Abstract
The plant, Stevia rebaudiana Bertoni (SrB), has been used for the treatment of diabetes in traditional medicine. Previously, we have demonstrated that long-term administration of the glycoside stevioside has insulinotropic, glucagonostatic, anti-hyperglycemic and blood pressure-lowering effects in type 2 diabetic animal models. The aim of this study was to elucidate if long-term administration of rebaudioside A, another glycoside isolated from the plant SrB, could improve glycemic control and lower blood pressure in an animal model of type 2 diabetes. We divided male Goto-Kakizaki (GK) rats into two groups which were fed a standard laboratory chow diet for eight weeks. The diet was supplemented with oral rebaudioside A (0.025 g/kg BW/day) in the experimental group. Blood glucose, weight, blood pressure and food intake were measured weekly. Animals were equipped with an intra-arterial catheter, and at week eight the conscious rats underwent an intra-arterial glucose tolerance test (IAGTT) (2.0 g/kg BW). During the IAGTT, the level of glucose, glucagon, and insulin responses did not differ significantly between the two groups. Fasting levels of glucose, glucagon, insulin or levels of blood lipids did not differ between the groups throughout the study period. We observed no effect on blood pressure or weight development. In conclusion, oral supplementation with rebaudioside A (0.025 g/kg BW/day) for eight weeks did not influence blood pressure or glycemic control in GK rats. Rebaudioside A failed to show the beneficial effects in diabetic animals previously demonstrated for stevioside.
Keywords: type 2 diabetes, rats, rebaudioside A, glycemic control, blood pressure
Background
Type 2 diabetes is a chronic disease with two hallmarks, i.e. insulin resistance and abnormal islet-cell function. Type 2 diabetes, together with hypertension, dyslipidemia and obesity, is an important feature of the metabolic syndrome [1, 2]. All elements of the metabolic syndrome contribute to the development of coronary artery disease (CAD), which is the cause of death among approx. 80% of type 2 diabetics. When type 2 diabetes has developed, adverse CAD outcomes may be reduced by a tight control of blood glucose and blood pressure [3, 4]. New approaches will be needed to achieve this goal, especially in low income countries.
Extracts of leaves of the plant Stevia rebaudiana Bertoni (SrB), have been used for years in traditional medicine in the treatment of diabetes [5]. The leaves of SrB contain at least 8 sweet steviol glycosides [5] of which the main constituents are stevioside and rebaudioside A [6]. Our group has previously shown that long-term treatment with stevioside has antidiabetic effects in the Goto-Kakizaki (GK) rat [7] and in the ZDF rat [8]. The GK rat is a non-obese animal model of type 2 diabetes characterized by a deficient insulin response to glucose in vivo and in vitro, as well as insulin resistance [9-12]. Interestingly, stevioside concomitantly influences another feature of the metabolic syndrome, i.e. blood pressure, which is lowered in the GK rat [7], in the ZDF rat [8], and in a non-diabetic hypertensive rat model [13], as well as in hypertensive non-diabetic humans [14, 15]. However, in theory any of the eight glycosides could be responsible for the anti-hyperglycemic effect demonstrated for extracts of the SrB. A promising candidate is rebaudioside A, which is present in the leaves at a level of about 3-8 % of the total dried weight. We have recently shown that rebaudioside A possesses a potent insulinotropic property in a dose- and glucose-dependent manner in isolated mouse islets [16]. Our hypothesis was that long-term administration of rebaudioside A in the GK rat could improve the glucose metabolism and normalize blood pressure.
Material and Methods
Animals
24 eleven-week-old male GK rats (Bomholtgård Breeding and Research Center, Ry, Denmark) were randomized into 2 groups, 12 animals in each group, and fed a standard laboratory animal chow diet (Altromin 1324, Altromin, Lage, Germany). In group A the chow diet was supplemented with rebaudioside A (0.025 g/kg BW/day) diluted in 8 ml drinking water twice daily. (Groups are referred to as A: Rebaudioside A and B: Control hereafter). The animals had free access to tap water.
Before entering the experiment, all the animals were fed with the standard chow diet for laboratory rats (Altromin). The experiments were performed in accordance with the Danish Council on Animal Care.
Rebaudioside A
The rebaudioside A used in this study was supplied by Wako Pure Chemical Industries Ltd., Osaka, Japan, and was tested to be 97.8% pure rebaudioside A by reversed phase HPLC (Danish Institute of Agricultural Sciences, Aarslev, Denmark). The chromatographic conditions for the HPLC analysis were the same as described previously for the separation of stevioside, rebaudioside A and their metabolites from SrB extracts [17].
Measurements during the study period
Weight and food consumption were monitored throughout the study period. Blood glucose, insulin and glucagon were measured once every week after overnight fasting. Blood was drawn from the tip of the tail, using micro hematocrit tubes (Na-heparinized), after gently preheating the tail. After centrifugation, plasma was collected and frozen for further analysis.
Systolic blood pressure was monitored weekly with automatic system 209002 (TSE GmbH, Bad Homburg, Germany), with the animals placed in a restrainer. The animals were trained in the restrainer prior to the experiment.
Intra arterial glucose tolerance test
After seven weeks of dietary treatment, the animals were equipped with an intravascular catheter for blood sampling and infusion as described elsewhere [7]. In brief, the animals were anaesthetized by subcutaneous injection of 1 ml/kg rat of a mixture of 0.08 mg/ml fentanyl citrate and 2.5 mg/ml fluanisone (Janssen Pharmaceutica N.V., Beerse, Belgium) and 1.25 mg/ml midazolam (Dumex-Alpharma A/S, Oslo, Norway). A catheter (Tygon Microbore Tubing, Norton Performance Plastics, UK) was inserted into the right carotid artery, and exteriorized at the neck of the animal. The catheter was filled with 0.9% saline containing 10 U/ml heparin (Løvens Kemiske Fabrik, Ballerup, Denmark). After surgery 0.08 mg Naloxone (DuPont Pharmaceuticals Ltd., Hertfordshire, UK) was injected intramuscularly. Animals were caged individually hereafter, and continued on the respective diets. Patency of the catheter was secured by daily flushing with 0.2 ml of the saline/heparin solution. After five days of recovery, the animals underwent an intra-arterial glucose tolerance test (IAGTT).
Animals were fasted 14 hours (water ad libitum) prior to the IAGTT, which started at 08.30 a.m. The animals were conscious and allowed to move freely in separate plastic cylinders during the experiment. The catheter was connected to a tubing (Tygon Microbore Tubing) allowing blood sampling and infusion of glucose. Glucose (2.0 g D-glucose/kg BW) was prepared as a 30% D-glucose solution in 0.9% saline, and infused as a bolus over 20 sec.
Blood samples were withdrawn at 15 minutes before and immediately prior to the glucose infusion, and hereafter at time points 2, 5, 10, 15, 20, 30, 45, 60, 90, 120, and 180 min after glucose infusion. 200 μl blood samples were taken on chilled tubes containing heparin/aprotinin, and centrifuged (3500 g 60 sec., 4ºC), and plasma was frozen for subsequent analysis of insulin, glucose, glucagon, FFA, triglycerides and cholesterol. The blood cells drawn from the animals were resuspended in 0.9% saline (in an equal volume of plasma drawn from each sample), and re-infused to prevent volume depletion.
Assays
Blood glucose was determined using the Glucose Oxidase method (GOD-PAP, Boehringer Mannheim, Germany). Insulin was determined by radioimmunassay with a guinea-pig anti-porcine insulin antibody (PNILGP4, Novo Nordisk, Bagsvaerd, Denmark) and mono-125I-(Tyr A14) labeled human insulin (Novo Nordisk) as tracer and rat insulin (Novo Nordisk) as standard. Free and bound radioactivity was separated using ethanol. Inter- and intra assay variation was below 10%. Rebaudioside A at the concentrations studied did not interfere with the insulin assay.
Triglycerides, free fatty acids (FFA) and total cholesterol were determined using colorimetric kits (Boehringer Mannheim). The animals continued on the same treatments after the IAGTT.
Statistical Analysis
Data are expressed as mean ± SEM. Two-sample t-test was used to compare differences between groups and a p-value of less than 0.05 was considered statistically significant. The area under the curve (AUC) was calculated using the trapezoidal method.
Results
Effects of long-term treatment with rebaudioside A on glucose metabolism determined with an intra-arterial glucose tolerance test in type 2 diabetic Goto-Kakizaki rats
We did not detect differences in fasting levels of plasma glucose (A: 15.5 ± 0.6 vs. B: 15.5 ± 0.9 mmol/l, p = 0.9 ) (Figure 1A), insulin (A: 1.5 ± 0.1 vs. B: 1.4 ± 0.2 ng/ml, p = 0.6) (Figure 1B) or glucagon (A: 123.2 ± 4.7 vs. B: 129.0 ± 6.2 pg/ml, p = 0.5) (Figure 1C) between the two groups before the glucose injection (2.0 g kg-1 BW). After the glucose injection, no significant difference between the groups in the level of plasma glucose was found, either during the first 30 minutes (AUC0-30min: A: 1045 ± 33 vs. B: 1107 ± 25 mmol/l x 30 minutes, p = 0.2) or for the whole 180-min period (AUC0-180min: A: 3940 ± 129 vs. B: 4115 ± 155 mmol/l x 180 minutes, p = 0.4).
Figure 1.
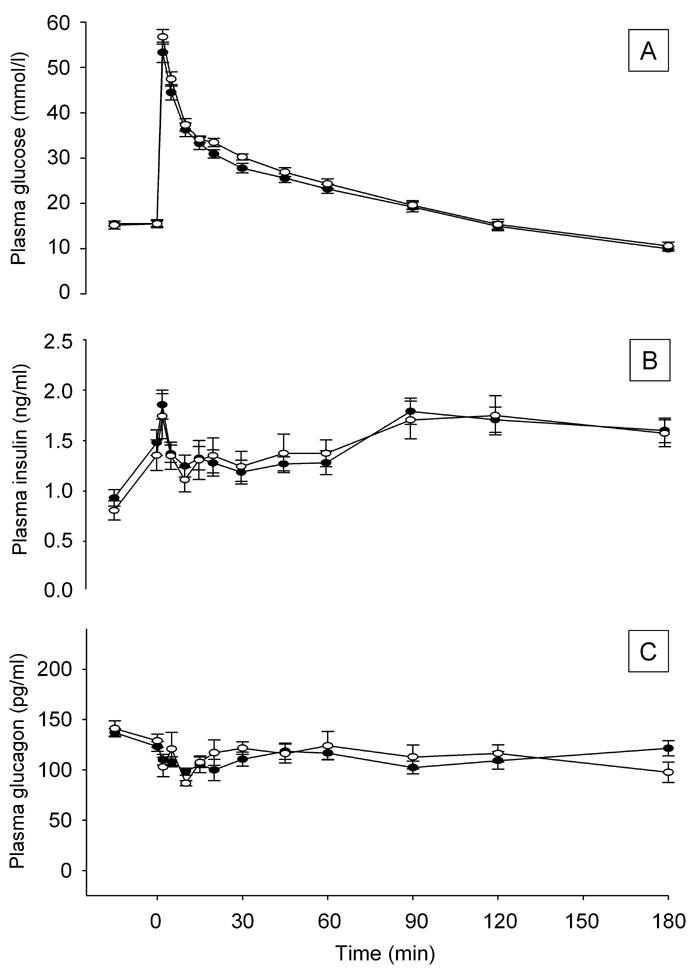
Effect of rebaudioside A treatment on plasma glucose levels (A), plasma insulin responses (B) and plasma glucagon (C) during IAGTT performed after 8 weeks treatment in overnight fasted GK-rats. (●) Standard chow diet + rebaudioside A, (○) standard chow diet. Data shown as mean ± SEM (n = 11-12 in each group).
First-phase insulin response, defined as 0-30 minutes, was similar in the two groups (AUC0-30min: A: 40 ± 3 vs. B: 40 ± 4 ng/ml x 30 minutes, p = 0.8). Insulin responses were similar for the IAGTT period overall for the two groups (AUC0-180min: A: 235 ± 11 vs. B: 238 ± 18 ng/ml x 180 minutes, p = 0.9).
The level of plasma glucagon, measured as AUC, did not differ between groups during the IAGTT. Thus, we did not detect any effect of rebaudioside A on the glucagon level (AUC0-30min: A: 3153 ± 139 vs. B: 3329 ± 192 pg/ml x 30 minutes, p = 0.5) (AUC0-180min: A: 21978 ± 628 vs. B: 22353 ± 1201 pg/ml x 180 minutes, p = 0.9).
Effects of long-term treatment with rebaudioside A on fasting blood glucose in type 2 diabetic Goto-Kakizaki rats
Figure 2 shows changes in blood glucose development in the two groups overall (Figure 2A). There was a general decline in the level of fasting blood glucose in both the rebaudioside A-treated and the control group. The level of glucose was lower in the rebaudioside A-treated group at some time points denoted by an asterisk (*p < 0.05), but the overall area under the curve did not differ significantly for the study period.
Figure 2.
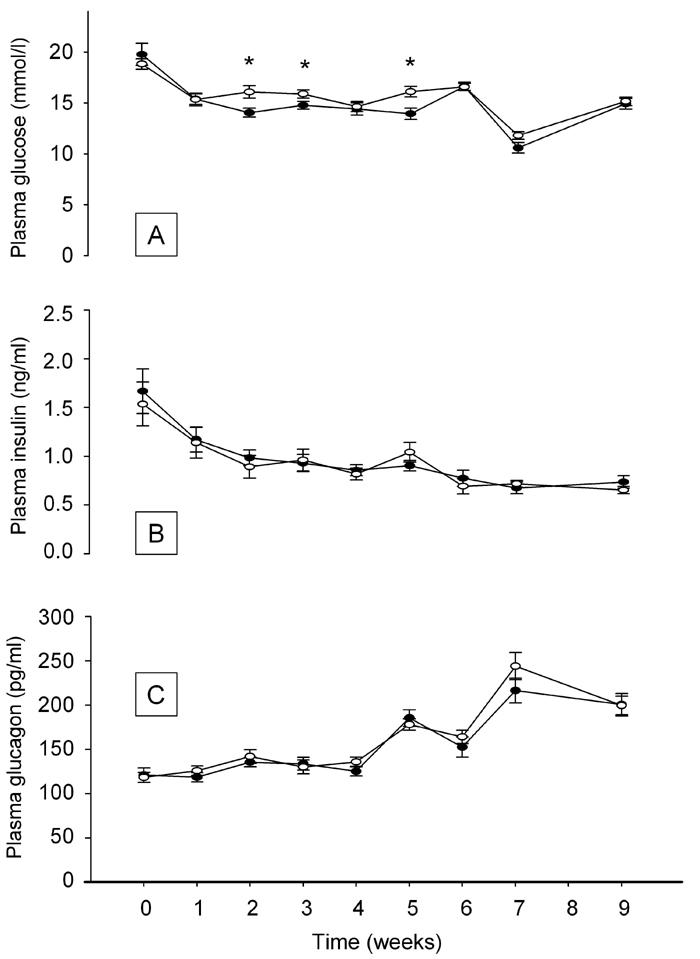
Effect of rebaudioside A treatment on plasma glucose (A), insulin (B) and glucagon (C) levels measured throughout the treatment period. (●) Standard chow diet + rebaudioside A, (○) standard chow diet. Data shown as mean ± SEM (n = 11-12 in each group). Asterisk denotes *p < 0.05 versus control at same time point.
The level of plasma insulin (Figure 2B) declined in the same manner in both the rebaudioside A and the control group. Corresponding to the decline in insulin level, we observed an increase in the level of fasting glucagon (Figure 2C). There were no significant differences in insulin or glucagon levels between the two groups at any time point.
Effects of long-term treatment with rebaudioside A on blood pressure, food consumption and body weight development in type 2 diabetic Goto-Kakizaki rats
Figure 3A indicates that initial systolic blood pressure was similar in the two groups. We observed an overall decrease in systolic blood pressure (p < 0.05) for the whole study period. The lowering of blood pressure occurred in both groups, and the average blood pressure was similar between the groups at all time points, apart from at six weeks.
Figure 3.
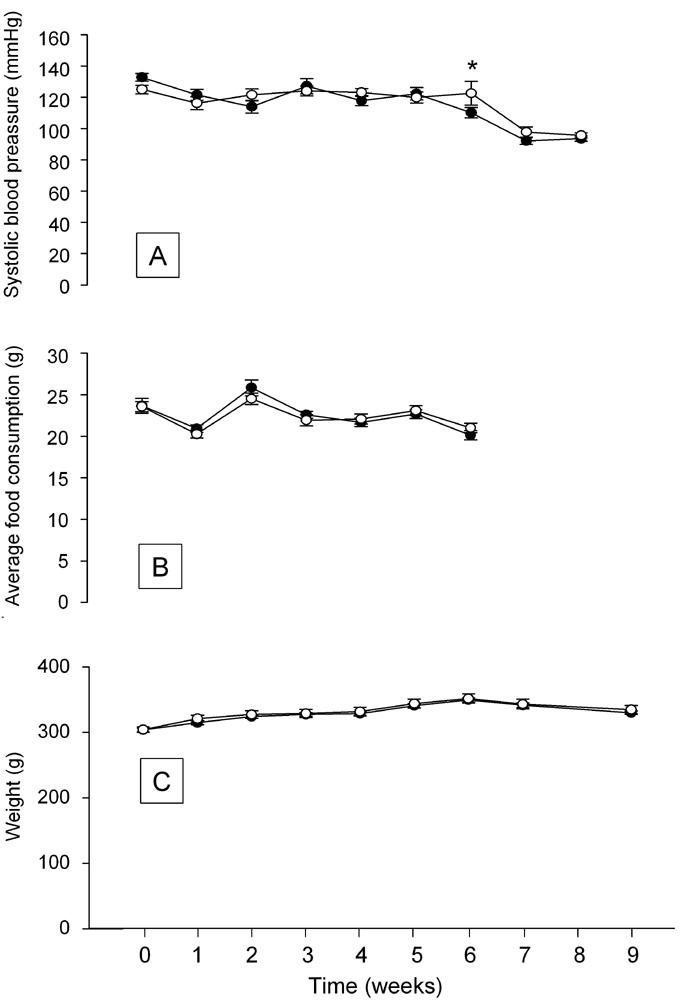
Effects of rebaudioside A treatment on systolic blood pressure (A), food consumption (B) and body weight (C). (●) Standard chow diet + rebaudioside A, (○) Standard chow diet. Data shown as mean ± SEM. (n = 11-12 animals in each group). Asterisk (*) denotes p < 0.05 versus control at same time point.
The average food consumption was similar in the two groups (Figure 3B). Measurement of food consumption stopped prior to operation at week 7.
Average body weight did not differ between the two groups throughout the study period and tended to reach a maximum around week 6 (Figure 3C).
Effects of long-term supplementation with rebaudioside A on fasting plasma levels of FFA, triglycerides and cholesterol in type 2 diabetic Goto-Kakizaki rats
The levels of fasting lipids (Table 1) did not differ between the two groups prior to the feeding experiment and we did not observe any significant changes in levels of FFA, triglycerides or total cholesterol between the groups at the end of the study.
Table 1. Blood lipid levels.
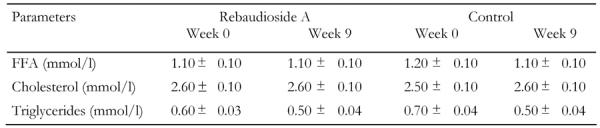
Levels of blood lipids measured after overnight fast prior to and after 9 weeks treatment with rebaudioside A. Data shown as mean ± SEM (n = 11-12 animals per group). There are no significant differences between groups or over time.
Discussion
We tested whether 8 weeks administration of the glycoside rebaudioside A could improve the glucose metabolism and lower blood pressure in the GK rat, effects which we have previously demonstrated for stevioside. Recently, we have shown that rebaudioside A has a potent insulinotropic effect in a dose- and glucose-dependent manner in isolated mouse islets [16]. The present study is, to our knowledge, the first to handle potential in vivo effects of the glycoside rebaudioside A in the GK rat.
Throughout the treatment period, we measured the level of fasting glucose, insulin and glucagon on a weekly basis. Apart from a significantly lower level of fasting blood glucose at weeks 2, 3 and 5, we did not observe any differences in the development of fasting glucose, insulin or glucagon concentrations during the treatment period. We found a tendency towards a decline in the level of fasting glucose and insulin throughout the study period, but the decline was similar in both groups. The development of fasting level of glucagon was similar in both the rebaudioside A-treated and the control group.
After eight weeks supplementation with rebaudioside A, we found similar levels of fasting glucose, insulin and glucagon in both the treated and the control group. These findings are in accordance with what we have previously demonstrated after long-term treatment with stevioside in this animal model [7].
When challenged with glucose during the IAGTT we did not detect any differences between the groups regarding either the responding glucose level or the secretion of insulin or glucagon at any time point. The AUC was similar in the two groups for the whole 3-hour period overall. It should, however, be kept in mind that the insulin and glucagon concentrations are derived from peripheral blood sampling. Consequently, we cannot exclude that the secretion of the pancreatic hormones has changed at the level of the portal vein, an effect that might have disappeared in the periphery due to dilution.
We have previously demonstrated increasing insulin concentrations and a suppression of glucagon both during the glucose tolerance test in long-term stevioside-treated conscious GK rats [7], and after a single bolus injection of stevioside in anesthetized GK rats [18]. The present study does not support our working hypothesis that these effects could also be found for rebaudioside A and even be more convincing than previously demonstrated for stevioside.
The lipid profiles did not differ significantly between the groups throughout the study period. Thus, the level of plasma FFA, triglycerides and total cholesterol remained unchanged. This finding was expected and is in accordance with our previous findings after long-term stevioside treatment [8].
Type 2 diabetes is a chronic metabolic disorder that not only results from insulin resistance and reduced first-phase insulin secretion, but is further characterized by a relative glucagon excess and a pancreatic alpha-cell dysfunction [19]. The suppression of circulating glucagon concentrations reduces blood glucose levels [20], supporting that agents inhibiting the glucagon secretion or action are beneficial for patients with type 2 diabetes. In experimental diabetes, an abnormal alpha-cell function is characterized by an impaired response to glucose and certain metabolites that probably results from a specific defect in glucose recognition [21]. The abnormal alpha-cell function appears not to be ascribed to insulin deficiency per se, but rather to an abnormal metabolic state secondary to insulin deficiency [22]. In the present study we did not detect any effect on glucagon levels and this is in contrast to our previous study with stevioside [7] where we found a suppression of the glucagon level during the first 30 minutes of the glucose tolerance test in GK rats. On the other hand, we have not previously been able to demonstrate this effect after long-term treatment in the Zucker Diabetic Fatty rat [8]. The discrepancy in results may be ascribed to differences in the rat type or the severity of diabetes; however different properties of the glycosides cannot be ruled out.
As can be seen from Figure 3C, the average weight development was similar in the two groups throughout the study period. Figure 3B shows similar food consumption between the groups. Thus, we conclude that the glycoside rebaudioside A supplementation does not affect weight development which corroborates with our previous finding of similar weight development in stevioside-treated GK rats [7].
The initial systolic blood pressure was not different between the two groups (Figure 3A). Subsequently, we observed a significant lowering of the systolic blood pressure in both groups, being most obvious from week 5 onwards. This contrasts with our previous findings for stevioside in the GK [7] and ZDF rat [8]. The lowering of blood pressure in the control group also differed from our previous findings in GK rats [7]. We do not have any obvious explanation for this discrepancy. Numerous studies have until now demonstrated a blood pressure-lowering effect of stevioside in animal models [13, 23] and in humans [14, 15].
Since rebaudioside A has been found to be about 100 times more insulinotropic in vitro than stevioside [16], the obvious question arises: Why does rebaudioside A not possess beneficial effects on blood glucose, islet hormone secretion and blood pressure?
Both stevioside and rebaudioside A are heat- and pH-stable [24, 25] and none of the digestive enzymes of humans or animals seem to be able to degrade stevioside into the aglycone steviol [26, 27]. In contrast, there are indications that stevioside and rebaudioside A are totally degraded to steviol when incubated with intestinal microflora from rats [28], pigs [29] and humans [17]. However, these studies have been carried out ex vivo with microbial specimens collected from the intestine. Recently, a study with orally administered steviol and stevia mixture in rats showed a rapid absorption of steviol per se and a more delayed appearance of steviol in the plasma when administered as a stevia mixture [30]. Koyama et al. further suggest that there are no species differences in the metabolism of steviol between rats and humans [30]. In contrast, Simonetti and co-workers suggested in a preliminary report that intact stevioside is absorbed from the intestine when given orally as a stevia mixture to human volunteers, and steviol-glucuronide seems to be the only metabolite present in plasma and urine [31]. Consequently, there are discrepancies in the literature on the fate of glycosides after oral administration. Altogether, there is convincing evidence, at least in the rat, that the glycosides are degraded to steviol and then absorbed.
In the present study we used the same dose (g/kg BW) of rebaudioside A as we have previously used for stevioside in the GK rat [7]. If we assume that stevioside and rebaudioside A are degraded in the intestine prior to absorption, our choice of dose would result in a 16% reduced concentration of active metabolites for rebaudioside A compared to stevioside. However, a 16 % relative reduction in daily intake of steviol can probably not explain the total lack of those promising effects previously demonstrated after stevioside administration in GK rats [7]. Unfortunately, we are unable to measure the level of rebaudioside A or its metabolites in the circulation, which could help to answer this question.
We conclude that, in the present study, oral administration of rebaudioside A does not act on two of the main features of the metabolic syndrome as previously demonstrated for stevioside, i.e. blood glucose and blood pressure. In the light of our previous in vitro study [16] this appears puzzling and we cannot rule out that the uptake of rebaudioside A has been hampered. Studies on the pharmacokinetics and pharmacodynamics of rebaudioside A are therefore urgently needed.
Acknowledgments
The study was supported by the Danish Medical Research Council; Institute of Experimental Clinical Research, Aarhus University; Aarhus Amtssygehus Forskningsfond; Research Foundation of Aarhus University, The Faculty of Health Science, Aarhus University; The A.P. Møller Foundation for the Advancement of Medical Science and the Novo Nordisk Foundation. The authors wish to thank Lene Trudsø, Kirsten Eriksen, Tove Skrumsager and Dorthe Rasmussen for skilful technical assistance.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Department of Noncommunicable Disease Surveillance; Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998. pp. 837–853. [PubMed]
- 4.UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 5.Soejarto DD, Kinghorn AD, Farnsworth NR. Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness. J Nat Prod. 1982;45:590–599. doi: 10.1021/np50023a013. [DOI] [PubMed] [Google Scholar]
- 6.Shibata H, Sawa Y, Oka T, Sonoke S, Kim KK, Yoshioka M. Steviol and steviol-glycoside: glucosyltransferase activities in Stevia rebaudiana Bertoni - purification and partial characterization. Arch Biochem Biophys. 1995;321:390–396. doi: 10.1006/abbi.1995.1409. [DOI] [PubMed] [Google Scholar]
- 7.Jeppesen PB, Gregersen S, Rolfsen SE, Jepsen M, Colombo M, Agger A, Xiao J, Kruhoffer M, Orntoft T, Hermansen K. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism. 2003;52:372–378. doi: 10.1053/meta.2003.50058. [DOI] [PubMed] [Google Scholar]
- 8.Dyrskog SEU, Jeppesen PB, Colombo M, Abudula R, Hermansen K. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in ZDF rats. Metabolism. 2005 doi: 10.1016/j.metabol.2005.03.026. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat) Adv Exp Med Biol. 1988;246:29–31. doi: 10.1007/978-1-4684-5616-5_4. [DOI] [PubMed] [Google Scholar]
- 10.Ostenson CG, Khan A, Abdel-Halim SM, Guenifi A, Suzuki K, Goto Y, Efendic S. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic GK rat. Diabetologia. 1993;36:3–8. doi: 10.1007/BF00399086. [DOI] [PubMed] [Google Scholar]
- 11.Movassat J, Saulnier C, Serradas P, Portha B. Impaired development of pancreatic beta-cell mass is a primary event during the progression to diabetes in the GK rat. Diabetologia. 1997;40:916–925. doi: 10.1007/s001250050768. [DOI] [PubMed] [Google Scholar]
- 12.Metz SA, Meredith M, Vadakekalam J, Rabaglia ME, Kowluru A. A defect late in stimulus-secretion coupling impairs insulin secretion in Goto-Kakizaki diabetic rats. Diabetes. 1999;48:1754–1762. doi: 10.2337/diabetes.48.9.1754. [DOI] [PubMed] [Google Scholar]
- 13.Chan P, Xu DY, Liu JC, Chen YJ, Tomlinson B, Huang WP, Cheng JT. The effect of stevioside on blood pressure and plasma catecholamines in spontaneously hypertensive rats. Life Sci. 1998;63:1679–1684. doi: 10.1016/s0024-3205(98)00439-1. [DOI] [PubMed] [Google Scholar]
- 14.Chan P, Tomlinson B, Chen YJ, Liu JC, Hsieh MH, Cheng JT. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br J Clin Pharmacol. 2000;50:215–220. doi: 10.1046/j.1365-2125.2000.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh MH, Chan P, Sue YM, Liu JC, Liang TH, Huang TY, Tomlinson B, Chow MS, Kao PF, Chen YJ. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: a two-year, randomized, placebo-controlled study. Clin Ther. 2003;25:2797–2808. doi: 10.1016/s0149-2918(03)80334-x. [DOI] [PubMed] [Google Scholar]
- 16.Abudula R, Jeppesen PB, Rolfsen SE, Xiao J, Hermansen K. Rebaudioside A potently stimulates insulin secretion from isolated mouse islets: Studies on the dose-, glucose-, and calcium-dependency. Metabolism. 2004;53:1378–1381. doi: 10.1016/j.metabol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Gardana C, Simonetti P, Canzi E, Zanchi R, Pietta P. Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora. J Agric Food Chem. 2003;51:6618–6622. doi: 10.1021/jf0303619. [DOI] [PubMed] [Google Scholar]
- 18.Jeppesen PB, Gregersen S, Alstrup KK, Hermansen K. Stevioside induces antihyperglycemic, insulinotropic and glucagonostatic effects in vivo: Studies in diabetic Goto-Kakizaki (GK) rats. Phytomedicine. 2002;9:9–14. doi: 10.1078/0944-7113-00081. [DOI] [PubMed] [Google Scholar]
- 19.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27:1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 20.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. doi: 10.1210/jcem.85.11.6993. [DOI] [PubMed] [Google Scholar]
- 21.Hermansen K. Characterisation of the abnormal pancreatic D and A cell function in streptozotocin diabetic dogs: studies with D-glyceraldehyde, dihydroxyacetone, D-mannoheptulose, D-glucose, and L-arginine. Diabetologia. 1981;21:489–494. doi: 10.1007/BF00257791. [DOI] [PubMed] [Google Scholar]
- 22.Hermansen K, Schmitz O, Orskov H. Reversal of D- and A-cell insensitivity to glucose in alloxan diabetic dogs by treatment with the artificial beta-cell (Biostator) Diabetes. 1985;34:260–266. doi: 10.2337/diab.34.3.260. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YH, Liu JC, Kao PF, Lee CN, Chen YJ, Hsieh MH, Chan P. Antihypertensive effect of stevioside in different strains of hypertensive rats. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65:1–6. [PubMed] [Google Scholar]
- 24.Chang SS, Cook JM. Stability studies of stevioside and rebaudioside A in carbonated beverages. J Agric Food Chem. 1983;31:409–412. [Google Scholar]
- 25.Toyoda K, Matsui H, Shoda T, Uneyama C, Takada K, Takahashi M. Assessment of the carcinogenicity of stevioside in F344 rats. Food Chem Toxicol. 1997;35:597–603. doi: 10.1016/s0278-6915(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 26.Hutapea AM, Toskulkao C, Buddhasukh D, Wilairat P, Glinsukon T. Digestion of stevioside, a natrual sweetener, by various digestive enzymes. J Clin Biochem Nutr. 1997;23:177–186. [Google Scholar]
- 27.Wingard RE Jr, Brown JP, Enderlin FE, Dale JA, Hale RL, Seitz CT. Intestinal degradation and absorption of the glycosidic sweeteners stevioside and rebaudioside A. Experientia. 1980;36:519–520. doi: 10.1007/BF01965774. [DOI] [PubMed] [Google Scholar]
- 28.Koyama E, Kitazawa K, Ohori Y, Izawa O, Kakegawa K, Fujino A, Ui M. In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food Chem Toxicol. 2003;41:359–374. doi: 10.1016/s0278-6915(02)00235-1. [DOI] [PubMed] [Google Scholar]
- 29.Geuns JM, Augustijns P, Mols R, Buyse JG, Driessen B. Metabolism of stevioside in pigs and intestinal absorption characteristics of stevioside, rebaudioside A and steviol. Food Chem Toxicol. 2003;41:1599–1607. doi: 10.1016/s0278-6915(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 30.Koyama E, Sakai N, Ohori Y, Kitazawa K, Izawa O, Kakegawa K, Fujino A, Ui M. Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem Toxicol. 2003;41:875–883. doi: 10.1016/s0278-6915(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 31.Simonetti P, Gardana C, Bramati L, Pietta P. In: Geuns JM, Buyse JG, Eds. The Safety of Stevioside. Euprint ed.; 2004. Bioavailability of stevioside from Stevia Rebaudiana in human volunteers: preliminary report; pp. 51–62. [Google Scholar]


