Abstract
BACKGROUND: Pre-emptive pancreas-kidney transplanttation is increasingly considered the best therapy for irreversible chronic kidney disease (CKD) in type 1 diabetics. However, the best approach in the wait for transplantation has not yet been defined. AIM: To evaluate our experience with a low-protein (0.6 g/kg/day) vegetarian diet supplemented with alpha-chetoanalogues in type 1 diabetic patients in the wait for pancreas-kidney transplantation. METHODS: Prospective study. Information on the progression of renal disease, compliance, metabolic control, reasons for choice and for drop-out were recorded prospectively; the data for the subset of patients who underwent the diet while awaiting a pancreas-kidney graft are analysed in this report. RESULTS: From November 1998 to April 2004, 9 type 1 diabetic patients, wait-listed or performing tests for wait-listing for pancreas-kidney transplantation, started the diet. All of them were followed by nephrologists and diabetologists, in the context of integrated care. There were 4 males and 5 females; median age 38 years (range 27.9-45.5); median diabetes duration 23.8 years (range 16.6-33.1), 8/9 with widespread organ damage; median creatinine at the start of the diet: 3.2 mg/dl (1.2-7.2); 4 patients followed the diet to transplantation, 2 are presently on the diet, 2 dropped out and started dialysis after a few months, 1 started dialysis (rescue treatment). The nutritional status remained stable, glycemia control improved in 4 patients in the short term and in 2 in the long term, no hyperkalemia, acidosis or other relevant side effect was recorded. Proteinuria decreased in 5 cases, in 3 from the nephrotic range. Albumin levels remained stable; the progression rate was a loss of 0.47 ml/min of creatinine clearance per month (ranging from an increase of 0.06 to a decrease of 2.4 ml/min) during the diet period (estimated by the Cockroft-Gault formula). CONCLUSIONS: Low-protein supplemented vegetarian diets may be a useful tool to slow CKD progression whilst awaiting pancreas-kidney transplantation.
Keywords: diabetes, CKD, low-protein diet, alpha-chetoanalogues, renal clearances, compliance, pre-emptive transplantation
Introduction
Pre-emptive pancreas-kidney transplantation is increasingly considered the best therapy for irreversible kidney damage in type 1 diabetes mellitus; the progressive improvements have led to an ever more precocious wait-listing of type 1 diabetic patients [1, 2].
Several transplant centers accept on the waiting list patients with serum creatinine above 2 mg/dl (or even at lower levels) in the presence of nephrotic syndrome [3]. In these cases, the support therapy for the renal disease is usually considered as playing a minor role, since transplantation is seen as the resolution of the clinical problem. However, a good metabolic balance is important whilst awaiting transplantation and stabilization of the clinical situation is of obvious clinical relevance, especially in cases with full-blown nephrotic syndrome.
Despite these encouraging early transplant options, referral to the nephrologist is still commonly performed at advanced stages of renal failure [4-6], and diabetic patients are often seen shortly before they need to start dialysis. In these cases, gaining a few months of time may allow the patients to undergo kidney-pancreas transplantation and thus to skip the dialysis phase, with very positive effects on the quality of life and possibly also on the clinical conditions; indeed, death rates are markedly increased after the start of renal replacement therapy (RRT) [3, 5, 6].
Nevertheless, the best approach whilst awaiting kidney-pancreas transplantation has not yet been defined. While the universal measures, such as good glycemia control, correction of dyslipidemia, strict control of hypertension, discontinuation of smoking or other voluptuary habits, are advocated in all cases, the role of diet (a more specific nephrological tool) is controversial in the context of diabetic nephropathy management, mainly for the fear of malnutrition [7-10].
A systematic review on the Cochrane library was favorable towards low-protein diets in diabetic patients with advanced kidney damage, but also stated that the level of available evidence is relatively low and the number of patients reported is still limited; this can be seen as an indirect reflection of the perplexities in clinical settings about low-protein diets in diabetic patients. Furthermore, the level of protein restriction, the acceptable compliance level in clinical practice and the long-term outcomes remain to be defined [11].
Lastly, the studies cited in the review were performed before the widening of indications for pre-emptive transplantation; indeed, the presence of alternative endpoints and goals may change the attitude towards prescription of a diet, i.e. no longer as a tool to slow progression to dialysis but as a means to stabilize the clinical conditions whilst awaiting transplantation.
The "menu" of diets available in CKD is quite varied, ranging from minimal protein restriction (0.8 g/kg/day), usually requiring a different distribution of quantity and quality of usual food during the day, to very complex "artificial" diets (0.3 g/kg/day of proteins), combining the use of aprotic food with a vegetarian regimen and essential amino or chetoacid supplementation; the compromise being diets at 0.6 g/kg/day of proteins, attainable either with the use of at least some aprotic foods or supplementing a vegetarian diet with essential aminoacids or chetoanalogues [11-13].
In this paper, we describe our experience with the implementation of a well-known concept (low-protein, supplemented vegetarian diet, supplying 0.6 g/kg/day of proteins, without need for aprotic foods, in type 1 diabetic patients with chronic kidney disease [12, 13]), in a new setting (patients wait-listed or being evaluated for wait-listing for kidney-pancreas transplantation).
Patients and methods
Setting of the study
The study was performed in the Outpatient Units and Day Hospital of the Chair of Nephrology, University of Turin, Italy. The center runs an outpatient network for CKD patients with early referral criteria [14, 15]. Diabetic patients are followed in strict and co-ordinated co-operation with the Diabetic Care Units of the University Hospital. In the period 1998-2004, our unit followed about 50 type 1 diabetic patients at different stages of CKD.
Choice of diet
The option of an alpha-chetoanalogue-supplemented low-protein vegetarian diet [12, 13] is proposed to all patients with progressive renal disease and/or with nephrotic syndrome not responsive to the usual therapies (ACE-inhibitors, ATII receptor-inhibitors or their association). In fact, there are three therapeutic options: no diet; conventional low-protein diet (usually requiring protein-free substitutes); low-protein vegetarian diet with alpha-chetoanalogues. The last consists of a vegetarian diet with a protein intake of 0.6 g/kg/day, supplemented with alpha-chetoanalogues (1-2 pills per 10 kg of body weight). The diet has a low phosphate content; calories and sodium, vitamin D, folic acid and erythropoietin prescription are individually tailored. Calcium, vitamin B12 and iron supplementation are routinely added [12, 13].
At least one free meal (and occasionally up to three) is allowed to improve long-term compliance. The control schedule includes at least one clinical control, with blood tests, every other month; in late CKD phases, controls are intensified up to once weekly.
Clinical data
Comorbidities, reasons for choice and for drop-out, nutritional status (subjective global assessment, SGA) and functional score (Karnofsky scale) were evaluated at the start of the diet and at the end of the diet period; all scores and parameters were recorded by the usual nephrologist caregiver (GBP) [16, 17].
Laboratory parameters
All biochemical parameters considered were assessed in the same settings (Laboratory of the Chair of Nephrology and General Laboratory of the University Hospital). Creatinine was assessed by the Jaffe rate method (variation coefficient 1.9%). BUN (blood urea nitrogen) was analysed by the enzymatic (urease) method; the coefficient of variation, according to the urea level, ranged from 1.9% at 114 mg/dl to 9% at 14 mg/dl. Clearances were calculated by the Cockroft and MDRD formula and based on 24 hours of urine collection [18]. Compliance was evaluated from urinary BUN, according to the Mitch formula (nitrogen intake was calculated as urea nitrogen appearance plus a non-urea nitrogen value of 0.031 g nitrogen/kg body weight) [19]. Total urinary proteins were measured by Pyrogallol Red-Molybdate at acidic pH (2.5).
Statistical analysis
The patient data were recorded in Excel and transferred to SPSS Inc., Chicago (version 11.5), for analysis. Descriptive analysis was performed as appropriate (median and range in the case of non-parametric data, mean and standard deviation in the case of a parametric distribution). The progression rate was analysed as loss of ml/min of creatinine clearance per month.
Results
The cases
Table 1 shows the main clinical and demographic characteristics of the patients who underwent at least one trial of the alpha-chetoanalogue-supplemented low-protein vegetarian diet whilst awaiting kidney-pancreas transplantation.
Table 1. Main clinical and biochemical data at baseline and at referral (patients are sorted by the start of the diet).
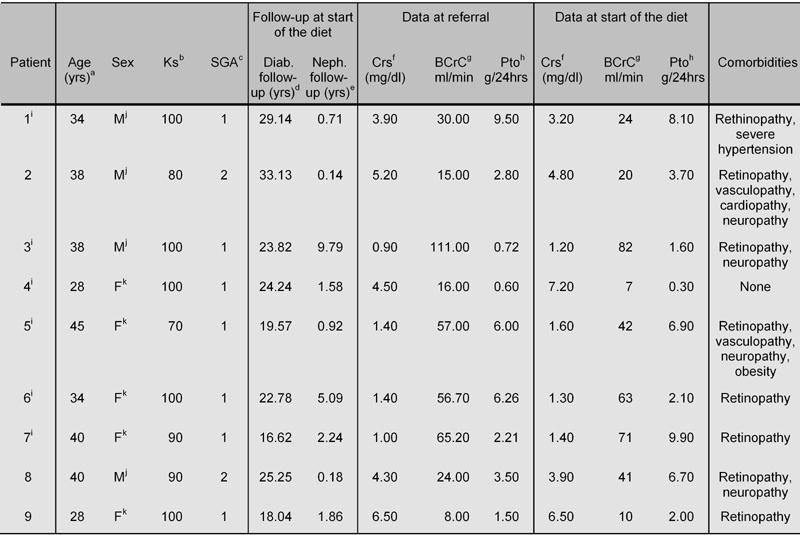
a Age at start of the diet. b Functional score (Karnofsky). c Nutritional score (Subjective Global Assessment). d Diabetes follow-up at the start of the diet. e Nephrology follow-up at the start of the diet. f Serum creatinine. g Blood creatinine clearance. h Proteinuria. i Renal biopsy. j Male. k Female.
The presence of diabetes-related comorbidity was the rule (neuropathy, severe visual impairment, vascular disease); only one patient was spared, and her renal disease was an uncommon one (non-hypertensive nephrosclerosis) without significant pathological signs of diabetic nephropathy. Diabetic nephropathy was the cause of renal disease in all the other cases. A kidney biopsy was performed in 6 cases (5 diabetic nephropathy, 1 nephrosclerosis).
The duration of diabetes was long in all patients (median 23.8, range 16.6-33.1 years). Referral occurred at different stages of CKD (at referral, serum creatinine: median 3.9, range 0.9-6.5 mg/dl; at the start of the diet: median 3.2, range 1.2-7.2 mg/dl); therefore, the duration of regular nephrological follow-up before the start of the diet was highly scattered (median 1.58, range 0.02-9.79 years).
Reasons for choice, reasons for drop-out and diet discontinuation
Medical advice was the main reason for choosing this diet, mainly in the hope of avoiding dialysis; the possibility of eating "normal" bread and pasta and the simplicity of the diet (avoiding some foods and not having to weigh others) were other reasons for choosing this (Table 2).
Table 2. Reasons for choice and for diet discontinuation, and outcome.
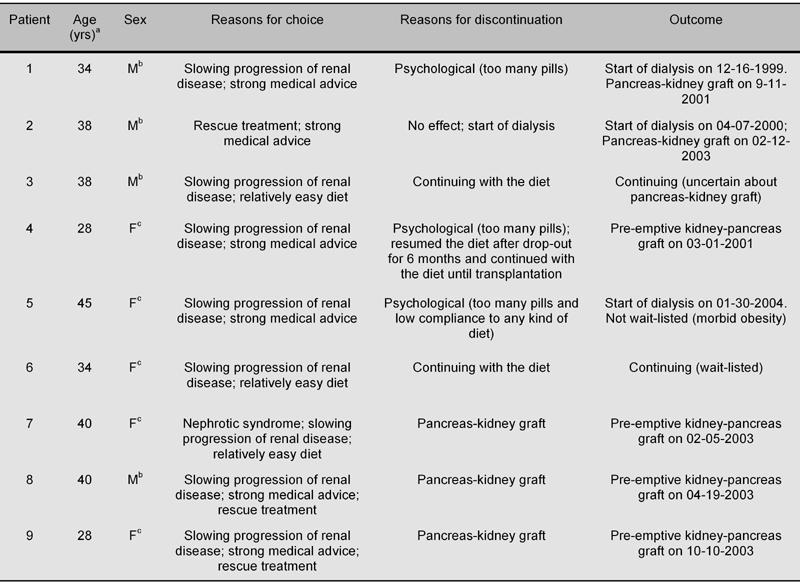
a Age at start of the diet. b Male. c Female.
The reason for drop-out was psychological in the 3 patients who discontinued the diet, i.e. the need to take many more "pills" every day. One of them, however, resumed the diet as a rescue treatment and was successfully grafted (kidney-pancreas graft) after approximately 6 months.
Another patient, presently wait-listed for a kidney-pancreas graft, had a baby during the diet period. Although she continued with moderate protein restriction (0.8 g/kg/day, supplemented with alpha-chetoanalogues) as a means of reducing the hyperfiltration state of pregnancy, the biochemical and functional data of the pregnancy period were not considered in the present analysis. No clinical side effect was a main or concomitant reason for discontinuation (Table 2).
Progression of renal disease and pattern of proteinuria
The diet achieved the aim of allowing the patient to arrive at pancreas-kidney transplantation without prior dialysis in 4 cases, and is still being followed in another 2 cases: one patient is wait-listed for a pancreas-kidney graft, while the second interrupted the wait-list tests because he was well adapted to the diet and preferred to continue with this therapy. The diet was ineffective in only one case, a rescue treatment in which the diet was started at a very late stage of CKD (Table 1) while a vascular access for hemodialysis was being prepared; it was quickly discontinued due to the occurrence of a full-blown uremic syndrome.
The patterns of proteinuria and of serum creatinine are reported in Figures 1 and 2. The data are scattered, reflecting the usual clinical heterogeneity of patients with chronic kidney disease.
Figure 1.
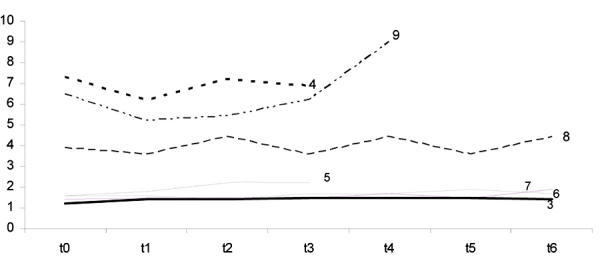
Serum Creatinine level at different time intervals (up to 1 year) in 7 patients with follow-up of at least 4 months on a low protein, supplemented vegetarian diet. Ordinate: serum creatinine (sCr mg/dl). t0: baseline. Each t point: controls at 2 months interval. Patient numbers correspond to numbers in tables.
Figure 2.
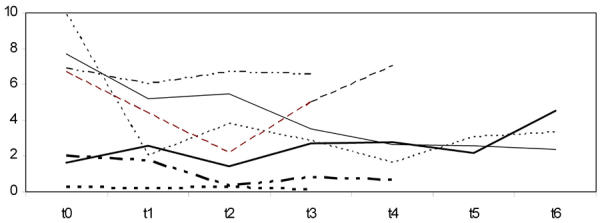
Proteinuria (g/24 hours) at different time intervals in 7 patients with follow-up of at least 4 months on a low protein, supplemented vegetarian diet. Ordinate: Proteinuria (Pto g/24 hr). t0 = baseline. Each t point: controls at 2 months interval. Patient numbers correspond to numbers in tables.
When only the patients who had followed the diet for at least 4 months (7 cases) were considered, the median progression rate was a loss of 0.51 ml/min of creatinine clearance per month (ranging from an increase of 0.33 to a decrease of 1.13 ml/min), based on 24 hours urine collection and a loss of 0.47 ml/min of creatinine clearance per month (ranging from an increase of 0.06 to a decrease of 2.4 ml/min) by the Cockroft and Gault formula.
Proteinuria showed a positive response to the diet, with a non-significant decreasing trend (median at baseline 2.5 g/24h, range 0.3-9.9, versus 2.1 g/24h, range 0.3-6.6, at the second control p = 0.128), although there were wide swings, occasionally concomitant with impairment-improvement of diabetes control (Figure 2).
Compliance, metabolic control and support therapy
Six of the nine patients were considered as cases with very low compliance (less than 50% of the drugs regularly taken); despite this, the diet was successfully continued in two and resumed in one.
In the 7 patients on the diet for at least 4 months, the protein intake (calculated with the Mitch formula) was 0.75 g/kg/day (range 0.49-1.92) at the first control, 0.88 (range 0.56-1.13) at the second, and 0.71 (range 0.58-0.95) at the last control.
Support therapy included ACE-inhibitors in 6 cases, ATII receptor inhibitors in 4 cases, and both of them in 4 cases, while 3 patients were on anti-dyslipidemia agents.
None of the feared side effects (impaired diabetes control, hyperkalemia and malnutrition) occurred during the diet period in these patients. No patient was severely malnourished; the Karnofsky and SGA scores remained stable in all cases (Table 1).
The main metabolic data (HbA1c, serum albumin and total proteins, cholesterol and triglycerides) at the different time intervals are reported in Table 3.
Table 3. Biochemical data at baseline and during follow-up.
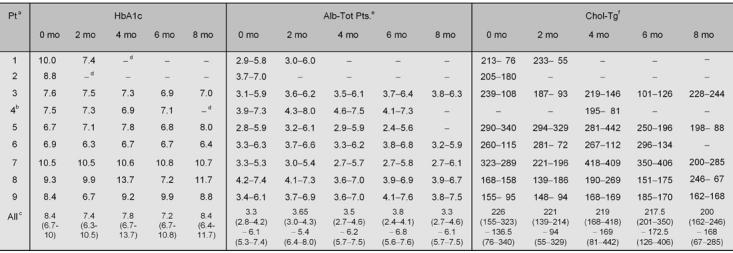
a Patient. b The second, longer period of diet was considered. c Median (range). d Patient retired. e Serum albumin and total proteins. f Cholesterol-triglycerides
Discussion
About twenty years after the Saint Vincent declaration, which set the ambitious goal of preventing CKD in diabetic patients, CKD is still an unmet challenge in this population [20-22]. In the meantime, the advances in pancreas-kidney and pancreas transplantation have profoundly changed the approach to younger type 1 diabetics: nephrological care can now be focused on the best timing and support in the wait for transplantation instead of on slowing the progression towards end-stage renal disease as much as possible [1-3].
However, this new approach has raised new questions and uncertainties about the role and goals of CKD therapy in type 1 diabetic patients. Most of the therapies commonly used for CKD have multiple goals: anti-hypertensives and anti-dyslipidemia agents are used not only to slow CKD progression, but also to prevent cardiovascular impairment; optimal diabetic care is used to slow all forms of progressive end-organ damage (ocular, nervous and vascular, as well as renal). In contrast, diet is usually considered a "pure" nephrological therapy and its role can be a matter of criticism if a kidney graft is being considered, mainly because of the fear of malnutrition [7-11].
However, this minimalist attitude may fail to take into account at least two main points: the first is that patients are still often referred relatively late for nephrological follow-up, while the second is the importance of diet as a metabolic stabilizer and not only in slowing renal function [12, 13].
Both points are exemplified by the case series reported here, that evaluated the possibility of implementing a low protein vegetarian diet, supplemented with alpha-chetoanalogues in the particular subset of patients awaiting pancreas-kidney transplantation. In fact, 4/9 patients were referred to the nephrological team at a late stage of CKD, when gaining a few months of renal function may actually allow the patient to avoid dialysis. Interestingly, in three of these cases, serum creatinine decreased after the start of the diet and stabilized thereafter; all three patients received a pre-emptive pancreas-kidney graft. Even though a general conclusion cannot be drawn from so few cases, these data are in keeping with the suggestion of a positive haemodynamic effect of a supplemented vegetarian diet on the failing kidney vasculature [23].
The diet was followed by 6/9 patients, with relatively good compliance: in the 7 patients on the diet for at least 4 months, the protein intake was 0.75 g/kg/day at the first control, and 0.71 at the last control, a reasonable compromise between a normoprotein diet and the 0.6 g/kg/day prescription. An interesting aspect suggested by these data is the gradual compliance, mostly evident in a patient whose daily protein intake decreased progressively from 1.92 to 1.01 to 0.95 g/kg/day, probably motivated by a decreasing 24h proteinuria (from 6.7 to 4.4 to 2.2 g/day). No important side effects occurred and glycaemia control was practically unaffected by the diet (Table 3). The main problems encountered by the patients were psychological, linked to the need to add several pills to the already complex therapeutic regimen; this was the cause of drop-out in three cases (although one patient resumed the diet six months later). However, the possibility to employ "normal" non-aproteic carbohydrates, without the need for weighing food was highly appreciated by the patients.
In such a small subset of patients, the analysis of the progression rate can only give an approximate indication of the trends, allowing us only to put the data in a written context; within these limits, the median progression rate with Cockroft Gault formula was a loss of 0.47 ml/min of creatinine clearance per month during the diet period.
It is somewhat difficult to put these data in the context of literature reports: in fact, these patients are the result of strong negative selection (4/9), since they were referred late for nephrological care or wait-listed for a pre-emptive graft, in all cases following the clinical impression of an aggressive and progressive disease, with severe and widespread end-organ damage. However, the data are within the "best" range reported for cases with late nephro-diabetological referral [24].
An interesting, albeit insignificant, trend was observed for proteinuria, which decreased in 5 cases with at least 2 months of follow-up. This effect, usually reported with very low-protein diets, may play a very important role in the management of advanced diabetic nephropathy, and indeed is one of the therapeutic targets in slowing CKD progression [23].
In conclusion, within the limits shared by all small non-randomised studies, this report suggests that a low-protein vegetarian diet supplemented with alpha-chetoanalogues may be well tolerated and devoid of serious side effects also in type 1 diabetic patients wait-listed for pancreas kidney transplantation: the availability of different therapeutic armamentaria may be of great importance in this critical phase for tailoring therapy, thus increasing the patients' chances of avoiding dialysis treatment.
Acknowledgments
Thanks to Dr. Peter Christie, for his careful linguistic revision.
References
- 1.Stratta RJ, Taylor RJ, Ozaki CF, Bynon JS, Miller SA, Knight TF, Fischer JL, Neumann TV, Wahl TO, Duckworth WC. A comparative analysis of results and morbidity in type I diabetics undergoing pre-emptive versus postdialysis combined pancreas-kidney transplantation. Transplantation. 1993;55:1097–1103. doi: 10.1097/00007890-199305000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Sudan D, Sudan R, Stratta R. Long-term outcome of simultaneous kidney-pancreas transplantation: analysis of 61 patients with more than 5 years follow-up. Transplantation. 2000;69:550–555. doi: 10.1097/00007890-200002270-00015. [DOI] [PubMed] [Google Scholar]
- 3.Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation. 2000;70:1736–1746. doi: 10.1097/00007890-200012270-00013. [DOI] [PubMed] [Google Scholar]
- 4.Kessler M, Frimat L, Panescu V, Briancon S. Impact of nephrology referral on early and midterm outcomes in ESRD: EPidemiologie de l'Insuffisance REnale chronique terminale en Lorraine (EPIREL): results of a 2-year, prospective, community-based study. Am J Kidney Dis. 2003;42:474–485. doi: 10.1016/s0272-6386(03)00805-9. [DOI] [PubMed] [Google Scholar]
- 5.Chantrel F, Enache I, Bouiller M, Kolb I, Kunz K, Petitjean P, Moulin B, Hannedouche T. Abysmal prognosis of patients with type 2 diabetes entering dialysis. Nephrol Dial Transplant. 1999;14:129–136. doi: 10.1093/ndt/14.1.129. [DOI] [PubMed] [Google Scholar]
- 6.Stoves J, Bartlett CN, Newstead CG. Specialist follow up of patients before end stage renal failure and its relationship to survival on dialysis. Postgrad Med J. 2001;77:586–588. doi: 10.1136/pmj.77.911.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey JN. Diabetic nephropathy. BMJ. 2002;325:59–60. doi: 10.1136/bmj.325.7355.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupisti A, Licitra R, Chisari C, Stampacchia G, D'Alessandro C, Galetta F, Rossi B, Barsotti G. Skeletal muscle and nutritional assessment in chronic renal failure patients on a protein-restricted diet. J Intern Med. 2004;255:115–124. doi: 10.1046/j.0954-6820.2003.01245.x. [DOI] [PubMed] [Google Scholar]
- 9.Aparicio M, Chauveau P, De Precigout V, Bouchet JL, Lasseur C, Combe C. Does nutrition affect the prognosis of aged hemodialysis patients? Nephrologie. 2002;23:77–83. [PubMed] [Google Scholar]
- 10.Kopple JD, Berg R, Houser H, Steinman TI, Teschan P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int. 1989;27:S184–S194. [PubMed] [Google Scholar]
- 11.Waugh NR, Robertson AM. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev 2000. 2:CD002181. 2000. [DOI] [PubMed]
- 12.Barsotti G, Navalesi R, Giampietro O, Ciardella F, Morelli E, Cupisti A, Mantovanelli A, Giovannetti S. Effects of a vegetarian, supplemented diet on renal function, proteinuria, and glucose metabolism in patients with 'overt' diabetic nephropathy and renal insufficiency. Contrib Nephrol. 1988;65:87–94. doi: 10.1159/000415752. [DOI] [PubMed] [Google Scholar]
- 13.Barsotti G, Ciardella F, Morelli E, Cupisti A, Mantovanelli A, Giovannetti S. Nutritional treatment of renal failure in type 1 diabetic nephropathy. Clin Nephrol. 1988;29:280–287. [PubMed] [Google Scholar]
- 14.Piccoli GB, Grassi G, Mezza E, Gai M, Iacuzzo C, Bechis F, Biancone L, Jeantet A, Dani F, Cavallo Perin P, Segoloni GP. Early referral of Type 2 diabetic patients: are we ready for the assault? Nephrol Dial Transplant. 2002;17:1241–1247. doi: 10.1093/ndt/17.7.1241. [DOI] [PubMed] [Google Scholar]
- 15.Piccoli GB, Soragna G, Mezza E, Putaggio S, Motta D, Gai M, Anania P, Fop F, Bermond F, Burdese M et al. Lost to follow-up: the bottleneck of early referral to renal units? Med Sci Monit. 2003;9:493–499. [PubMed] [Google Scholar]
- 16.Laszlo J. In: Wyngaarden JB, Smith LH Jr (eds). Cecil Textbook of Medicine. WB Saunders, PA: 1988. Oncology; pp. 1082–1089. [Google Scholar]
- 17.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr. 1987;11:8–13. doi: 10.1177/014860718701100108. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 19.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 20.Viberti GC, Mogensen CE, Passa P, Bilous R, Mangili R. In: Mogensen CE (ed). Kluwer Academic Publishers. Boston: 1994. St Vincent Declaration, 1994. Guidelines for the prevention of diabetic renal failure in the kidney and hypertension in diabetes mellitus. [Google Scholar]
- 21.Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 22.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–S85. [PubMed] [Google Scholar]
- 23.Teschan PE, Beck GJ, Dwyer JT, Greene T, Klahr S, Levy AS, Mitch WE, Snetselaar LG, Steinman TI, Walser M. Effect of a ketoacid-aminoacid-supplemented very low protein diet on the progression of advanced renal disease: a reanalysis of the MDRD feasibility study. Clinical Nephrol. 1998;50:273–283. [PubMed] [Google Scholar]
- 24.Joss N, Paterson KR, Deighan CJ, Simpson K, Boulton-Jones JM. Diabetic nephropathy: how effective is treatment in clinical practice? Q J Med. 2002;95:41–49. doi: 10.1093/qjmed/95.1.41. [DOI] [PubMed] [Google Scholar]


