HeLa cytoplasmic extracts that were depleted of endogenous PCBP2 by RNA affinity chromatography (Blyn et al, 1997). The activity of these extracts in poliovirus translation was routinely restored to mock-depleted levels by adding recombinant PCBP2. However, some poly(rC)-depleted extracts were not restored to mock-depleted levels upon the addition of recombinant PCBP2 protein (unpublished observations), suggesting that other factors required for translation may have been depleted by the RNA affinity chromatography. We examined different poly(rC)-depleted HeLa extracts (depleted of endogenous PCBP2, as determined by Western blot analysis; data not shown) for the presence of SRp20 by Western blot analysis and found that all mock-depleted cytoplasmic extracts contained detectable levels of SRp20 (Figure 3A, lanes 1 and 3), whereas some poly(rC)-depleted extracts did not contain SRp20 (Figure 3A, compare lanes 2 and 4). As shown in Figure 3B, both poly(rC)-depleted extracts were deficient in translation of a luciferase reporter construct driven by the poliovirus IRES in the absence of exogenously added recombinant PCBP2. However, only one of the extracts could be restored to high levels of poliovirus translation by the addition of recombinant PCBP2 (S10 #2; Figure 3B). Unlike S10 #1, this extract still contained detectable levels of SRp20, as determined by Western blot analysis (refer to Figure 3A). These data demonstrate that the ability of cytoplasmic extracts to support poliovirus translation correlates with SRp20 protein levels.
Figure 3.

Poliovirus translation in HeLa cell cytoplasmic extracts that lack SRp20. (A) SRp20 in poly(rC)-depleted HeLa S10 extracts. A Western blot using a monoclonal antibody to SRp20 shows the analysis of different HeLa S10 extracts that were either mock-depleted (lanes 1 and 3) or poly(rC) depleted (lanes 2 and 4). S10 #1 and S10 #2 were derived from two different cytoplasmic extracts prepared and depleted independently. Protein size markers from the SDS–polyacrylamide gel used to generate this blot are indicated to the right of panel. (B) Poliovirus translation in HeLa S10 extracts that lack PCBP and SRp20. In vitro translation assays in HeLa cytoplasmic S10 extracts were carried out using luciferase reporter RNAs preceded by the poliovirus 5′ NCR. Increasing amounts of recombinant PCBP2 were added to HeLa extracts that were depleted of PCBP but contained SRp20 (cross-hatched bars, S10 #2) or to extracts that were depleted of both PCBP and SRp20 (black bars, S10 #1). Translation activity is depicted as relative light units of firefly luciferase.
We predicted that depletion of SRp20 from our cellular extracts varies because of differences in depletion efficiency and the concentration of SRp20 in different S10 preparations. To specifically address the hypothesis that SRp20 is required in concert with PCBP2 for poliovirus translation, we used HeLa cell extracts depleted of both PCBP2 and SRp20 and subsequently added back recombinant PCBP2 and SRp20 to test their involvement in IRES-dependent translation. As shown by the Western blot analysis displayed in Figure 4A, these extracts were significantly depleted of both PCBP2 (left panel, compare lane 2 to lane 3) and SRp20 (right panel, compare lane 5 with lane 6); however, overall protein levels remained the same as demonstrated by the levels of actin shown in both panels. These depleted extracts displayed a marked decrease in poliovirus translation (as measured by a luciferase reporter driven by the poliovirus IRES) when compared to the mock-depleted extract (approximately 5.5 × 103 relative light units for depleted extracts versus approximately 1.7 × 107 relative light units for mock-depleted extracts; data not shown). As shown in Figure 4B, poliovirus translation was increased to intermediate levels when either recombinant PCBP2 (approximately 30 × depleted levels) or recombinant SRp20 (approximately 10 × depleted levels) was added alone; however, when the recombinant proteins were added together, they resulted in a more than additive rescue in poliovirus translation (approximately 100 × depleted levels; Figure 4B). Our data suggest that both PCBP2 and SRp20 are required for efficient poliovirus translation, and these proteins may function cooperatively in IRES-mediated translation.
Figure 4.
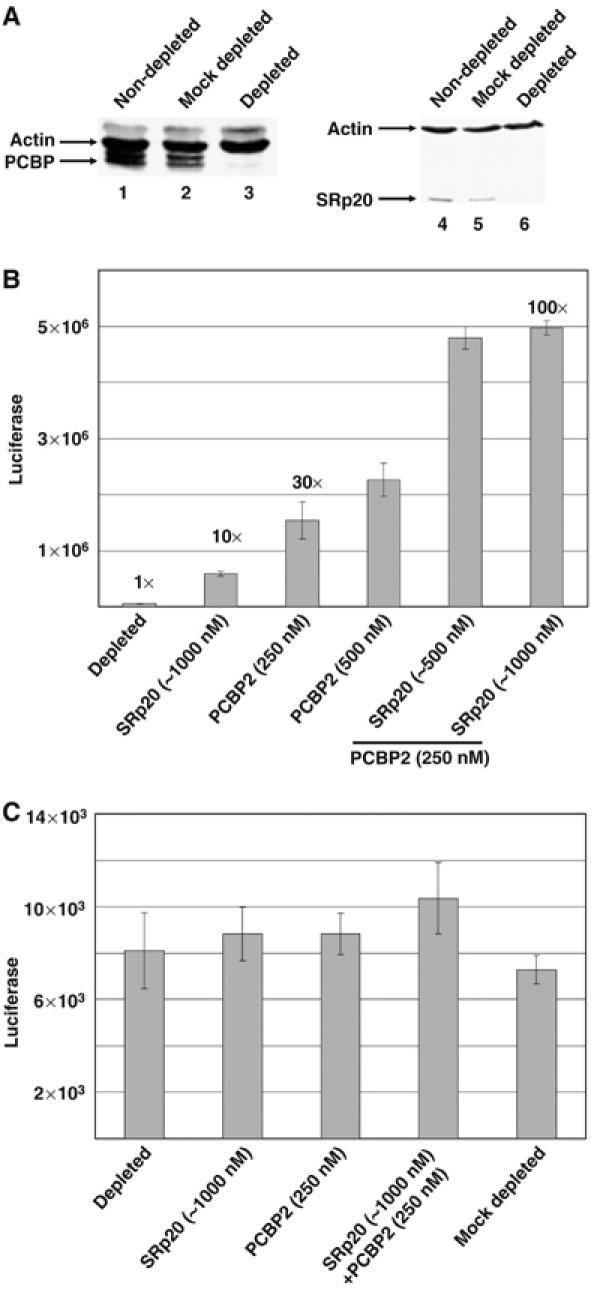
Cooperative activity of PCBP2 and SRp20 in poliovirus IRES-mediated translation. (A) Poly(rC) RNA affinity chromatography depletion of PCBP and SRp20 from HeLa S10 extracts. Western blot analysis was carried out using a polyclonal antibody to PCBP (left panel) or a monoclonal antibody to SRp20 (right panel). Both blots were also probed with a monoclonal antibody to actin (Santa Cruz Biotechnology) to normalize for possible nonspecific depletion effects. Nondepleted (lanes 1 and 4), mock-depleted (lanes 2 and 5) and poly(rC)-depleted extracts (lanes 3 and 6) were examined for the levels of SRp20 or PCBP2. (B) In vitro translation assays of poliovirus luciferase reporter RNA (5′PVLuc) in poly(rC)-depleted HeLa extracts depleted of both PCBP and SRp20. Translations without recombinant proteins added were arbitrarily set at 1. Increasing amounts of recombinant SRp20, PCBP2 or both proteins were added to the translation reactions, and the fold stimulation over depleted levels is shown above the bars. Translation activity is depicted as relative light units of firefly luciferase. (C) In vitro translation of capped mRNA in depleted S10. Translation assays in HeLa extracts depleted of PCBP and SRp20 were carried out using an in vitro transcribed, 5′-capped luciferase reporter RNA. Recombinant proteins were added as described for (B), above. Translation activity is depicted as relative light units of firefly luciferase.
It has been previously shown that PCBP2 is not required for cap-dependent translation (Walter et al, 1999). To test the possible role of SRp20 in cap-dependent translation, we carried out translation assays in poly(rC)-depleted extracts with a capped mRNA that encodes the firefly luciferase protein. These in vitro transcribed mRNAs contain a short 5′ NCR upstream of the luciferase-coding region and a 5′ cap structure. As shown in Figure 4C, poly(rC) depletion and the addition of recombinant PCBP2 and/or SRp20 had no significant effect on translation of capped mRNAs, confirming previously published results that PCBP2 (Walter et al, 1999) and SRp20 are not involved in cap-dependent translation (Sanford et al, 2004).
The functional data shown thus far were derived through the depletion of both PCBP2 and SRp20 to examine cap-independent translation. As depletion of SRp20 alone from HeLa cell extracts has been unsuccessful despite several attempts using RNA affinity depletion, immuno-depletion and magnesium precipitation (data not shown), we attempted to block the function of SRp20 using an alternative approach. A monoclonal antibody that specifically recognizes SRp20 (but not other SR proteins) was used to inhibit SRp20 in functional assays. Using the in vitro translation of a luciferase reporter construct containing the 5′ NCR of poliovirus RNA in HeLa cytoplasmic extracts, we added increasing amounts of the SRp20 monoclonal antibody and tested its ability to inhibit SRp20 function (Figure 5). Addition of SRp20 antibody caused a significant decrease in poliovirus translation (to approximately 50%; Figure 5, black bars) when compared with the control reaction with no antibody added (Figure 5, white bar) or when mouse IgG was added as a negative control (Figure 5, gray bars). These data suggest that when SRp20 is inhibited by a monoclonal antibody, there is a decrease in poliovirus cap-independent translation.
Figure 5.
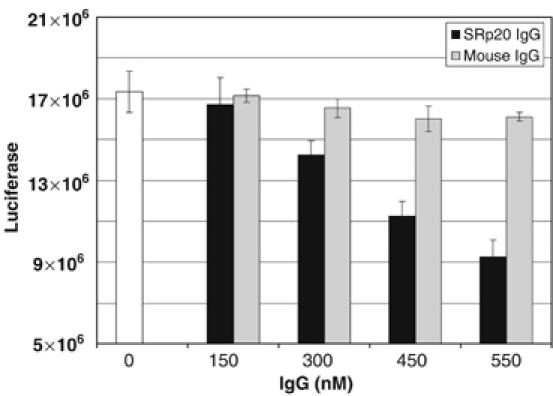
Downregulation of SRp20 decreases poliovirus translation in HeLa cell lysates. In vitro translation assays were carried out in HeLa cytoplasmic S10 extracts using a poliovirus 5′ NCR luciferase construct (5′PVLuc). Increasing amounts of IgG from a mouse monoclonal antibody to SRp20 were added to translation reactions (black bars). Normal mouse IgG was added as a negative control to examine the effect of nonspecific IgG on translation (gray bars). Translations without IgG added were set as 100% (open bar). Translation activity is depicted as relative light units of firefly luciferase.
IRES-mediated translation is inhibited in HeLa cells treated with SRp20-specific short interfering RNAs (siRNAs)
To analyze the function of SRp20 in poliovirus translation in cultured human cells, we utilized RNA interference (RNAi) to specifically downregulate SRp20 protein expression and examined these cells for their ability to translate poliovirus-luciferase reporter constructs. siRNAs were utilized to target a unique sequence in the coding region of SRp20 that was chosen from a library of previously generated and tested sequences that efficiently downregulate SRp20 mRNA expression. For these RNAi studies, we utilized two different SRp20 siRNA oligonucleotide pairs that were transfected separately into HeLa cells, and the cells were subsequently examined for SRp20 protein expression levels and poliovirus translation. To confirm that SRp20 siRNA treatment would not inhibit cap-dependent translation, we transfected untreated and siRNA-treated cells with a capped mRNA encoding the luciferase protein to measure translation efficiency. The levels of cap-dependent translation were similar in cells treated with SRp20 siRNAs and a nonspecific, control siRNA (data not shown).
To test the effect of SRp20 downregulation on poliovirus IRES-mediated translation specifically, we utilized a dicistronic RNA construct that uses cap-dependent translation to synthesize Renilla luciferase and the poliovirus IRES to synthesize firefly luciferase (Figure 6A). This dicistronic construct was transfected into cells treated with SRp20 siRNAs or nonspecific siRNA as a negative control, and poliovirus IRES activity was measured as the ratio of firefly luciferase to Renilla luciferase over background luciferase levels (determined using a construct without the poliovirus IRES). Treatment of the cells with a nonspecific, control siRNA resulted in poliovirus IRES activity∼12-fold above background levels (Figure 6C, control siRNA). Upon treatment with 1.6 μg of SRp20-specific siRNAs per 35-mm plate of HeLa cells, there was an ∼ 2-fold decrease in poliovirus IRES activity, correlating with the 40–50% decrease we observed in SRp20 protein expression (Table I and Figure 6B and C). We observed a similar inhibitory effect at lower concentrations of siRNA as well (data not shown; refer to Materials and methods). RNAi caused a significant decrease in SRp20 protein expression in HeLa cells and a concomitant decrease in poliovirus translation; however, cap-dependent translation was not affected (refer to Table I). These data suggest that SRp20 is involved in poliovirus translation in HeLa cells.
Figure 6.
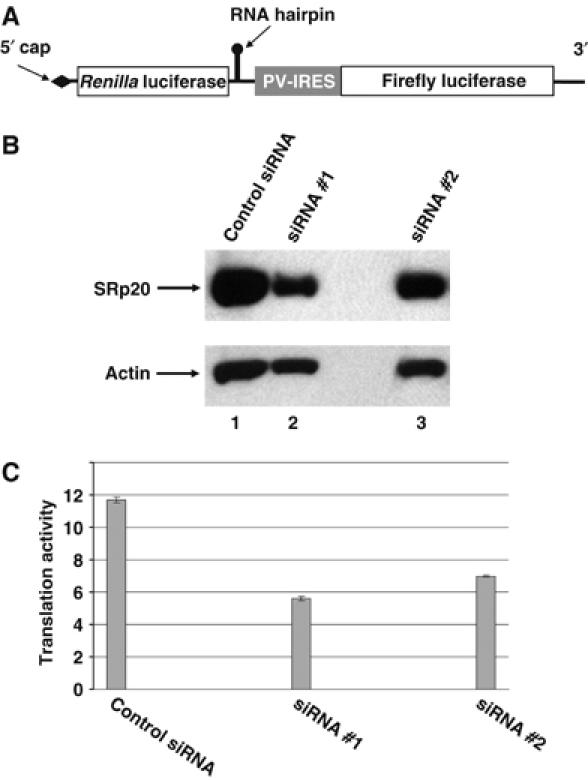
Poliovirus translation in HeLa cells downregulated for SRp20 by RNAi. (A) Schematic of the dicistronic poliovirus-IRES construct. The dicistronic RNA used to measure poliovirus IRES activity contains two cistrons separated by the poliovirus IRES. The 5′ end of the RNA contains a cap structure followed by the first cistron encoding Renilla luciferase, and an RNA hairpin is inserted downstream of the first cistron to prevent ribosome read-through. This hairpin sequence is followed by the poliovirus IRES, which drives translation of the firefly luciferase cistron. (B) Western blot analysis of SRp20 following RNAi treatment. HeLa cells grown in 35-mm plates were transfected with 1.6 μg of two different siRNA duplexes specific for SRp20 (as well as a nonspecific, control siRNA duplex) and examined by Western blot analysis for SRp20 protein levels using a monoclonal antibody to SRp20 (Zymed Laboratories). Off-target protein levels were assayed by Western blot using a polyclonal antibody to actin (Santa Cruz Biotechnology). The lane designations depicted above the figure correspond to the siRNA samples shown on the translation graph in panel C (below). (C) Decreased expression of SRp20 in HeLa cells inhibits poliovirus IRES-dependent translation compared with cap-dependent translation. The siRNA duplex set is indicated on the x-axis. Poliovirus translation activity is represented as the ratio of firefly luciferase (cap-independent) to Renilla luciferase (cap-dependent) which is presented as fold above background. Background levels were measured in lysates from HeLa cells that were transfected with reporter constructs lacking the poliovirus IRES. Error bars shown in the figure represent the root mean square of the ratios.
Table 1.
Translation activity of a poliovirus dicistronic construct following SRp20 siRNA treatment
| Control siRNA | SRp20 siRNA #1 | SRp20 siRNA #2 | |
|---|---|---|---|
| Rluc cap-dependent | 8467±921a | 8907±104 | 7130±401 |
| Fluc IRES-mediated | 2949±483 | 1603±24 | 1540±87 |
| aValues (±s.d. from the mean) shown are derived from Rluc (Renilla luciferase) or Fluc (firefly luciferase) assays on samples representing 10% of the harvested cell lysate. | |||
Discussion
IRES-mediated translation of picornavirus RNA requires cellular proteins present in the cytoplasm of infected cells. PCBP2 is one cellular protein that has been demonstrated to bind to the 5′ NCR of poliovirus RNA and mediate translation initiation. PCBP2 has been shown to bind the poliovirus IRES as a dimer, and this dimer–RNA interaction is required for translation of the viral RNA (Bedard et al, 2004). However, the mechanism by which PCBP2 or other noncanonical translation factors mediate ribosome recruitment is not yet known. PCBP2 may function in poliovirus translation initiation by recruiting ribosomes through protein–protein interactions with ribosomal proteins or other cellular proteins that may provide a bridging interaction with ribosomal subunits. To identify cellular proteins that interact with PCBP2, we carried out a yeast two-hybrid screen of a HeLa cell cDNA library. In our initial library screen we identified SRp20, a noncanonical translation factor candidate. It should be noted that previous yeast two-hybrid studies with the murine version of PCBP had identified another SR protein (9G8) as an interacting partner (Funke et al, 1996), and a yeast two-hybrid screen with human PCBP2 (also known as hnRNP E2) identified other hnRNP proteins as binding partners (Kim et al, 2000). SRp20 had not previously been shown to function in cellular or viral translation; however, SR proteins including SRp20 have been detected as cosedimenting with ribosomes in sucrose gradients (Sanford et al, 2004). We confirmed the interaction of PCBP2 and SRp20 in vitro using biochemical assays and showed that the KH3 domain of PCBP2 (which had previously been shown to be important for poliovirus translation; Walter et al, 2002) is necessary for its interaction with SRp20. We also found that the RS domain of SRp20 is required for its interaction with PCBP2. This domain had been previously shown to mediate several of the protein–protein interactions already defined for SR proteins (Manley and Tacke, 1996). Furthermore, the interaction between PCBP2 and SRp20 was detected in cytoplasmic extracts made from poliovirus-infected HeLa cells, providing evidence for the localization of such complexes in the cytoplasm of human cells where both translation and viral RNA replication occur.
To test the function of SRp20 in poliovirus translation, we utilized several in vitro approaches. When recombinant SRp20 or PCBP2 was added alone to in vitro translation assays in extracts of HeLa cells depleted of SRp20 and PCBP2, there was a low to modest level of rescue of poliovirus translation, respectively. However, when both SRp20 and PCBP2 were added to the depleted extracts, there was more than an additive rescue of poliovirus translation. Our data suggest that these two cellular proteins function cooperatively in poliovirus cap-independent translation initiation. In addition, the addition of SRp20 IgG, but not a nonspecific mouse IgG, caused a dose-dependent decrease in translation in vitro, confirming that SRp20 has a role in poliovirus translation.
We analyzed the role of SRp20 in poliovirus translation in cultured HeLa cells using RNAi to decrease SRp20 protein expression. Double-stranded complementary RNA oligonucleotides were utilized to target the SRp20 coding sequence for specific degradation through the RNAi pathway (Hannon and Rossi, 2004; Huppi et al, 2005). Following incubation with the SRp20 siRNA oligonucleotides, HeLa cells displayed a specific decrease in SRp20 protein expression as determined by Western blot analysis. Using a dicistronic reporter assay that controls for factors such as transfection efficiency, RNA stability and nonspecific metabolic changes within the cell, we observed a specific decrease in translation of a reporter protein driven by the poliovirus IRES that correlated with the decrease in SRp20 protein expression. This result provides additional evidence that SRp20 has a specific role in poliovirus IRES-mediated translation, but not cap-dependent translation in cultured HeLa cells.
SR proteins have been studied extensively for their roles in different aspects of cellular gene expression, including mRNA processing, export and more recently, translation. There are several examples of SR proteins with multiple functions that may link various steps in both cellular and viral gene expression. For example, herpes simplex virus utilizes interactions between viral and cellular proteins in various steps of viral gene expression. The herpes simplex virus protein ICP27 has been shown to interact with SR proteins and SR protein kinases, interactions that are required for export of unspliced herpesvirus mRNAs and result in the downregulation of cellular pre-mRNA splicing and nuclear export (Sciabica et al, 2003; Sandri-Goldin, 2004). ICP27 has also been shown to be associated with translation initiation factors (Fontaine-Rodriguez et al, 2004) and may function in translation of the viral mRNA encoding VP16 (Ellison et al, 2005), possibly via its interaction with SR proteins. Thus, the SR protein family contains multifunctional proteins that are involved in several aspects of both viral and cellular gene expression. Our data suggest that one shuttling SR protein, SRp20, is required for poliovirus cap-independent translation and raise the possibility that SRp20 is involved in the cap-independent translation of other viral or cellular mRNAs.
It should be noted that for the translation assays as well as the protein–protein interaction experiments described in this study, we have not assessed the phosphorylation state of either SRp20 or PCBP2, both of which are known to be phosphorylated in human cells. However, the SRp20 used in our studies was derived either from expression of recombinant baculovirus in cultured insect cells or by in vitro translation in rabbit reticulocyte lysate, both of which are known to phosphorylate proteins (e.g., refer to (Suragani et al, 2006) and (Joshi et al, 1995), respectively). As recombinant PCBP2 was expressed in bacteria, it is unlikely that this protein is phosphorylated. Unphosphorylated PCBP2 binds to poly(rC) homopolymers with a greater affinity than its phosphorylated counterpart (Leffers et al, 1995), and this recombinant form of PCBP2 has been shown to be active in binding to poliovirus RNA and in restoring translation activity in poly(rC)-depleted extracts (Blyn et al, 1996; Walter et al, 1999). Finally, it has been demonstrated that SR protein-specific kinases are localized to the cytoplasm of HeLa cells (Ding et al, 2006), suggesting that phosphorylation of SRp20 may occur in the cytoplasmic S10 extracts used in our in vitro translation assays for poliovirus IRES function.
PCBP2 functions in translation of RNAs from picornaviruses other than poliovirus, including human rhinovirus, coxsackievirus and hepatitis A virus (Graff et al, 1998; Walter et al, 1999). Based upon this conservation in function, we predict that the role of SRp20 will be conserved among these picornaviruses. Little is known about PCBP2 and cap-independent translation of cellular mRNAs; however, there is evidence suggesting that PCBP2 interacts with the IRES element found in c-myc mRNAs and may function in translation initiation (Evans et al, 2003). Future studies may reveal that both PCBP2 and SRp20 have a conserved role in ribosome recruitment for IRES-mediated translation of both viral and cellular RNAs.
In preliminary studies, we found that SR proteins other than SRp20 can interact with PCBP2 in GST pull-down assays (unpublished data), raising the question as to whether other SR proteins might function in poliovirus cap-independent translation. Given that the levels of SRp20-specific RNAi inhibition of poliovirus IRES-mediated reporter gene expression correlated with the extent of SRp20 protein expression, it does not appear that other SR proteins are able to functionally substitute for SRp20 in poliovirus translation in transfected HeLa cells. However, the nuclear versus cytoplasmic distribution of SR proteins may change during the course of a viral infection, as has been shown with other nuclear hnRNP proteins that are redistributed from the nucleus to the cytoplasm during a poliovirus or human rhinovirus infection of HeLa cells (Gustin and Sarnow, 2001, 2002). In addition, it was recently shown that one SR protein (ASF/SF2) has different requirements for its functions in the nucleus versus those in the cytoplasm and that the RS domain is not required for its role in cap-dependent translation in the cytoplasm (Sanford et al, 2005b). Our data show a requirement for the RS domain of SRp20 in binding to PCBP2, indirectly implicating its role in poliovirus cap-independent translation and highlighting the differences in mechanisms utilized for IRES-mediated translation versus cap-dependent translation.
Identifying and characterizing the interaction of SRp20 with PCBP2 has provided new insights into cap-independent translation mechanisms and identified a novel role for SRp20 in this process. Given our yeast two-hybrid results and co-immunoprecipitation data, it is highly likely that SRp20 and PCBP2 form a stable complex in the cytoplasm of uninfected human cells. It will be of interest to determine if this complex plays a role in any of the known cellular functions of PCBPs (also known as αCP's or hnRNP E's), which include formation of stability complexes with the 3′ noncoding regions of specific mRNAs (Holcik and Liebhaber, 1997), inhibition of mRNA translation (Ostareck et al, 1997; Perrotti et al, 2002), and a role in apoptosis and cell cycle arrest (Zhu and Chen, 2000); reviewed in Makeyev and Liebhaber (2002). A putative role for SRp20 in any of these important regulatory functions would further amplify the ever-expanding repertoire of activities ascribed to SR proteins, including the role we have described for SRp20 in IRES-mediated translation initiation.
Materials and methods
Plasmid design
pT220-460, which contains the cDNA for stem–loop IV RNA of the poliovirus IRES downstream of the T7 promoter, pQE30-PCBP2, the wild-type PCBP2 bacterial expression vector and p5′PVLuc, which encodes the 5′ noncoding region of poliovirus RNA followed by the luciferase coding region, have been previously described (Dildine and Semler, 1992; Blyn et al, 1996; Walter et al, 1999).
PCBP2-coding sequences from pQE30-PCBP2 were cloned into the GAL4-binding domain fusion plasmid, pGBT-9 (Clontech). The HeLa cDNA library utilized for screening is present in the pGAD-GH GAL4 activation domain plasmid (Clontech). The GST-PCBP2 plasmid was generated by cloning the PCBP2-coding sequence from pQE30-PCBP2 into the pGEX-2T vector (Pharmacia). PCBP2 truncations were generated by PCR amplification of pQE30-PCBP2 and were inserted into the pGAD-424 vector.
Yeast two-hybrid assays and β-galactosidase activation assays
The yeast two-hybrid assay was performed as described in the Matchmaker protocol (Clontech). The bait plasmid containing the complete PCBP2-coding sequence cloned into the GAL4-binding domain expression vector and the HeLa cDNA library were co-transformed into HF7c yeast. Transformants were plated on media lacking tryptophan and leucine to detect transformation efficiency and additionally without histidine to detect interactions. For determination of β-galactosidase activity in isolated yeast transformants, colorimetric assays were performed as described in the Matchmaker (Clontech) protocol using filter lift and liquid assays. Enzyme activity was determined by calculating Miller units, as described (Miller, 1972).
Purification of recombinant PCBP
Purification of his-tagged PCBP2 proteins was performed essentially as described by Parsley et al (1997). Before use in functional assays, the recombinant proteins were dialyzed overnight in initiation factor buffer (Brown and Ehrenfeld, 1979). Concentration and purity of protein preparations were determined by Bradford protein assay (Bio-Rad) and SDS–PAGE, respectively. Proteins used in these experiments were at least 90% pure, as estimated by Coomassie staining. GST fusion proteins were expressed from the pGEX-4T vectors (Pharmacia Biotech). GST proteins were purified as described (Bedard et al, 2004).
Purification of recombinant SRp20
Sf9 cells (derived from the fall armyworm Spodoptera frugiperda) were infected with a recombinant baculovirus expressing a his-tagged version of SRp20, as described (Barnard and Patton, 2000) and incubated for 48 h under permissive growth conditions. Cell harvests and protein purification steps were carried out essentially as described (Barnard and Patton, 2000).
GST pull-down assays
GST pull-down assays were carried out with recombinant GST-PCBP2 and in vitro-translated 35S-methionine-labeled SRp20, as described (Bedard et al, 2004). Plasmid vectors for in vitro transcription of SRp20 and ΔRS-SRp20 cDNA sequences were a generous gift from Kathryn Sciabica and Rozanne Sandri-Goldin (Sciabica et al, 2003).
HeLa cell cytoplasmic extract preparation, depletion, and in vitro translation
Cytoplasmic extracts were prepared as described (Brown and Ehrenfeld, 1979; Barton and Flanegan, 1993; Walter et al, 2002). S10 extracts were depleted (and mock-depleted) of PCBP and SRp20 by RNA affinity chromatography with poly(rC) agarose essentially, as described (Bedard et al, 2004). In vitro translation experiments were carried out at 30°C for 75 min, as described by Walter et al (1999).
Luciferase assays
Luciferase values were measured in triplicate as previously described (Jang et al, 2004), using a SIRIUS luminometer (Berthold Detection Systems), and similar results were observed in three or four independent experiments.
RNA interference
HeLa cell monolayers in 35-mm plates were grown to 60% confluency (approximately 6 × 105 cells) and transfected with either 0.4, 0.8 or 1.6 μg of dsRNA oligonucleotides (Ambion) using TransMessenger reagent (Qiagen). Following transfection, cells were grown for 24 h, trypsinized, diluted 1:3 in fresh medium and grown to 60% confluency. Cells were then transfected with a dicistronic luciferase reporter RNA with the poliovirus 5′ NCR driving the downstream firefly luciferase gene and incubated for 6 h. Passive lysis buffer (200 μl) was then added to each monolayer, incubated for 15 min at room temperature, and cells were harvested. Samples from each monolayer were used in dual luciferase assays and Western blot analysis with an SRp20 monoclonal antibody (Neugebauer and Roth, 1997; Zymed Laboratories) or an actin polyclonal antibody (Santa Cruz Biotechnology).
SRp20 siRNA oligonucleotide sequences used were:
5′ GGAAAUAGAAGACAGUUUG 3′ sense #1
5′ CAAACUGUCUUGUAUUUCC 3′ antisense #1
5′ GGUCCCUUUCUAGAGAUAG 3′ sense #2
5′ CUAUCUCUAGAAAGGGACC 3′ antisense#2.
Acknowledgments
We thank James Patton for the gift of recombinant baculovirus expressing SRp20. We are indebted to Rozanne Sandri-Goldin and members of her laboratory for advice regarding SR proteins and to Klemens Hertel for his valuable insights into SR protein functions. We thank Rozanne Sandri-Goldin and Klemens Hertel for critical reading of the manuscript. In addition, we thank Hung Nguyen and MyPhuong Tran for expert technical assistance. KMB was a predoctoral trainee of Public Health Service training grant AI 07319. This work was supported by the Public Health Service Grant AI 26765 from the National Institutes of Health.
References
- Barnard DC, Patton JG (2000) Identification and characterization of a novel serine–arginine-rich splicing regulatory protein. Mol Cell Biol 20: 3049–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DJ, Flanegan JB (1993) Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol 67: 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard KM, Walter BL, Semler BL (2004) Multimerization of poly(rC) binding protein 2 is required for translation initiation mediated by a viral IRES. RNA 10: 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham GJ, Sonenberg N (2000) Picornavirus RNA translation: roles for cellular proteins. Trends Microbiol 8: 330–335 [DOI] [PubMed] [Google Scholar]
- Blaustein M, Pelisch F, Tanos T, Munoz MJ, Wengier D, Quadrana L, Sanford JR, Muschietti JP, Kornblihtt AR, Caceres JF, Coso OA, Srebrow A (2005) Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol 12: 1037–1044 [DOI] [PubMed] [Google Scholar]
- Blyn LB, Swiderek KM, Richards O, Stahl DC, Semler BL, Ehrenfeld E (1996) Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci USA 93: 11115–11120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn LB, Towner JS, Semler BL, Ehrenfeld E (1997) Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol 71: 6243–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BA, Ehrenfeld E (1979) Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology 97: 396–405 [DOI] [PubMed] [Google Scholar]
- Brunner JE, Nguyen JHC, Roehl HH, Ho TV, Swiderek KM, Semler BL (2005) Functional interaction of heterogeneous nuclear ribonucleoprotein C with poliovirus RNA synthesis initiation complexes. J Virol 79: 3254–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR (1997) Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol 138: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres JF, Screaton GR, Krainer AR (1998) A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev 12: 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildine SL, Semler BL (1992) Conservation of RNA–protein interactions among picornaviruses. J Virol 66: 4364–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JH, Zhong XY, Hagopian JC, Cruz MM, Ghosh G, Feramisco J, Adams JA, Fu XD (2006) Regulated cellular partitioning of SR protein-specific kinases in mammalian cells. Mol Biol Cell 17: 876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison KS, Maranchuk RA, Mottet KL, Smiley JR (2005) Control of VP16 translation by the herpes simplex virus type 1 immediate-early protein ICP27. J Virol 79: 4120–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, Mitchell SA, Spriggs KA, Ostrowski J, Bomsztyk K, Ostarek D, Willis AE (2003) Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo. Oncogene 22: 8012–8020 [DOI] [PubMed] [Google Scholar]
- Fontaine-Rodriguez EC, Taylor TJ, Olesky M, Knipe DM (2004) Proteomics of herpes simplex virus infected cell protein 27: association with translation initiation factors. Virology 330: 487–492 [DOI] [PubMed] [Google Scholar]
- Funke B, Zuleger B, Benavente R, Schuster T, Goller M, Stevenin J, Horak I (1996) The mouse poly(C)-binding protein exists in multiple isoforms and interacts with several RNA-binding proteins. Nucleic Acids Res 24: 3821–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamarnik AV, Andino R (1997) Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA 3: 882–892 [PMC free article] [PubMed] [Google Scholar]
- Graff J, Cha J, Blyn LB, Ehrenfeld E (1998) Interaction of poly(rC) binding protein 2 with the 5′ noncoding region of hepatitis A virus RNA and its effects on translation. J Virol 72: 9668–9675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR (2000) Sorting out the complexity of SR protein functions. RNA 6: 1197–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR (2005) Coordinated control of splicing and translation. Nat Struct Mol Biol 12: 1022–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin KE, Sarnow P (2001) Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J 20: 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin KE, Sarnow P (2002) Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol 76: 8787–8796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ, Rossi JJ (2004) Unlocking the potential of the human genome with RNA interference. Nature 431: 371–378 [DOI] [PubMed] [Google Scholar]
- Hertel KJ, Graveley BR (2005) RS domains contact the pre-mRNA throughout spliceosome assembly. Trends Biochem Sci 30: 115–118 [DOI] [PubMed] [Google Scholar]
- Holcik M, Liebhaber SA (1997) Four highly stable eukaryotic mRNAs assemble 3′ untranslated region RNA–protein complexes sharing cis and trans components. Proc Natl Acad Sci USA 94: 2410–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Steitz JA (2001) Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell 7: 899–905 [DOI] [PubMed] [Google Scholar]
- Huang Y, Steitz JA (2005) SRprises along a messenger's journey. Mol Cell 17: 613–615 [DOI] [PubMed] [Google Scholar]
- Huppi K, Martin SE, Caplen NJ (2005) Defining and assaying RNAi in mammalian cells. Mol Cell 17: 1–10 [DOI] [PubMed] [Google Scholar]
- Jackson RJ (2005) Alternative mechanisms of initiating translation of mammalian mRNAs. Biochem Soc Trans 33: 1231–1241 [DOI] [PubMed] [Google Scholar]
- Jang GM, Leong LE, Hoang LT, Wang PH, Gutman GA, Semler BL (2004) Structurally distinct elements mediate internal ribosome entry within the 5′-noncoding region of a voltage-gated potassium channel mRNA. J Biol Chem 279: 47419–47430 [DOI] [PubMed] [Google Scholar]
- Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E (1988) A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol 62: 2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B, Cai AL, Keiper BD, Minich WB, Mendez R, Beach CM, Stepinski J, Stolarski R, Darzynkiewicz E, Rhoads RE (1995) Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J Biol Chem 270: 14597–14603 [DOI] [PubMed] [Google Scholar]
- Kim JH, Hahm B, Kim YK, Choi M, Jang SK (2000) Protein–protein interaction among hnRNPs shuttling between nucleus and cytoplasm. J Mol Biol 298: 395–405 [DOI] [PubMed] [Google Scholar]
- Leffers H, Dejgaard K, Celis JE (1995) Characterisation of two major cellular poly(rC)-binding human proteins, each containing three K-homologous (KH) domains. Eur J Biochem 230: 447–453 [PubMed] [Google Scholar]
- Makeyev AV, Liebhaber SA (2002) The poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8: 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley JL, Tacke R (1996) SR proteins and splicing control. Genes Dev 10: 1569–1579 [DOI] [PubMed] [Google Scholar]
- Miller JH (1972) Experiments in Molecular Genetics. New York: Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- Neugebauer KM, Roth MB (1997) Distribution of pre-mRNA splicing factors at sites of RNA polymerase II transcription. Genes Dev 11: 1148–1159 [DOI] [PubMed] [Google Scholar]
- Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW (1997) mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89: 597–606 [DOI] [PubMed] [Google Scholar]
- Parsley TB, Towner JS, Blyn LB, Ehrenfeld E, Semler BL (1997) Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA 3: 1124–1134 [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N (1988) Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334: 320–325 [DOI] [PubMed] [Google Scholar]
- Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, Iervolino A, Condorelli F, Gambacorti-Passerini C, Caligiuri MA, Calabretta B (2002) BCR-ABL suppresses C/EBPalpha expression through inhibitory action of hnRNP E2. Nat Genet 30: 48–5811753385 [Google Scholar]
- Sandri-Goldin RM (2004) Viral regulation of mRNA export. J Virol 78: 4389–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JR, Ellis J, Caceres JF (2005a) Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem Soc Trans 33: 443–446 [DOI] [PubMed] [Google Scholar]
- Sanford JR, Ellis JD, Cazalla D, Caceres JF (2005b) Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. Proc Natl Acad Sci USA 102: 15042–15047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JR, Gray NK, Beckmann K, Caceres JF (2004) A novel role for shuttling SR proteins in mRNA translation. Genes Dev 18: 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciabica KS, Dai QJ, Sandri-Goldin RM (2003) ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J 22: 1608–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Kan JL, Green MR (2004) Arginine-serine-rich domains bound at splicing enhancers contact the branchpoint to promote prespliceosome assembly. Mol Cell 13: 367–376 [DOI] [PubMed] [Google Scholar]
- Suragani RN, Ghosh S, Ehtesham NZ, Ramaiah KV (2006) Expression and purification of the subunits of human translational initiation factor 2 (eIF2): phosphorylation of eIF2alpha and beta. Protein Expr Purif 47: 225–233 [DOI] [PubMed] [Google Scholar]
- Tacke R, Manley JL (1999) Determinants of SR protein specificity. Curr Opin Cell Biol 11: 358–362 [DOI] [PubMed] [Google Scholar]
- Walter BL, Nguyen JH, Ehrenfeld E, Semler BL (1999) Differential utilization of poly(rC) binding protein 2 in translation directed by picornavirus IRES elements. RNA 5: 1570–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter BL, Parsley TB, Ehrenfeld E, Semler BL (2002) Distinct poly(rC) binding protein KH domain determinants for poliovirus translation initiation and viral RNA replication. J Virol 76: 12008–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windgassen M, Sturm D, Cajigas IJ, Gonzalez CI, Seedorf M, Bastians H, Krebber H (2004) Yeast shuttling SR proteins Npl3p, Gbp2p, and Hrb1p are part of the translating mRNPs, and Npl3p can function as a translational repressor. Mol Cell Biol 24: 10479–10491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Maniatis T (1993) Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75: 1061–1070 [DOI] [PubMed] [Google Scholar]
- Zahler AM, Lane WS, Stolk JA, Roth MB (1992) SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev 6: 837–847 [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen X (2000) MCG10, a novel p53 target gene that encodes a KH domain RNA-binding protein, is capable of inducing apoptosis and cell cycle arrest in G(2)-M. Mol Cell Biol 20: 5602–5618 [DOI] [PMC free article] [PubMed] [Google Scholar]


