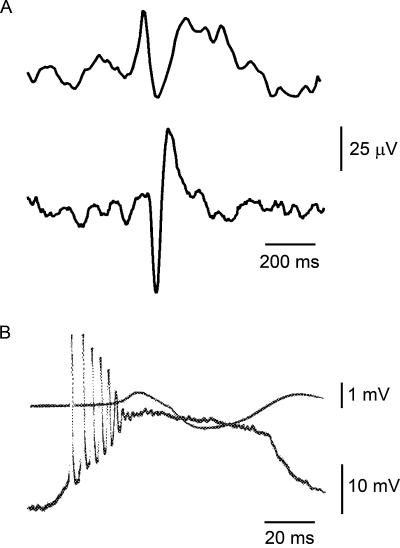
Intracellular recordings in the 1960s revealed that interictal spikes in models of focal epilepsy, which resemble those in the EEG of epilepsy patients, are due to large, long-lasting neuronal depolarizations that evoke high-frequency action potential firing (see Fig. 1). Such single neuron electrophysiological events are referred to as paroxysmal depolarization shifts (PDSs). It is estimated that a spike, detectable by EEG scalp electrodes, reflects the synchronous discharge of PDSs by several million neurons over a time span of 30–75 milliseconds (3). Two divergent hypotheses were proposed to account for the PDSs. The “synaptic theorists” viewed the PDSs as outsized synaptic potentials resulting from excessive synchronous synaptic activation of essentially normal neurons. In contrast, the “epileptic neuron theorists” proposed that the excitability properties of the neurons exhibiting PDSs are altered such that normal synaptic drive results in the abnormal PDS response. Ultimately, proponents of the synaptic view prevailed (4); however, new developments have shaken confidence in this conclusion. It is now apparent that many idiopathic epilepsies are due to genetically determined ion channel defects. Epileptic discharges in these conditions truly may result from neurons with altered intrinsic excitability properties. Moreover, it seems that ion channels that mediate the intrinsic bursting properties of neurons can be altered in acquired epileptogenesis (5). In an unexpected twist, recent evidence suggests that PDSs may not be dependent upon circuit or intrinsic abnormalities of neurons at all, but rather are generated, at least in some cases, by the release of neuroactive substances, most notably glutamate, from astrocytes (6).
FIGURE 1.
A: Scalp EEG recordings of interictal spikes. Reprinted with permission from Prog Neurobiol (1). Copyright Elsevier Science Ltd. 2001. B: One of the earliest examples of an intracellularly recorded paroxsysmal depolarization shift in an “acute penicillin focus” in the cat cortex. Upper superimposed tracing shows the field response recorded at the cortical surface. Adapted with permission from Exp Neurol (2). Copyright Elsevier Science (USA) 1964.
Just as the debate on the electrophysiological underpinnings of the PDSs is being reopened, a new question has come to the fore: Are interictal spikes more than just epiphenomena? Specifically, does interictal activity trigger seizures or, alternately, keep them under control, and does interictal activity contribute to the evolution of the epileptogenic process? The arguments are laid out in two reviews in this issue of Epilepsy Currents. Staley and Dudek propose the hypothesis that interictal spikes promote epileptogenesis, noting that they arise in the latent period before spontaneous seizures in experimental epilepsy models. The authors speculate that persistent abnormal interictal activity over the course of time leads to the formation of abnormal excitatory connections and also triggers synaptic plasticity mechanisms that strengthen excitatory circuits. Both of these factors are presumed to reinforce the epileptic state.
Avoli et al. take the opposite point of view, arguing persuasively (based on a wealth of data from experimental animals and brain slices treated with the convulsant 4-aminopyridine) that while interictal spikes resemble the spiking that occurs during seizures, they are independent events from ictal discharges that may in some situations reduce the propensity for seizures. In fact, contrary to the popular notion that interictal spikes trigger seizures, spike quantification studies in epileptic patients indicate that the frequency of interictal spikes does not change or even decreases before the onset of a seizure. Additional evidence presented by Avoli et al. demonstrates that when interictal discharges are eliminated, seizure discharges emerge, suggesting that interictal spikes have anticonvulsant properties.
Clearly, this debate is more than just of theoretical interest. If Staley and Dudek are correct, then suppressing interictal spiking in a brain-injured individual may prevent the subsequent development of epilepsy. Indeed, Staley and Dudek speculate that it may even be possible to reverse the epileptogenic process by suppressing spiking in an individual who is already exhibiting spontaneous seizures. In contrast, Avoli et al. would argue that there are some situations in which suppression of interictal spikes may be exactly the wrong approach, as this would lead to an increase in seizures. If they are correct, electrical stimulation of certain circuits in a way that simulates interictal activity may be a useful anticonvulsant strategy. At the moment, insufficient data are available to decide between the starkly different perspectives presented in this point–counterpoint. However, both sets of authors admit that some types of interictal spikes could be friend and others foe, depending upon the type of spike and the nature of the circuit in which they occur. Also, it is worth remembering that the divergent hypotheses are based on experiments with entirely different experimental systems: rats experiencing status epilepticus, on one hand, and brain slices treated with 4-aminopyridine, on the other. The relevancy of either model to the human situation is uncertain. Ultimately, there may be truth on both sides. In any case, the reviews should encourage the fresh thinking and new experiments that are needed to uncover the true implications of interictal spikes.
References
- 1.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto H, Ajmone Marsan C. Cortical cellular phenomena in experimental epilepsy: Interictal manifestations. Exp Neurol. 1964;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- 3.Cavazos JE, Sanchez R. Pathophysiology of seizures and epilepsy. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy. Scientific Foundations of Clinical Practice. New York: Marcel Dekker; pp. 5–20. [Google Scholar]
- 4.Johnston D, Brown TH. The synaptic nature of the paroxysmal depolarizing shift in hippocampal neurons. Ann Neurol. 1984;16 Suppl:S65–S71. doi: 10.1002/ana.410160711. [DOI] [PubMed] [Google Scholar]
- 5.Dudek FE, Rogawski MA. The epileptic neuron redux. Epilepsy Curr. 2002;2:151–152. doi: 10.1046/j.1535-7597.2002.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]