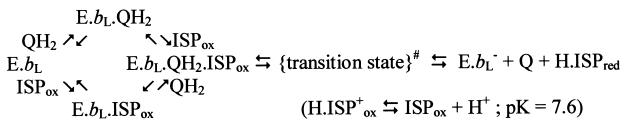Abstract
Quinol oxidation by the bc1 complex of Rhodobacter sphaeroides occurs from an enzyme–substrate complex formed between quinol bound at the Qo site and the iron–sulfur protein (ISP) docked at an interface on cytochrome b. From the structure of the stigmatellin-containing mitochondrial complex, we suggest that hydrogen bonds to the two quinol hydroxyl groups, from Glu-272 of cytochrome b and His-161 of the ISP, help to stabilize the enzyme–substrate complex and aid proton release. Reduction of the oxidized ISP involves H transfer from quinol. Release of the proton occurs when the acceptor chain reoxidizes the reduced ISP, after domain movement to an interface on cytochrome c1. Effects of mutations to the ISP that change the redox potential and/or the pK on the oxidized form support this mechanism. Structures for the complex in the presence of inhibitors show two different orientations of Glu-272. In stigmatellin-containing crystals, the side chain points into the site, to hydrogen bond with a ring hydroxyl, while His-161 hydrogen bonds to the carbonyl group. In the native structure, or crystals containing myxothiazol or β-methoxyacrylate-type inhibitors, the Glu-272 side chain is rotated to point out of the site, to the surface of an external aqueous channel. Effects of mutation at this residue suggest that this group is involved in ligation of stigmatellin and quinol, but not quinone, and that the carboxylate function is essential for rapid turnover. H+ transfer from semiquinone to the carboxylate side chain and rotation to the position found in the myxothiazol structure provide a pathway for release of the second proton.
The bc1 complex family of enzymes plays a central role in all the main pathways of energy conversion, and the photosynthetic apparatus of Rhodobacter sphaeroides exemplifies one of the simplest of these (1–5). This system is convenient experimentally because of the ease with which electron transfer can be initiated by illumination (6). X-ray crystallographic structures of mitochondrial complexes (7–10) contain at their core the three catalytic subunits, cytochrome (cyt) b, cyt c1, and the Rieske iron–sulfur protein (ISP), common to the bacterial enzymes (11–13). Homology models of these show that the catalytic superstructure is highly conserved, as had been expected from studies of the mechanism, which is essentially the same in the two systems (1–5). The bc1 complex catalyzes the oxidation of quinol and the reduction of cyt c (or c2) through a modified Q cycle (1–6, 14–17). Two separate internal electron transfer chains connect three catalytic sites. At one site, heme c1 is oxidized by cyt c2. Two catalytic sites in cyt b are involved in oxidation or reduction of ubiquinone. In the bifurcated reaction at the quinol-oxidizing site (the Qo site), one electron from quinol is passed to the ISP, which transfers it to cyt c1, while the semiquinone produced is oxidized by another chain consisting of the two b hemes of cyt b. At the quinone-reducing site (Qi-site), electrons from the b-heme chain are used to generate quinol. The integration of the oxidation and reduction reactions with the release or uptake of protons in the aqueous phases allows the complex to pump protons across the membrane.
The reaction at the Qo site determines the unique functional characteristics of the bc1 complex. The bifurcation of electrons between high- and low-potential chains is the crucial event through which the free-energy drop between the quinol pool and oxidized acceptor is used to generate a proton gradient. The efficiency of this process is therefore of primary importance in energy conversion. The complex has evolved to maximize the efficiency, and it achieves a remarkable partitioning in which the second electron is passed almost exclusively to the low-potential heme bL, despite the more favorable redox gradient provided by the high-potential chain bH.
In this paper, we identify the pathways by which protons are released from the Qo site on quinol oxidation. The mechanism we propose is based on an analysis of the binding of inhibitors at the Qo site as shown by the structures, and on the pH dependence of activation barriers in the partial reactions of the site, of turnover, and of the redox properties of the centers. Our mechanism accounts for the functional modifications on mutation of a highly conserved glutamate of the -PEWY- loop (named from the sequence in single-letter code) of cyt b, and of residues of the ISP that lead to changes in redox potential and pK, which previously had seemed paradoxical.
EXPERIMENTAL PROCEDURES
Structures of the complex from chicken heart mitochondria have been discussed elsewhere (8). Refined structures have also been solved at similar resolution for the chicken complexes with myxothiazol or β-methoxyacrylate (MOA)-stilbene bound. Refinement data for deposited structures (1bcc, 2bcc, and 3bcc) are included in the files, and data for other structures are similar and will be published elsewhere.
Modeling of the quinones at the Qo site was performed with sculpt (18) (Interactive Simulations, acquired by MDL Information Systems, San Leandro, CA) running on a PC computer. After removal of the coordinate data for the incumbent inhibitor, the quinone abstracted from PDB file 4rcr (photochemical reaction center) was positioned at the Qo site to mimic the inhibitor. To model the occupancy of the distal domain, the stigmatellin-containing structure was used. For occupancy of the proximal domain, the myxothiazol structure was used. After preliminary manipulation, the quinone was allowed to relax with the protein frozen to force conformation to the site volume. In the distal position, the quinone head was constrained by tethering the two carbonyl O atoms, one to Nɛ of His-161 and one to Oɛ1 of Glu-272. This tethered relaxation resulted in distances suitable for H bonds, which were represented by adding H atoms to mimic the ligation of the quinol. Water molecules in the putative water chain were based on a model constructed for molecular dynamics simulation (39), with some relaxation to accommodate residue changes in different structures.
Kinetic measurements and determination of activation energies were essentially as described previously (17). Measurement of redox potentials for cytochromes (15, 16) and the ISP (19) were as described in the references. Mutagenesis was performed essentially as described in ref. 20, and measurements of EPR spectra of the ISP were as described in ref. 21. Details of mutagenic primers will be described elsewhere.
RESULTS
Inhibitor and Substrate Complexes at the Qo Site.
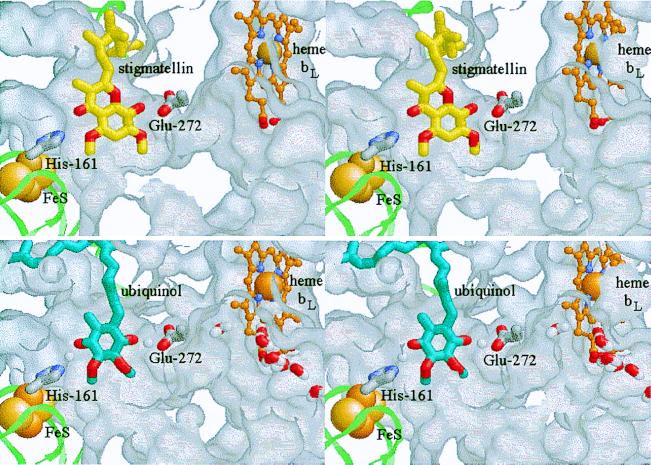
The binding of stigmatellin at the Qo site is shown in Fig. 1 Upper. The model is based on the chicken mitochondrial complex with stigmatellin (PDB ID 2bcc), and further refinement of the crystals containing stigmatellin and antimycin previously described (ref. 8, deposited as 3bcc). The Qo site has a bifurcated volume, with lobes distal from and proximal to heme bL. The inhibitor is constrained at the distal end of the binding pocket, as previously observed, through its interaction with the ISP. However, in the revised configuration, stigmatellin is rotated by 180o compared with that in 3bcc (8). The inhibitor is liganded by a bond between His-161 of the ISP and the carbonyl O atom of the chromone ring. His-161 is also a ligand to the 2Fe–2S cluster and has been identified as the group responsible for the pK of ≈7.6 on the oxidized ISP (ISPox) (22, 23). In contrast to published structures (7–9), a second ligand to stigmatellin has now been identified, provided by Glu-272 of the highly conserved -PEWY- loop. The carboxylate of the side chain of Glu-272 is found within H-bonding distance of the -OH group of the second ring of stigmatellin, diagonally across from the carbonyl group. To bind to the inhibitor, the side chain has rotated from the position in the absence of inhibitor, where it points out of the site. The side chain is also found in the “out” position when myxothiazol or MOA-stilbene binds at the site, and rotation out of the binding domain is necessary to accommodate these inhibitors. In the “out” position, the carboxylate group forms part of the exterior-facing surface of a cavity reaching into the protein from the aqueous phase on the P side of the structure.
Figure 1.
Stigmatellin binding as a model for the enzyme–substrate complex. (Stereo pairs.) The ISP is shown as a pale green ribbon, with the 2Fe–2S cluster as space-filling spheres. cyt b is represented by the exterior surface of the protein, mapped with a 1.4-Å probe. The protein has been cut away to reveal the Qo-site volume. The side chains of His-161 (ISP) and Glu-272 (cyt b) are shown as Corey–Pauling–Koltun (CPK) colored tube models, and heme bL as a ball-and-stick model, with the Fe atom space-filling and C atoms in orange. (Upper) The binding of stigmatellin (C atoms yellow, O atoms red; from PDB 2bcc). (Lower) The enzyme–substrate complex: ubiquinol is shown with C atoms blue, O atoms red. A putative water chain (bottom right) occupies a channel in cyt b leading from the external aqueous phase to the heme bL binding pocket and the Qo pocket. See Experimental Procedures for model building and the text for discussion. These and the native and myxothiazol- and quinone-containing structures are available as supplementary material for interactive viewing through a Chime tutorial at www.pnas.org.
Lancaster and Michel (24) have suggested that in reaction centers, stigmatellin might mimic an intermediate of the reaction cycle—either the neutral semiquinone or the quinol anion, QH−. Link (25) has proposed that in the bc1 complex, stigmatellin mimics a complex between semiquinone and ISPred. We have suggested that stigmatellin at the Qo site mimics the enzyme–substrate complex between quinol and ISPox and shows the site of its formation (26, 27). In Fig. 1 Lower, we show a model of this reaction complex, in which quinol has been inserted into the stigmatellin structure in place of the inhibitor. The second reaction partner in formation of this complex is the mobile domain (head) of the ISP (8, 26, 27). We have suggested that movement of the head between interfaces on cyt c1 and cyt b is essential for catalysis (8, 10, 26, 27). Because of this movement, the head of the ISPox acts as a second substrate (Scheme S1).
Scheme 1.
Kinetic Assays of Substrate Binding.
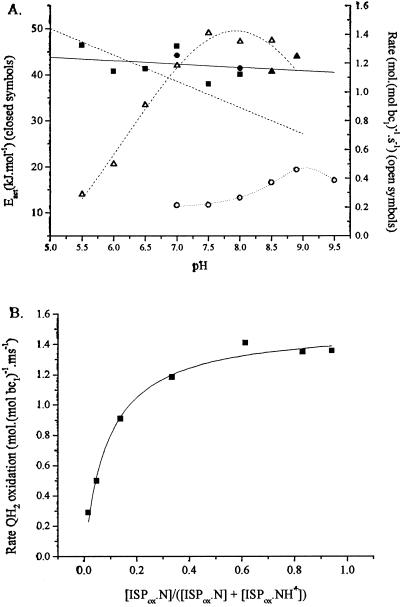
The two binding processes can be distinguished by varying one or other substrate. Since other steps are not rate limiting, the rate of quinol oxidation can be measured by following the rate of reduction of either cyt bH (at pH <8.0) or cyt bL (at pH >8.5) in the presence of antimycin, which blocks electron transfer out of the cyt b chain (15–17). For the same reason, the rate can also be assayed in the absence of inhibitor by measuring the electrogenic processes associated with electron transfer through the b-cytochrome chain, through the electrochromic carotenoid change (6, 15, 28, 29). Using any of these approaches, we can vary [QH2] through redox titration at fixed pH. We can vary [ISPox] separately by changing pH, while adjusting redox poise so as to keep the initial [QH2] constant (Fig. 2A). This latter approach depends on our suggestion that the species needed to bind in formation of the reaction complex is the dissociated ISPox (19, 27).
Figure 2.
Kinetic parameters for partial reactions. (A) Filled symbols (left-hand axis): Dependence of activation energy on pH for quinol oxidation. Each point represents a separate set of experiments in which the reaction was measured as a function of temperature and the data were analyzed with an Arrhenius plot. Reactions measured: ■, reduction of cyt bH (pH values <8.5), or ▴, reduction of cyt bL (pH values ≥8.5), with quinol pool 30% reduced; ●, slow phase of electrochromic change, with quinol pool 90% reduced. Solid line, linear fit to data; dashed line, slope of −5.7 kJ⋅mol−1. Open symbols (right-hand axis), rate of quinol oxidation: ▵, native bc1 complex (average of two experiments, assayed by cyt bH reduction); ○, mutant Y156W (at 2 × scale, assayed through slow phase of electrochromic change, quinone pool 30% reduced). (B) The dependence of rate of quinol oxidation on concentration of ISPox. Data from A for the pH dependence of cyt bH reduction have been replotted to show the variation of rate with dissociated [ISPox], calculated using a pK of 7.6 on ISPox.
For both substrates, the rate of quinol oxidation was dependent on substrate concentration. For changes in [QH2], we have previously shown that the rate saturates with the pool still partially oxidized, indicating a ≈30-fold tighter binding of QH2 than Q (15, 17). The rate as a function of the calculated concentration of dissociated ISPox ([ISPox]) is shown in Fig. 2B, and it also shows a saturation curve. The curve is shifted so that the half-maximal rate occurs at pH ≈6.4, well below the pK at 7.6. From this, it seems likely that ISPox is also preferentially bound in the reaction complex.
For neither substrate did the activation energy change with substrate concentration (Fig. 2A). We have previously shown that the activation energy was the same with the quinone pool either ≈50% reduced or initially oxidized (17). From the data it can be seen that the activation energy was the same whether the pool was 30% or 90% reduced, and whether the reaction was measured through reduction of heme bH or heme bL, or through the electrogenic reactions, and was independent of pH under all these conditions. In these experiments, because of the variation of Em of the quinone/quinol couple with pH or temperature, the Eh at each pH was adjusted so that the initial concentration of quinol in any set was the same before flash activation. Since [ISPox] varies with pH in the range below the pK, the lack of effect of pH on activation energy also shows that [ISPox] did not effect the activation barrier (Fig. 2A). These results show that the step with a high activation energy was after formation of the enzyme–substrate complex, as previously noted by Crofts and Wang (17).
Effects of Mutation.
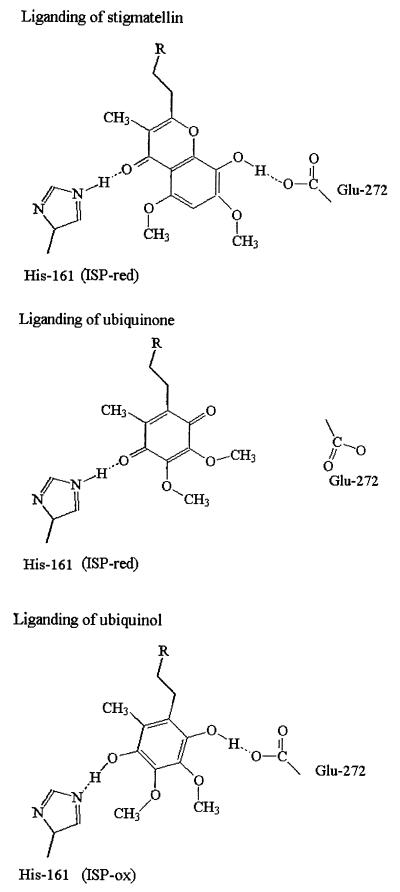
Mutations at the residue equivalent to Glu-272 in the Rb. sphaeroides bc1 complex (E295D, -G, and -Q) show an inhibited rate of quinol oxidation (ref. 30 and see Table 1), and mutants at the equivalent residue in the b6f complex (the related enzyme in oxygenic photosynthetic chains) are also inhibited (31). The E295G and E295Q mutants were most severely affected. The two more active strains (E295D and G) both showed resistance to stigmatellin (reported in ref. 3). This latter property is now explained by the liganding function identified here. Interactions between ISP and the occupant of the site have been studied through the change induced in the EPR spectrum of the 2Fe–2S center (21). We have suggested that this gx = 1.800 signal is diagnostic of complex formation between a single quinone occupant and the reduced subunit (26). In both E295G and E295Q strains, the gx = 1.800 signal appeared normal, showing that quinone could interact readily with ISPred despite the mutational change and the strongly inhibited electron transfer (Table 1). This finding suggests that although the glutamate is a ligand to stigmatellin (as seen in Fig. 1 Upper), it does not stabilize the binding of quinone. If Glu-272 provided a ligand to quinol, we might expect some effect on binding of substrate. In E295D the apparent Km was marginally greater, but in E295G, it was substantially higher (2.4-fold) than wild type, in line with a weak liganding role (Table 1). The quinol oxidation in E295Q was so inhibited that no reliable Km could be determined. On the basis of these effects, and the structural information, we propose that the ligation of stigmatellin, quinone, and quinol is as shown in Fig. 3.
Table 1.
Mutations at Glu-295
| Mutant* | gx† value | gx ampl. | Rate,‡ % | Relative Km§ | Inhibitor resist.¶ |
|---|---|---|---|---|---|
| Wild type | 1.80 | Large | 100 | 1.0 | None |
| E295D | — | — | 11 | 1.54 | Stig |
| E295G | 1.80 | Large | 4.9 | 2.4 | Stig |
| E295Q | 1.81 | Large | <4 | — | — |
Numbering from Rb. sphaeroides sequence; E295 is equivalent to E272 in chicken.
gx value; band in the EPR spectrum of the ISP, centered at 1.80 in wild type, and associated with interaction between quinone and ISPred.
Relative rate; the rate measured at Eh 110 mV, normalized to the wild type and expressed as a percentage.
Relative Km; the Km value determined from Lineweaver–Burke plots in which the concentration of QH2 was estimated from the Eh and a value for Em,pH of 90 mV. The molar concentration depends on the ratio Qtot:bc1, which is in the range 20–60, depending on assumptions. Assuming 30 Qtot:bc1, and 1 mM bc1 in the membrane, Km for wild type was 1.66 mM.
Inhibitor resistance; measured by titration of the amplitude of cyt b reduction in the presence of antimycin. Resistance to stigmatellin is indicated by Stig.
Figure 3.
Liganding of Qo-site occupants. Liganding of stigmatellin and quinone with ISPred and of quinol with ISPox. Residues are numbered as in ref. 8.
DISCUSSION
Release of the First Proton.
Rich (32), from physicochemical studies of reactions between quinols and quinones in aqueous and aprotic media, suggested that oxidation of quinol must proceed through prior dissociation to the quinol anion, QH−. Because of the high pK of the first dissociation (pK >11.3), binding of quinol would likely be accompanied by a release of the first proton through stabilization by the protein. Brandt and colleagues (33, 34) have suggested an alternative model based on the observation that the activation energy measured in a steady-state assay showed a strong pH dependence, decreasing with increase in pH up to pH >9.5. This observation was interpreted as showing that dissociation of QH2 to QH−, with the pK of the unbound quinol, was the process giving the high barrier.
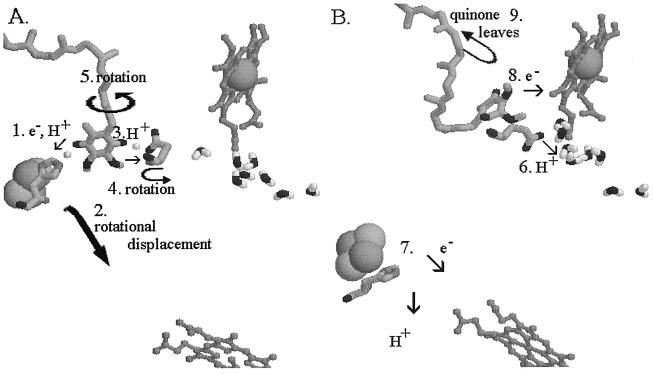
We have measured the pH dependence of the rates and activation energies of the kinetically accessible partial reactions of quinol oxidation (ref. 27, and see Fig. 2A), under conditions in which the initial concentrations of quinol and oxidant were the same (15–17, 27). In contrast to Brandt and Okun (33), we see no variation of activation energy for any of the partial reactions over the pH range 5.5 to 8.9. However, as with the mitochondrial complexes, we observed a strong dependence on pH of the rate of quinol oxidation (ref. 27 and Fig. 2)). In our experiments this was measured under single-turnover conditions (6, 15), and analysis of the partial reactions showed that oxidation of quinol (rather than of the components of the high-potential chain) was the pH-dependent step. This increase of rate with pH was observed only in the acidic range, up to pH ≈7.5 (27, 35). In view of the pK ≈7.6 on the oxidized ISP measured through redox titrations as a function of pH (19, 35), we suggested that this was a more likely candidate to account for the pH dependence than the dissociation of quinol suggested by Brandt and Okun (33), and we proposed that formation of the reaction complex must therefore require the dissociated form (19, 27). This provides a pathway for release of the first proton from quinol, as shown in the overall mechanism we propose for the Qo site in Fig. 4. Release occurs through H transfer from quinol to ISP (Fig. 4A, process 1), followed by movement of ISP extrinsic domain to the cyt c1 reaction interface (process 2), and oxidation of H⋅ISPred with release of H+ (Fig. 4B, process 7). We recognize that the H-transfer process might be complex. The slow electron transfer (k ≈ 1.5⋅103 s−1) over ≈7 Å is of interest because the high activation barrier (45–65 kJ⋅mol−1) is likely in this step. The barrier could be contributed by any combination of the separate elements (electron transfer, H+ transfer, and bond cleavage) involved. We will discuss the energy landscape of quinol oxidation in greater detail elsewhere (S.H., N.U., M.G.-K., and A.R.C., unpublished work). Snyder and Trumpower (36) have also briefly noted a possible role for dissociation of His-161 in proton processing.
Figure 4.
Mechanism proposed for reactions at the Qo site after formation of the reaction complex. (A) The reaction complex between QH2 bound in the distal domain and ISPox bound at the interface on cyt b. The small white spheres show the H atoms of the H bonds, in both of which the quinol is the donor. The protein structure is that for the stigmatellin complex. (B) The reaction complex has dissociated to products, and the ISPred has moved to a position close to cyt c1. The protein structure is that for the myxothiazol complex. Numbers indicate the sequence of reactions. In the text, italic numbers refer to the numbered processes.
To test this hypothesis, we have looked for mutant strains in which the pK on the ISPox is modified. In a set of mutants constructed at Y165 (Y156 in Rb. sphaeroides), we have identified one strain, Y156W, in which the pK on ISPox is shifted to higher pH (pK ≈8.5). The strain also showed a lowered midpoint potential (Em ≈198 mV) and a reduced rate of quinol oxidation. This mutant showed a shift in pH optimum for quinol oxidation to higher pH, as expected from our hypothesis (Fig. 2A). As in similar work with yeast bc1 complex (37), the rate of quinol oxidation among mutant strains was dependent on the Em value of the ISP. The decline in rate with decrease in Em, ISP supports the idea that the activation barrier is in this electron transfer step (17, 38).
Release of the Second Proton.
The protein structures in Fig. 4 are those from the stigmatellin-containing (Fig. 4A) or myxothiazol-containing (Fig. 4B) crystals. In these models, the inhibitor has been replaced by quinol (Fig. 4A; see also Fig. 1 Lower), or quinone (Fig. 4B), constrained to the volume of the inhibitor-occupied site. As discussed above, it seems likely that stigmatellin might mimic the binding of both quinone (through the carbonyl H bond to His-161) and of quinol (through the hydroxyl H bond to Glu-272), as shown in Fig. 3. This possibility suggests a pathway for release of the second proton from the site. We propose that the H bond between Glu-272 and the stigmatellin -OH involves the dissociated glutamate and mimics a similar H bond formed on binding quinol. Release of the second proton would then involve H+ transfer to Glu-272 to form the neutral acid (Fig. 4A, process 3), followed by rotation of the side chain (process 4), and release of the proton (Fig. 4B, process 6). Because, in the stigmatellin structure, the Glu-272 side chain constricts the proximal domain of the Qo site (see Fig. 1 Upper), occupancy of this domain by inhibitor requires rotation of the side chain out of the site (process 4), as shown in Fig. 4B. If the semiquinone must move into the proximal domain before oxidation (process 5) as we have suggested (8, 26, 27), then rotation of the side chain would also have to occur before this could happen. We suggest that before rotation, the side chain must be protonated by dissociation of the neutral semiquinone to form the semiquinone anion, which would move to the proximal domain. The semiquinone anion would then be the species donating an electron to heme bL (process 8). Because movement of neither species includes a substantial vectorial displacement across the low-dielectric phase, the electrogenic contribution would be minimal, as seen experimentally (28). The structures show that rotation of the side chain to the “out” position brings the polar group to the surface of a channel into cyt b from the aqueous phase (process 6), which also connects to the heme bL propionates. It seems likely that this channel contains a water chain to facilitate transfer of the proton from Glu-272 to the exterior, as suggested in ref. 39 and shown by the waters modeled in Figs. 1 Lower and 4. Support for a role of Glu-272 in proton processing also comes from the work from Joliot’s lab on mutants in Chlamydomonas at the equivalent residue, which were modified in electron transfer, and in the kinetics of electrogenic events measured through the electrochromic response (31).
Supplementary Material
Acknowledgments
We thank Prof. P. L. Dutton and H. Ding for help with EPR spectroscopy of the Glu-295 mutants. We acknowledge with gratitude the support for this research provided by National Institutes of Health Grants GM 35438 (to A.R.C.) and DK 44842 (to E.A.B.) and by the Office of Health and Environmental Research, U.S. Department of Energy, under contract DE-AC03-76SF00098 (E.A.B.).
ABBREVIATIONS
- bc1 complex
ubihydroquinone:cytochrome c oxidoreductase (EC 1.10.2.2)
- bL and bH
low-potential and higher-potential hemes of cytochrome b
- cyt
cytochrome
- ISP
Rieske-type iron–sulfur protein
- ISPox and ISPred
oxidized and reduced forms of the ISP
- MOA-stilbene
substituted stilbene with β-methoxyacrylate as the pharmacologically active group
- Q
quinone form of ubiquinone-10
- QH2
hydroquinone, or quinol, form of ubiquinone-10
- Qi site
quinone-reducing site of the bc1 complex
- Qo site
hydroquinone-oxidizing site of the bc1 complex
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The atomic coordinates for the chicken mitochondrial bc1 complex have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID codes: native, 1bcc; with stigmatellin, 2bcc; with stigmatellin and antimycin, 3bcc).
References
- 1.Cramer W A, Soriano G M, Ponomarev M, Huang D, Zhang H, Martinez S E, Smith J L. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:477–508. doi: 10.1146/annurev.arplant.47.1.477. [DOI] [PubMed] [Google Scholar]
- 2.Nitschke W, Muehlenhoff U, Liebl U. In: Photosynthesis: A Comprehensive Treatise. Raghavendra A, editor. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 285–304. [Google Scholar]
- 3.Brasseur G, Sami Saribas A, Daldal F. Biochim Biophys Acta. 1996;1275:61–69. doi: 10.1016/0005-2728(96)00051-5. [DOI] [PubMed] [Google Scholar]
- 4.Degli Esposti M, De Vries S, Crimi M, Ghelli A, Patarnello T, Meyer A. Biochim Biophys Acta. 1993;1143:243–271. doi: 10.1016/0005-2728(93)90197-n. [DOI] [PubMed] [Google Scholar]
- 5.Brandt U, Trumpower B L. CRC Crit Rev Biochem. 1994;29:165–197. doi: 10.3109/10409239409086800. [DOI] [PubMed] [Google Scholar]
- 6.Crofts A R, Wraight C A. Biochim Biophys Acta. 1983;726:149–186. [Google Scholar]
- 7.Xia D, Yu C-A, Kim H, Jia-Zhi Xia J-Z, Kachurin A M, Zhang L, Yu L, Deisenhofer J. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Huang L-S, Shulmeister V M, Chi Y-I, Kim K-K, Hung L-W, Crofts A R, Berry E A, Kim S-H. Nature (London) 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 9.Iwata S, Lee J W, Okada K, Lee J K, Iwata M, Rasmussen B, Link T A, Ramaswamy S, Jap B K. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 10.Crofts A R, Berry E A. Curr Opin Struct Biol. 1998;8:501–509. doi: 10.1016/s0959-440x(98)80129-2. [DOI] [PubMed] [Google Scholar]
- 11.Ljungdahl P O, Pennoyer J D, Robertson D E, Trumpower B L. Biochim Biophys Acta. 1987;891:227–241. doi: 10.1016/0005-2728(87)90218-0. [DOI] [PubMed] [Google Scholar]
- 12.Andrews K M, Crofts A R, Gennis R B. Biochemistry. 1990;29:2645–2651. doi: 10.1021/bi00463a004. [DOI] [PubMed] [Google Scholar]
- 13.Guergova-Kuras M, Salcedo-Hernandez R, Bechmann G, Kuras R, Gennis R B, Crofts A R. Protein Expression Purif. 1998;15:370–380. doi: 10.1006/prep.1998.1018. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P. J Theor Biol. 1976;62:327–367. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 15.Crofts A R, Meinhardt S W, Jones K R, Snozzi M. Biochim Biophys Acta. 1983;723:202–218. doi: 10.1016/0005-2728(83)90120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crofts A R. In: The Enzymes of Biological Membranes. Martonosi A N, editor. Vol. 4. New York: Plenum; 1985. pp. 347–382. [Google Scholar]
- 17.Crofts A R, Wang Z. Photosynth Res. 1989;22:69–87. doi: 10.1007/BF00114768. [DOI] [PubMed] [Google Scholar]
- 18.Surles M, Richardson J, Richardson D, Brooks F. Protein Sci. 1994;3:198–210. doi: 10.1002/pro.5560030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugulava N, Crofts A R. FEBS Lett. 1998;440:409–413. doi: 10.1016/s0014-5793(98)01493-8. [DOI] [PubMed] [Google Scholar]
- 20.Yun C-H, Wang Z, Crofts A R, Gennis R B. J Biol Chem. 1992;267:5901–5909. [PubMed] [Google Scholar]
- 21.Ding H, Robertson D E, Daldal F, Dutton P L. Biochemistry. 1992;31:3144–3158. doi: 10.1021/bi00127a015. [DOI] [PubMed] [Google Scholar]
- 22.Iwata S, Saynovits M, Link T A, Michel H. Structure. 1996;4:567–579. doi: 10.1016/s0969-2126(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 23.Kuila D, Schoonover J R, Dyer R B, Batie C J, Ballou D, Fee J A, Woodruff W H. Biochim Biophys Acta. 1992;1140:175–183. doi: 10.1016/0005-2728(92)90007-o. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster C R, Michel H. Structure. 1997;5:1339–1359. doi: 10.1016/s0969-2126(97)00285-2. [DOI] [PubMed] [Google Scholar]
- 25.Link T A. FEBS Lett. 1997;412:257–264. doi: 10.1016/s0014-5793(97)00772-2. [DOI] [PubMed] [Google Scholar]
- 26.Crofts A R, Barquera B, Gennis R B, Kuras R, Guergova-Kuras M, Berry E A. In: The Phototrophic Prokaryotes. Peschek G A, Loeffelhardt W, Schmetterer G, editors. New York: Plenum; 1999. pp. 229–239. [Google Scholar]
- 27.Crofts A R, Berry E A, Kuras R, Guergova-Kuras M, Hong S, Ugulava N. In: Photosynthesis: Mechanisms and Effects. Garab G, editor. Vol. 3. Dordrecht, The Netherlands: Kluwer; 1999. pp. 1481–1486. [Google Scholar]
- 28.Glaser E G, Crofts A R. Biochim Biophys Acta. 1984;766:322–333. doi: 10.1016/0005-2728(84)90248-2. [DOI] [PubMed] [Google Scholar]
- 29.Venturoli G, Fernandez-Velasco J G, Crofts A R, Melandri B A. Biochim Biophys Acta. 1988;935:258–272. doi: 10.1016/0005-2728(86)90070-8. [DOI] [PubMed] [Google Scholar]
- 30.Crofts A R, Barquera B, Bechmann G, Guergova M, Salcedo-Hernandez R, Hacker B, Hong S, Gennis R B. In: Photosynthesis: From Light to Biosphere. Mathis P, editor. Vol. 2. Dordrecht, The Netherlands: Kluwer; 1995. pp. 493–500. [Google Scholar]
- 31.Zito F, Finazzi G, Joliot P, Wollman F-A. Biochemistry. 1998;37:10395–10403. doi: 10.1021/bi980238o. [DOI] [PubMed] [Google Scholar]
- 32.Rich P R. Biochim Biophys Acta. 1981;637:28–33. [Google Scholar]
- 33.Brandt U, Okun J G. Biochemistry. 1997;36:11234–11240. doi: 10.1021/bi970968g. [DOI] [PubMed] [Google Scholar]
- 34.Brandt U. Biochim Biophys Acta. 1998;1365:261–268. doi: 10.1016/s0005-2728(98)00078-4. [DOI] [PubMed] [Google Scholar]
- 35.Link T A. Biochim Biophys Acta. 1994;1185:81–84. [Google Scholar]
- 36.Snyder C, Trumpower B L. Biochim Biophys Acta. 1998;1365:125–134. doi: 10.1016/s0005-2728(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 37.Denke E, Merbitzzahradnik T, Hatzfeld O M, Snyder C H, Link T A, Trumpower B L. J Biol Chem. 1998;273:9085–9093. doi: 10.1074/jbc.273.15.9085. [DOI] [PubMed] [Google Scholar]
- 38.Van Doren S R, Gennis R B, Barquera B, Crofts A R. Biochemistry. 1993;32:8083–8091. doi: 10.1021/bi00083a005. [DOI] [PubMed] [Google Scholar]
- 39.Izrailev, S., Crofts, A. R., Berry, E. A. & Schulten, K. (1999) Biophys. J., in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.