Abstract
BACKGROUND and OBJECTIVES: The renin-angiotensin system plays a critical role in circulatory homoeostasis. Evidence has emerged that suggests a pathologic role for angiotensin II in patients with kidney disease. Losartan is an antagonist of angiotensin II and blocks the angiotensin II type-1 receptor. Thus it may reduce proteinuria and delay the progression of renal disease in diabetic nephropathy. We investigated the effects of losartan on the mRNA expressions of membrane-type3 matrix metalloproteinases (MT3-MMP) and the tissue inhibitor of metalloproteinase-2 (TIMP-2) in diabetic kidneys in order to evaluate degradation and remodeling of the extracellular matrix. METHODS: Male Wistar rats were divided into 3 groups. Group A was the control group containing healthy rats (n = 11), group B comprised diabetic rats without any therapy (n = 11), and group C consisted of diabetic rats treated with losartan (n = 9). 24-hr urine samples were collected in order to measure urinary albumin excretion (UAE). After a period of 18 weeks, the kidneys were extracted from all rats in order to measure the mRNA expressions of MT3-MMP, TIMP-2 and transforming growth factor-β1 (TGF-β1) by RT-PCR. We also examined the glomerular basement membrane thickening and the mesangial matrix (MM) density (MM area/mesangial area). RESULTS: The expression of renal MT3-MMP mRNA in group B (1.37 ± 0.96) was significantly higher than that in group A (0.75 ± 0.34, p < 0.05), but also significantly higher than in group C (0.75 ± 0.30, p < 0.05). Similarly, the mRNA expression of renal TIMP-2 in group B (0.73 ± 0.37) was significantly increased compared to that in group A (0.32 ± 0.19, p < 0.05), but also higher than in group C (0.34 ± 0.17, p < 0.05). In addition, subjects in group B showed abundant TGF-β1 mRNA expression and UAE compared to groups A and C, as well as significantly higher glomerular basement membrane thickening and MM density (all p < 0.05). CONCLUSIONS: We conclude that MT3-MMP and TIMP-2 production in the renal cortex of diabetic kidneys is increased. Losartan can prevent the development of diabetic nephropathy by decreasing MT3-MMP and TIMP2 production in diabetic kidneys.
Keywords: diabetic nephropathy, losartan, TIMP-2, MT3-MMP, TGF-β1
Introduction
Extracellular matrix (ECM) accumulation and glomerular basement membrane (GBM) thickening are the dominant histological abnormalities found in early diabetic nephropathy (DN). It has been shown that increased synthesis of GBM collagens and mesangial matrix (MM) proteins play a key role in the pathogenesis of DN. Recent studies have demonstrated that their decreased degradation also contributes to the pathogenesis of DN [1]. Matrix metalloproteinases (MMPs) are proteolytic enzymes and belong to a group of proteinases which degrade GBM and MM components. Membrane-type matrix metalloproteinases (MT-MMPs), including MT1-MMP, MT2-MMP, MT3-MMP and so on, induce the correct location of MMPs on the cell surface and activate them [2]. The tissue inhibitor of metalloproteinases (TIMP), a natural inhibitor of MMPs, can reduce MMP activity and thus prevent the degradation of GBM and MM proteins [2].
Angiotensin II (Ang II) is assumed to increase intracellular reactive oxygen species in renal cells and thus contribute to the development and progression of diabetic renal injury [3]. A recent study has also shown that MMP-2 and TIMP mRNA levels were elevated in Ren2 rats exhibiting renal damage [4]. Losartan is an antagonist of Ang II type 1 receptor (AT1R). Clinical data support the view that losartan prevents the development and progression of DN [5]. However, the mechanisms underlying its protective effect remain unclear. In order to investigate the role of Ang II and the response to angiotensin-converting enzyme inhibition on MMPs and TIMPs, we examined the effect of losartan on the mRNA expressions of MT3-MMP and TIMP-2 in the kidneys of diabetic rats.
Materials and methods
Preparation of the diabetic rat model
48 male Wister rats weighing 220 g to 260 g were divided randomly into 3 groups. 12 rats were normal control subjects (group A). The others were injected with streptozotocin (STZ, Sigma-Aldrich, St. Louis, MO) dissolved in the citric acid (0.1 mmol/l, pH 4.5) intraperitoneally at a dose of 60 mg/kg body weight. We regarded rats as diabetic when fasting blood glucose (FBG) remained above 13.9 mmol/l. Then, 19 diabetic rats were fed with losartan at a dose of 20 mg/kg body weight once a day (group C), and the remaining 17 diabetic rats received no treatment (group B). The blood glucose in the tail vein was measured once a week using a One Touch II glucometer (LifeScan, Milpitas, CA). The diabetic rats were injected with 4-8 U of NPH insulin (Lilly, Indianapolis, IN) subcutaneously once a day to keep the FBG level between 16.7 and 26.0 mmol/l. Each animal was assessed at an interval of 10 to 20 days for body weight and 24-hr urinary volume.
Management of samples
At the end of the 18th wk, all rats were anaesthetized with ether and the kidneys were removed from these animals. The kidneys were washed with cold normal saline (4°C) and the capsules were peeled off carefully. A fraction of renal cortices were put into fixative solution to prepare the sections. 24-hr urine samples were collected before the rats were sacrificed and stored at -40°C for later albumin detection.
Reverse transcription-polymerase chain reaction (RT-PCR)
The total RNA was extracted from the rat kidneys using RNeasy Midi Kit (Qiagen, Hilden, Germany), and was reversely transcribed (RT). Then, 10 μg of the RT product was used to perform PCR. The primers were as follows: MT3-MMP: sense 5'-ATCTTGGCCTTATGCC-3', antisense 5'-CGCATTGTCTTGTCAGG-3', yielding a 717 bp product; TIMP-2: sense 5'-GGCAAGATGCACATTACC-3', antisense 5'-TTGACATCCCTTCCTGGA-3', yielding a 386 bp product; TGF-β1 sense 5'-TGAGTGGCTGTCTTTTGACG-3', antisense 5'-TTCTCTGTGGAGCTGAAGCA-3', yielding a 294 bp product. The primers for β-actin used for internal control were 5'-AGGCCAACCGCGAGAAGATGAC-3' (sense) and 5'-GTACATGGTGGTGGTGCCGCCAGAC-3' (antisense), yielding a 581 bp product. 10μl PCR products were electrophoresed through an ethidium bromide-stained 2% agarose gel, and the band intensity was analyzed using quantitative scanning densitometry. The ratio of MT3-MMP, TIMP-2 and TGF-β1 to β-actin served as the levels of mRNA expressions.
Ultramicrostructure of the glomeruli
The ultramicrostructure of the glomeruli was examined by transmission electron microscopy (TEM). The MM density (MM area/mesangial area) was used as a proxy for the MM area. More than 5 MM areas were observed, and the average of the respective density measurements was used to determine the MM density. The values of the GBM width were calculated from the average width of more than 5 GBMs.
Measurement of urine albumin excretion (UAE)
24-hr urine samples were collected during the course of the study. Urine albumin was measured daily by ELISA (Rat Albumin Enzyme Immunoassay Kit, SPIbio, France).
Statistical analysis
The results were expressed as mean ± SD. Statistical analysis was performed using one-way ANOVA, preceded by normal transformation if data were not normally distributed.
Results
Preparation of the diabetic rat model
During the course of the investigation several rats were lost from the total rat population of the study groups. One rat in group A and one in group B died as a result of fighting amongst the rats. Two rats in group B and four in group C were discarded because of the failure to induce diabetic conditions. One rat in group B and two in group C were discarded due to restored blood glucose; one in group B and three in group C died from systemic failure. One rat in group B and one in group C died of unknown causes. Thus, by the end of the 18th week, there were 11 rats in group A, 11 in group B and 9 in group C.
The average FBG values in group B (23.46 ± 1.39 mmol/l) and group C (23.85 ± 1.34 mmol/l) were above 13.9 mmol/l and significantly higher than those in group A (4.74 ± 0.62 mmol/l). The difference between group B and group C was not significant. The increments of body weight in group B (56.8 ± 8.3 g) and in group C (54.3 ± 10.2 g) were significantly lower than in group A (161.8 ± 20.4 g). The average urinary volumes in group B (54.6 ± 14.8 ml/day) and C (50.8 ± 12.0 ml/day) were significantly increased compared to that in group A (8.14 ± 1.75 ml/day). The gathered data confirmed the successful implementation of the diabetic model.
mRNA expression
Under rational PCR conditions, at least one specifically amplified band in each sample clearly identified MT3-MMP, TIMP-2 and TGF-β1 (Figure 1). All expressions of MT3-MMP mRNA, TIMP-2 and TGF-β1 in group B were significantly higher than those in group A, and in group C significantly lower than those in group B (Table 1).
Figure 1.
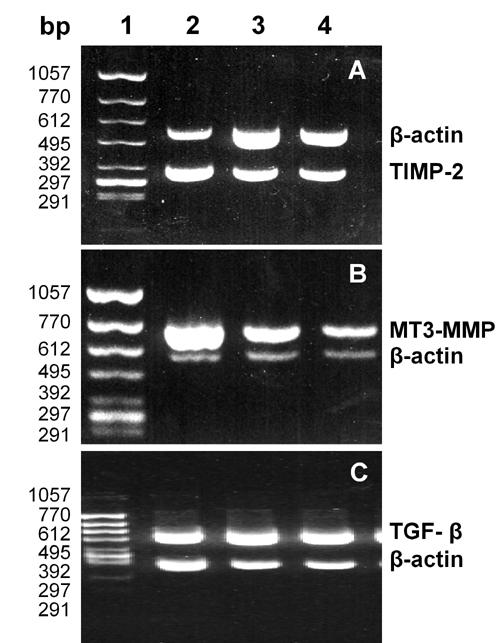
Electrophoresis of the RT-PCR products of TIMP-2 (A), MT3-MMP (B) and TGF-β (C) mRNA. Lane 1: DNA marker, ΦX174HincII digest. Lane 2: diabetic rat without therapy. Lane 3: normal rat. Lane 4: diabetic rat treated with losartan.
Table 1. Comparison of MT3-MMP, TIMP-2 and TGF-β1 mRNA levels and UAE.
Data are mean ± SD. F: one-way ANOVA value. Group A: normal healthy control rats. Group B: diabetic rats without therapy. Group C: diabetic rats treated with losartan. * compared with group A, p < 0.05; # compared with group B, p < 0.05, and compared with group A , p < 0.1.
Glomerular ultramicrostructure under TEM
Both the width of GBM (532 ± 108 nm) and the MM density (56.4 ± 6.8) in group B were significantly higher than the respective values in group A (width of GBM: 312 ± 25 nm, p < 0.01; MM density: 33.9 ± 5.2, p < 0.01) (Figure 2). The same measurements in group C (width of GBM: 316 ± 33 nm, p < 0.01; MM density: 35.4 ± 6.7, p < 0.01) were significantly lower than in group B. We could not detect significant differences in these measurements between group A and group C. The focal GBM thickening in diabetic rats was observed in group B, but not in groups A and C.
Figure 2.
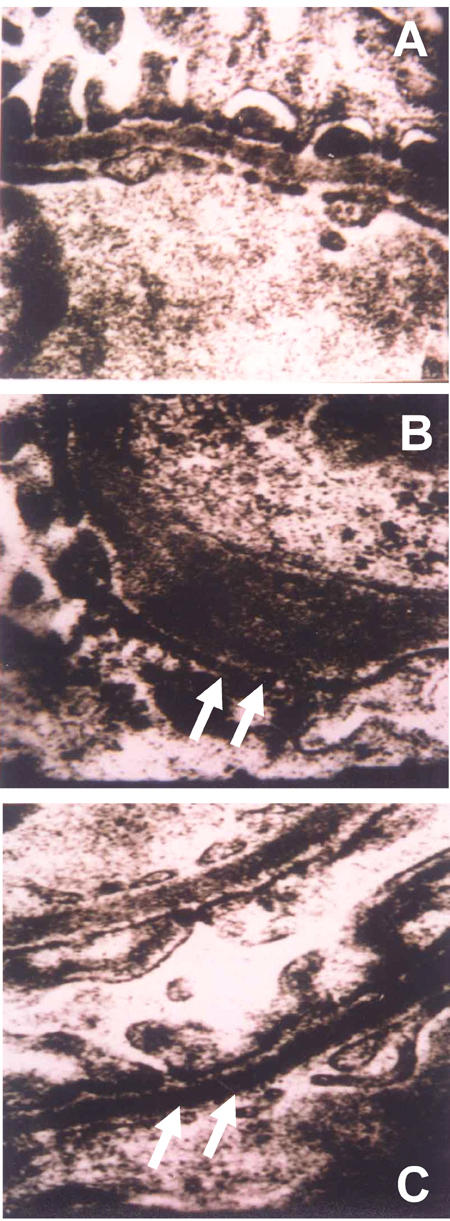
A: GBM of normal rats. B: GBM of diabetic rats without treatment. The rats exhibited significant GBM thickening. C: GBM of diabetic rats treated with losartan. No significant thickening compared to normal healthy rats could be observed. The GBMs of rats in groups A and C were without pathological findings. All images taken under TEM (x30,000).
Measurement of UAE
The UAE in group B was significantly higher than that in groups A and C (Table 1).
Discussion
In this study, we examined whether losartan is able to slow the development of DN by antagonizing AT1R. We found a decrease in the width of GBM and the mesangial area in diabetic rats. In STZ-induced diabetic rats, the expression of glomerular MMPs decreased in the rats that showed mesangial proliferation, but remained unchanged in the rats without mesangial proliferation [6]. Lupia et al. observed reduced expression of MMP2 in mesangial cells of diabetic NOD mice [7], which is consistent with the finding made in glomeruli of patients with type-2 diabetes mellitus [8]. Decreased MMP2 reduces the degradation of ECM protein, which results in an accumulation of ECM. As most other studies have demonstrated that the expression or activity of MMPs decreases in renal tissues or mesangial cells under hyperglycemic conditions, this suggests that MMPs may be involved in the pathogenesis of DN.
Few publications have investigated the expression of MT3-MMP mRNA in diabetic renal tissue and the effect of losartan on MT3-MMP mRNA expression in diabetic kidneys. Our study showed that the expression of MT3-MMP mRNA was increased in the kidneys of rats with DN. Another important finding from our study is that losartan not only delays the development of DN but also down-regulates MT3-MMP mRNA in diabetic kidneys. However, the function of up-regulated MT3-MMP mRNA in diabetic kidneys is not yet clear. Romanic and co-workers reported that the tubular expression of MT5-MMP is increased and the activity of MMP2 is elevated in diabetic patients [9]. This led to the hypothesis that the observed changes could be relative to tubular epithelial cell atrophy under diabetic conditions.
We did not determine the expression of other MMPs in our study, so that it remains unknown whether other MMP activity is increased and occurs together with upregulated MT3-MMP mRNA. Therefore, we cannot be sure whether our findings relate to tubular epithelial cell atrophy under diabetic conditions. MT3-MMP may contribute to the degradation of GBM collagen and the mesangial matrix via the induction of cell location and activation of MMP2 [10]. We consider that the increased expression of MT3-MMP mRNA in diabetic kidneys has a compensatory effect that may prevent kidneys in DN-positive rats from renal damage. When DN was delayed by losartan treatment, this compensatory change diminished. This inference still requires further evidence.
Increased TIMP-1 expression was found in the glomeruli of STZ-induced diabetic rats [11] and mesangial cells cultured in high glucose [7]. However, there are controversial findings regarding TIMP-2. MecLennan et al. [12] observed no change to TIMP-2 expression in mesangial cells cultured in high glucose. Interestingly, Caenazzo and others reported that the MMP-2/TIMP-2 ratio was decreased in mesangial cells under high glucose conditions [13], which means that TIMP-2 expression was increased. In contrast, our results show increased renal TIMP-2 mRNA in STZ-diabetic rats with DN, and this change can be reversed by losartan treatment. Thus, increased TIMP-2 may inhibit the activity of MMP-2 and other MMPs and decrease the degradation of ECM proteins. This suggests that TIMP-2 plays a critical role in the pathogenesis of DN. Losartan may exert a beneficial effect on DN partly through decreasing TIMP-2 expression.
Despite the observation that losartan decreases TIMP-2 mRNA expression in diabetic kidneys, the mechanisms underlying this effect remain unknown. Some data demonstrated that Ang II increased TGF-β1 expression in kidneys and TGF-β1 regulated the expression of many MMPs [14]. Therefore, Ang II may regulate the expression of many MMPs via TGF-β1. Our study verifies that losartan can downregulate TGF-β1 mRNA in diabetic kidneys. So we hypothesize that losartan decreases TIMP-2 mRNA via decreasing TGF-β1. However, no studies have proven the relation between Ang II and TIMP-2. Thus, it remains unclear whether the effect of losartan is direct or indirect via the reduction of blood pressure. Further study is needed in order to answer these open questions.
References
- 1.McLennan SV, Fisher EJ, Yue DK, Turtle JR. High glucose concentration causes a decrease in mesangium degradation. A factor in the pathogenesis of diabetic nephropathy. Diabetes. 1994;43(8):1041–1045. doi: 10.2337/diab.43.8.1041. [DOI] [PubMed] [Google Scholar]
- 2.Davies M, Martin J, Thomas GJ, Lovett DH. Proteinases and glomerular matrix turnover. Kidney Int. 1992;41(3):671–678. doi: 10.1038/ki.1992.103. [DOI] [PubMed] [Google Scholar]
- 3.Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54(3):775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolbrinker J, Markovic S, Wehland M, Melenhorst WB, van Goor H, Kreutz R. Expression and response to Angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with Renin-dependent hypertension. J Pharmacol Exp Ther. 2006;316(1):8–16. doi: 10.1124/jpet.105.093112. [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–868. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 6.Reckelhoff JF, Tygart VL, Racusen LC, Dzielak DJ. Glomerular metalloprotease activity in streptozotocin-treated rats and in spontaneously diabetic rats (BB/DP) Life Sci. 1994;55(12):941–950. doi: 10.1016/0024-3205(94)00540-0. [DOI] [PubMed] [Google Scholar]
- 7.Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes. 1999;48(8):1638–1644. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- 8.Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia. 1997;40(12):1449–1454. doi: 10.1007/s001250050848. [DOI] [PubMed] [Google Scholar]
- 9.Romanic AM, Burns-Kurtis CL, Ao Z, Arleth AJ, Ohlstein EH. Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. Am J Physiol Renal Physiol. 2001;281(2):F309–317. doi: 10.1152/ajprenal.2001.281.2.F309. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57(10):2055–2260. [PubMed] [Google Scholar]
- 11.Shankland SJ, Ly H, Thai K, Scholey JW. Glomerular expression of tissue inhibitor of metalloproteinase (TIMP-1) in normal and diabetic rats. J Am Soc Nephrol. 1996;7(1):97–104. doi: 10.1681/ASN.V7197. [DOI] [PubMed] [Google Scholar]
- 12.McLennan SV, Martell SY, Yue DK. High glucose concentration inhibits the expression of membrane type metalloproteinase by mesangial cells: possible role in mesangium accumulation. Diabetologia. 2000;43(5):642–648. doi: 10.1007/s001250051353. [DOI] [PubMed] [Google Scholar]
- 13.Caenazzo C, Garbisa S, Onisto M, Zampieri M, Baggio B, Gambaro G. Effect of glucose and heparin on mesangial alpha 1(IV)COLL and MMP-2/TIMP-2 mRNA expression. Nephrol Dial Transplant. 1997;12(3):443–448. doi: 10.1093/ndt/12.3.443. [DOI] [PubMed] [Google Scholar]
- 14.Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J Am Soc Nephrol. 1999;10(4):790–795. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]


