Abstract
Orally ingested glucose leads to a greater insulin response compared to intravenously administered glucose leading to identical postprandial plasma glucose excursions, a phenomenon referred to as the "incretin effect". The incretin effect comprises up to 60% of the postprandial insulin secretion and is diminished in type 2 diabetes. One of the very important gastrointestinal hormones promoting this effect is glucagon-like peptide 1 (GLP-1). It only stimulates insulin secretion and normalizes blood glucose in humans under hyperglycemic conditions, therefore it does not cause hypoglycemia. Other important physiological actions of GLP-1 are the inhibition of glucagon secretion and gastric emptying. It further acts as a neurotransmitter in the hypothalamus stimulating satiety. In vitro and animal data demonstrated that GLP-1 increases β-cell mass by stimulating islet cell neogenesis and by inhibiting apoptosis of islets. In humans, the improvement of β-cell function can be indirectly observed from the increased insulin secretory capacity after GLP-1 infusions. GLP-1 represents an attractive therapeutic principle for type 2 diabetes. However, native GLP-1 is degraded rapidly upon exogenous administration and is therefore not feasible for routine therapy. The first long-acting GLP-1 analog ("incretin mimetic") Exenatide (Byetta®) has just been approved for type 2 diabetes therapy. Other compounds are being investigated in clinical trials (e.g. liraglutide®, CJC1131®). Dipeptidyl-peptidase IV inhibitors (DPP-IV inhibitors; e.g. Vildagliptin®, Sitagliptin®) that inhibit the enzyme responsible for incretin degradation are also under study.
Keywords: type 2 diabetes, incretin, incretin mimetics, GLP-1, DPP-IV inhibitors, vildagliptin, sitagliptin
Gastrointestinal hormones and the incretin effect
Zunz and La Barre hypothesized as early as 1929 that gastrointestinal factors stimulate insulin secretion after a meal [1]. Physiological studies in the second half of the 20th century showed then that orally administered glucose evoked a greater insulin response than an intravenously administered glucose infusion calculated to lead to exactly the same serum glucose excursions. This difference in the insulin response was named the "incretin effect" and the gastrointestinal hormones stimulating insulin secretion after oral glucose ingestion were called "incretins" [2-4]. The metabolic, neural and hormonal effects of the small intestine on the endocrine pancreas are referred to as "entero-insular axis" (Figure 1). Approximately 30 - 60% of the C-peptide and 80 - 90% of the insulin response after an oral glucose load are conveyed by incretin hormones in non-diabetic subjects, depending on the amount of glucose. In type 2 diabetes, the incretin effect is reduced or even absent [3, 5].
Figure 1. The Enteroinsular Axis.
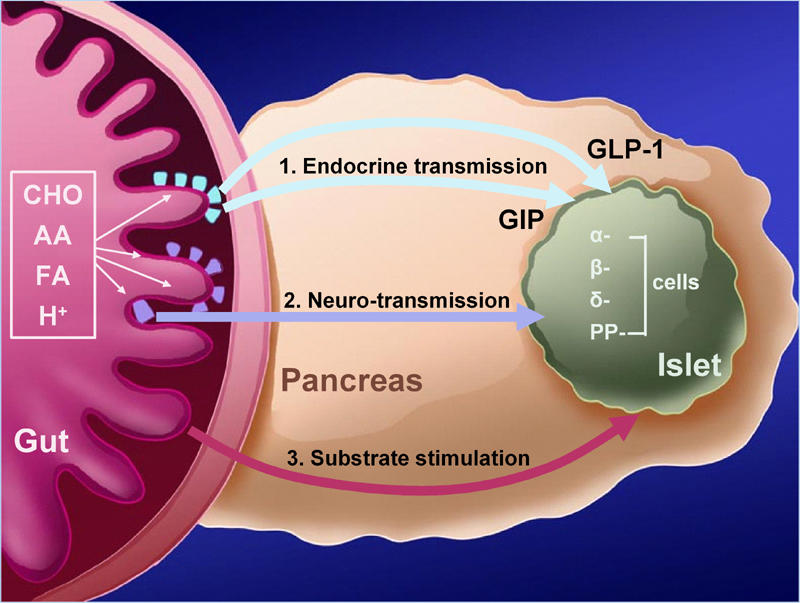
Postprandially, insulin secretion is directly stimulated by substrates, neuro-transmission (through entero-pancreatic nerves activated by chymus and intestinal distension), and by strong endocrine stimulation through incretin hormones. CHO: carbohydrates. AA: amino acids. FA: fatty acids. H+: hydrogen from gastric acid production. α-cells produce glucagon, β-cells insulin, δ-cells somatostatin, and PP-cells pancreatic polypeptide. (Modified according to [2]).
Glucose-dependent insulinotropic peptide (GIP) (also referred to as gastric inhibitory polypeptide) secreted from the entero-endocrine K-cells in the jejunum was the first incretin hormone discovered in 1971, which accounts for approximately 60% of the total incretin effect [4, 6]. In 1985, another important incretin hormone, glucagon-like peptide 1 (GLP-1) was discovered and shortly thereafter found to be a physiological incretin in humans [4, 7, 8].
GLP-1 is a product of the proglucagon gene. It is generated by tissue-specific post-translational processing of proglucagon. The gene is expressed in the A-cells of the pancreas, the neuroendocrine L-cells of the small intestine and the hypothalamus. Biologically active peptides are formed by post-translational cleavage of proglucagon in the previously specified tissues. GLP-1 is mainly formed in the small intestine, whereas glucagon is the major product of the proglucagon processing in the pancreas [8]. GLP-1 is recognized as a potentiator of glucose-induced insulin secretion and substantially contributes to the incretin effect. Plasma concentrations of GLP-1 increase six- to eight-fold after a carbohydrate meal [7]. The physiological relevance of GLP-1 and GIP as incretin hormones is further emphasized by studies with knockout mice which are lacking the GIP or GLP-1 receptor [9, 10].
GLP-1 and the incretin effect in type 2 diabetes
Interestingly, of the two important incretins, GIP has lost most of its insulinotropic potency in type 2 diabetic patients [11, 12]. In contrast, GLP-1 still effectively stimulates insulin secretion in these patients [13-15]. The underlying cause for the diverging properties of GIP and GLP-1 regarding the altered incretin effect in type 2 diabetes is not completely understood. The promising therapeutic potential of GLP-1 as a pharmacological tool for treating type 2 diabetes was proposed in the 1990s, along with the further characterization of the incretin effect [15, 16]. In contrast to other insulinotropic agents, e.g. the sulfonylureas, the insulinotropic effect of GLP-1 depends even more closely on the actual glucose concentration providing the possibility of glucose normalization without the risk of hypoglycemias [15, 17]. As well as the glucose lowering effect via the stimulation of insulin secretion, GLP-1 has a variety of additional physiological effects that may be advantageous in type 2 diabetes therapy.
GLP-1 and its effect on glucagon secretion
GLP-1 inhibits glucagon secretion in vitro and in vivo [18, 19]. In type 2 diabetes, excessive glucagon secretion in relation to the plasma glucose stimulates hepatic glycogenolysis and therefore contributes to fasting hyperglycemia [20]. In type 2 diabetic patients, infusions of GLP-1 lead to a significant suppression of glucagon secretion together with a normalization in fasting plasma glucose [15]. GLP-1 administration however, does not impair the glucagon counter-regulatory response to hypoglycemia, since the glucagon secretion is glucose-dependent [21].
GLP-1 and the regulation of satiety – central effects in the hypothalamus and peripheral effects in delaying gastric emptying
In the central nervous system, GLP-1 acts as a neurotransmitter in the hypothalamus and stimulates satiety directly. Intraceribroventricular GLP-1 application lead to decreased food intake in rodents [22]. Peripherally, various gastrointestinal functions are influenced directly and indirectly by GLP-1. Gastric emptying after meals is slowed, in addition GLP-1 inhibits gastric acid secretion [23]. In humans, a continuous subcutaneous infusion of GLP-1 over six weeks lead to significant weight loss due to reduced calorie intake attributable to increased feelings of satiety [22, 24]. Whether the effects of GLP-1 on satiety in humans are mainly mediated by the retardation of gastric emptying through a feedback loop or are centrally mediated is still under investigation [25, 26].
GLP-1 and its effect on β-cell mass and function
Experiments in rodent models as well as in vitro studies demonstrate an increase of β-cell mass after long-term administration of GLP-1 due to a stimulation of islet cell neogenesis [27-29] from precursor cells on the one hand, and due to an inhibition of apoptosis of β-cells on the other [28, 30]. The restoration of some β-cell function parameters can be detected indirectly from the increased insulin secretory capacity in humans receiving GLP-1. However, an increase of β-cell mass cannot be directly quantified without invasive surgical techniques in humans [31]. In isolated human islets, glucose-dependent insulin secretion and islet cell morphology is significantly improved, when the islets are incubated with GLP-1 [32]. The reason for the expansion of β-cell mass by GLP-1 is due to the inhibition of apoptotic signaling pathways and the stimulation of signaling pathways leading to a proliferation of β-cells [28, 30, 32]. The mRNA levels for Bcl-2 and caspase 3 as markers for apoptotic activity are down regulated in human islets incubated with GLP-1 [32].
GLP-1 as a potential therapeutic principle in the treatment of type 2 diabetes
The incretin effect is known to be reduced in patients with type 2 diabetes, resulting in inappropriately low insulin secretion following oral ingestion of nutrients [33]. More recent studies have indicated that GLP-1 secretion is also impaired in these subjects, suggesting that a reduced meal-related GLP-1 response may contribute to the decreased incretin effect [34]. GLP-1 is effective in patients with type 2 diabetes, increasing insulin secretion and normalizing both fasting and postprandial blood glucose when given as a continuous intravenous infusion (Figure 2) [15], even in subjects with advanced type 2 diabetes long after sulfonylurea secondary failure [35]. Unexpectedly, the effects of a single subcutaneous injection of GLP-1 were disappointing. Although high plasma levels of immunoreactive GLP-1 were achieved, insulin secretion rapidly returned to pre-treatment values and blood glucose concentrations were not normalized [36]. Nevertheless, the effect of repeated subcutaneous administration on fasting blood glucose is as good as that of intravenous administration [36], while continuous subcutaneous administration for 6 weeks reduces fasting and postprandial glucose concentrations and lowers HbA1c concentrations [24].
Figure 2.
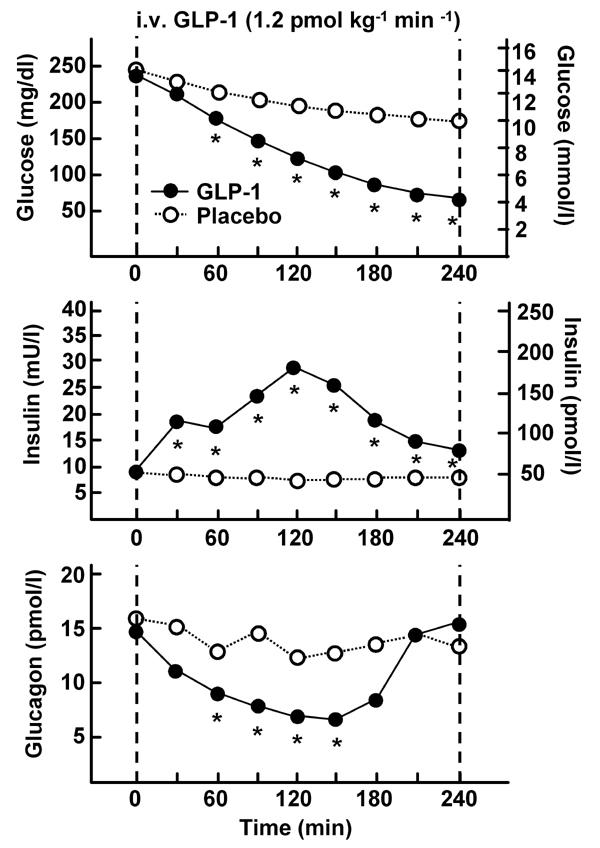
The figure represents plasma glucose, insulin, and glucagon levels after intravenous administration of 1.2 pmol kg-1 min-1 GLP-1 in 10 type 2 diabetic patients. The time interval of continued GLP-1 infusion (●) and placebo administration (○) was 240 min. Asterisk (*) indicates a significance level of p < 0.0001 (modified according to [15]).
The multiple actions of GLP-1 may constitute a new and attractive therapeutic principle for the treatment of type 2 diabetes by improving the postprandial metabolic situation and eliminating hypoglycemic events (Table 1) [24]. The risk of hypoglycemia observed in patients treated with GLP-1 is minimal because GLP-1 only stimulates insulin secretion under hyperglycemic conditions. Intravenous infusions of GLP-1 are able to normalize plasma glucose in patients with type 2 diabetes. Furthermore, hepatic glucose production is lowered by GLP-1 due to the inhibition of glucagon secretion (Figure 2) [15]. A 6-week continuous subcutaneous infusion of GLP-1 significantly decreased glucagon levels and improved glycemic control, HbA1c decreased by 1.3% [24]. Furthermore, the patients treated with GLP-1 lost approximately 2 kg in weight (Figure 3) [24, 37].
Table 1. Favorable effects of incretin mimetics in type 2 diabetes therapy.

Figure 3. Blood glucose and body weight at baseline, after 1 week and after 6 weeks treatment with either a continuous subcutaneous infusion of GLP-1 or placebo.
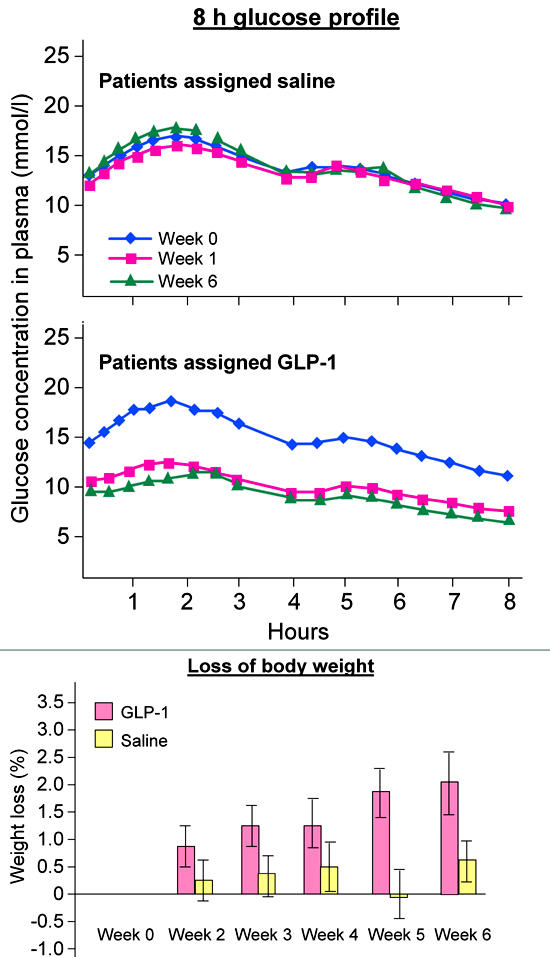
The graphs show blood glucose profiles over a day during week 0, 1 and 6, the bars represent the loss in body weight in percentages (modified according to [24]).
GLP-1 and its limitations in type 2 diabetes therapy
A possible explanation for the short-lived effectiveness of single subcutaneous injections of GLP-1 was indicated when it was shown that GLP-1 (and the other incretin, GIP) was metabolized by plasma in vitro and that the enzyme dipeptidyl peptidase-IV (DPP-IV) was capable of mediating this degradation [38, 39]. Only 20% of the GLP-1 administered during a continuous intravenous infusion is estimated to reach circulation intact as the active form [24, 39]. DPP-IV cleaves peptides with a penultimate amino acid residue alanine or proline leaving biologically inactive split products [38].
Generally, either DPP-IV-resistant peptides that bind to the GLP-1 receptor and show GLP-1-like biological effects or substances inhibiting the DPP-IV could be used to utilize the therapeutic potential of GLP-1 [40].
For this reason, long acting GLP-1 analogs (also referred to as "incretin mimetics") that are resistant to degradation by DPP-IV, are currently being evaluated for clinical use or already being introduced into clinical practice [40-43].
Advantages of "incretin mimetics" and synthetic GLP-1 analogs as new therapeutic agents
Currently, the first incretin mimetic already available in the U.S.A. for the therapy of type 2 diabetic patients not optimally controlled with oral agents is exenatide (Byetta®, Eli Lilly & Amylin Pharamaceuticals). It is the synthetic form of a naturally occurring peptide called exendin-4 that was originally found in the salivary gland of the Gila monster (Heloderma suspectum). Exenatide has a high amino acid sequence identity to GLP-1 and is not degraded by DPP-IV (Figure 4). It binds to the GLP-1 receptor in vitro with a higher affinity than GLP-1 and shows similar gluco-regulatory effects as native GLP-1 [44]. In clinical studies, a twice-daily subcutaneous administration of exenatide resulted in a significant improvement of glycemic control without weight gain and an improvement in β-cell function without causing hypoglycemia in monotherapy (Figure 5) [45, 46]. Hypoglycemia occurred only in patients receiving exenatide and unadjusted doses of sulfonylureas [31, 46-49].
Figure 4. Native GLP-1 and possible molecular changes to create long-acting incretin mimetics.
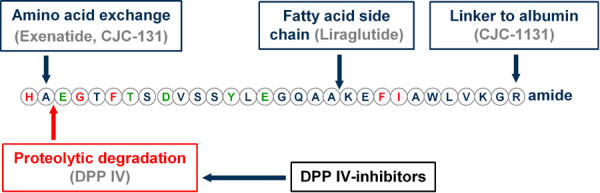
The amino acid sequence of native GLP-1 is shown in the one-letter code. Also, the N-terminal cleavage site of DPP-IV is depicted by an arrow. The schematic molecular changes of the currently available incretin mimetics are shown.
Figure 5. Glycemic control in subjects with type 2 diabetes treated with metformin and a sulfonylurea plus exenatide or placebo (ITT population).
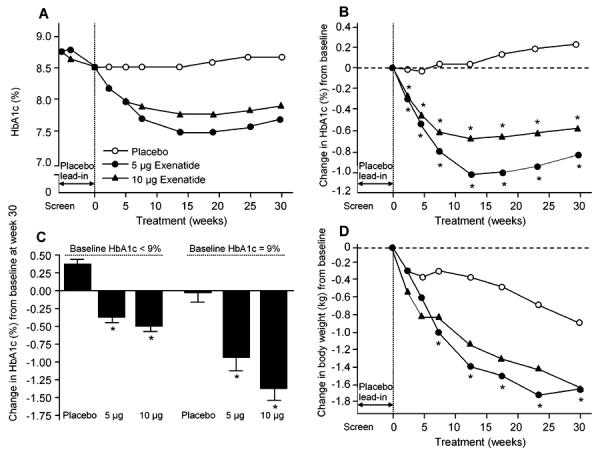
A: HbA1c values over the course of the study (raw data). B: Change in HbA1c over 30 weeks. * Adjusted p < 0.0001 compared with placebo. Week 30 changes in HbA1c values from baseline were -0.77 ± 0.08% (10-µg arm; adjusted p < 0.0001 vs. placebo), -0.55 ± 0.07% (5-µg arm; adjusted p < 0.0001 vs. placebo), and 0.23 ± 0.07% (placebo arm). C: Week 30 change in HbA1c stratified by baseline HbA1c. For subjects with baseline HbA1c < 9%, baseline HbA1c values were 7.92 ± 0.04% (n = 169), 7.91 ± 0.04% (n = 172), and 7.94 ± 0.04% (n = 172) for the 10-µg exenatide, 5-µg exenatide, and placebo arms, respectively. The corresponding values for subjects with baseline HbA1c ≥ 9% were 9.86 ± 0.07% (n = 72), 9.75 ± 0.07% (n = 73), and 9.75 ± 0.07% (n = 75). D: Effects of exenatide on body weight. Subjects in the 10-µg exenatide b.i.d. treatment arm received 5 µg exenatide b.i.d. during weeks 0-4. Subjects in all treatment arms were maintained on metformin-sulfonylurea therapy. * p < 0.001 compared with placebo treatment. Data are means ± SE (reproduced from [49]).
The synthetic GLP-1 analog liraglutide (NN2211) (Novo Nordisk pharmaceuticals) is also DPP-IV resistant and possesses a biologically longer half-life than native GLP-1 due to the addition of a fatty acid side chain to the peptide molecule (Figure 4). The mechanism of protraction is a combination of albumin binding and self-association, resulting in slow absorption from subcutis, stability against dipeptidyl-peptidase IV, and a long plasma half-life. Due to its prolonged action (t0.5 = 13 h) it is suitable for once-daily injection. Liraglutide also improves plasma glucose and HbA1c [37, 50]. A recent study reported only one hypoglycemic event in 135 patients (0.074%) receiving liraglutide compared to four of 26 patients (15.4%) receiving sulfonylurea. Another study reported 2.8% of subjects receiving liraglutide experiencing hypoglycemia compared to 5.8% of the group receiving metformin [37, 51]. Recent studies using primary neonatal rat islets showed that native GLP-1 and liraglutide similarly inhibited both cytokine- and free fatty acid-induced apoptosis in a dose-dependent manner, suggesting that liraglutide may be useful for retaining β-cell mass in both type 1 and type 2 diabetic patients [52].
CJC-1131 (under development by ConjuChem) is an analog of GLP-1 with a very long half-life due to its DPP-IV resistance and covalent binding to serum albumin (Figure 4).
All the compounds mentioned lead to an increase of β-cell mass and an improvement of β-cell function in experimental animal models [52-55].
Unlike insulin treatment that requires substantial dose adjustment, there is likely to be a standard therapeutic incretin dose for most patients. Dosing of the incretin mimetics and GLP-1 analogs will probably be uncomplicated because the probability of hypoglycemia is low. Nausea (not more than experienced with metformin therapy) [40, 41, 47] may be observed during the beginning of treatment, but can be controlled with mild antiemetic drugs and usually ceases within a few days. The only disadvantage is that all compounds must be administered subcutaneously, as non-peptidergic incretin mimetics for oral use are not yet available [40].
All GLP-1 analogs and incretin mimetics mentioned in this review have been safe and well tolerated in clinical trials [40, 41]. Nausea occurred in some patients at the beginning of therapy, but it was usually mild and did not lead to a discontinuation of treatment [47]. Hypoglycemia was less common compared to patients receiving oral anti-glycemic medication in these trials [31, 37, 47].
DPP-IV inhibitors
The therapeutic principle of GLP-1 can also be implemented by inhibiting GLP-1 degradation. Support for this approach to therapy also comes from the observations that glucose tolerance is improved in animals in which the enzyme has been genetically deleted [56] and in animals treated with DPP-IV inhibitors [57]. Various substances with DPP-IV inhibition properties that have a good bioavailability after oral ingestion are currently being tested in pre-clinical and clinical trials. One compound already in phase III clinical trials is LAF237, vildagliptin® (Novartis Pharma). In clinical studies, vildagliptin lowered HbA1c in type 2 diabetic patients not treated sufficiently with metformin [58].
In contrast to the "incretin mimetics", the weight loss observed with therapy using these substances is not observed with the treatment of DPP-IV inhibitors that seem to be weight neutral. On the other hand, therapy with DPP-IV inhibitors does cause nausea as a side effect, which is observed in therapy with "incretin mimetics". The application of DPP-IV inhibitors retards endogenous GLP-1 degradation, but there is still some uncertainty as to whether all effects of DPP-IV inhibitors are mediated by the prolongation of the biological half-life of the peptide [59-61]. One puzzling finding might support this: in patients with type 2 diabetes, concentrations of active GLP-1 after meal ingestion are doubled by DPP-IV inhibition (compared with placebo), and glucose control improves [58]. In contrast, when similar increases in GLP-1 levels are produced by exogenous infusion, these have little or no effect on insulin secretion or glucose levels [11]. This suggests that mediators other than GLP-1 may contribute to the therapeutic effect of DPP-IV in-hibition. For instance, DPP-IV inhibition also blocks the inactivation of the other major incretin hor-mone, gastric inhibit-tory peptide (GIP) [59]. Furthermore, various neuropeptides may contribute to the actions of DPP-IV inhibitors in diabetes. These are biologically active peptides that are localized to islet nerve terminals and function as neurotransmitters; some may be sub-strates for DPP-IV [59]. One neuropep-tide of potential importance is pituitary adenylate cyclase-acti-vating peptide (PAC AP), which is localized to islet nerves and has several actions relevant to glucose homeostasis [62]. For example, PACAP is a powerful stimulator of insulin secretion and may, like GLP-1, be of importance for islet mass. PACAP may play a leading role in contributing to the prandial, neurally dependent cephalic phase of insulin secretion. Furthermore, it enhances glucose uptake in adipocytes and augments the antilipolytic action of insulin [59, 63]. Since PACAP is also a substrate for DPP-IV, it is reasonable to speculate that this neuropeptide may contribute to the therapeutic benefits of DPP-IV inhibition. However, it is not yet known whether neuropeptides such as PACAP are substrates of DPP-IV in humans under physiological conditions, and this remains a weakness in this line of argument [59].
Because DPP-IV is involved in the degradation of many peptide hormones, the action of DPP-IV is less specific than "incretin mimetics". Along with this, the long-term immunological effects of DPP-IV inhibitors in humans are not yet known, since DPP-IV is also expressed on lymphocytes as CD 26 [64, 65].
In summary, the therapeutic principle of GLP-1 using "incretin mimetics" is a new and attractive treatment option with multiple favorable actions for type 2 diabetes (see Table 1) [40, 41].
Perspectives
The therapeutic principle of GLP-1 with the multiple mode of action besides its glucose-normalizing effect adds a new and attractive perspective to diabetes therapy. Since "incretin mimetics" are peptides, they have to be injected. This fact and their potential costs will probably give them a place in clinical practice for patients who have failed on oral therapy and in whom insulin therapy is not an alternative due to weight problems or possible hypoglycemia. Theoretically, GLP-1-like agents may also be useful in slowing the progression of type 2 diabetes or to be used as anti-obesity agents [66], but here life-style intervention and metformin are also effective [67]. DPP-IV inhibitors have the advantage of being oral (and maybe less costly) agents, but their multiple effects are currently not completely elucidated [59]. So far, only data from clinical trials covering a time frame of little more than one year are available. Long term effects of "incretin mimetics" and DPP-IV inhibitors e.g. on β-cell proliferation and on the brain have to be followed in clinical practice.
References
- 1.Zunz E, La Barre J. Contributions a l’étude des variatins physiologiques de la sécrétion interne du pancréas: relations entre les sécrétions externe et interne du pancréas. Arch Int Physiol. 1929;31:20–44. [Google Scholar]
- 2.Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 3.Creutzfeldt W. Entero-insular axis and diabetes mellitus. Horm Metab Res. 26(Suppl 1992):13–18. [PubMed] [Google Scholar]
- 4.Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul Pept. 2002;107:1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 5.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Dryburgh JR. A gastric inhibitory polypeptide II. The complete amino acid sequence. Can J Biochem. 1971;49:867–872. doi: 10.1139/o71-122. [DOI] [PubMed] [Google Scholar]
- 7.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. Proglucagon processing in porcine and human pancreas. J Biol Chem. 1994;269:18827–18833. [PubMed] [Google Scholar]
- 9.Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier JJ, Hucking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes. 2001;50:2497–2504. doi: 10.2337/diabetes.50.11.2497. [DOI] [PubMed] [Google Scholar]
- 13.Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992;326:1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 14.Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992;15:270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 15.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 16.Holst JJ. Glucagon-like peptide-1, a gastrointestinal hormone with a pharmaceutical potential. Curr Med Chem. 1999;6:1005–1017. [PubMed] [Google Scholar]
- 17.Vilsboll T, Krarup T, Madsbad S, Holst JJ. No reactive hypoglycaemia in Type 2 diabetic patients after subcutaneous administration of GLP-1 and intravenous glucose. Diabet Med. 2001;18:144–149. doi: 10.1046/j.1464-5491.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama T, Komatsu R, Namba M, Watanabe N, Itoh H, Tarui S. Glucagon-like peptide-1 (7-36 amide): a potent glucagonostatic and insulinotropic hormone. Diabetes Res Clin Pract. 1988;5:281–284. doi: 10.1016/s0168-8227(88)80063-9. [DOI] [PubMed] [Google Scholar]
- 19.Meier JJ, Nauck MA. The potential role of glucagon-like peptide 1 in diabetes. Curr Opin Investig Drugs. 2004;5:402–410. [PubMed] [Google Scholar]
- 20.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 21.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- 22.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 23.Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–2725. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 24.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 25.Gutzwiller JP, Degen L, Matzinger D, Prestin S, Beglinger C. Interaction between GLP-1 and CCK-33 in inhibiting food intake and appetite in men. Am J Physiol Regul Integr Comp Physiol. 2004;287:R562–567. doi: 10.1152/ajpregu.00599.2003. [DOI] [PubMed] [Google Scholar]
- 26.Gutzwiller JP, Degen L, Heuss L, Beglinger C. Glucagon-like peptide 1 (GLP-1) and eating. Physiol Behav. 2004;82:17–19. doi: 10.1016/j.physbeh.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Perfetti R, Hui H. The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res. 2004;36:804–810. doi: 10.1055/s-2004-826167. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 29.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL. Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia. 2004;47:478–487. doi: 10.1007/s00125-004-1327-5. [DOI] [PubMed] [Google Scholar]
- 31.Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 32.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 33.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 34.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 35.Nauck MA, Sauerwald A, Ritzel R, Holst JJ, Schmiegel W. Influence of glucagon-like peptide 1 on fasting glycemia in type 2 diabetic patients treated with insulin after sulfonylurea secondary failure. Diabetes Care. 1998;21:1925–1931. doi: 10.2337/diacare.21.11.1925. [DOI] [PubMed] [Google Scholar]
- 36.Nauck MA, Wollschlager D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Willms B. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 37.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 38.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 39.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 40.Meier JJ, Gallwitz B, Nauck MA. Glucagon-like peptide 1 and gastric inhibitory polypeptide: potential applications in type 2 diabetes mellitus. BioDrugs. 2003;17:93–102. doi: 10.2165/00063030-200317020-00002. [DOI] [PubMed] [Google Scholar]
- 41.Joy SV, Rodgers PT, Scates AC. Incretin Mimetics as Emerging Treatments for Type 2 Diabetes (January) Ann Pharmacother. 2004;39(1):110–118. doi: 10.1345/aph.1E245. [DOI] [PubMed] [Google Scholar]
- 42.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 43.Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul Pept. 2005;128:135–148. doi: 10.1016/j.regpep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- 45.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 46.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 47.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 48.Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62:173–181. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 49.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 50.Mark M. NN-2211 Novo Nordisk. IDrugs. 2003;6:251–258. [PubMed] [Google Scholar]
- 51.Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27:1915–1921. doi: 10.2337/diacare.27.8.1915. [DOI] [PubMed] [Google Scholar]
- 52.Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Sturis J, Gotfredsen CF, Romer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 55.Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jette L, Benquet C, Drucker DJ. Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52:751–759. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- 56.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reimer MK, Holst JJ, Ahren B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol. 2002;146:717–727. doi: 10.1530/eje.0.1460717. [DOI] [PubMed] [Google Scholar]
- 58.Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 59.Ahren B. What mediates the benefits associated with dipeptidyl peptidase-IV inhibition? Diabetologia. 2005;48:605–607. doi: 10.1007/s00125-005-1706-6. [DOI] [PubMed] [Google Scholar]
- 60.Nauck MA, El-Ouaghlidi A. The therapeutic actions of DPP-IV inhibition are not mediated by glucagon-like peptide-1. Diabetologia. 2005;48:608–611. doi: 10.1007/s00125-005-1704-8. [DOI] [PubMed] [Google Scholar]
- 61.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48:612–615. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 62.Filipsson K, Kvist-Reimer M, Ahren B. The neuropeptide pituitary adenylate cyclase-activating polypeptide and islet function. Diabetes. 2001;50:1959–1969. doi: 10.2337/diabetes.50.9.1959. [DOI] [PubMed] [Google Scholar]
- 63.Akesson L, Ahren B, Manganiello VC, Holst LS, Edgren G, Degerman E. Dual effects of pituitary adenylate cyclase-activating polypeptide and isoproterenol on lipid metabolism and signaling in primary rat adipocytes. Endocrinology. 2003;144:5293–5299. doi: 10.1210/en.2003-0364. [DOI] [PubMed] [Google Scholar]
- 64.Villhauer EB, Brinkman JA, Naderi GB, Burkey BF, Dunning BE, Prasad K, Mangold BL, Russell ME, Hughes TE. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem. 2003;46:2774–2789. doi: 10.1021/jm030091l. [DOI] [PubMed] [Google Scholar]
- 65.Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibition as an approach to the treatment and prevention of type 2 diabetes: a historical perspective. Biochem Biophys Res Commun. 2002;294:1–4. doi: 10.1016/S0006-291X(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 66.Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: therapeutic perspectives. Eur J Pharmacol. 2002;440:269–279. doi: 10.1016/s0014-2999(02)01434-6. [DOI] [PubMed] [Google Scholar]
- 67.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]


