Abstract
T cell activation is a complex process that requires a multitude of interactions between antigen-presenting cells (APC) and T cells. The primary signal is provided via the binding of the antigen (Ag) presented by the major histocompatibility complex (MHC) on an APC and the T cell receptor (TCR). This signal determines the specificity of the immune response but it is not sufficient to mount an effective antigen-specific immune response; co-signals are additionally required for that purpose. These co-signals are costimulatory pathways that can be either positive or negative and consequently determine the nature of the immune response. The B7-1/2/CD28 costimulatory axis is one of the most extensively studied positive signaling pathways, and it has been shown that this signal leads to a robust T cell activation, proliferation and survival. In this article we discuss the recently described PD-1/PD-L1/PD-L2 costimulatory axis, whose role in pancreatic autoimmunity is only just becoming more deeply understood. The blockade or deficiency of PD-1 leads to an exacerbation of diabetes, signifying that the role of PD-1 is to provide negative signals to T cells. On the other hand, the PD-1 ligand, PD-L1, has been shown to provide both positive and negative signals. The prediction of the existence of a non-PD-1 receptor on T cells capable of transmitting positive signals further adds to the complex nature of this costimulatory pathway.
Keywords: type 1 diabetes, costimulation, autoimmunity, PD-1, PD-L1, PD-L2
Basic properties of PD-1 and PD ligands
PD-1 (programmed death-1) is a type 1 transmembrane protein and its extracellular region contains a single immunoglobulin V (IgV) domain. Its cytoplasmic region has two tyrosines, each of which constitute an immunoreceptor tyrosine-based inhibition motif (ITIM) and an immunoreceptor tyrosine-based switch motif (ITSM) [1]. It is the ITSM that is required for the inhibitory activity of PD-1. PD-1 exists as a monomer on cell surfaces due to the lack of membrane proximal cysteine [2]. Co-localization of PD-1 with TCR/CD28 on T cells is essential for its inhibitory function that involves the CD28-mediated activation of phosphatidylinositol-3-kinase (PI3K) [3]. PD-1 can be induced, not only on CD4 and CD8 T cells, but also on B cells and myeloid cells. NK-T cells have also been shown to express low levels of PD-1. During thymic development, PD-1 is predominantly expressed on CD4-CD8- T cells and also on double negative γδ T cells [4]. On the other hand, there is also some evidence in support of the role of PD-1 as a regulator of positive selection [5]. PD-1-deficient mice exhibit an overactivation of immune responses and thus supports the development of autoimmune diseases in dependence of the genetic background [6-8]. Also, PD-1 knock-out mice display a more vigorous T cell response as compared to normal controls [9]. These findings suggest that the engagement of PD-1 on T cells predominantly leads to the generation of negative signals.
PD-1 has two ligands, namely PD-L1 (B7-H1) and PD-L2 (B7-DC), and their similarity with B7 molecules prompted their identification using databased search [10-12]. PD ligands are type 1 transmembrane proteins with IgV and IgC domains in their extracellular region. PD-L2 has been shown to have an affinity for PD-1 that is two to six times higher than that of PD-L1 [2]. These PD ligands show a distinct pattern of expression; PD-L1 is more widely expressed than PD-L2 [10-13]. PD-L1 is expressed on T and B cells, dendritic cells and macrophages and also becomes upregulated upon activation [14-16]. Interestingly, PD-L1 has also been shown to be expressed by non-hematopoietic cells including endothelial cells in the heart, β-cells in the pancreas, and also in non-lymphoid organs namely lung, muscle and placenta [14, 16-19]. The expression of PD-L1 in non-lymphoid tissues suggests a potential regulatory role of PD-L1 in regulating autoreactive T and B cells in target organs. On the other hand, PD-L2 is more restricted and its expression can be observed in dendritic cells and macrophages [14-16]. There is also evidence that the expression of PD-L1 and PD-L2 can be influenced by Th1 and Th2 cytokines, such as IFN-γ and IL-4, which have been shown to upregulate PD-L1 and PD-L2, respectively [20].
Evidence for an additional costimulatory receptor for PD-L1 and PD-L2
A growing quantity of data suggests that at least one additional receptor for both PD-L1 and PD-L2 exists and significant observations have been made which strongly support this notion. In PD-1-deficient mice, neither PD-L1- nor PD-L2-associated costimulatory activity is affected [21, 22]. In a tumor model, Liu et al. have shown that PD-L2 can bind to PD-1-deficient T cells and target cells are killed in the absence of PD-1 [23]. This seems to explain the observations made by several groups reporting that positive costimulation may be delivered to T cells by PD-L1 and PD-L2 [12, 13, 24]. The existence of another positive costimulatory molecule makes the PD-1/PD ligand axis more versatile. Therefore, it is tempting to speculate that PD ligands control the way the immune response develops, depending on which receptor they bind to. Via PD-1 the PD ligands provide negative signals and, upon binding to the yet unidentified receptor(s), may provide the positive signals to T cells (Figure 1).
Figure 1.
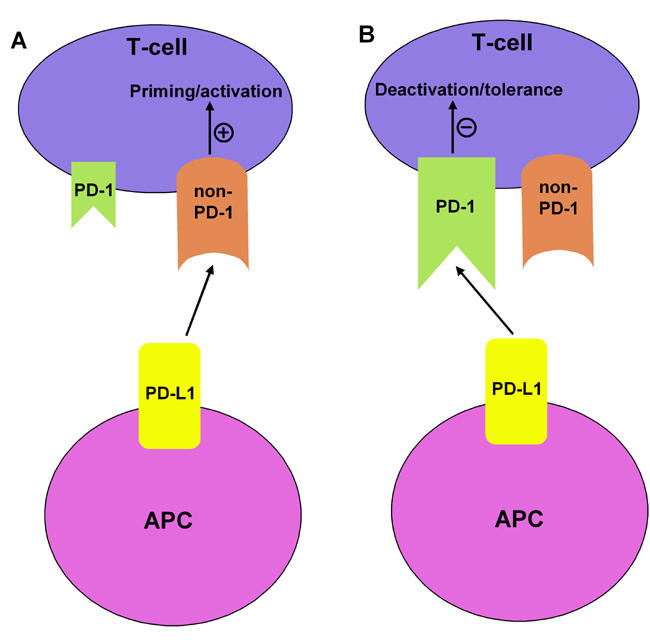
The positive or negative costimulatory behavior of PD-L1 may be governed by the relative availability of PD-1 and non PD-1 receptors on T cells. (A) Although the exact immune nature of the non-PD-1 receptor is not known, it is tempting to speculate that, at an early stage of immune response, PD-L1 (in conjunction with other costimulatory molecules like B7-2 etc.) may help deliver positive activating signals via non PD-1. (B) As the T cells become activated, PD-1 is upregulated, which in turn can bind to PD-L1 and transmit negative deactivating signals into the T cells. Furthermore, the interplay between PD-1/non-PD-1/PD-L1 may be guided by the activation status of T cells, the type of immune cells involved and the nature/stage of the disease.
The manipulation of the PD-1/PD-L pathway as a potential therapy against autoimmunity
Although the exact roles played by the individual members of the PD-1/PD-L pathway are not clear, emerging data nevertheless provide interesting clues as to how the PD-1/PD-L pathway can be manipulated for a wide variety of disease conditions besides autoimmunity, including infectious diseases, asthma, cancer and transplantation. However, this article is constricted to discussing existing data, in the field of autoimmunity and type 1 diabetes in particular.
PD-1 and type 1 diabetes
The negative costimulatory function of PD-1 in type 1 diabetes models was first examined by Ansari et al. who reported that a blockade of PD-1 exacerbates diabetes in prediabetic non-obese diabetic (NOD) mice (1 to 10 weeks of age), which was also associated with increased IFN-γ producing GAD-reactive splenocytes [25]. In addition, Ansari and colleagues also showed that, in mice treated with CTLA-4, exacerbation was prevented only in neonates. This means that, contrary to CTLA-4, PD-1 regulates not only the initial phase of the disease, but also controls it at the effector phase. Recently, Wang et al. investigated PD-1-deficiency in mice with a NOD background and confirmed earlier studies involving the PD-1 blockade, also reporting the strong Th1 polarization of cells infiltrating islets [26]. Furthermore, this new study by Wang and co-workers takes a deeper look at the role of PD-1 in type 1 diabetes. It proposed that PD-1KO mice might be an efficient model to study type 1 diabetes, owing to a 100% penetrance of the disease in these mice. This, in turn, makes it easier to identify the responsible genes and/or the analyses of the immunological function of each diabetes-susceptible locus, which otherwise is more difficult owing to the incomplete penetrance of diabetes in the NOD mouse model. Consequently, they have identified two previously unrecognized dominant susceptible loci, Iddp1 and Iddp2 (i.e. Idd under PD-1 deficiency). The authors also proposed the notion that autoimmune disease in different genetic backgrounds, together with PD-1-deficiency, can have the consequence that PD-1-deficiency may exaggerate the genetic predisposition of autoimmune diseases, such as diabetes in NOD mice.
The role of PD-L1 and PD-L2 in modulating anti-islet immune responses
The negative costimulatory role of PD-1 in the context of type 1 diabetes is clearer than the role of its ligands. A blockade of PD-L1 in NOD mice exacerbates the disease, leading to increased insulitis, overt diabetes, and higher frequencies of GAD-reactive T cells than with a PD-1 blockade. Interestingly, this effect was also observed in otherwise resistant male NOD mice, suggesting a strong immunomodulatory role of the PD-1/PD-L1 pathway. Thus it suggests that PD-1/PD-L1 interactions may provide critical negative signals which in turn dampen the anti-islet immune response [25] and, in its absence, self-reactive T cells may acquire a more pathogenic phenotype. Moreover, the expression of PD-L1 has been seen on islet-infiltrating inflammatory cells and also on islet cells in diabetic NOD mice [14, 25]. This means that PD-L1 may play an important role in the regulation of autoreactive T cell responses at the level of the target organ.
The role of islet-associated PD-L1 expression is becoming more widely understood, and it appears to be complex. This aspect can be exemplified by the study conducted by Subudhi et al. [27]. Contrary to the belief that PD-L1 provides negative stimulation, this study came along with an unexpected finding showing that PD-L1 may be a potential positive costimulator. Upon expression of PD-L1 on islet cells under the control of insulin promoter (RIP-PD-L1), mice exhibited spontaneous autoimmune diabetes associated with marked insulitis and efficient priming of CD8 T cells. These data are thought-provoking and indicate that target organ expression of PD-L1 can initiate anti-islet T cell responses. The susceptibility of islets expressing PD-L1 to autoimmune attacks was not found to be due to a possible vulnerability of islets for apoptosis and loss of function as the islet structure, morphology and long term function in vivo were not found to be significantly affected compared to controls. The exacerbation of autoimmunity due to PD-L1 expression in islets was not prevented by anti-PD-1 treatment, suggesting that PD-L1-mediated enhancement of T cell responses is independent of PD-1.
Several possibilities have been suggested as to whether PD-L1 is a positive or a negative costimulator. Indeed, the PD-L1 blocking model is quite different from the islet PD-L1 expression model in the sense that the former systemically blocks PD-L1 from interacting with its ligand and the latter has a more reductionist approach, concentrating on the target organ itself. The timing and location of PD-L1 expression may be important, along with a potential bi-directional signaling associated with PD-L1. It is also tempting to speculate that PD-L1 may bind to the yet unidentified receptor (as described above), thereby leading to the generation of potentially pathogenic signals. It is still an open question and needs further investigation.
Data describing the role of PD-L2 in influencing the generation of anti-islet immune responses are still at a very early stage. PD-L2 blockade in female NOD mice did not significantly affect the incidence of diabetes and no PD-L2 staining was observed in NOD islets [25], suggesting that endogenous PD-L2 may not have a large role to play. However, the generation of PD-L2-deficient NOD mice may shed more light on the role of PD-L2 from the perspective of both central and peripheral tolerance.
Conclusion
One of the central issues to be addressed regarding the role of PD-1/PD ligands in regulating T cell responses in general, and pancreatic autoimmunity in particular, is the identification of the non-PD-1 receptor, which has been predicted to transmit positive signals to T cells. PD-1, on the other hand, has been widely recognized as a negative costimulator. Furthermore, the dual nature of PD-L1 in providing both positive and negative signals and its exact role in autoimmunity may depend upon the nature of the disease, the type of immune cells involved, the activation status of the T cells, relative levels of PD-1 and non-PD-1 molecules on T cells (Figure 1), and the genetic background of the mice. Recently, PD-L1 and PD-L2 have been shown to differentially regulate the susceptibility and chronic progression of experimental autoimmune encephalomyelitis in a strain-specific manner [28]. The identification of the PD-1/PD ligand costimulatory axis underlines the dynamism and complexity associated with the regulation of T cell immune responses. There appears to be an interesting parallel between the frequently studied B7/CD28 family of costimulatory molecules and the PD-1/PDL axis. The PD-1 and non-PD-1 pair of negative and positive costimulators mirrors the CD28 and CTLA-4 pair of molecules. Thus any particular costimulatory axis comes with its own checks and balances, where one molecule helps priming and/or activation and the other controls the unrequested hyper-activation of immune response. Indeed, much work has still to be done to fully elucidate the individual roles played by PD-1/PD ligands in controlling autoimmunity, which might pave the way for the identification of new therapeutic strategies.
Acknowledgments
Deepak Yadav is a recipient of a postdoctoral fellowship from the Juvenile Diabetes Research Foundation International (JDRF, 2005).
References
- 1.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Schwartz JC, Guo X, Bhatia S, Cao E, Lorenz M, Cammer M, Chen L, Zhang ZY, Edidin MA, Nathenson SG, Almo SC. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191:891–898. doi: 10.1084/jem.191.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 9.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 12.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 14.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 16.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 17.Wiendl H, Mitsdoerffer M, Schneider D, Melms A, Lochmuller H, Hohlfeld R, Weller M. Muscle fibres and cultured muscle cells express the B7.1/2-related inducible co-stimulatory molecule, ICOSL: implications for the pathogenesis of inflammatory myopathies. Brain. 2003;126:1026–1035. doi: 10.1093/brain/awg114. [DOI] [PubMed] [Google Scholar]
- 18.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 19.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 20.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 25.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo JC, Anders RA, Sun Y, Chen L, Wang Y, Alegre ML, Fu YX. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Differential role of programmed death-1 ligand and programmed death-2 ligand in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]


