Abstract
There is considerable controversy concerning the theoretical basis of retrograde amnesia (R.A.). In the present paper, we compare medial temporal, medial plus lateral temporal, and frontal lesion patients on a new autobiographical memory task and measures of the more semantic aspects of memory (famous faces and news events). Only those patients with damage extending beyond the medial temporal cortex into the lateral temporal regions showed severe impairment on free recall remote memory tasks, and this held for both the autobiographical and the more semantic memory tests. However, on t-test analysis, the medial temporal group was impaired in retrieving recent autobiographical memories. Within the medial temporal group, those patients who had combined hippocampal and parahippocampal atrophy (H+) on quantified MRI performed somewhat worse on the semantic tasks than those with atrophy confined to the hippocampi (H−), but scores were very similar on autobiographical episodic recall. Correlational analyses with regional MRI volumes showed that lateral temporal volume was correlated significantly with performance on all three retrograde amnesia tests. The findings are discussed in terms of consolidation, reconsolidation, and multiple trace theory: We suggest that a widely distributed network of regions underlies the retrieval of past memories, and that the extent of lateral temporal damage appears to be critical to the emergence of a severe remote memory impairment.
The neural bases of retrograde amnesia and long-term memory functions remain key issues in cognitive neuroscience. In particular, the time course of medial temporal lobe (MTL) involvement in the storage and retrieval of remote memories has not been resolved. The present study examines performance across a range of measures of remote memory in amnesic patients with focal medial temporal lobe pathology, more widespread temporal lobe damage, and frontal lobe damage. The purpose is to test theoretical predictions concerning the nature of retrograde amnesia.
Despite a proliferation of studies on memory and its disorders over the last three to four decades, many of the critical issues are still widely debated (Kopelman 2002, 2006). There are two main theoretical positions currently discussed in the literature regarding the role of MTL structures in the retrieval of remote memories, each of which makes distinct predictions about how remote memory is affected by the physical location and extent of brain damage. According to the consolidation model (Zola-Morgan and Squire 1990; Squire 1992; Squire and Alvarez 1995), episodic (autobiographical events) and semantic memories are processed in the same way, initially dependent on the hippocampal formation, but over time they become represented in the neocortex, independently of medial temporal structures. In contrast, multiple trace theory (Nadel and Moscovitch 1997, 2001; Nadel et al. 2000; Moscovitch et al. 2006) proposes a critical distinction between semantic and episodic memory. Retrieval of episodic (and spatial) memories is claimed to always be dependent on MTL structures, including the hippocampus. However, although the hippocampus contributes to the formation and assimilation of semantic memories (Moscovitch et al. 2006), remote memory for such information (e.g., general aspects of past events, such as names of people and places, and for personal semantics) is thought to operate independently of the hippocampus (Nadel et al. 2000).
A central claim of consolidation theory is that only recently acquired episodic and semantic memories are compromised in patients with damage restricted to the hippocampus. Where damage has extended beyond the hippocampus to include other MTL regions (i.e., parahippocampal, perirhinal, and entorhinal cortices), more remote memories might be affected, but a steep temporal gradient will still occur (Rempel-Clower et al. 1996; Reed and Squire 1998). Finally, when damage extends into anterolateral temporal regions, the storage areas themselves will be affected, resulting in a much more temporally extensive remote memory loss. However, unless the damage is so widespread that the entire medial and lateral temporal lobes are bilaterally destroyed, consolidation theory would still predict relative preservation of the most remote memories, i.e., a temporal gradient. The proposal that damage restricted to the hippocampus should have no effect on the storage and retrieval of remote memories raises important questions. First, is a memory trace ever actually “stored” within the hippocampus? If so, then there must be some process by which the memory is transferred (or replicated) from the hippocampus to the temporal neocortex. Second, does the form of the reallocated memory change or stay the same?
In contrast to the consolidation model, multiple trace theory (MTT) proposes that the hippocampi or medial temporal lobes are continuously involved in the storage and retrieval (reactivation) of episodic memories, whatever their age. Although some studies of memory-disordered patients provide support for this claim (e.g., Moscovitch et al. 1999, 2006; Nadel et al. 2000; Viskontas et al. 2000), it is clear that the duration of retrograde amnesia varies widely in investigations of patients described as having focal medial temporal lobe damage, ranging from very brief retrograde memory loss to a virtually complete inability to recall any information from any premorbid time period (for reviews, see Kopelman and Kapur 2001; Spiers et al. 2001). For example, a patient described as having selective hippocampal damage (patient “V.C.”) was reported to have an extensive and temporally ungraded retrograde amnesia (Cipolotti et al. 2001), while others (e.g., patients “R.B.” and “G.D.”), with apparently similar pathology, were described as having a retrograde deficit limited to 1–2 yr (Zola Morgan et al. 1986; Rempel-Clower et al. 1996).
It is also of note that functional neuroimaging studies have similarly produced conflicting findings. On the one hand, several recent functional activation studies in healthy volunteers have provided evidence that the hippocampus and related medial temporal lobe structures are more activated during the retrieval of recent compared with remote episodic memories (Haist et al. 2001; Niki and Luo 2002; Piefke et al. 2003; Mayes et al. 2004). These investigations have used autobiographical or famous faces tasks, and the findings could be interpreted as consistent with consolidation theory. On the other hand, other studies indicate that medial temporal structures (and the hippocampi in particular) are significantly activated in retrieving both recent and remote autobiographical memories (Maguire et al. 2001; Ryan et al. 2001; Piolino et al. 2002; Maguire and Frith 2003), although the pattern of activations within the hippocampi may vary between recent and remote memory activations (Gilboa et al. 2004). Such findings would seem more consistent with the predictions of multiple trace theory. Bernard et al. (2004) made a similar finding in a task involving the recognition of famous faces. To the extent that the retrieval of famous faces taps remote semantic memory, this finding counters the argument that the hippocampi are selectively involved in remote episodic memory (Nadel and Moscovitch 1997; Tulving and Markowitsch 1998), although it has been argued that retrieval of such semantic information can elicit autobiographical memories that might explain the hippocampal activations (Moscovitch et al. 2006).
Another perspective on the nature of memory traces has recently been proposed that contradicts the consolidation view that the stability of a memory trace is tightly linked to its age. In a landmark fear conditioning study in rats, Nader et al. (2000) presented evidence that during retrieval from long-term memory, the synapses holding a memory trace are somehow uncoupled, such that a process of reconsolidation, requiring new protein, is necessary for that memory to be returned to an inactive, stable state. In this and subsequent studies (Debiec et al. 2002; Nader 2003), these investigators have rekindled interest in earlier reports that the process of reactivating stable long-term memories returns them to a labile state, sensitive to disruption and/or alteration (e.g., Misanin et al. 1968; Lewis 1979; Mactutus et al. 1979). Other groups have since published findings consistent with this general picture (e.g., Land et al. 2000; Sara 2000; Kida et al. 2002). One implication of these studies is that a stable memory trace is not the final end point of the encoding process, but that all memories are dynamic and subject to alteration. Although it may be difficult to formally test the predictions of reconsolidation theory in human studies of remote memory, the emphasis on complex, dynamic, and interactive processes may be instructive when considering the conflicting empirical evidence for the “traditional” view that there are two qualitatively different memory states.
In previous investigations by our group, we compared the retrograde amnesias of patients with diencephalic, temporal lobe, or frontal lesions (Kopelman et al. 1999). We found differing patterns of temporal gradient across these patient groups, together with evidence of an important retrieval component to the deficit but no major access/storage differences between the frontal and the temporal lobe groups. Laterality comparisons indicated that right temporal and frontal lobe pathology disproportionately impaired autobiographical memory retrieval, whereas left temporal damage more closely affected what was termed the lexical-semantic “labeling” of remote memories (Kopelman et al. 1999). None of these findings could easily be “explained” by a simple or unmodified consolidation or multiple trace account. A subsequent analysis of MRI regional brain volumes in these 40 patients tested a fundamental claim of MTT—namely, that the degree of damage to the medial temporal cortex would correlate with the severity and temporal extensiveness of remote autobiographical memory loss (Kopelman et al. 2003). The findings supported the view that widespread neural networks are involved in the storage and retrieval of autobiographical and other remote memories. Brain volume measures in critical structures could account for 60% of variance on autobiographical memory measures in diencephalic patients and for 60%–68% of variance in patients with frontal lesions. Significant correlations with medial temporal lobe volume were found only in the diencephalic group, in whom they were thought to reflect the distal effects of thalamic changes, but not in patients whose principal pathology was in the temporal lobes (herpes encephalitis and hypoxic cases). This latter finding was inconsistent with one of the main predictions of multiple trace theory (but see Gilboa et al. 2005). Other observations failed to support consolidation theory, suggesting again that the empirical evidence does not appear to lend full support to either of these theories (Kopelman et al. 2003).
In the present investigation, we have explored these issues further using new tests in a different group of patients. In particular, we compare remote memory performance in patients with focal medial temporal lobe damage with that in patients with more widespread (medial and lateral) temporal lobe damage in order to examine key outstanding issues:
Is damage to the medial temporal lobes sufficient to produce impairment in the recall of remote memories?
Does medial temporal lobe damage affect only remote autobiographical memory? Or does it also affect the more semantic aspects of remote memory? Or is more lateral temporal lobe damage necessary to produce retrograde amnesia in either domain?
Are there distinct patterns of remote memory impairment associated with damage to the hippocampi in isolation versus the involvement of neighboring (parahippocampal) structures, as measured on quantified structural MRI?
For comparative purposes, we have also included a group of frontal lobe amnesic patients to explore the neural correlates of remote memory performance in more detail.
How do the findings relate to the claims of consolidation, reconsolidation, and multiple trace theory?
Results
Remote memory in patients with medial temporal, lateral temporal, and frontal lesions
Famous faces
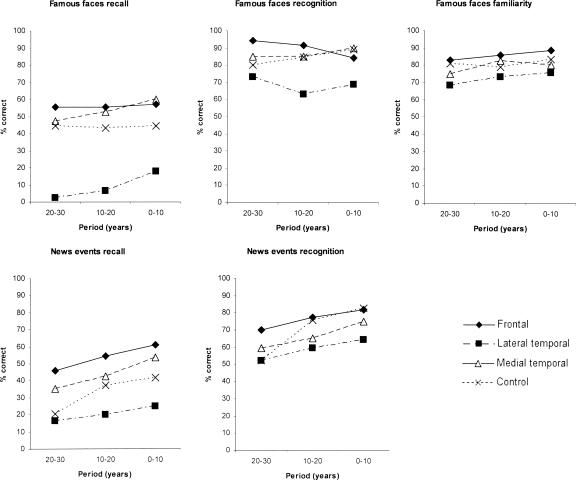
Figure 1 shows the results of the famous faces recall, recognition, and familiarity tasks in the upper panel. The “lateral” temporal lesion group performed worst across all time periods on recall and recognition, but particularly the former. We conducted a repeated measures multivariate analysis of variance of Group × Condition (recall, recognition, familiarity) × Time Period (10-yr block). The Group effect was significant (F3,22 = 3.54, P < 0.05). The Group × Task interaction effect was also significant (F6,44 = 2.67, P < 0.05). This latter effect was driven primarily by a disproportionately severe deficit in recall relative to familiarity and recognition in the “lateral” temporal patients. The Group × Task × Period interaction was not significant (F12,88 = 0.79, N.S.). We then examined each task individually.
Figure 1.
Retrograde memory performance on famous faces (recall, recognition, familiarity) and news events (recall, recognition) tests in patient groups and controls at each time period (years pre-onset).
Recall
There was a statistically significant main effect of Group (F3,22 = 5.80, P < 0.01), but there was no significant Group × Period interaction (F6,44 = 0.20, N.S.). Planned comparisons revealed that the lateral temporal group scored significantly below controls (F1,14 = 1.31, P < 0.05), but that the other two patient groups did not differ significantly from controls.
Recognition
There was no significant Group effect (F3,22 = 2.26, N.S.) or Group × Period effect (F6,44 = 0.97, N.S.).
Familiarity
There were no differences among the groups (F3,22 = 0.60, N.S.) and no significant interaction effect (Group × Period interaction: F6,44 = 0.19, N.S.).
As a final analysis of the data, independent samples t-tests were carried out to compare each patient group with controls at each period on the three measures. On recall, the lateral temporal patients produced significantly poorer scores in the two most remote periods (P < 0.01) with a near-significant effect in the most recent period (P < 0.06). On recognition, there was a nonsignificant trend for the lateral temporal patients to show impairment at the most recent period (0–10 yr; P < 0.10). No differences were found between medial temporal or frontal patients and controls (P > 0.10 in all comparisons). As mentioned below (see Materials and Methods), these findings are very unlikely to have reflected the way our controls’ data were analyzed: Matching the seven (out of 19) patients whose amnesia had lasted >5 yr with seven controls in terms of the half-decades analyzed would have made very minimal difference as the control curves on this test were essentially flat (Fig. 1).
News events
Figure 1 (lower panel) shows the findings on the news events task, recall, and recognition. Again, the “lateral” temporal lesion group performed poorest across all time periods in both tasks.
A repeated measures multivariate analysis of variance was carried out involving the variables Group × Task (recall, recognition) × Time Period (10-yr block). The Group effect was not significant (F3,22 = 1.79, N.S.). There was a marginally significant Group × Task effect (F3,22 = 3.00, P = 0.05), but the Group × Task × Period effect was not significant (F6,44 = 0.31, N.S.). This pattern is consistent with the result on the famous faces test, the “lateral” temporal group performing disproportionately badly at recall testing. However, when we examined the recall and recognition tasks individually, there was only a nonsignificant trend for the groups to differ in terms of news events recall (F3,22 = 2.28, P = 0.10) and the Group × Period interaction was also not statistically significant (F6,44 = 0.28, N.S.). On recognition testing there was no significant difference between the groups (F3,22 = 1.8, N.S.) and no significant Group × Period interaction (F6,44 = 1.06, N.S.).
As a final analysis of the data, independent samples t-tests were carried out to compare each patient group with controls at each period on recall and recognition. No differences were found between patient and control group performance on recall (P > 0.1 in all comparisons). On recognition, the lateral temporal patients were impaired only on the most recent period (0–10 yr; P < 0.05), consistent with a temporal gradient (relative to control group performance) in this group. No differences were found between medial temporal or frontal patients and controls (P > 0.10 in all comparisons). These generally negative findings are unlikely to have reflected the way our controls were analyzed because, as mentioned below (see Materials and Methods), matching the seven (out of 19) patients whose amnesia had lasted >5 yr with seven controls in terms of the half-decades analyzed would have diminished rather than increased the probability of obtaining significant differences at the earliest time point.
Autobiographical memory
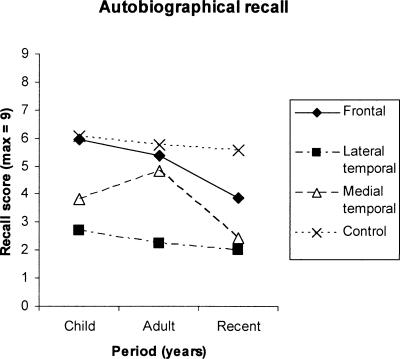
Figure 2 shows the findings on the autobiographical episodic recall test. The “Child” and “Adult” data points are based on all patients who performed this test and the “Recent” data point on only those patients for whom this data point was entirely retrograde. Control participants showed a relatively flat curve across the three time periods. Compared with this, the “lateral” temporal lesion patients performed very poorly and did not show a temporal gradient relative to controls in their performance. The frontal lesion group showed a pattern consistent with a temporal gradient, in that performance was undifferentiated from controls at the most remote period, but this group showed relatively poor performance in the recent period. The medial temporal group’s performance was intermediate between the frontal and the “lateral” temporal lesion groups.
Figure 2.
Autobiographical recall scores (max = 9) in patient groups and controls across three time periods (Recent, Adulthood, Childhood).
On examining statistically only those patients with “retrograde” data at each data point, there was a main effect of Group (F3,11 = 4.92, P < 0.05), but the Group × Time Period interaction was not statistically significant. Examining all participants who performed this test on the first two data points (Childhood, Adult) only, there was a highly significant main effect of Group (F3,18 = 8.15, P = 0.001), but again the Group × Time Period interaction was not significant (F3,18 = 1.64, N.S.). Planned comparisons for this latter analysis showed that the lateral temporal group differed significantly from controls in terms of main effect (F1,9 = 38.24, P < 0.001) but that the other two patient groups did not differ significantly from controls.
As a final analysis of the data, independent samples t-tests were carried out at each data point to compare each patient group with controls. Lateral temporal patients showed significantly poorer recall performance at all periods (P < 0.01). There was a nonsignificant trend for the medial temporal patients to show poorer performance at recalling events from the most remote period (P < 0.10), but they did not differ significantly from controls at the intermediate (Young Adult) period. However, the medial temporal group performed significantly worse than controls at the most recent period (P < 0.05), therefore providing partial evidence for a temporal gradient in this group. Consistent with this, the difference between the medial temporal group’s score for the Young Adult time period and their Recent score was statistically significant on a t-test (P < 0.05), but other comparisons within this group were not significant. Frontal patients showed a nonsignificant trend to differ from controls on recall scores at the most recent period only (P < 0.10).
Further investigation of the role of medial temporal lobe structures in memory retrieval
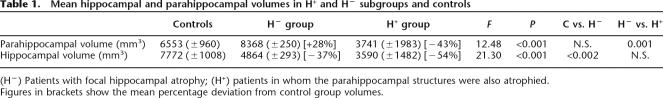
Inspection of the quantified MRI measurements revealed that the medial temporal group could be subdivided into a subgroup of three patients (H−) in whom the hippocampi alone appeared to be atrophied and a subgroup of two patients (H+) in whom the parahippocampal structures were also atrophied (see Table 1; Fig. 5, below). Although the small sample sizes render formal statistical comparisons unreliable, we visually examined these data (and used descriptive statistics) in order to determine whether there might be a differential effect of hippocampal versus more widespread medial temporal damage on remote memory performance.
Table 1.
Mean hippocampal and parahippocampal volumes in H+ and H− subgroups and controls
(H−) Patients with focal hippocampal atrophy; (H+) patients in whom the parahippocampal structures were also atrophied.
Figures in brackets show the mean percentage deviation from control group volumes.
Figure 5.
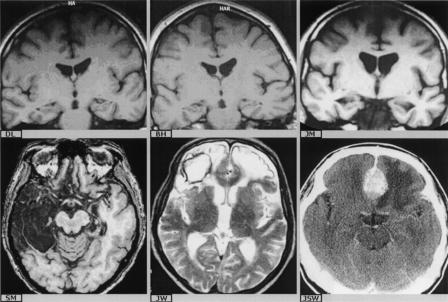
(Top row) Representative coronal sections showing hippocampal atrophy only (H−) in patients “D.L.” and “B.H.” (cerebal hypoxia), and parahippocampal and hippocampal atrophy (H+) in patient “J.M.” (cerebral hypoxia). (Bottom row) Axial sections showing bilateral medial temporal lobe pathology and extensive right antero-lateral pathogy in patient “S.M.” (herpes encephalitis) and bilateral frontal pathology in patients “J.W.” (contusion and hemorrhage) and “J.S.W.” (meningioma).
Table 1 shows the mean hippocampal and parahippocampal volumes in these two subgroups relative to healthy controls. F values from One-Way Analyses of Variance and their significance values are shown, together with the results of (C vs. H− and H− vs. H+) Bonferroni post hoc tests. The table shows that the H− subgroup did not differ significantly from controls in terms of total (left and right) parahippocampal volume, but that the H+ subgroup showed significantly smaller parahippocampal volumes than the H− subgroup (P < 0.001); the H+ group also differed significantly from controls (P < 0.02). In contrast, both H+ and H− subgroups showed significantly smaller mean hippocampal volumes than controls (P < 0.001, P < 0.002, respectively), but they did not differ significantly from one another with respect to mean hippocampal volumes. As these medial temporal subgroups are small and the findings in these analyses are uncorrected for duration of amnesia, we have included descriptive statistics only.
Famous faces
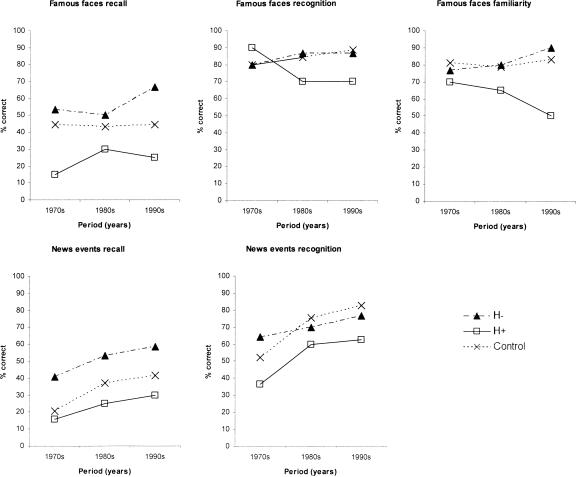
Figure 3 (top panel) presents the data for the H−, H+, and control groups on each of the three tasks. There were consistent trends across the two patient subgroups. On recall, the H− patients were able to recall >50% of the names of famous personalities from the recent and remote periods. In contrast, the H+ patients recalled correctly only 15% of faces from the remote period and 25% of faces from the recent period. There was no suggestion of a gradient difference between the H+ and H− subgroups. On recognition, the mean scores of the two subgroups were closer, and on familiarity, there was a suggestion of a temporal gradient in the H+ group. Figure 3 also shows that the H− group scored at or above the control means. The H+ subgroup scored more consistently below the control means, but at or within 1 S.D. below the controls at each data point (except recognition for the 1970s, where they scored above the control mean).
Figure 3.
A comparison of patients with focal hippocampal atrophy (H−) and additional parahippocampal atrophy (H+) on famous faces and news events tasks at each time period.
News events
Figure 3 (bottom panel) shows that the H+ patients also performed poorer than the H− patients across both news events recall and recognition with very similar gradients. Again the H− group performed at or above the control means. The H+ subgroup scored below the control means, but within 1 S.D. at all points except recognition for the 1990s, where they scored just greater than 1 S.D. below the controls.
Autobiographical memory
Figure 4 shows the autobiographical test results. Both patient groups showed a similar pattern of performance on episodic recall, in particular showing a decrease in performance for the most recent period relative to the preceding (Young Adult) period. Both H− and H+ scored just greater than 1 S.D. (and <2 S.D.) below the control means for the Childhood and Recent periods, and within 1 S.D. of the controls for the Young Adult period.
Figure 4.
A comparison of patients with focal hippocampal atrophy (H−) and additional parahippocampal atrophy (H+) on autobiographical recall of events at each time period.
Correlations between remote memory performance and regional brain volumes across patients
Correlational analyses were performed on the patients’ data. For the semantic tests, these were based on the mean scores across the time periods in the three decades pre-onset. For the autobiographical test, the mean value across the first two time periods (Childhood, Young Adult) was used in all participants to prevent any confounding by anterograde memories.
Table 2 presents the correlation coefficients of performance on the famous faces test with regional brain volumes across patients. Given an a priori hypothesis that smaller volumes would be associated with poorer test scores, we adopted a one-tailed criterion for statistical significance. Recall performance was significantly associated with temporal and frontal lobe brain volumes. The temporal lobe correlations were somewhat stronger in the left hemisphere, particularly for the medial temporal measurements, perhaps relating to the verbal demands of the task. There were no significant correlations between recognition scores on the famous faces task and brain volumes. Familiarity scores were significantly associated only with medial temporal volume in the right hemisphere, and with right hippocampal and parahippocampal volumes taken individually. Left hippocampal/parahippocampal volumes did not correlate significantly with familiarity performance.
Table 2.
Correlations between performance on the famous faces task and regional brain volumes
*P < 0.05; **P < 0.01; one-tailed.
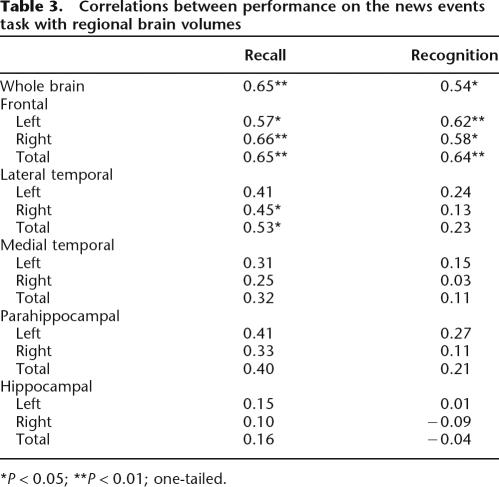
Table 3 shows that, on news events recall, there was a significant association between whole brain volume and both recall and recognition performance. In addition, there were significant correlations with left, right, and total frontal volume and with right and total lateral temporal lobe volumes, but not with any of the medial temporal measures. On recognition testing, there were significant correlations with frontal lobe volume but not with temporal lobe volume. There were no significant laterality effects.
Table 3.
Correlations between performance on the news events task with regional brain volumes
*P < 0.05; **P < 0.01; one-tailed.
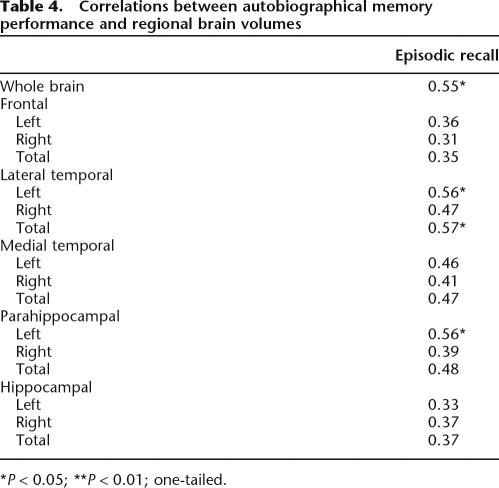
Table 4 shows correlations between regional brain volumes and autobiographical episodic recall. There were no significant correlations with frontal lobe volumes. Total lateral temporal and left lateral temporal (but not right) volume was correlated with episodic recall. Left parahippocampal volume was also significantly correlated with episodic recall. The episodic recall–hippocampal volume correlations were not significant.
Table 4.
Correlations between autobiographical memory performance and regional brain volumes
*P < 0.05; **P < 0.01; one-tailed.
Discussion
Several key findings have emerged from the present study. First, only those patients with damage extending beyond medial temporal cortex into lateral temporal regions showed severe impairments on free recall on the more semantic remote memory tests (famous faces and news events). In contrast, where damage was confined to medial temporal cortical areas, recall performance did not differ significantly from the controls. On autobiographical episodic recall, only the lateral temporal group showed statistically significant impairment on our overall (ANOVA) analyses. However, on t-test analysis of performance at the individual data points, the medial temporal group showed significant impairment (P < 0.05) at the most recent data point, consistent with other reports of a brief, time-limited retrograde memory loss in patients with medial temporal lesions (Zola-Morgan et al. 1986; Kapur and Brooks 1999). This finding contrasts with those reports that have described a severe and temporally extensive retrograde amnesia in apparently similar cases (Viskontas et al. 2000, 2002; Cipolotti et al. 2001). In general, there were only small and nonsignificant differences in recognition memory performance between the patient groups and the controls, although the lateral temporal group was significantly impaired (P < 0.05) on news events for the most recent time period (0–10 yr) and showed a similar trend on famous faces. The generally better performance by the patient groups (including the lateral temporal group) on recognition than recall tests is suggestive of a retrieval component to the deficit, although a possible ceiling effect in the controls might also contribute to/account for this difference on the famous faces test.
Secondly, a comparison of the medial temporal subgroups revealed that the patients with medial temporal lobe damage extending beyond the hippocampus into parahippocampal structures (H+) performed consistently below those with focal hippocampal atrophy (H−) and healthy controls on both the famous faces and news events tests, just as they had also performed worst on anterograde tests (Kopelman et al. 2006). However, the H+ group’s scores were at or within 1 S.D. of the control means, and this finding must be interpreted cautiously in such small subgroups. On the autobiographical memory measure, both medial temporal subgroups performed consistently below controls at all periods—just over 1 S.D. below the control means for the Childhood and Recent periods—and there was minimal difference between the two subgroups (Fig. 4). There have been very few such comparisons in the previous literature. Reed and Squire (1998) compared two patients with pathology apparently confined to the hippocampi (equivalent to our H− subgroup, but MRI data were available for only one of these cases) with two patients with more widespread temporal lobe damage, involving other medial temporal lobe structures and beyond (more like our “lateral” temporal lobe group): The former group showed a time-limited retrograde loss, involving events/facts from the decade before onset, and the latter a more extensive (but still temporally graded) retrograde amnesia. Subsequently, Bayley et al. (2003) and Manns et al. (2003) have examined six hippocampal cases, for whom relative but not absolute hippocampal volumes were given for five of the cases: The findings were broadly consistent with those of Reed and Squire (1998). Most recently, Bayley et al. (2005) have reported further findings, showing normal autobiographical memory scores in these five cases and abnormal scores in three patients with additional neocortical atrophy. New quantified MRI data indicated that three of the first group were, in fact, H− in our terms and two were H+, although inspection of the published images indicates that the latter group (whose diagnosis was viral encephalitis) also had bilateral temporal polar signal alteration, which our H+ group (with hypoxic damage) did not. On the basis of reviews of the literature, Fujii et al. (2000) and Moscovitch et al. (2006) have argued that damage to the “hippocampus proper” (the CA fields) causes little or no retrograde amnesia, whereas damage to the “hippocampal complex” (broadly, the medial temporal lobes) is required to produce a severe retrograde amnesia. In addition, Buchanan et al. (2005) recently carried out an H−/H+ comparison in terms of the bias to produce pleasant or unpleasant memories to word cues: The H− group produced a normal pattern of responding, whereas the H+ group appeared to produce fewer unpleasant (but a normal number of pleasant) memories.
Thirdly, our correlational analyses, carried out in patients with relatively focal lesions, supported the view that a widespread, distributed network of brain regions is involved in retrieval from remote memory. Recall of famous faces was correlated with frontal, lateral, and medial temporal volumes (predominantly in the left hemisphere, presumably because of the requirement to name these faces). On news events, recall correlated significantly with left and right frontal and with right and total lateral temporal volumes, and recognition was correlated with left and right frontal lobe volumes. Autobiographical episodic recall correlated only with total lateral and left lateral temporal lobe volumes, and with left parahippocampal volume; correlations with hippocampal volumes were nonsignificant. The significant correlations found between lateral temporal volumes and recall performance across famous faces, news events, and autobiographical memory tasks might be considered consistent with the consolidation view that there is no critical distinction in the neural underpinnings of semantic and episodic remote memory. However, the correlational findings also suggested that retrieval from semantic memory (recall of famous faces and, to a lesser extent, news events) might also be contingent on the integrity of frontal lobe processes.
With respect to the issues outlined in the introduction, our findings generally suggest that damage to the medial temporal lobes in isolation is not sufficient to produce significant impairment in the retrieval of remote memories, either in terms of autobiographical memory tests or on tasks (famous faces, famous news events) examining the more semantic aspects of remote memory. This finding is broadly consistent with observations obtained by Buchanan et al. (2005) using a very different type of task. However, t-test analysis at individual data points did indicate some impairment at the most recent time point on the autobiographical test only. This latter finding is consistent with those studies that have found only a brief retrograde amnesia in similar patients (Reed and Squire 1998; Kapur and Brooks 1999; Bayley et al. 2003, 2005; Manns et al. 2003), but not with those studies that have obtained a much more extensive retrograde memory loss (Viskontas et al. 2000, 2002; Cipolotti et al. 2001). There was a nonsignificant trend for patients with more extensive medial temporal lobe damage (involving neighboring, parahippocampal structures) to show a worse performance on the more semantic remote memory tasks (famous faces, famous news events) than those with hippocampal damage in isolation, as others have suggested there would be (e.g., Rempel-Clower et al. 1996; Moscovitch et al. 2006). The relatively preserved autobiographical and remote memory performance in the MTL group, relative to patients with more extensive temporal lobe pathology, suggests that the lateral temporal regions are more critically involved than the MTL in both autobiographical and semantic remote memory retrieval; the correlational findings were consistent with this. There may be various reasons why differences in the temporal extent of retrograde amnesia have been reported in medial temporal cases, other than just lesion location and extent. These might include differences in the measures used, in task “difficulty,” and in the validation and matching of the salience of test material across time periods.
In contrast to several earlier reports that frontal pathology can in itself produce an extensive retrograde amnesia (Baddeley and Wilson 1986; Kopelman 1991; Della Sala et al. 1993; Mangels et al. 1996; Kopelman et al. 1999, 2003), we found no evidence for a remote memory impairment on any of the tests, except for a nonsignificant trend for this group to perform worse than controls at “recent” autobiographical memories. However, the correlational analyses indicated significant correlations between frontal lobe volumes and recall performance on famous faces and news events (but not autobiographical memory), consistent with the involvement of frontal processes in retrieval from remote memory. These results raise the possibility that, although frontal (and medial temporal) regions may form part of a distributed neural network subserving memory retrieval, damage to the frontal cortex needs to be quite extensive and exceed a certain critical volume before a significant remote memory impairment can be observed.
With respect to the claims of the competing theories, it has generally been assumed in the past that a temporal (or “Ribot”) gradient is a marker of consolidation (Squire and Alvarez 1995; Meeter and Murre 2004), and that “flat” remote memory curves are more likely to favor MTT (Nadel and Moscovitch 1997) or a retrieval deficit (Sanders and Warrington 1971). However, it has at times been argued that a temporal gradient can be consistent with a retrieval deficit (Weiskrantz 1985) or that a flat curve is consistent with a consolidation deficit (Rempel-Clower et al. 1996), at least in mild cases where tests are insensitive to pick up a brief R.A. Hence, the criteria for differentiating between these theories are not completely agreed. In the present investigation, there was only partial evidence for temporal gradients across our analyses (evident on comparing t-test findings at individual data points on some tests, but not in terms of significant interaction effects on ANOVA), a finding that, according to most accounts, challenges the conception of the long-term consolidation of memories. The general lack of clear temporal gradients in this study is broadly consistent with previous observations by Kopelman et al. (1999), which showed only gentle temporal gradients in patients with temporal lobe or frontal lobe lesions across autobiographical and news events tasks, whereas Korsakoff patients (not included in the present study) showed much steeper gradients. On the other hand, our findings also present a challenge for multiple trace theory, which predicts significant remote memory impairment, particularly in autographical recall, in patients with relatively restricted medial temporal lobe lesions. The correlational analyses, in which there was only limited evidence for an association between autobiographical memory scores and medial temporal lobe volumes, also failed to support the predictions of MTT (cf. the findings of Kopelman et al. 2003 in a differing set of patients of focal lesion patients, but see Gilboa et al. 2005 in Alzheimer patients).
How do these findings square with recent neuroimaging evidence for medial temporal lobe activation during retrieval of even the most remote memories (Maguire et al. 2001; Ryan et al. 2001; Piolino et al. 2002; Bernard et al. 2004; Gilboa et al. 2004)? Our view is that the present findings can accommodate these observations. Regions within the hippocampi and medial temporal cortices may, indeed, be involved in remote memory retrieval, but this is not to say that these regions are either critical or necessary. Thus, we found an association of medial temporal cortical volumes with recall performance on the famous faces test, yet patients with medial temporal lobe involvement performed at the same level as did the controls. In contrast, the consistently significant correlations found between lateral temporal lobe volumes and recall performance on the semantic and autobiographical measures were accompanied by significantly poorer performance by the patients with lateral temporal damage relative to controls. Consistent with our position, recent studies of reconsolidation offer a further means for reconciling consolidation theory and MTT. If reconsolidation of an old memory occurs in the hippocampus at retrieval (or, alternatively, encoding of the events of a testing session), but the hippocampus is not involved in the expression of a remote memory, this could explain why the majority of neuroimaging studies show hippocampal activation during remote memory recall, and also the finding that hippocampal/medial temporal damage alone does not affect the retrieval of remote memories (cf. Debiec et al. 2002).
In summary, patients with medial temporal damage were relatively unimpaired in the recall of autobiographical and “more” semantic information, and, against the predictions of both hypotheses, there were relatively weak and inconsistent (across tasks) correlations between medial temporal lobe volumes and remote memory performance. There was support for consolidation theory in that only those patients with lateral temporal involvement showed severe difficulties in retrieval of remote autobiographical and semantic information, and (on t-test analysis) the medial temporal group showed significant impairment in retrieval of recent autobiographical memories. On the other hand, patients with damage in either the medial or lateral temporal lobes showed generally “flat” temporal gradients, which most researchers would interpret as more likely to be consistent with multiple trace theory. Overall, our present findings appear to indicate that the extent of lateral temporal damage is particularly critical to the emergence of a severe remote memory impairment, perhaps as part of a more widely distributed network underlying the retrieval of past memories (cf. Kopelman et al. 2003; Bayley et al. 2005). Outstanding issues concern, and future research should investigate, the extent of regional specialization within these circuits and how they relate to specific aspects of episodic and semantic memory.
Materials and Methods
Participants—clinical and MRI description
Medial temporal lesion group
Five patients were selected on the basis of significant anterograde memory loss and MRI evidence that regional brain atrophy was restricted to the medial temporal lobe structures. The atrophy was attributable to acute hypoxic episodes in three of these patients, all of whom had become amnesic within 3 yr before testing. A fourth patient had experienced an acute encephalopathy of uncertain origin in her teens, associated with presumed hypoxia and subsequent left-sided mesial temporal sclerosis and partial seizures. The fifth patient had suffered complex partial seizures over a period of many years, but developed an identified memory impairment 3 yr before testing, following a severe bout of seizures. In all cases, the atrophy was bilateral. Figure 5 shows coronal sections from the brains of these patients, revealing medial temporal lobe atrophy, confined to the hippocampi (top row, left and middle) and involving both hippocampal and parahippocampal structures (top row, right).
Temporal lobe lesion group
These patients were chosen on the basis of their all having significant anterograde memory impairments, in association with MRI evidence of extensive medial and antero-lateral temporal lobe damage. In this study, these patients are sometimes referred to as the “lateral” temporal lobe group to distinguish them from those with pathology confined to the medial temporal lobes, but it should be understood that this group’s pathology also involved medial temporal lobe structures. Of the seven patients selected for this group, five had been diagnosed with (antibody-confirmed) herpes encephalitis. In four of these patients, there was evidence of temporal lobe damage in both hemispheres, although the extent of damage was predominantly left-lateralized in three patients and predominantly right-lateralized in one patient; the remaining patient (patient “D.J.”) showed unilateral left temporal lobe damage, as previously described by Stanhope and Kopelman (2000). In all except D.J., the signal alteration on MRI implicated the medial temporal lobes bilaterally (in D.J. unilaterally), involving the hippocampi and parahippocampal structures including the entorhinal, perirhinal, and parahippocampal cortices. In the more affected hemisphere, the signal alteration involved the antero-lateral temporal lobe cortex (see Fig. 5, bottom row, left). Two further patients were included in this group. One patient had suffered an encephalitic illness at age 20, resulting in residual temporal lobe epilepsy. The other patient had had a temporal lobe abscess at age 17, and following a series of seizures at age 34, she subsequently developed (predominantly) verbal memory impairment. An MRI carried out at this time showed a large left temporal CSF-filled lesion, involving medial and lateral temporal lobe structures. Four of these patients had become amnesic 10–12 yr before testing, and three had become amnesic within the last 5 yr.
Frontal lesion patients
Seven patients with focal frontal lesions and deficits on measures of executive function were recruited (for example MRIs, see Fig. 5, bottom row, middle and right). All showed some “frontal” behavioral symptoms such as apathy, irritability, emotional lability, or disinhibition. In two patients, the pathology resulted from acute head injury and associated contusions and hematomas, worse on the right than the left. Another two patients had undergone surgery for removal of tumors: one, a left frontal meningioma arising from the planum sphenoidale, which had been only partially resected; the other, a transfrontal craniotomy for removal of a pituitary tumor resulting in right anteromedial frontal damage. There were a further two cases with frontal infarcts. In one of these patients, the damage was restricted to the left hemisphere, but the other patient showed bilateral frontal signal alteration: both showed pronounced “frontal” behavioral changes. Finally, one patient had suffered a large right frontal cerebral abscess following a tooth infection, and he showed extensive residual signal alteration in the right prefrontal cortex on MRI. Of these patients, five had become amnesic within the last 5 yr, and the other two became amnesic 7 and 14 yr before testing.
Controls
Healthy control participants (N = 9) were recruited from a local further education college as well as nonclinical staff in the hospital, matched as closely as possible to the total patient group for age, sex, NART-R (National Adult Reading test revised edition [Nelson and Willison 1991]), and years of education.
Quantitative structural MRI
MRI scans were axially acquired on a 1.5T Philips scanner, using a protocol of T1 and T2 weighted gradient and PD echo 3D volume data sets. The slice thickness was 1.5 mm and the matrix size was 256 × 256, giving a voxel size of 1.3 mm3. An HP735 graphics workstation was used to segment (delineate) brain structures of interest across sequential MR slices. The data were analyzed using a hierarchical segmentation program, allowing detailed volumetric assessment. The program incorporates visualization, manipulation, and storage/retrieval functions in its interface, and segmentation tools include a multislice 2D hierarchical segmentation program, a 2D polyline tool for drawing a sequence of connected straight lines, and a 3D plane cutting tool. Quantitative structural MRI measurements of the left and right temporal lobes, antero-lateral temporal lobes, medial temporal lobes, and hippocampi were taken from planimetric measurements determined according to the anatomical definitions and segmentation criteria described in detail by Colchester et al. (2001) and Kopelman et al. (2001, 2003).
In brief, segmentations were carried out on coronal brain sections in order to measure hippocampal volume and medial temporal (combined hippocampal and parahippocampal) volume. The boundary definitions for the hippocampus were closely similar to those described by Mori et al. (1997) except that the subiculum was included as part of the hippocampus. Anteriorly, the alveolar covering of the hippocampus provided a border with the amygdala. The posterior limit was the coronal slice in which the fornix emerged from the fimbria of the hippocampus, just anterior to the splenium of the corpus callosum. Thus, the hippocampal measurement included the CA fields, dentate gyrus, and subiculum. The “medial temporal” measurements used the same anterior and posterior margins, but, in the coronal plane, segmentations were taken from the subiculum around the cortical surface of the parahippocampal gyrus and then deep into the collateral (rhinal) sulcus until it met the inferolateral point of the hippocampus. Thus, our parahippocampal measurements were derived after subtraction of hippocampal from medial temporal volume and incorporated the perirhinal, entorhinal, and parahippocampal cortices.
The anterior (temporal pole), lateral, and inferior boundaries of the temporal lobe were defined by cerebro-spinal fluid (C.S.F.). The medial boundary was followed up into the suprasellar cistern and into the choroidal fissure. The superior boundary was segmented by viewing coronal slices sequentially from front to back. Once the temporal stem was encountered, the superomedial margin was defined by a line from the inferior fundus of the circular sulcus of the insula to the most lateral invagination of the choroidal fissure. The posterior boundary was formed with reference to three points: the superomedial limit of the central sulcus, the posterior commissure, and an explicitly defined “approximation” of the preoccipital notch (Colchester et al. 2001). The “lateral” temporal lobe measurement was obtained after subtraction of the “medial temporal” lobe volume from the “temporal lobe” volume. Thus, the boundaries of the “lateral” temporal lobe incorporated the temporo-polar region as well as the superior, middle, and inferior temporal lobe gyri, and the anterior portion of the fusiform gyrus.
For the frontal lobes, the superior, medial, and lateral surface is bounded by C.S.F. Additional boundaries were defined on serial coronal sections commencing at the front and working backward. In the anterior sections, the inferior surface was bounded by subarachnoid space. Working posteriorly, once the temporal stem was present, segmentation involved drawing a polyline from the inferolateral edge of the frontal operculum into the sylvian fissure to the insula then upward into the fundus of the circular sulcus. From there a straight line was drawn to the superolateral tip of the lateral ventricle. The roof of the lateral ventricle was followed to the midline. This procedure was continued until the posterior limits of the frontal lobe were reached. The posterior boundary was formed by a plane defined by three points: the superomedial limit of the central sulcus, the posterior commissure, and the infero-lateral limit of the central sulcus from which a line was drawn to the sylvian fissure and their junction was the third landmark. This pragmatic definition (Colchester et al. 2001) incorporated virtually the entire frontal lobe and the anterior cingulate. For further details, see Colchester et al. (2001).
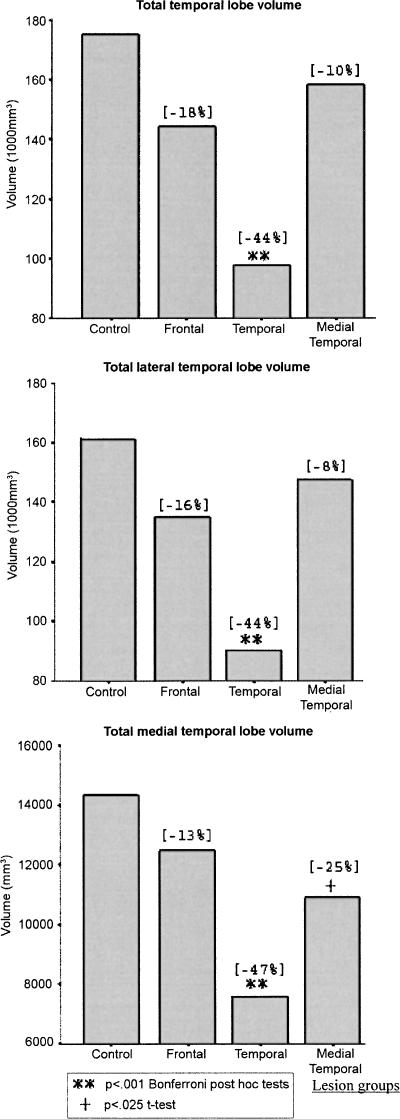
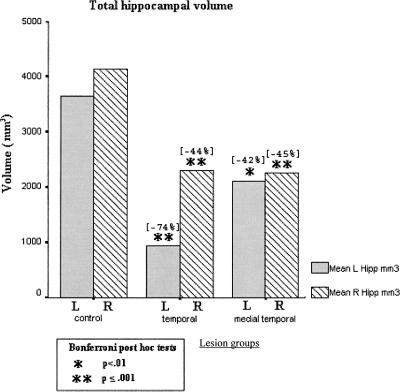
Figure 6 shows total, lateral, and medial temporal lobe mean volumes in the patient groups, relative to a reference control sample (N = 10) (Colchester et al. 2001; Kopelman et al. 2003) who did not differ significantly from the present control group in terms of mean age, sex ratio, or NART-R IQ. It shows that the temporal lobe lesion group showed significant atrophy across total temporal lobe, total lateral temporal, and total medial temporal volumes. The frontal lesion and medial temporal lesion groups did not differ significantly from controls in terms of total temporal lobe or lateral temporal volumes. The medial temporal lesion group showed a mean medial temporal lobe volume approximately halfway between the controls and the temporal lobe lesion group: They differed significantly from controls in terms of mean medial temporal volume on a t-test (t = 2.86, P < 0.025), but not on a Bonferroni post hoc test following one-way ANOVA across all four groups. Figure 7 shows that the medial temporal lesion group and the temporal lesion group both showed highly significant atrophy in terms of left and right hippocampal volumes. In both Figures 6 and 7, the percentage reduction in mean patient volumes is indicated relative to the mean of the control volumes. In particular, the mean reduction in hippocampal volume in our medial temporal group was 42% on the left (compared with 39% in Bayley et al. [2005’s three hypoxic patients) and 45% on the right (compared with 39%).
Figure 6.
Volumetric measures of temporal lobe structures for controls and each patient group. Figures in brackets show the mean percentage deviation from control group volumes.
Figure 7.
Left and right hippocampal volumes for controls and the two temporal lobe groups (temporal and medial temporal). Figures in brackets show the mean percentage deviation from control group volumes.
Background neuropsychological findings
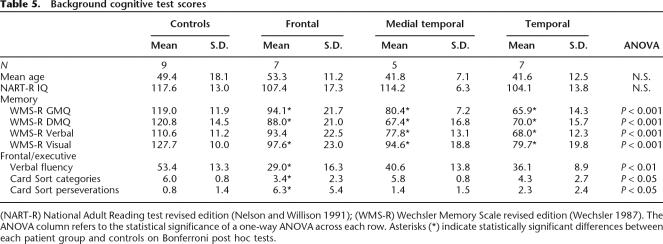
Background cognitive test scores were collected and are summarized in Table 5. On a measure of estimated premorbid IQ (NART-R) (Nelson and Willison 1991), there were no differences among the groups (F3,24 = 1.54, N.S.). However, there were significant group differences on all memory scores (see Table 5). There were significant differences across the groups for the general memory index (F3,24 = 17.91, P < 0.001), delayed memory index (F3,24 = 16.05, P < 0.001), as well as the individual visual and verbal indexes. In terms of Bonferroni post hoc tests, all patient groups performed significantly more poorly than controls on the general memory, delayed memory, and visual memory subtests (P < 0.05). On verbal memory, the control group scores differed significantly from both temporal lobe lesion groups (P < 0.05) but not from the frontal lesion group. The “lateral” temporal lobe lesion patients performed more poorly than the frontal lesion patients on general memory and the verbal memory subtests (P < 0.05). The two temporal lesion groups did not differ significantly from each other on any of the memory tests. The mean WMS-R general memory index in the medial temporal group (80.4) was broadly similar to that of Bayley et al. (2005)’s three hypoxic patients (74.0), and that of the “lateral temporal” group (65.9) similar to their MTL+ subgroup (62.7). Moreover, the medial temporal group showed a mean NART-R minus WMS-R General Memory decrement of 33.8 points, and a mean NART-R minus WMS-R Delayed Memory decrement of 46.8 points.
Table 5.
Background cognitive test scores
(NART-R) National Adult Reading test revised edition (Nelson and Willison 1991); (WMS-R) Wechsler Memory Scale revised edition (Wechsler 1987). The ANOVA column refers to the statistical significance of a one-way ANOVA across each row. Asterisks (*) indicate statistically significant differences between each patient group and controls on Bonferroni post hoc tests.
Table 5 also shows significant differences in card-sorting categories (F3,24 = 3.31, P < 0.05) and perseverations (F3,24 = 4.55, P < 0.05), and on verbal fluency (F3,24 = 4.84, P < 0.01) with the control group performing best and the frontal lesion group performing worst in each case. On Bonferroni post hoc tests, the frontal lesion group performed significantly worse than controls (in each case P < 0.05). Neither the “lateral” temporal nor the medial temporal lesion group differed significantly from the controls.
Experimental tests and procedures
Famous faces
Pictures of 50 persons who became famous between 1960 and 1999 were selected, with 10 chosen from each of the five decades. Where possible, personalities were chosen who were famous in the United Kingdom during a relatively discrete period of time (e.g., Jomo Kenyatta, Freddy Laker, John Smith) rather than those whose fame spanned several decades such as Winston Churchill or Paul McCartney. By taking this approach, we were confident that we were tapping memory for faces from a particular period.
For each famous face, we also selected three photographs of unfamiliar faces of the same gender and a similar age. All items were resized to the same dimensions and converted (where necessary) to monochrome.
Memory was assessed on three measures: recall, forced-choice recognition, and familiarity.
For each item, the familiarity test was administered first, in which patients were asked to select the face that was most familiar from among three nonfamous foils. Then, the recall task was given, in which a famous face was presented in isolation, and the participant was asked to name the person. Finally, the recognition test was administered: While the famous face was in view, the participant was asked to select the famous person’s name from among three foils—one famous foil and two nonfamous foils (e.g., [a] Harold Macmillan; [b] Antony Eden; [c] Peter Jefferies; [d] Gordon Marks). The same order of questioning was used on all trials (familiarity followed by recall followed by recognition), and all data were collected for an item before the next item was presented. Guessing was encouraged where necessary.
For each measure, scoring was carried out with reference to the date of onset of each patient’s amnesia to ensure that the test was truly of “retrograde” amnesia. Thus, all personalities identified as famous after the onset of amnesia in each individual case were excluded from the analysis. Results are reported for the three decades before the onset of amnesia. Scores for personalities famous during the 10 yr before the onset of amnesia formed the most recent data point. The next data point comprised personalities famous between 10 and 20 yr before onset of amnesia, and so on. In practice, this meant that data were taken from the 1970s to 1990s in 12 patients, from the 1960s to 1980s in six patients, and from 1956 to 1985 in one patient. In the case of healthy controls, scoring was in terms of 10-yr “blocks” preceding the date of testing (1970s to 1990s), and scores for the 1950s and 1960s were ignored. We used this control comparison even for the seven out of 19 patients whose amnesia had lasted for more than 5 yr (see above) because (1) the individual controls had not been recruited to match each individual patient on a pair-by-pair basis, and (2) the control curves were essentially flat across all conditions (see Results section and Fig. 1), even when findings from the 1960s and 1950s were included.
Famous news events picture task
This test was adapted and updated from that used by Kopelman and colleagues (Kopelman 1989; Kopelman et al. 1999). Pictures of 50 famous news events that occurred between 1950 and 1999 were selected, with 10 events chosen for each of the five decades. All pictures were presented in monochrome. Memory was assessed on two measures:
Recall. The patient was presented with each picture and asked to identify and describe the event depicted.
Forced-choice recognition. For each of the 50 events, four choices were presented orally and in printed form. Of these, there were two unfamiliar (fictitious) events, one true event unrelated to the picture (alternative familiar), and the target. Guessing was encouraged where necessary.
Using the same method outlined for the famous faces test, scoring was carried out with reference to the date of onset of each patient’s amnesia to ensure that the test was truly of “retrograde” amnesia. Thus, all events that occurred after the onset of amnesia in each individual case were excluded from the analysis. Scores for events occurring during the 10 yr before the onset of amnesia formed the most recent data point. The next data point comprised events that occurred between 10 and 20 yr before onset of amnesia, and so on. This procedure resulted in a total of three data points, extending back 30 yr. Again, this meant that data were taken from the 1970s to 1990s in 12 patients, from the 1960s to 1980s in six patients, and from 1956 to 1985 in one patient. In the case of healthy controls, scoring was in terms of the three 10-yr “blocks” preceding the date of testing, and items from the 1950s and 1960s were ignored. As before, we used this control comparison even for the seven out of 19 patients whose amnesia had lasted more than 5 yr (see above) because the individual controls had not been selected to match each individual patient on a pair-by-pair basis. It should be noted that, as the control curves were gently rising across both conditions (see Results section and Fig. 1), our method would have slightly increased, rather than diminished, the a priori probability of obtaining significant differences at the earliest time period.
The same order of questioning was used in all trials (recall followed by recognition), and all data were collected for an item before the next item was presented.
Autobiographical memory
This was a new test, modeled on the Autobiographical Memory Interview (AMI) (Kopelman et al. 1989), but using the same cues across each time period and a system of progressive prompting. Before the administration of this test, interviews were carried out with each patient’s spouse or other family member so that verifiable information could be collected for each of the measures. Data for the most recent period (see above) were included for subsequent analysis only for those patients who became amnesic within 5 yr prior to testing to ensure that retrograde (rather than anterograde) memory was being assessed. As part of this process, we also returned to the raw data to ensure that the memories did, indeed, tap retrograde memories (rather than the period between amnesia onset and testing). In other words, the most recent time point is based only on those subjects for whom this reflects purely retrograde memory.
Each participant was asked to recall an event in each of three categories: (1) at home; (2) at school/college/hospital; (3) on a journey or holiday. These category cues were repeated across each of three time periods above (Childhood, Early Adult, Recent/last 5 yr). The participant was progressively prompted to produce memories to these same prompts across each of these time periods to facilitate recall of a recorded event. For example, if the participant was unable to recall any event from a given category, the experimenter provided an initial cue (e.g., “involving your brother [or sister, mother, father, etc., depending on the specific memory provided by family members]”), followed by further, more specific cues (e.g., “involving your brother where you were hurt [or injured, etc.]”) (up to three prompts) in order to help elicit a target memory. In all cases, each prompt provided one additional element relevant to the memory being tested. In this way, a similar and equivalent test structure was used for all patients (and the number of prompts was recorded), even though the actual memories being tapped were unique to each participant.
Scoring was according to the descriptive richness of the memory produced (amount of correct information recalled) and its specificity in time and place on a scale of 0–3, analogous to the AMI and irrespective of the number of prompts required. All episodes were scored independently by M.D.K., P.B., and J.B. In terms of inter-rater reliability, >80% of episodes were given the same scores by all three raters, and 100% of episodes were given the same marks by at least two of the three raters. In the latter case, the mark agreed on by two of the raters was chosen.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.265906
References
- Baddeley A.D., Wilson B. Amnesia, autobiographical memory, and confabulation. In: Rubin D.C., editor. Autobiographical memory. Cambridge University Press; Cambridge, UK: 1986. pp. 225–252. [Google Scholar]
- Bayley P.J., Hopkins R.O., Squire L.R. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Bayley P.J., Gold J.J., Hopkins R.O., Squire L.R. The neuroanatomy of remote memory. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F.A., Bullmore E.T., Graham K.S., Thompson S.A., Hodges J.R., Fletcher P.C. The hippocampal region is involved in successful recognition of both remote and recent famous faces. Neuroimage. 2004;22:1704–1714. doi: 10.1016/j.neuroimage.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Tranel D., Adolphs R. Emotional autobiographical memories in amnesic patients with medial temporal lobe damage. J. Neurosci. 2005;25:3151–3160. doi: 10.1523/JNEUROSCI.4735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolotti L., Shallice T., Chan D., Fox N., Scahill R., Harrison G., Stevens J., Rudge P. Long-term retrograde amnesia: The crucial role of the hippocampus. Neuropsychologia. 2001;39:151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Colchester A.C.F., Kingsley D., Lasserson D., Kendall B.E., Bello F., Rush C., Stevens T., Goodman G., Heilpern G., Stanhope N., et al. Structural MRI volumetric analysis in patients with organic amnesia, 1: Methods and comparative findings across diagnostic groups. J. Neurol. Neurosurg. Psychiatry. 2001;71:13–22. doi: 10.1136/jnnp.71.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J., LeDoux J.E., Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Della Sala S., Laiacona M., Spinnler H., Trivelli C. Autobiographical recollection and frontal damage. Neuropsychologia. 1993;31:823–839. doi: 10.1016/0028-3932(93)90131-i. [DOI] [PubMed] [Google Scholar]
- Fujii T., Moscovitch M., Nadel L. Memory consolidation, retrograde amnesia, and the temporal lobe. In: Cermak L.S., editor. Memory and its disorders. Vol. 2. Elsevier Science; Amsterdam: 2000. pp. 223–250. [Google Scholar]
- Gilboa A., Winocur G., Grady C.L., Hevenor S.J., Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gilboa A., Ramirez J., Kohler S., Westmacott R., Black S.E., Moscovitch M. Retrieval of autobiographical memory in Alzheimer’s disease: Relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15:535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Haist F., Gore J.B., Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat. Neurosci. 2001;4:1139–1145. doi: 10.1038/nn739. [DOI] [PubMed] [Google Scholar]
- Kapur N., Brooks D.J. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. 1999;9:247–254. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kida S.S., Josselyn S.A., Ortiz S.P., Kogan J.H., Chevere I., Masushige S., Silva A.J. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D. Remote and autobiographical memory, temporal context memory and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D. Frontal dysfunction and memory deficits in the alcoholic Korsakoff syndrome and Alzheimer-type dementia. Brain. 1991;114:117–137. [PubMed] [Google Scholar]
- Kopelman M.D. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D. Retrograde memory loss. In: Miller B., Goldenberg G., editors. Handbook of clinical neurology: Neuropsychology and behaviour. Elsevier Ltd; Edinburgh, UK: 2006. [Google Scholar]
- Kopelman M.D., Kapur N. The loss of episodic memories in retrograde amnesia: Single-case and group studies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1409–1422. doi: 10.1098/rstb.2001.0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman M.D., Wilson B.A., Baddeley A.D. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D., Stanhope N., Kingsley D. Retrograde amnesia in patients with diencephalic, temporal lobe or frontal lesions. Neuropsychologia. 1999;37:939–958. doi: 10.1016/s0028-3932(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D., Lasserson D., Kingsley D., Bello F., Rush C., Stanhope N., Stevens T., Goodman G., Heilpern G., Kendall B. Structural MRI volumetric analysis in patients with organic amnesia, 2: Correlations with anterograde memory and executive tests in 40 patients. J. Neurol. Neurosurg. Psychiatry. 2001;71:23–28. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman M.D., Lasserson D., Kingsley D.R., Bello F., Rush C., Stanhope N., Stevens T.G., Goodman G., Buckman J.R., Heilpern G. Retrograde amnesia and the volume of critical brain structures. Hippocampus. 2003;13:879–891. doi: 10.1002/hipo.10140. [DOI] [PubMed] [Google Scholar]
- Kopelman M.D., Bright P., Buckman J., Fradera A., Yoshimasu H., Colchester A.C.F. Recall and recognition memory in amnesia: Patients with hippocampal, medial temporal, temporal lobe or frontal pathology. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.10.005. (in press) [DOI] [PubMed] [Google Scholar]
- Land C., Bunsey M., Riccio D.C. Anomalous properties of hippocampal lesion induced retrograde amnesia. Psychobiology. 2000;28:476–485. [Google Scholar]
- Lewis D.J. Psychobiology of active and inactive memory. Psychol. Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- Mactutus C.F., Riccio D.C., Ferek J.M. Retrograde amnesia for old (reactivated) memory: Some anomalous characteristics. Science. 1979;204:1319–1320. doi: 10.1126/science.572083. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Frith C.D. Ageing affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1–13. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Henson R.N.A., Mummery C.J., Frith C.D. Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport. 2001;12:441–444. doi: 10.1097/00001756-200103050-00004. [DOI] [PubMed] [Google Scholar]
- Mangels J., Gershberg F.B., Shimamura A.P., Knight R.T. Impaired retrieval from remote memory in patients with frontal lobe damage. Neuropsychology. 1996;10:32–41. [Google Scholar]
- Manns J.R., Hopkins R.O., Squire L.R. Semantic memory and the human hippocampus. Neuron. 2003;38:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Mayes A.R., Montaldi D., Spencer T.J., Roberts N. Recalling spatial information as a component of recently and remotely acquired episodic or semantic memories: An fMRI study. Neuropsychology. 2004;18:426–441. doi: 10.1037/0894-4105.18.3.426. [DOI] [PubMed] [Google Scholar]
- Meeter M., Murre J.M.J. Consolidation of long-term memory: Evidence and alternatives. Psychol. Bull. 2004;130:843–857. doi: 10.1037/0033-2909.130.6.843. [DOI] [PubMed] [Google Scholar]
- Misanin J.R., Miller R.R., Lewis D.J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:203–204. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Mori E., Yoneda Y., Yamashita H., Hirono H., Ikeda M., Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer’s disease: An MRI volumetric study. J. Neurol. Neurosurg. Psychiatry. 1997;63:214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M., Yaschyshyn T., Ziegler M., Nadel L. Remote episodic memory and amnesia: Was Endel Tulving right all along? In: Tulving E., editor. Memory, consciousness and the brain: The Tallinn conference. The Psychology Press; New York: 1999. pp. 331–345. [Google Scholar]
- Moscovitch M., Nadel L., Winocur G., Gilboa A., Rosenbaum R.S. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nadel L., Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L., Moscovitch M. The hippocampal complex and long-term memory revisited. Trends Cogn. Sci. 2001;5:228–230. doi: 10.1016/s1364-6613(00)01664-8. [DOI] [PubMed] [Google Scholar]
- Nadel L., Samsonovich A., Ryan L., Moscovitch M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- Nader K., Schafe G.E., LeDoux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nelson H.E., Willison J.R. National adult reading test. NFER-Nelson; Windsor, UK: 1991. 2d ed. [Google Scholar]
- Niki K., Luo J. An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. J. Cogn. Neurosci. 2002;14:500–507. doi: 10.1162/089892902317362010. [DOI] [PubMed] [Google Scholar]
- Piefke M., Weiss P.H., Zilles K., Markowitsch H.J., Fink G.R. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Piolino P., Desgranges B., Benali K., Eustache F. Episodic and semantic remote autobiographical memory in ageing. Memory. 2002;10:239–258. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Reed J.M., Squire L.R. Retrograde amnesia for facts and events: Findings from four new cases. J. Neurosci. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower N.L., Zola S.M., Squire L.R., Amaral D.G. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L., Nadel L., Keil K., Putnam K., Schnyer D., Trouard T., Moscovitch M. Hippocampal complex and retrieval of recent and very remote autobiographical memories: Evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus. 2001;11:707–714. doi: 10.1002/hipo.1086. [DOI] [PubMed] [Google Scholar]
- Sanders H.I., Warrington E.K. Memory for remote events in amnesic patients. Brain. 1971;94:661–668. doi: 10.1093/brain/94.4.661. [DOI] [PubMed] [Google Scholar]
- Sara S.J. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Spiers H.J., Maguire E.A., Burgess N. Hippocampal amnesia. Neurocase. 2001;7:357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr. Opin. Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Stanhope N., Kopelman M.D. Art and memory: A 7-year follow-up of herpes encephalitis in a professional artist. Neurocase. 2000;6:99–110. [Google Scholar]
- Tulving E., Markowitsch H.J. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Viskontas I.V., McAndrews M.P., Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J. Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas I.V., McAndrews M.P., Moscovitch M. Memory for famous people in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychology. 2002;16:472–480. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale–Revised: Manual. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Weiskrantz L. On issues and theories of the human amnesic syndrome. In: Weinberger N.M., et al., editors. Memory systems of the brain. Guilford Press; New York: 1985. pp. 380–415. [Google Scholar]
- Zola-Morgan S., Squire L.R. The primate hippocampal formation: Evidence for a time-limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L.R., Amaral D.G. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to the field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]