Abstract
BACKGROUND: Diet has long been associated with a risk of insulin resistance and poor glycemic control. We sought to investigate the association between food groups and indices of glycemic control in adults without type 2 diabetes and cardiovascular disease. METHODS: During 2001 - 2002 we randomly enrolled 1514 men (18-87 years old) and 1528 women (18-89 years old) without evidence of cardiovascular disease from the Attica area of Greece. Of them, 118 men and 92 women were excluded from the present analysis due to a history of diabetes mellitus (type 2). Fasting blood glucose and insulin levels were measured, while dietary habits were evaluated through a semi-quantitative food frequency questionnaire. RESULTS: Red meat consumption was positively associated with hyperglycemia (p = 0.04), hyperinsulinemia (p = 0.04), and HOMA levels (p = 0.03), even after adjusting for BMI and various other potential confounders. The intake of fruits, vegetables, legumes, yogurt and other dairy products was not associated with levels of glycemic control indices. CONCLUSIONS: A higher consumption of red meat and its products may aggravate hyperinsulinemia and insulin resistance in non-diabetic people.
Keywords: insulin, glycemic control, metabolic syndrome, diet
Introduction
Dietary habits have long been associated with the management and/or prevention of various metabolic disorders, such as insulin resistance, obesity, type 2 diabetes, as well as with the development of cardiovascular diseases [1, 2]. In particular, the adoption of a dietary pattern characterized by a high intake of red meat, besides other components, including refined grain products, snacks, sweets and fried foods, is believed to contribute to the increased prevalence of type 2 diabetes worldwide [3-5]. In contrast, the adoption of a dietary pattern that is characterized by a high consumption of non-refined cereals, fruits and vegetables, a moderate intake of dairy produce, poultry and fish, and a low intake of red meat, typical of the Mediterranean diet, is believed to contribute through various mechanisms towards a reduction in the prevalence of type 2 diabetes, metabolic syndrome and cardiovascular disease [6-8].
Insulin resistance is a major constituent of the metabolic syndrome - characterized by central fat, hyperglycemia, hyperinsulinemia and dyslipidemia - and has been associated with the development of type 2 diabetes and cardiovascular disease [9]. In recent years, several clinical and epidemiological studies have attempted to associate various food intakes with the risk of insulin resistance, type 2 diabetes and cardiovascular disease [1-9]. Some studies showed that the consumption of red meat, particularly processed meat, increases the risk of insulin resistance and type 2 diabetes, whereas there is controversy over poultry. Moreover, other studies have suggested that a high fish and seafood consumption may reduce the risk of type 2 diabetes in populations with a prevalence of obesity, while others have found no benefits [10]. In addition, results from the Health Professionals Follow-up Study indicate that a high intake of low-fat dairy or skimmed milk products, but not whole milk, was associated with a lower risk of insulin resistance and diabetes mellitus among men [11]. Yet, the British Women's Heart and Health Study has found that avoiding milk is associated with a reduced insulin resistance [12]. Finally, it is generally accepted that a high intake of fruits, vegetables and legumes is associated with a reduced risk of insulin resistance, type 2 diabetes and cardiovascular disease [13].
Since the results from previous studies on the effects of food groups on indices of glycemic control appear conflicting, we sought to evaluate the association of dietary habits with fasting blood glucose and insulin levels in men and women without diabetes mellitus and cardiovascular disease.
Methods
Population of the study
From May 2001 to December 2002, 4056 inhabitants of the Attica region, which includes 78% urban and 22% rural areas, were randomly selected and asked to enroll into the "ATTICA" study; 3042 of them agreed to participate (75% participation rate). All participants were interviewed by trained staff (cardiologists, general practitioners, dieticians and nurses) who used standard questionnaires that evaluated lifestyle habits and various socio-demographic, clinical and biological characteristics. 5% of the men and 3% of the women were excluded from the study because they reported a history of cardiovascular or any other atherosclerotic disease, or chronic viral infections. Moreover, participants were required not to have had a cold or flu, an acute respiratory infection, dental problems or any type of surgery during the past few weeks. In this work, we also excluded 118 men and 92 women who had type 2 diabetes mellitus. A clinically confirmed history of diabetes was also recorded and all participants underwent the fasting plasma glucose test. In accordance with the American Diabetes Association diagnostic criteria, participants with blood glucose levels greater than 125 mg/dl were classified as having diabetes (excluded), while those with glucose levels between 100 and 125 mg/dl were classified as having impaired fasting glucose (IFG) [14]. Patients with type 1 diabetes were also excluded from the analysis due to their small sample size (i.e. < 1% of the study's population).
The study was approved by the Medical Research Ethics Committee of the First Cardiology Clinic of Athens University and was carried out in accordance with the Declaration of Helsinki (1989) of the World Medical Association.
Dietary assessment
The participant’s usual dietary intake over the year preceding enrollment was assessed by a validated, semi-quantitative food frequency questionnaire, including 156 foods and beverages commonly consumed in Greece [15]. We asked all participants to report the daily or weekly average intake of several food items that they consumed. Then, the frequency of consumption was quantified approximately in terms of the number of times per month this food was consumed. Moreover, based on a special database that incorporated food composition tables, we also calculated total energy, protein, carbohydrate and total fat (saturated, monounsaturated, polyunsaturated) intake.
Socio-demographic and lifestyle variables
Current smokers were defined as those who smoked at least one cigarette per day; former smokers were defined as those who had stopped smoking for at least one year and the rest of the participants were defined as non-smokers. Occasional smokers (less than 7 cigarettes per week) were recorded and combined with current smokers due to their small sample size. For a more accurate evaluation of smoking habits we calculated the pack-years (cigarette packs per day multiplied by the number of years of smoking), adjusted for a nicotine content of 0.8 mg/cigarette. For the ascertainment of physical activity status we developed an index of weekly energy expenditure using frequency (times per week), duration (in minutes per time) and intensity of sports or other habits related to physical activity. Intensity was gradated in qualitative terms such as: light (expended calories < 4 kcal/min, i.e. walking slowly, cycling stationary, light stretching etc.), moderate (expended calories 4-7 kcal/min, i.e. walking briskly, cycling outdoors, swimming with moderate effort etc.) and high (expended calories < 7 kcal/min, i.e. walking briskly uphill, long-distance running, cycling fast or racing, swimming fast crawl etc.). Participants who did not report any physical activities were defined as sedentary. For the rest of the participants we calculated a combined score by multiplying the weekly frequency, duration and intensity of physical activity.
Anthropometric, clinical and biochemical characteristics
The participant’s standing height and weight were recorded, and the body mass index (BMI) was calculated as weight (in kilograms) divided by standing height (in meters squared). According to standard guidelines, those with a BMI greater that 24.9 kg/m2 were defined as overweight,and those with a BMI greater than 29.9 kg/m2 were defined as obese. Arterial blood pressure was measured three times at the end of the physical examination with the subject in a sitting position. People with systolic/diastolic blood pressure levels < 140/90 mmHg or who were under special medication were defined as having hypertension. Blood samples were collected from the antecubital vein between 8 and 10 a.m., in a sitting position, after 12 h of fasting and avoidance of alcohol. Cholesterols and triglyceride levels were also measured in all participants, using the colorimetric enzymic method in a Technicon automatic analyzer RA-1000 (Dade-Behring Marburg GmbH, Marburg, Germany). Total cholesterol levels < 200 mg/dl or use of lipid-lowering agents classified participants as having hypercholesterolemia. Blood glucose levels (in mg/dl) were measured immediately with a Beckman Glucose Analyzer (Beckman Instruments, Fullerton, CA, USA). Serum insulin concentrations (μU/ml) were assayed by means of radioimmunoassay (RIA100, Pharmacia Co., Erlangen, Germany). Precision was 12% for low (3 μU/ml) and 5% for high (90 μU/ml) serum levels. The intra-assay coefficient of variation was 9% and the limit of detection was 3 μU/ml. Insulin resistance was assessed by the calculation of the homeostasis model assessment (HOMA-R) approach (glucose in mg/dl x insulin in μU/ml / 22.5), while for the calculation of insulin secretory capacity (HOMA-B) we used the formula: (fasting insulin in μU/ml) x 3.33 / (fasting glucose in mg/dl - 3.5).
Further details about the methods used in the "ATTICA" study may be found in the study literature [16].
Statistical analysis
Power analysis showed that the number of enrolled participants is adequate to evaluate two-sided standardized differences between the subgroups of the study and the investigated parameters greater than 0.5, achieving a statistical power greater than 0.90 at a 5% probability level (p-value).
Continuous variables are presented as mean values ± standard deviation. Categorical variables are presented as absolute and relative frequencies. Associations between categorical variables were evaluated by means of the chi-squared test, while differences between categorical and several biochemical, clinical and nutritional variables were tested using the Student's t-test and the Mann-Whitney criterion (for the normally-distributed and the skewed variables, respectively). Due to multiple comparisons we used the Bonferroni correction in order to account for the increase in type 1 error. A multiple linear regression model was applied to test the association between food intake and the investigated biomarkers of glycemic control, after controlling for several potential confounders. Normality of the residuals was tested using the Shapiro-Wilk test. Co-linearity between independent variables was evaluated through the Variance Inflation Factor, and serial autocorrelation was tested through the Durbin-Watson criterion. The fitness levels of the subjects were graphically evaluated, i.e. standardized residuals against fitted values. All reported p-values are based on two-sided tests and compared to a significance level of 5%. SPSS 12 (SPSS Inc., Chicago, IL, USA) software was used for all the statistical calculations.
Results
Table 1 illustrates the various characteristics of the participants. As we can see, male and female subjects were of a similar age, had similar rates of physical inactivity and family history of diabetes, while there were significantly more men with obesity, hypertension and hypercholesterolemia than women. Moreover, the men were more frequently smokers and had a higher education status than the women. Furthermore, men had higher fasting glucose, insulin and HOMA levels.
Table 1. Gender-specific characteristics of participants free of diabetes and cardiovascular disease.
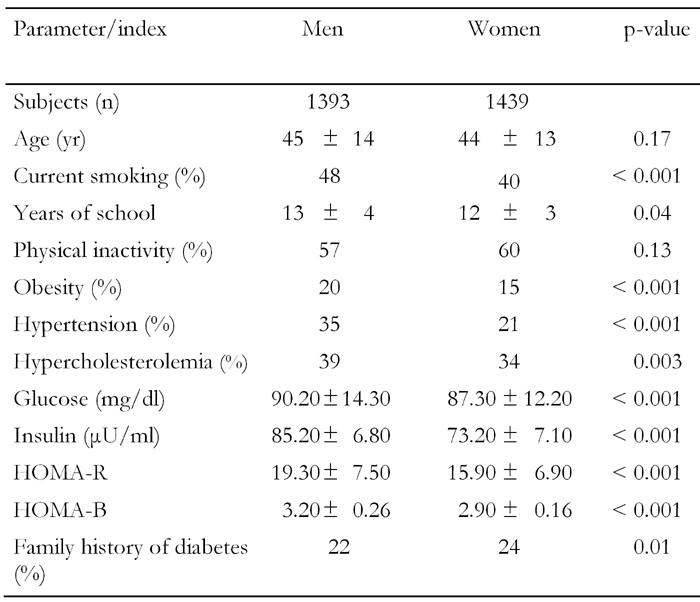
Data are mean ± SD. HOMA: homeostasis model assessment. Calculation of the HOMA-R-value: (glucose in mg/dl) x (insulin in μU/ml) / 22.5. Calculation of HOMA-B: (fasting insulin in μU/ml) x 3.33 / (fasting glucose in mg/dl - 3.5).
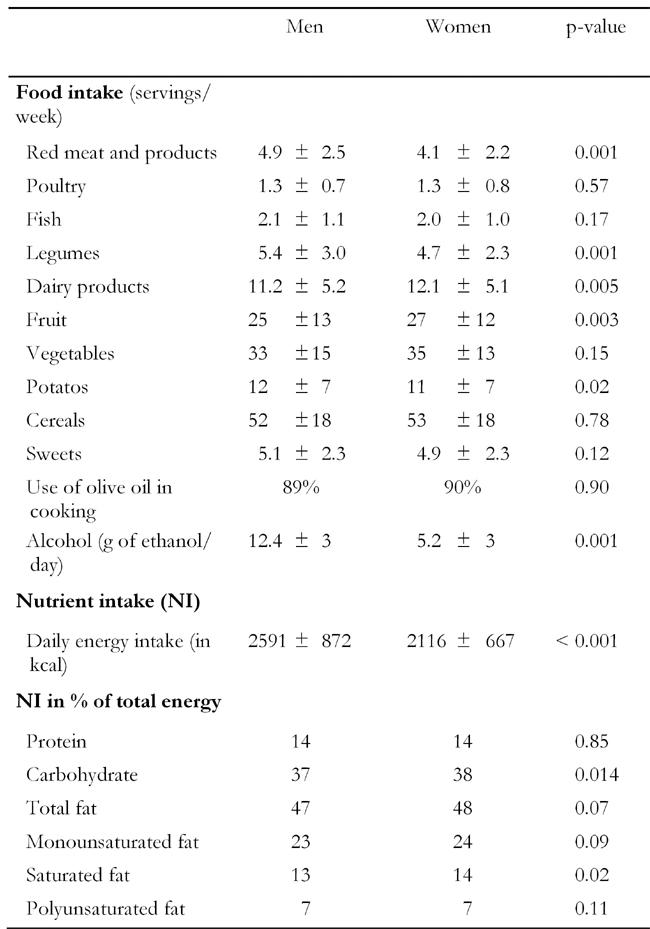
Table 2 illustrates food consumption and nutrient intake in men and women. Although some significant differences were observed in dietary habits between men and women, most of these differences could be attributed to the large sample size. However, we could say that men consumed more servings of red meat and meat products, potatoes, legumes and alcoholic beverages and less portions of fruit and dairy products than women (at p < 0.05). Moreover, although men had a higher energy intake than women, no significant differences between genders were observed in the macronutrient intake.
Table 2. Weekly food consumption and nutrient intake in men and women.
Dietary analysis revealed that protein and carbohydrate intakes were not associated with fasting blood glucose, fasting insulin, insulin resistance or insulin secretory capacity, after adjusting for age and sex (Table 3). Initially, fat intake was inversely correlated with fasting insulin and HOMA-R levels, but when we also adjusted for the BMI of the participants we observed that the fat intake was no longer associated with glycemic control indices (data not presented in text or tables).
Table 3. Partial correlation coefficients between indices of glycemic control and macronutrient intake.
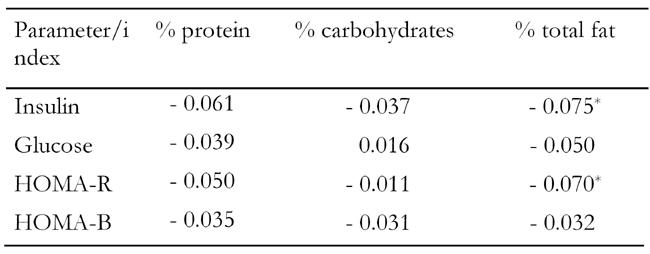
Correlation coefficients were adjusted for age and sex. HOMA: homeostasis model assessment. HOMA-R: assessment of insulin resis-tance. HOMA-B: assessment of insulin secretory capacity. * p < 0.05.
Further food group analysis showed that red meat consumption was positively correlated with insulin levels and insulin resistance, but not blood glucose, after adjusting only for age and sex (Table 4). However, multiple regression analysis revealed that red meat intake was positively associated with insulin, HOMA-R, and with blood glucose, after adjusting for gender, age, BMI, clinical characteristics and various other potential confounders. Specifically, for every serving of red meat consumed per day, a 0.42 mg/dl increase in glucose and a 0.32 μU/ml increase in insulin levels were observed (Table 5). Fish intake was also positively correlated with blood glucose, insulin and HOMA (Table 4); however, this association became insignificant after we adjusted for BMI and other potential confounders (Table 5). Although there was a strong trend for the consumption of vegetables to be positively correlated with glucose and insulin (Table 4), this association became insignificant after we adjusted for the BMI of the participants. Moreover, HOMA-B was positively correlated with fish and potato intake (Table 4), but when age, sex and BMI were taken into account this relationship became insignificant. All other foods, such as yogurt, poultry, feta cheese, fruits, and potatoes were not associated with any of the indices of glycemic control in all participants.
Table 4. Partial correlations coefficients that evaluated the relationships between indices of glycemic control and consumption of various foods.
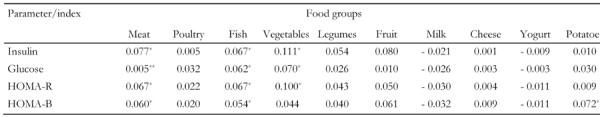
Correlation coefficients were adjusted for age and sex. HOMA: homeostasis model assessment. HOMA-R: assessment of insulin resistance. HOMA-B: assessment of insulin secretory capacity. * p < 0.05. ** p < 0.01.
Table 5. Results from linear regression analysis that evaluated the association between glycemic control indices and meat, fish and whole milk.
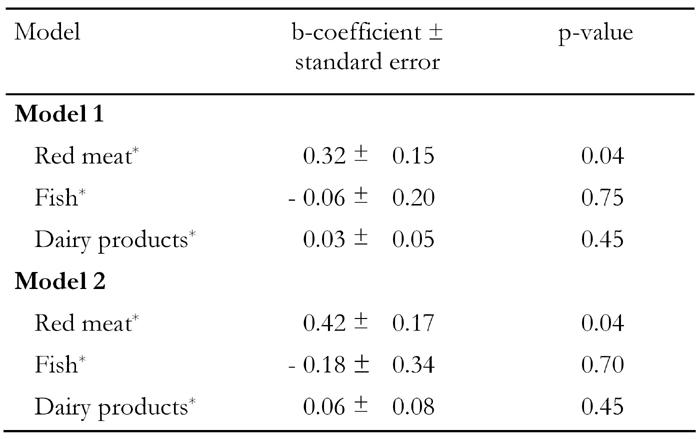
Model 1: insulin is dependent variable. Model 2: glucose is dependent variable. Both models were adjusted for age, gender, BMI, physical activity status, smoking habits, years of school, presence of hypertension and hypercholesterolemia. * Servings per week.
Discussion
Our study indicates that a higher consumption of red meat may aggravate indices of glycemic control in subjects without diabetes, which may lead to the insulin resistance-related development of chronic diseases.
The observed relationship between red meat and hyperglycemia, hyperinsulinemia and insulin sensitivity has been consistent with the findings of other studies [17, 18]. The total fat content of red meat, particularly saturated fat, is believed to be one of the main contributors to the increased prevalence of insulin resistance and type 2 diabetes [19]. We observed an inverse correlation between total fat intake and indices of glycemic control in subjects without diabetes, although BMI-dependent. However, less than one third of the total fat intake was saturated and only a portion of saturated fat was derived from meat consumption. Therefore, further investigation is needed to conclude if the high saturated fat content of read meat has a major involvement in the insulin resistance process. Furthermore, we did not observe an association between protein intake and indices of glycemic control and thus, we cannot claim that the adverse association of red meat consumption with indices of glycemic control was mediated through its high protein content, but it may be that cholesterol content or certain cooking or processing practices such as nitrates and nitrites, trans fatty-acids, glycotoxins or heme-iron load may be responsible [20, 21]. However, it was not the purpose of this study to investigate these parameters.
We did not observe a significant association between the consumption of chicken with indices of glycemic control, and this is consistent with the findings of other studies [22]. In fact, results from the Nurses' Health Study showed that frequent poultry intake was associated with a moderately decreased risk of type 2 diabetes [23]. Nevertheless, in the diabetic population of the ATTICA study we observed a trend for a positive correlation of chicken consumption with insulin resistance. A large fraction of the population who has been diagnosed with diabetes follows advice to substitute read meat with poultry and in that case most of their saturated fat is derived from this food. More studies are required in order to investigate the relation of poultry consumption with insulin resistance both in diabetic and non-diabetic people [24].
In our study, we also observed an inverse correlation between total fat intake and indices of glycemic control in subjects without diabetes. The diets of our participants contained high amounts of fat, predominantly monounsaturated fatty acids (MUFA), in the form of olive oil. Data from epidemiological and controlled metabolic studies have shown that consumption of MUFAs has been associated with improvements in dyslipidemia, even in glycemic control as long as it does not lead to a higher BMI [25-27]. This may be one of the reasons why the lipid profile of our participants was good, although their diets have some characteristics of a "westernized diet". Whether the consumption of a rich MUFA diet alone or in combination with other dietary components contributed to this lipid profile is not clear.
The fact that not all fats are the same is well known. The anti-inflammatory effect of n-3 fatty acids, obtained from fish oil is well established. As diabetes mellitus is considered to be related with a low-grade chronic inflammation, one could assume that fish consumption would be linked with diabetes prevention or control [28]. However, the effects of fish consumption on indices of glycemic control are inconsistent; some have suggested that fish consumption is associated with a decreased risk of type 2 diabetes and obesity and a lower incidence of both coronary heart disease and total mortality, due to its omega-3 fatty acid content, but others have not [29, 30]. We observed that fish consumption was positively associated with hyperglycemia, hyperinsulinemia, and insulin resistance in non-diabetic people, and that this association was mainly dependent on BMI status. This correlation was not found in the diabetic population of our study. The reason for this adverse association is not clear, but it may be that beta-cell function in obese non-diabetic subjects may already have been deteriorating and thus failing to normalize the fish intake-induced hyperglycemia. Furthermore, it has been proposed that when omega-3 fatty acids become an additional source of fatty acids, utilization of glucose may be inhibited, leading to hyperglycemia. Glauber et al. reported a 26% increase in hepatic glucose output with fish oil supplementation in patients with type 2 diabetes without any change in glucose uptake [31]. As fish is consumed often in Greece, it may significantly contribute towards increasing energy intake and BMI.
We do not reveal an association between milk consumption and glycemic indices in the non-diabetic subjects of our study, neither with yogurt nor cheese. In contrast, in our diabetic population, whole milk aggravated hyperinsulinemia and insulin sensitivity, which may be due to the insulinotropic properties of milk proteins [32]. Results from different studies are conflicting, some showing that milk and calcium intake is associated with a lower prevalence of hyperinsulinemia and insulin resistance, others that milk leads to a higher insulin resistance [33-35]. It is unknown whether similar results would be obtained with low-fat milk consumption and this needs additional examination.
The consumption of fruits, vegetables and legumes has been associated with a reduced risk of insulin resistance and type 2 diabetes, as already mentioned [13]. We, however, found no association between these foods and indices of glycemic control in subjects without diabetes. Interestingly, we observed a legume-induced increased insulin secretion, which was BMI status-dependent, selectively in subjects without diabetes. Although legumes have no saturated fat, are rich in fibers and have a low glycemic index, they are often consumed in Greek dishes with large quantities of olives, bread and other starches, and this may partially explain this BMI-dependent rise in glycemic indices. The potential insulinotropic properties of the protein content of legumes need to be examined in detail.
Limitations
This is a cross-sectional study that cannot provide causal relationships but only state hypotheses for future research. The evaluation of food habits was ascertained once, through a food frequency questionnaire. We cannot exclude the possibility of under-reporting or over-reporting, although subjects completed the food frequency questionnaires with the help and supervision of well-trained staff. Further dietary evaluation could increase the accuracy of our findings. Also, because of a high degree of statistical co-linearity, our ability to distinguish the effects of our foods of interest, such as red meat from intakes of its major components such as animal fat, animal protein and iron was limited. In addition, a limited variation of intakes of macronutrients and subtypes of total red meat, fish, milk, etc. in our subjects could lead to insufficient statistical power to detect significant association.
Conclusion
In summary, our study indicates that a higher consumption of total meat may lead to insulin resistance and related chronic disease development, such as obesity, diabetes and cardiovascular diseases. A higher consumption of read meat is a typical component of a "westernized" diet. It could be suggested that the total substitution of meat for cheese, yogurt and vegetable sources of proteins may ameliorate hyperinsulinemia and insulin resistance in non-diabetic subjects.
Acknowledgments
The ATTICA study is supported by research grants from the Hellenic Society of Cardiology (#1-HCS2002). The authors would like to thank the field investigators of the "ATTICA" study: Yannis Skoumas, Natassa Katinioti (physical examination), Akis Zeimbekis (physical examination), Spiros Vellas (physical examination), Efi Tsetsekou (physical/psychological evaluation), Dina Massoura (physical examination), Lambros Papadimitriou (physical examination), as well as the technical team: Marina Toutouza (principal investigator in biochemical analysis), Carmen Vassiliadou (genetic analysis), Manolis Kambaxis (nutritional evaluation), Konstadina Palliou (nutritional evaluation), Constadina Tselika (biochemical evaluation), Sia Poulopoulou (biochemical evaluation) and Maria Toutouza (database management).
References
- 1.Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Lindstrom J, Louheranta A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutr. 2004;7:147–165. doi: 10.1079/phn2003586. [DOI] [PubMed] [Google Scholar]
- 2.Villegas R, Salim A, Flynn A, Perry IJ. Prudent diet and the risk of insulin resistance. Nutr Metab Cardiovasc Dis. 2004;14:334–343. doi: 10.1016/s0939-4753(04)80023-1. [DOI] [PubMed] [Google Scholar]
- 3.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 4.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia. 2003;46:1465–1473. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- 5.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25:417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton P, Eckel RH, Howard BV. AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation. 2001;103:1823–1825. doi: 10.1161/01.cir.103.13.1823. [DOI] [PubMed] [Google Scholar]
- 7.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 8.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Jarrett RJ. The metabolic syndrome. Lancet. 2005;366(9501):1922. doi: 10.1016/S0140-6736(05)67779-3. [DOI] [PubMed] [Google Scholar]
- 10.Nkondjock A, Receveur O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab. 2003;29:635–642. doi: 10.1016/s1262-3636(07)70080-0. [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165:997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor DA, Ebrahim S, Timpson N, Davey Smith G. Avoiding milk is associated with a reduced risk of insulin resistance and the metabolic syndrome: findings from the British Women's Heart and Health Study. Diabet Med. 2005;22:808–811. doi: 10.1111/j.1464-5491.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Serdula M, Janket SJ, Cook NR, Sesso HD, Willett WC, Manson JE, Buring JE. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care. 2004;27:2993–2996. doi: 10.2337/diacare.27.12.2993. [DOI] [PubMed] [Google Scholar]
- 14.Association AD. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.Katsouyanni K, Rimm EB, Gnardellis C, Trichopoulos D. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int J Epidemiol. 1997;26:S118–S127. doi: 10.1093/ije/26.suppl_1.s118. [DOI] [PubMed] [Google Scholar]
- 16.Pitsavos C, Panagiotakos DB, Chrysohoou C, Stefanadis C. Epidemiology of Cardiovascular risk factors in Greece; aims, design and baseline characteristics of the ATTICA study. BMC Public Health. 2003;3:1–9. doi: 10.1186/1471-2458-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami K, Okubo H, Sasaki S. Effect of dietary factors on incidence of type 2 diabetes: a systematic review of cohort studies. J Nutr Sci Vitaminol. 2005;51:292–310. doi: 10.3177/jnsv.51.292. [DOI] [PubMed] [Google Scholar]
- 18.Duc Son le NT, Hanh TT, Kusama K, Kunii D, Sakai T, Hung NT, Yamamoto S. Anthropometric characteristics, dietary patterns and risk of type 2 diabetes mellitus in Vietnam. J Am Coll Nutr. 2005;24:229–234. doi: 10.1080/07315724.2005.10719469. [DOI] [PubMed] [Google Scholar]
- 19.Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Med Sci Monit. 2005;11:RA359–367. [PubMed] [Google Scholar]
- 20.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr. 2004;79:70–75. doi: 10.1093/ajcn/79.1.70. [DOI] [PubMed] [Google Scholar]
- 21.Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H. Glycotoxins: a missing link in the "relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes Care. 2002;25:1898–1899. doi: 10.2337/diacare.25.10.1898. [DOI] [PubMed] [Google Scholar]
- 22.Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59:441–448. doi: 10.1038/sj.ejcn.1602094. [DOI] [PubMed] [Google Scholar]
- 23.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidmann C, Colditz GA, Hu FB. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–684. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archer SL, Greenlund KJ, Valdez R, Casper ML, Rith-Najarian S, Croft JB. Differences in food habits and cardiovascular disease risk factors among Native Americans with and without diabetes: the Inter-Tribal Heart Project. Public Health Nutr. 2004;7:1025–1032. doi: 10.1079/PHN2004639. [DOI] [PubMed] [Google Scholar]
- 25.Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. 1998;67:577S–582S. doi: 10.1093/ajcn/67.3.577S. [DOI] [PubMed] [Google Scholar]
- 26.Bautista MC, Engler MM. The Mediterranean diet: is it cardioprotective? Prog Cardiovasc Nurs. 2005;20:70–76. doi: 10.1111/j.0889-7204.2005.04558.x. [DOI] [PubMed] [Google Scholar]
- 27.Hofman Z, van Drunen JD, de Later C, Kuipers H. The effect of different nutritional feeds on the postprandial glucose response in healthy volunteers and patients with type II diabetes. Eur J Clin Nutr. 2004;58:1553–1556. doi: 10.1038/sj.ejcn.1602007. [DOI] [PubMed] [Google Scholar]
- 28.Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–440. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Holness MJ, Greenwood GK, Smith ND, Sugden MC. Diabetogenic impact of long-chain omega-3 fatty acids on pancreatic beta-cell function and the regulation of endogenous glucose production. Endocrinology. 2003;144:3958–3968. doi: 10.1210/en.2003-0479. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 31.Glauber H, Wallace P, Griver K, Brechtel G. Adverse metabolic effect of omega-3 fatty acids in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1988;108:663–668. doi: 10.7326/0003-4819-108-5-663. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–1253. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–2932. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 34.Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 35.Hoppe C, Molgaard C, Vaag A, Barkholt V, Michaelsen KF. High intakes of milk, but not meat, increase s-insulin and insulin resistance in 8-year-old boys. Eur J Clin Nutr. 2005;59:393–398. doi: 10.1038/sj.ejcn.1602086. [DOI] [PubMed] [Google Scholar]


