Abstract
MAIN OBJECTIVES: The objective was to describe pre-gestational history and the maternal, fetal and neonatal outcome in pregnancies in women with pre-gestational type 2 diabetes during the period between 1992 and 2006 from one center in the Netherlands. METHODS: Patients attending the obstetric-diabetology outpatient clinic of a tertiary referral center were studied. This center also has a regular diabetes clinic and a community midwifery service. Patients were identified from the database. Maternal outcome (pre-eclampsia, pre-term delivery, Cæsarean section) and fetal and neonatal outcome (macrosomia, congenital malformations, perinatal mortality, neonatal hypoglycemia) were analyzed as well HbA1c levels, planning of pregnancy, gestational age at first antenatal visit and ethnic background. RESULTS: Sixty-six singleton pregnancies from 48 women were analyzed. Their age was 34 ± 5 yr, the BMI 31.7 ± 7.4 and the median duration of diabetes was 3 yr. 52% were Caucasian and 35% were of Moroccan descent. 49% did not complete secondary school. Moroccan descent was associated with a lower educational level and a BMI comparable with the whole study group. The proportion of planned pregnancies was approximately 70%. The mean HbA1c in the first trimester was 6.4 ± 1.1% and the gestational age at first visit was 10 ± 5 wk, in one-quarter before 6 wk. The prevalences of variables related to maternal and neonatal outcome were as follows: spontaneous abortion 13.6%, pre-eclampsia 8.9%, pre-term delivery 21.4%, spontaneous labor 25.0%, induced labor 48.2%, Cæsarean section 42.9%, macrosomia (≥90th percentile) 41.1%, severe hypoglycemia 41.5% and major congenital malformations 5.1%. CONCLUSIONS: Pre-gestational type 2 diabetes is associated with an increased incidence of adverse pregnancy outcome despite reasonable mean HbA1c level and despite a high frequency of planned pregnancies. Many women report relatively late. Improvement in the outcome requires more active peri-gestational specialist care and a tailored approach is required towards migrant communities.
Keywords: type 2 diabetes, pregnancy, congenital malformations, macrosomia, neonatal hypoglycemia
Introduction
Over the last few decades many studies have shown that women with type 1 diabetes mellitus still have a high incidence of adverse maternal, fetal and neonatal pregnancy outcome [1-9]. There is a strongly elevated risk of congenital malformations [1, 3, 6, 7], macrosomia [3, 7], and pre-eclampsia, [1, 7] as well as pre-term delivery and Cæsarean section [1, 6, 7]. Neonatal hypoglycemia occurs frequently during the first days after delivery [1]. Elevated plasma glucose levels are a major contributor to the development of congenital abnormalities [1, 5], pre-eclampsia [10, 11] and macrosomia [1, 12]. This has led to the adoption of general clinical policies aimed at reaching normoglycemia or near-normoglycemia.
Less is known of the pregnancy outcome in women with pre-gestational type 2 diabetes mellitus. During the last decade, five surveys have been published which show that maternal, fetal and neonatal outcomes are similar to, if not worse than those in pregnancies with pre-gestational type 1 diabetes [6, 13-17]. Pre-gestational type 2 diabetes is an emerging problem especially since type 2 diabetes has become a global epidemic [18]. This means that type 2 diabetes occurs with increasing frequency in younger age groups. Since, at the same time, the maternal age of first and subsequent pregnancies has risen in modern society, more and more women are confronted with the problems and burden of diabetes type 2 during pregnancy.
Type 2 diabetes in pregnancy is also associated with additional potentially harmful factors. A considerable number of women are of migrant origin and language and cultural barriers may exist, impairing adequate pre-gestational care and counseling [19]. This may result, for example, in late reporting for initial antenatal care. Medication poses additional problems. Not only are some women continuing to take oral glucose-lowering medication while pregnant, but a considerable number of women may also use anti-hypertensive and anti-hyperlipidemic drugs. Some of these drugs are associated with congenital malformations [20-22].
Although type 2 diabetes in pregnancy is related to adverse outcomes, relatively little detailed information is available concerning the issues mentioned. Since the incidence of type 2 diabetes is still relatively low, it takes a long time or a multi-center approach to collect numbers of patients sufficiently large to allow meaningful and detailed analysis. In this retrospective single center study, we performed an exploratory analysis of maternal, fetal and neonatal outcome in type 2 diabetes over the last 14 years with particular attention to the pre-gestational and early gestational period. This information is used for a larger prospective study.
Patients and methods
Patients
Patients with any form of diabetes attending the combined outpatient clinic for Obstetrics and Diabetology of the University Medical Centre Utrecht, in the Netherlands, were entered into a database. The department serves as a tertiary referral center but also has a regular diabetes service and a midwifery section working in the community. Patients with a diagnosis of type 2 diabetes mellitus, who delivered between July 1992 and July 2006, were identified for this study. Maternal, fetal and neonatal data are kept in this database. Patients had to have a diagnosis of pre-gestational type 2 diabetes based on a previous medical diagnosis and/or abnormal biochemical data and/or use of oral medication and/or insulin. Patients who were referred to our center based on their obstetric pathology were excluded, as they were first seen after 30 wk of gestation.
Methods
The following maternal, fetal and neonatal items were retrieved from the database.
Maternal parameters:
Age
Weight and length (body mass index (BMI))
Parity
Planning of pregnancy (use of folic acid and/or evidence of pre-gestational counseling and actions in the medical files)
Ethnic background (Caucasian (including Turkish), Moroccan, Asian, Negroid, others)
Gestational age at first antenatal visit (counted from the last of day of the last menstruation, thus normally a minimum of 4 wk)
Duration of clinical diabetes
Insulin use during pregnancy
Incidence of pre-eclampsia
Incidence of spontaneous abortion (before week 20)
Abortion due to severe malformations
Pre-term delivery (20-37 wk)
Fetal death
Mode of delivery
Fetal or neonatal delivery:
Congenital malformations (major malformations, defined as malformations which result in death, require major surgical intervention or result in physical handicaps including major cosmetic handicaps)
Birth weight
Macrosomia (defined separately as birth weight >4000g, or birth percentile ≥90 (large-for-gestational age (LGA)); severe macrosomia defined separately as birth weight >4500g, or birth percentile ≥97.7 (very-large-for gestational age (VLGA))
Neonatal hypoglycemia (plasma glucose ≤2.6 mmol/l)
Severe neonatal hypoglycemia (plasma glucose ≤2.0 mmol/l and/or use of intravenous glucose infusion)
Hyperbilirubinemia requiring at least one period of phototherapy
Statistical analysis
Results are given for continuous variables as mean and standard deviation with normal distribution and as median and 95th percentile (p95) in case of a skewed distribution. Categorical variables are given as percentages. Differences between groups are tested with the appropriate (non-)parametric tests for continuous variables with the χ2-test or Fisher’s exact test for categorical variables. The level of statistical significance is 0.05. Univariate analysis was performed with macrosomia as the dependent variable. Parameters with p < 0.10 were entered into a multivariate regression modal to test for independence if multiple determinants were found. A similar procedure was performed with severe neonatal hypoglycemia.
Results from the study population were compared with those from the general population [1]. According to this data, the normal incidence of pre-eclampsia is 1.05% of the general population, the pre-term delivery is 7.1% and Cæsarean section 12.0%. The normal perinatal mortality is 0.8%. According to the definition of gestational age given above, the incidence of LGA is 10% and the incidence of VLGA is 2.3% in the general population. The normal incidences of macrosomia (>4000 g) and severe macrosomia (>4500 g) are 16.8% and 4.0% respectively [23]. The incidences in the study group were compared with the incidences in the normal population using the single proportion test. Incidences of neonatal complications, hypoglycemia and hyperbilirubinemia are unknown in the Netherlands and therefore a comparison between the study group and the normal population could not be made. In this study, major congenital malformations are reported retrospectively. The incidence of all congenital malformations in the general population in the Netherlands is 2.6%, but there is no information available concerning a clear distinction between major and minor malformations. Therefore, in our study, no comparison was made with respect to this issue either.
Results
52 pregnant women with type 2 diabetes were seen at our clinic between 1992 and 2006. Four patients were excluded because they were referred from another hospital due to a complicated pregnancy - leaving 48 women. The total number of pregnancies studied was 66, all singleton pregnancies. The maternal characteristics are shown in Table 1. The mean age for the first pregnancy in our hospital was 34 yr with a range from 25 to 45 yr. Most women had had earlier pregnancies and already delivered one or more children. Many women were overweight with a BMI < 25 in 88% and a BMI < 30 in 55% of the cases. The diabetes was generally of short duration with a median of 3 yr. Hypertension occurred in a few patients, none had chronic complications. Using a crude classification of the level of formal education received, it showed that about half of the women had not received or completed secondary school and about 15% had completed university or vocational training.
Table 1. Characteristics of the participating women.
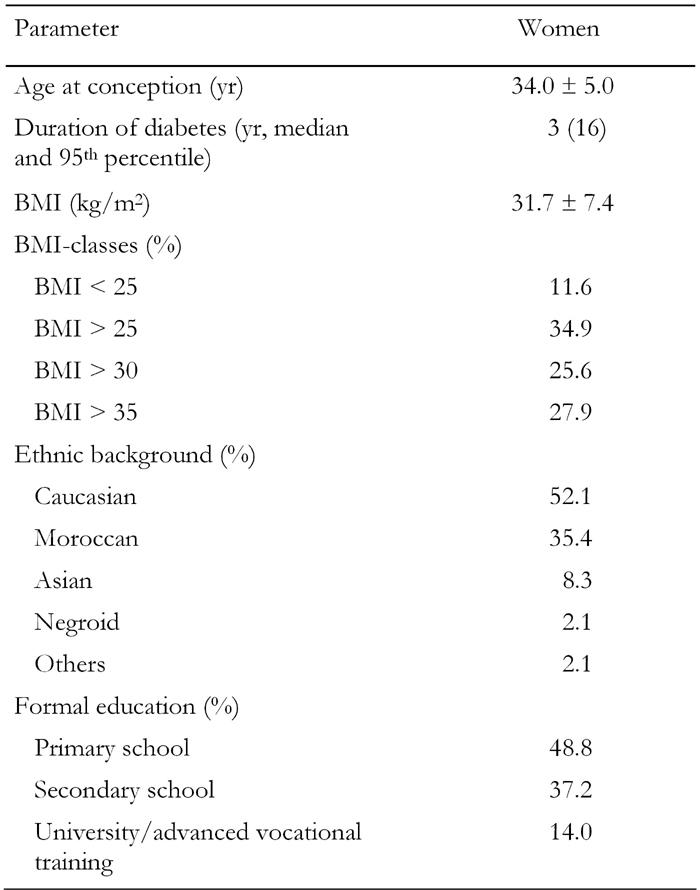
Data are mean ± SD or percent. BMI: body mass index.
There was a clear ethnic distribution in this population. A little more than half of the women were of Caucasian origin, women of Moroccan origin constituted another large group. Other ethnic backgrounds were relatively low in frequency. Comparing the two largest groups (Caucasian and Moroccan), there was no difference in BMI (32.7 ± 6.0 vs. 31.2 ± 4.5, Caucasians and Moroccans respectively, p = 0.4) nor in the distribution over the BMI classes. There was, however, a difference in formal education. Caucasian women had received or completed more advanced education than Moroccan women (p = 0.009).
The 48 women had 66 pregnancies. Planning pregnancy is one of the major issues in diabetic pregnancies. The majority of the 66 pregnancies were planned. Folic acid was used by 70% and more than 80% had done some or all the planning of their pregnancy. Any form of planning was associated with ethnic background: 93.8% of pregnancies in Caucasian women were planned compared to 68.2% in Moroccan women (p < 0.05). Similarly, secondary or higher education was associated with more planning (92.0% vs. 69.0%, p < 0.05) compared to primary education only. Closely related to the issue of planning is the timing of the first antenatal visit, see Figure 1. The mean gestational age at the first outpatient visit was 10 ± 5 wk. One quarter were seen before the second week (6th week calculated from the last menstrual period) and 70% were seen before 18 wk. Early presentation (before 12 weeks) was associated with Caucasian origin (85 vs. 56%, p = 0.02). Education was not significantly related.
Figure 1.
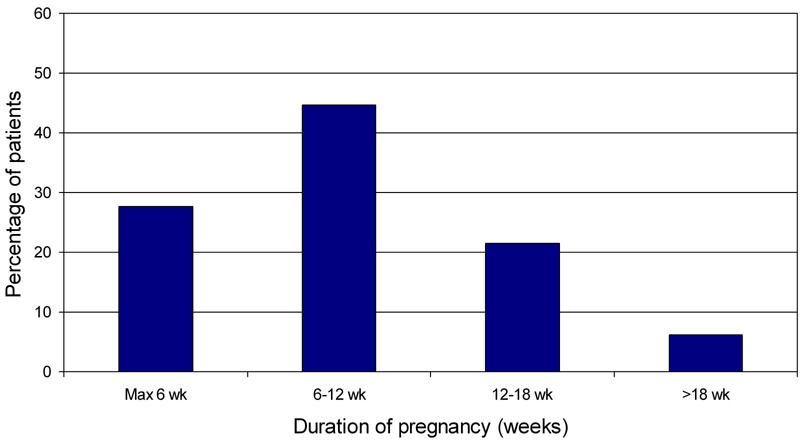
Gestational age at first antenatal presentation.
The maternal outcome is shown in Table 2. Pregnancy ended before 20 wk in 13.6% of patients. Eight women were on oral glucose-lowering drugs during early pregnancy and one on diet only. These 9 women were put on insulin too and thus all of the women were treated with insulin during pregnancy. The mean HbA1c value decreased steadily from 6.9% before pregnancy to 6.0% in the second trimester with a slight increase in the third trimester to 6.2% (Figure 2). The number of patients with HbA1c measurements varied in the different trimesters, so a formal statistical analysis was not performed. There were no cases of intrauterine fetal death. Pre-eclampsia occurred at an increased incidence but there were no cases of the HELLP-syndrome or eclampsia. The higher incidence of pre-eclampsia almost reached statistical significance (p = 0.07).
Table 2. Maternal pregnancy outcome.
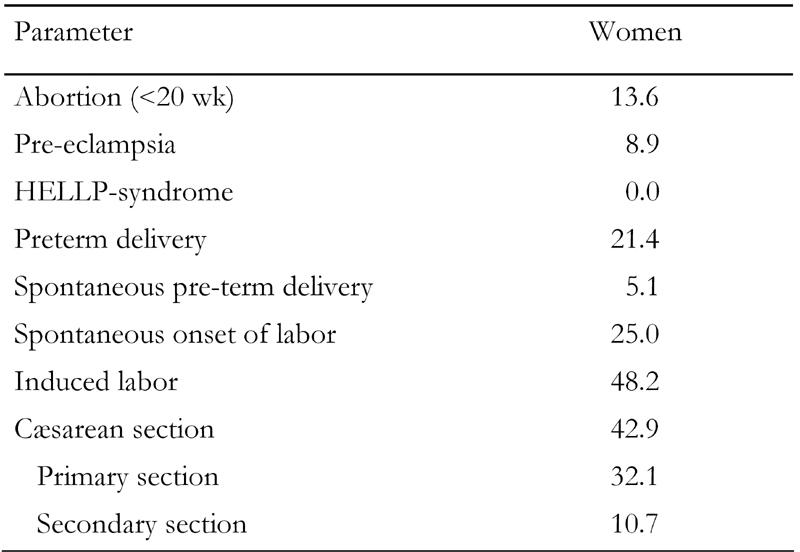
Data are in percent.
Figure 2.
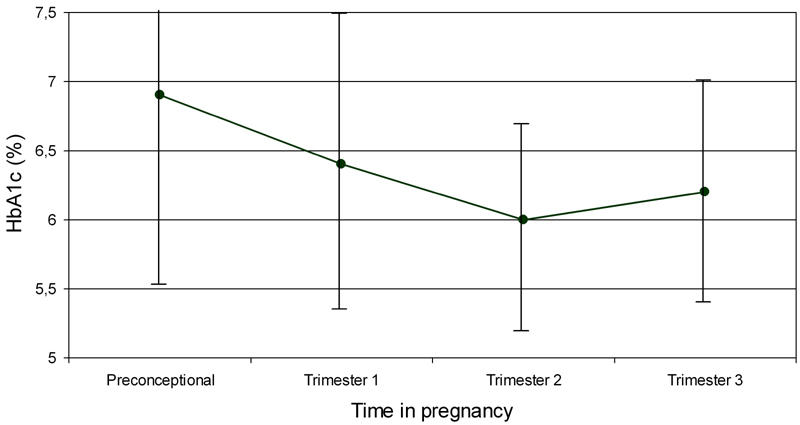
HbA1c-levels before and during pregnancy. Data are means ± SD.
Labor started spontaneously in 25%, in four cases needing oxytocin augmentation. Induced labor was required in 48.2% and a Cæsarean section was required in 42.9%. The incidence of Cæsarean section was significantly higher than in the normal population (p < 0.001). The majority of Cæsarean sections (78%) were planned before delivery. Pre-term delivery occurred in 21.4%. This was significantly higher than in the normal population (p < 0.02). Three of the 12 pre-term deliveries (25%) were spontaneous; the others were induced for medical reasons. There was no maternal mortality in this series.
The neonatal outcome is shown in Table 3. Major congenital malformations (one sacral teratoma, two cardiac malformations) were observed in 5.1% of the cases studied. None of the women were on oral medication during the first trimester. Macrosomia occurred in 41% when using the LGA definition, 14% had a birth weight of >p97.7 (VLGA). LGA occurred significantly more frequently in the study group compared with the normal population (p < 0.001). The higher incidence of VLGA just failed to reach statistical significance (p = 0.07). In general, the incidence of macrosomia with a birth weight of >4000g or >4500g were comparable with the normal population. Figure 3 shows the body weight distribution.
Table 3. Neonatal outcome.

Data are in percent.
Figure 3.
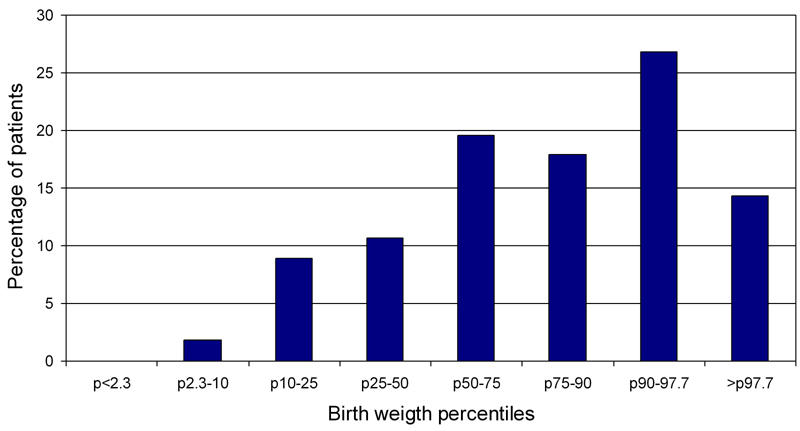
Distribution of birth weights.
Severe neonatal hypoglycemia occurred in approximately 40% and phototherapy for hyperbilirubinemia was given to one in five babies. Over all, perinatal mortality, spanning from 20 wk of gestation to 28 days after delivery was 3.6%, was not significantly different form the normal population (0.8%) probably also due to the effect of the small group size. The reasons and circumstances of the two cases of perinatal death are listed in Table 4. Macrosomia (≥p90) was associated with an increased frequency of Cæsarean section compared with birth weight (<p90) (61 vs. 30%, p < 0.03). A similar picture emerged with macrosmia defined as a birth weight of >4000 g (69 vs. 32%, p < 0.02).
Table 4. Perinatal mortality.

PROM: prelabor rupture of the membrane.
Associations of macrosomia, defined as a birth weight ≥p90, were sought. Macrosomia was only associated with higher maternal body length (macrosomia vs. no macrosomia: 167.6 ± 7.7cm vs. 162.4 ± 3.8 cm, p < 0.01) but not with pre-gestational maternal weight, BMI, gestational age at first visit, planning of pregnancy, use of folic acid, level of formal education or HbA1c in the different trimesters. Severe hypoglycemia occurred with higher frequency with a birth weight ≥p90 (57% vs. 31%) just failing to reach the conventional level of significance (p = 0.06). No other determinants were found.
Discussion
This study, which spanned a period of 14 years, shows that there is still an increased frequency of adverse pregnancy outcomes in women with type 2 diabetes with emphasis on an increased frequency of congenital malformations, pre-eclampsia, macrosomia and neonatal hypoglycemia. In the North-West European region studied, we could not observe a significant difference of pregnancies by women of Caucasian or Moroccan origin.
In this study, the outcome was compared with available national data for the general population. Since the aim was to determine the outcome in type 2 diabetes compared to the general, 'normal' population, we did not use the data from the population of other patients in this center because this population was selected specifically in view of the nature of this center. The group of type 2 diabetes patients was considered to be well-representative of the normal diabetes population, since many patients originated from the midwifery department or were seen in the general outpatient clinic.
The planning of pregnancy with pre-gestational optimization of glycemic control is of paramount importance. This study showed that a large majority of patients in our population did indeed plan their pregnancy. To define the planning of pregnancy, the use of folic acid or pre-gestational attention and early visits to the clinic were considered. Although folic acid is part of the recommended treatment in early pregnancy, its use in the general population of the Netherlands is much lower than the 70% seen in this study. Some kind of planning was seen in more than 80% of patients which was consistent with the high level of birth planning in the Dutch society. In comparison, only 5% of pregnancies in a comparable country such as Denmark was planned in a contemporary study [13].
Strict glycemic control is a critical factor for a good course and outcome of pregnancy in women with diabetes. In this study we took HbA1c as the indicator of overall control. The mean level, of those in whom this was known, was close to 7% before pregnancy, decreasing during pregnancy and slightly increasing in the last trimester. This pattern is well-known in pregnancy. The decrease in HbA1c during the first two trimesters is attributed to an increase in red cell production as well as to patients’ action to improve control. The HbA1c levels were comparable to the recent Danish study [13]. These results suggest that with targeted actions and good logistics, improved glycemic control is achievable.
Pre-gestational counseling means that the HbA1c-level should be maintained as low as possible, Furthermore, counseling should achieve the cessation of treatment with oral glucose-lowering medication, the starting or adjusting of insulin treatment if necessary and the discontinuation of other potentially teratogenic medication. A complicating factor may be that many patients with type 2 diabetes are treated by a general practitioner. In view of the complications of diabetes in pregnancy and the intensive end complex therapies required, all patients who express the desire to become pregnant should be referred for specialist care before conception. In addition, the issue of pregnancy should be discussed between all fertile women with type 2 diabetes and their treating physician, that being either a general practitioner or a medical specialist.
Strict glycemic control also means that women should be encouraged to come to the clinic as soon as possible, approxiamtely at week 4 to 5. The analysis of our group shows that three-quarters of women report only after six weeks.
When comparing our study group with other groups in published studies, the gestational age is much lower and the percentage of planned pregnancies is higher in our study group. In the Danish study, the mean gestational age at first visit was at week 13 [13]. Apart from increased attention to the problem of pre-gestational type 2 diabetes, this difference may also be due to logistical and cultural factors. A complicating factor may be that many women do not want to tell their employers and colleagues that they are pregnant in the first trimester and this limits the possibilities to achieve the beneficial regular life and work schedules. All these issues are a logical part of pre- and peri-gestational counseling. Interestingly, Towner et al. did not find an association between the incidence of congenital abnormalities and gestational age at first presentation [16]. However, glycemic control was poor in this compared to the other studies.
Our population has distinctive features in that it has a large group of women of Moroccan origin. Other studies have shown that there is a large proportion of non-Caucasian patients in women with type 2 diabetes and pregnancy and each country or region has its own migrant make-up [13-17]. Type 2 diabetes has a definite increased prevalence in Moroccan, Turkish, Negroid and Asian subjects [19]. The over-representation of patients of Moroccan origin could be due to an increased proneness for early type 2 diabetes in the Moroccan population, for example linked to higher BMI, less physical activity or as yet unknown factors. Ethnic background was related to educational level and planning of pregnancy in our study. This reinforces the fact that targeted education is another key point to be aware of. The relatively small sample precluded a detailed statistical analysis comparing Caucasian and non-Caucasian groups. The study by Vanger et al. carried out in Denmark reported higher incidences of adverse outcome in the non-Caucasian women, but these differences were not statistically significant in the small groups [24].
As was expected on the basis of earlier surveys, there was an increased incidence of adverse maternal and neonatal outcomes. In our study, spontaneous abortion occurred in 13.6% of the cases, which is comparable to the 11.3% incidence in the Danish study [13]. Pre-eclampsia has been linked to endothelial dysfunction and insulin resistance, thus the higher incidence is not surprising. This has also been shown in another study [13].
Macrosomia occured in approximately 40% according to the LGA definition. This number is comparable to other studies where it occurred in 56% and 47% of the cases [12, 13] and is roughly comparable with the incidence in women with type 1 diabetes [1, 7]. In our study group, only maternal height was associated with macrosomia which corresponds to an earlier study of a large non-diabetic population [25].
HbA1c was not related to fetal size. The low predictive power of HbA1c regarding fetal weight was described earlier in type 1 diabetes [26]. Glucose levels, especially the postprandial ones in the third trimester are well correlated with fetal weight, indicate that the HbA1c-value is a relatively insensitive indicator for glucose levels [27, 28]. This has been exemplified by Kyrne-Grebalski et al. who showed that hypo- and hyperglycemic episodes were frequent even with an HbA1c level between 6 and 7% [29]. More detailed analyses using continuous glucose measurements have documented extensive swings in glucose levels despite HbA1c levels generally being considered as acceptable [30, 31].
Major congenital malformations occurred with a high incidence, which is comparable with other studies [6, 13-15, 17]. An earlier study in type 2 diabetic women reported that 11.7% had major congenital abnormalities between 1987 and 1992 [16]. The pathogenesis is linked to glucose control during the first weeks of pregnancy. Due to the retrospective nature of our study and the late presentation of women, detailed information during this period is lacking. However, data is available to suggest that the women were not poorly controlled. Towner et al. found that peri-conceptional HbA1c was the only independent determinant of the incidence of congenital malformations [16]. In type 1 diabetes, the association between peri-gestational HbA1c and congenital malformations is well-established [1]. The non-randomized trial by Fuhrmann et al. is the best interventional proof of the necessity of strict peri-gestational glycemic control to reduce congenital malformations [32]. Recently, Temple et al. showed in an observational study that pre-gestational care was associated with a lower HbA1c in early pregnancy [33]. It still remains unclear to what degree maternal characteristics account for these differences in outcome. These factors relate for example to income, education and smoking. These observations have to be tested in women with type 2 diabetes.
Pre-term delivery occurred in about 20%, which is comparable with other studies [13]. Spontaneous pre-term delivery occurred in 5.3% of pregnancies >20 wk. It is remarkable that, in our study, two of three neonatal deaths occurred in women with pre-term ruptured membranes. Further study is needed to elucidate whether this is a real phenomenon or a chance finding.
Perinatal mortality seems to be increased and is comparable to that of the UK study (2.5%), the New Zealand study (3.9%) and the Danish study (6.7%) [13, 14, 17] and it is similar to that in women with type 1 diabetes. A considerable part is explicable by the congenital malformations [3]. In the New Zealand study, 33% of perinatal deaths were related to congenital abnormalities, as compared to 25% and 33% in type 1 diabetes [1, 7]. Other factors, such as stillbirth and difficult delivery also contribute to perinatal death, suggesting avenues for new potential preventive treatment.
The limitations of this study are based on the retrospective nature as well as the limited size and special ethnic composition of the study group. There is minimal contribution from the Hindu or East Asian population and the Afro-Caribbean population. Both groups have a relatively high prevalence of diabetes during the fertile period. An extension of this retrospective study that aims to include these groups is ongoing. Due to the limited size, differences between the study group and the normal population had to be quite large to reach statistical significance. This underscores the importance of a multi-center study.
In conclusion, pre-gestational type 2 diabetes is still associated with an increased incidence of adverse pregnancy outcome despite a mean HbA1c level within normal ranges and a high incidence of planned pregnancies. A considerable proportion of the women report at the 'normal' gestational age for the first antenatal visit, which is however late in diabetic pregnancies. Improvements in outcomes may be expected from more active specialist peri-gestational care, including pre-pregnancy referral to specialist care, and a tailored approach for women from migrant communities.
References
- 1.Evers IM, de Valk HW, Visser GH. Type 1 diabetic pregnancies are still at high risk for complications: outcome of a nationwide study. BMJ. 2004;328:908–915. doi: 10.1136/bmj.38043.583160.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawthorne G, Robson S, Ryall EA, Sen D, Roberts SH, Platt MP. Prospective population based survey of outcome in pregnancy in diabetic women: results of the Northern Diabetic Pregnancy Audit. BMJ. 1997;315:279–281. doi: 10.1136/bmj.315.7103.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, Platt MJ, Stanisstreet M, van Velszen D, Walkinshaw S. Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ. 1997;315:275–278. doi: 10.1136/bmj.315.7103.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temple R, Aldridge V, Greenwood R, Heyburn P, Sampson M, Stanley K. Association between outcome of pregnancy and glycemic control in early pregnancy in type 1 diabetes: population based study. BMJ. 2002;325:1275–1276. doi: 10.1136/bmj.325.7375.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suhonen L, Hulesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type 1 diabetes mellitus. Diabetologia. 2000;43:79–82. doi: 10.1007/s001250050010. [DOI] [PubMed] [Google Scholar]
- 6.Boulot P, Chabbert-Buffet N, d'Ercole C, Floriot M, Fontaine P, Fournier A, Gillet JY, Gin H, Grandperret-Vauthier S, Geudj AM et al. French multicentric survey of outcome of pregnancy in women with pregestational diabetes. Diabetes Care. 2003;26:2990–2993. doi: 10.2337/diacare.26.11.2990. [DOI] [PubMed] [Google Scholar]
- 7.Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, Beck-Nielsen H. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27:2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 8.Penney GC, Mair G, Pearson DW. Outcomes of pregnancies in women with type 1 diabetes in Scotland: a national population-based study. BJOG. 2003;110:315–318. [PubMed] [Google Scholar]
- 9.Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, Golightly S, Miller A. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales and Northern Ireland: population based study. BMJ. 2006;333(7560):177. doi: 10.1136/bmj.38856.692986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CD, Tan HY, Hong SF, Nickless NA, Copel JA. Strategies for reducing the frequency of preeclampsia in pregnancies with insulin-dependent diabetes mellitus. Am J Perinatol. 1996;13:265–268. doi: 10.1055/s-2007-994340. [DOI] [PubMed] [Google Scholar]
- 11.Hiilesmaa V, Suhonen L, Teramo K. Glycemic control is associated with pre-eclampsia but not with pregnancy-induced hypertension in women with type 1 diabetes mellius. Diabetologia. 2000;43:1534–1539. doi: 10.1007/s001250051565. [DOI] [PubMed] [Google Scholar]
- 12.Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992;10:1251–1257. doi: 10.2337/diacare.15.10.1251. [DOI] [PubMed] [Google Scholar]
- 13.Clausen TD, Mathiesen E, Ekbom P, Hellmuth E, Mandrup-Poulsen T, Damm P. Poor pregnancy outcome in women with type 2 diabetes. Diabetes Care. 2005;28(2):323–328. doi: 10.2337/diacare.28.2.323. [DOI] [PubMed] [Google Scholar]
- 14.Dunne F, Brydon P, Smith K, Gee H. Pregnancy in women with type 2 diabetes: 12 years outcome data 1990-2002. Diabet Med. 2003;20(9):734–738. doi: 10.1046/j.1464-5491.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- 15.Farrell T, Neale L, Cundy T. Congenital anomalies in the offspring of women with type 1, type 2 and gestational diabetes. Diabet Med. 2002;19:322–326. doi: 10.1046/j.1464-5491.2002.00700.x. [DOI] [PubMed] [Google Scholar]
- 16.Towner D, Kjos SL, Leung B, Montoro MM, Xiang A, Mestman JH, Buchanan TA. Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care. 1995;18:1446–1451. doi: 10.2337/diacare.18.11.1446. [DOI] [PubMed] [Google Scholar]
- 17.Cundy T, Gamble C, Townend K, Henley PG, MacPherson P, Roberts AB. Perinatal mortality in type 2 diabetes. Diabet Med. 2000;17:33–39. doi: 10.1046/j.1464-5491.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 18.Feig D, Palda VA. Type 2 diabetes: a growing concern. Lancet. 2002;359:1690–1692. doi: 10.1016/S0140-6736(02)08599-9. [DOI] [PubMed] [Google Scholar]
- 19.Weijers RN, Bekedam DJ, Oosting H. The prevalence of type 2 diabetes and gestational diabetes mellitus in an inner city multi-ethnic population. Eur J Epidemiol. 1998;14:693–699. doi: 10.1023/a:1007597623897. [DOI] [PubMed] [Google Scholar]
- 20.Piacquadio K, Hollingsworth DR, Murphy H. Effects of in-utero exposure to oral hypoglycaemic drugs. Lancet. 1991;338:866–869. doi: 10.1016/0140-6736(91)91512-s. [DOI] [PubMed] [Google Scholar]
- 21.Hellmuth E, Damm P, Molsted-Pedersen L. Congenital malformations in offspring of diabetic women treated with oral hypoglycaemic agents during embryogenesis. Diabet Med. 1994;11:471–474. doi: 10.1111/j.1464-5491.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 22.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ACE inhibitors. New Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 23. http://statine.cbs.nl/statweb. Central Bureau of Statistics, the Netherlands; [Google Scholar]
- 24.Vangen S, Stoltenberg C, Holan S, Moe N, Magnus P, Harris JR, Stray-Pedersen B. Outcome of pregnancy among immigrant women with diabetes. Diabetes Care. 2003;26:327–332. doi: 10.2337/diacare.26.2.327. [DOI] [PubMed] [Google Scholar]
- 25.Kirchengast S, Hartman B, Schweppe B, Husslein P. Impact of maternal body build characteristics on new born size in two different European populations. Hum Biol. 1998;70:761–774. [PubMed] [Google Scholar]
- 26.Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia in women with type 1 diabetes mellitus. Diabetologia. 2002;45:1484–1489. doi: 10.1007/s00125-002-0958-7. [DOI] [PubMed] [Google Scholar]
- 27.Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992;10:1251–1257. doi: 10.2337/diacare.15.10.1251. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, Aarons JH. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164:103–111. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 29.Kyne-Grzebalski D, Wood L, Marshall SM, Taylor R. Episodic hyperglycaemia in pregnant women with well-controlled type 1 diabetes mellitus: a major potential factor underlying macrosomia. Diabet Med. 1999;16:702–706. doi: 10.1046/j.1464-5491.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 30.Kerssen A, Evers IM, de Valk HW, Visser GH. Poor glucose control in women with type 1 diabetes mellitus and 'safe' hemoglogin A1c values in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2003;13(5):309–313. doi: 10.1080/jmf.13.5.309.313. [DOI] [PubMed] [Google Scholar]
- 31.Kerssen A, de Valk HW, Visser GH. Do HbA1c levels and the self-monitoring of blood glucose levels adequately reflect glycaemic control during pregnancy in women with type 1 diabetes mellitus. Diabetologia. 2006;49(1):25–28. doi: 10.1007/s00125-005-0057-7. [DOI] [PubMed] [Google Scholar]
- 32.Fuhrmann K, Reiher H, Semmler K, Fischer F, Fischer M, Glockner E. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6:219–223. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- 33.Temple RC, Aldridge VJ, Murphy HJ. Pre-pregnancy care and pregnancy outcome in women with type 1 diabetes. Diabetes Care. 2006;29:1744–1749. doi: 10.2337/dc05-2265. [DOI] [PubMed] [Google Scholar]


