Abstract
Using pharmacological techniques, it has been demonstrated that both consolidation and extinction of Pavlovian fear conditioning are dependent to some extent upon L-type voltage-gated calcium channels (LVGCCs). Although these studies have successfully implicated LVGCCs in Pavlovian fear conditioning, they do not provide information about the specific LVGCC isoform involved. Both of the major LVGCC subtypes found in the brain (Cav1.2 and Cav1.3) are targets of the pharmacological manipulations used in earlier work. In this study, we used mice in which the gene for the pore-forming subunit (α1D) Cav1.3 was deleted (Cav1.3 knockout mice) to elucidate its contribution to consolidation and extinction of conditioned fear. We find that Cav1.3 knockout mice exhibit significant impairments in consolidation of contextual fear conditioning. However, once sufficiently overtrained, the Cav1.3 knockout mice exhibit rates of extinction that are identical to that observed in wild-type mice. We also find that Cav1.3 knockout mice perform as well as wild-type mice on the hidden platform version of the Morris water maze, suggesting that the consolidation deficit in conditioned fear observed in the Cav1.3 knockout mice is not likely the result of an inability to encode the context, but may reflect an inability to make the association between the context and the unconditioned stimulus.
In Pavlovian fear conditioning, pairing a conditional stimulus (CS) with an aversive unconditional stimulus (US) results in a conditioned fear response. A fear response is said to be contextually conditioned when it is elicited by the context in which the US was delivered. In this case, the context serves as the CS (for recent review, see Fanselow and Poulos 2005). After contextual fear conditioning, extended exposure to the context in the absence of the US results in reduced probability and amplitude—or extinction—of the conditioned response. Both consolidation and extinction of conditioned fear have been demonstrated to be critically dependent on the amygdala (for review, see Maren 2003).
Recently, many studies have investigated the molecular basis of fear conditioning and its extinction in rodents. Molecules involved in synaptic plasticity within the amygdala have been of particular interest. In light of evidence that a form of long-term potentiation (LTP) that depends on L-type voltage-gated calcium channels (LVGCCs) exists in the amygdala (Weisskopf et al. 1999), multiple groups have explored the role of LVGCCs in fear conditioning and its extinction. In rats, blockade of LVGCCs in the lateral amygdala impairs consolidation of auditory conditioned fear (Bauer et al. 2002). Systemic blockade of LVGCCs in mice, however, does not impair the acquisition, consolidation, or expression of conditioned fear, but rather its extinction (Cain et al. 2002; Suzuki et al. 2004). Demonstration that infusions into the basolateral amygdala of an LVGCC antagonist block, whereas infusions of an LVGCC agonist facilitate, extinction of conditioned fear in mice provides additional support for the importance of LVGCCs in extinction of conditioned fear (Barad 2005).
Thus far, studies of the role of LVGCCs in fear conditioning and its extinction have relied solely on pharmacological techniques. Though these techniques are powerful in elucidating a prominent role for LVGCCs in conditioned fear, they do not allow for identification of the specific LVGCCs involved. There are two major subtypes of brain LVGCCs, Cav1.2 and Cav1.3, both of which are the targets of pharmacological manipulations. In this study, we use mice in which the gene for the pore-forming subunit (α1D) Cav1.3 L-VSCC subtype has been deleted (Cav1.3 knockout mice; a generous gift from D. James Surmeier, Northwestern University) to elucidate its contribution to consolidation and extinction of contextually conditioned fear.
Results
General neurological screen
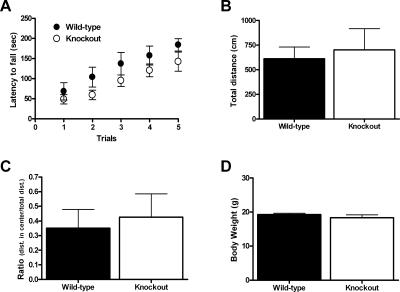
It has been previously reported that CaV1.3 homozygous knockout (KO) mice are by and large neurologically normal (Clark et al. 2003). Similarly, we found that deletion of CaV1.3 does not result in significant impairments in vestibular function, spontaneous locomotor activity, or anxiety-like behavior. CaV1.3 KO mice (n = 6) were not significantly different when compared with wild-type (WT) (n = 6) mice on the rotarod (Fig. 1A), as both groups exhibited significant improvement in performance as training progressed (F(1,10) = 27.1, P < 0.05 for effect of training) and there was no significant effect of genotype (F(1,10) = 2.35, P > 0.05). We did not find any significant difference between the CaV1.3 KO mice (n = 6) and their WT littermates (n = 5) in the open field (Fig. 1B). The total distance traveled by the CaV1.3 KO mice (702 ± 215 cm) was not significantly different when compared with WT mice (612 ± 118 cm; t(9) = 0.35, P > 0.05). In addition, we quantified the ratio of distance spent in the center of the open field as a function of the total distance traveled (Fig. 1C) as a measure of anxiety-like behavior (Crawley 1999). The ratio of center distance to total distance traveled in the open field did not differ between CaV1.3 KO mice (center: total ratio 0.426 ± 0.159) and WT mice (0.351 ± 0.127; t(9) = 0.36, P > 0.05), suggesting that deletion of CaV1.3 did not affect anxiety-like behavior. However, in contrast to the earlier report by Clark et al. (2003) we did not find any significant difference in body weight in the CaV1.3 KO mice (n = 7; 18.3 ± 0.83 g) when compared with WT littermates (n = 6; 19.3 ± 0.72; t(9) = 0.82, P > 0.05) (Fig. 1D).
Figure 1.
CaV1.3 knockout mice are neurologically normal. (A) Mice were placed on the accelerating rotarod for a maximum of 300 sec once a day for 5 d and the latency to fall was recorded for CaV1.3 knockout mice and wild-type littermates. The latency to fall for knockout mice was not significantly different from wild-type animals. (B,C) Exploratory behavior as measured in the open field was similar in CaV1.3 knockout mice and wild-type littermates both in the overall distance traveled (B) and in the ratio between the distance traveled in the center divided by the total distance traveled (C). (D) Average body weight was not significantly different between wild-type mice and the CaV1.3 knockout mice. All data are presented as mean ±SEM.
Cav1.3 KO mice are impaired on consolidation of contextually conditioned fear
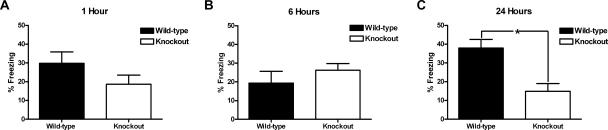
As outlined above, pharmacological blockade of L-type calcium channels disrupts consolidation of conditioned fear (Bauer et al. 2002); therefore, we sought to determine the relative contribution of the CaV1.3 isoform during the consolidation of conditioned fear. For these experiments, three separate groups of CaV1.3 KO mice and their WT littermate controls were conditioned with a single unsignaled footshock (see Materials and Methods). Results are shown in Figure 2. During conditioning, neither CaV1.3 KO nor WT mice in any of the three groups froze significantly during the 3 min preceding the shock. Each group was returned to the conditioning chambers at a different time point after conditioning. Mice returned to the conditioning chambers 1 h (n = 8 for KO and WT) or 6 h (n = 8 for KO and WT) after conditioning exhibited significant freezing compared with before conditioning (F(1,14) = 27.8, P < 0.05 and F(1,14) = 25.4, P < 0.05, respectively, for effect of conditioning); however, there was no effect of genotype at either the 1 h (F(1,14) = 2.2, P > 0.05; Fig. 2A) or 6 h (F(1,14) = 1.4, P > 0.05; Fig. 2B) time point. Similarly, mice returned to the conditioning chambers 24 h (n = 8 and 11 for KO and WT, respectively) after conditioning exhibited significant freezing (F(1,17) = 64.5, P < 0.05 for effect of conditioning) compared with before conditioning. However, at the 24-h time point, the CaV1.3 KO mice exhibited significantly less freezing (14.9% ± 4.2%) when compared with their WT littermates (37.9% ± 4.6%; Fig. 2C) with a significant main effect of genotype (F(1,17) = 12.2, P < 0.05) and training x genotype interaction (F(1,17) = 13.1, P < 0.05). It is important to note that deletion of CaV1.3 had no effect on post-shock freezing measured for 30 sec after the footshock in the 24-h group. Both KO and WT mice exhibited substantial post-shock freezing (20.9% ± 9.2% for KO and 26.7% ± 6.3% for WT; F(1,17) = 19.0, P < 0.05 for effect of training); however, there was no main effect of genotype on post-shock freezing (F(1,17) = 0.2, P > 0.05) and the effect of the shock did not interact with genotype (F(1,17) = 0.3, P > 0.05), suggesting that foot-shock sensitivity and US processing were not disrupted in KO mice. The absence of an effect of genotype immediately following the shock or at 1 and 6 h after training suggests that acquisition of contextually conditioned fear is intact in CaV1.3 KO mice. However, that an effect of genotype arises 24 h after training argues that CaV1.3 KO mice are impaired with respect to consolidation of contextually conditioned fear.
Figure 2.
Deletion of CaV1.3 disrupts normal consolidation, but not acquisition, of contextual fear conditioning. (A) CaV1.3 knockout mice and wild-type littermates exhibit similar levels of freezing 1 h following a trial of contextual fear conditioning. (B) CaV1.3 knockout mice and wild-type littermates exhibit similar levels of freezing 6 h following a trial of contextual fear conditioning. (C) CaV1.3 knockout mice exhibit significantly less freezing when compared with their wild-type littermates 24 h after a conditioning trial. *P < 0.05 for post hoc comparison between genotypes. All data are presented as mean ±SEM.
We also obtained similar results with mice in a C57BL/6 genetic background; however, the deficit was somewhat more pronounced. After 2 d of context conditioning (one unsignaled shock per day, as above) both the CaV1.3 KO (n = 8) and WT mice (n = 11) exhibited an increase in conditioned fear on day 3 (F(2,34) = 25.9, P < 0.05 for the effect of training). However, the average percent time freezing in the CaV1.3 KO mice after 2 d of training (13.8 ± 2.8%) was significantly less than that observed for the WT mice (33.5% ± 7.4%). A repeated measures ANOVA revealed a significant effect of genotype (F(1,17) = 5.0, P < 0.05) and a significant interaction between genotype and training (F(2,34) = 4.2, P < 0.05). Thus, in two different genetic backgrounds, deletion of the CaV1.3 pore-forming subunit results in disrupted contextual fear conditioning. These results are consistent with previous experiments demonstrating that L-type calcium channel antagonists disrupt consolidation of conditioned fear when infused directly into the lateral amygdala (Bauer et al. 2002).
Extinction of contextually conditioned fear is normal in Cav1.3 KO mice
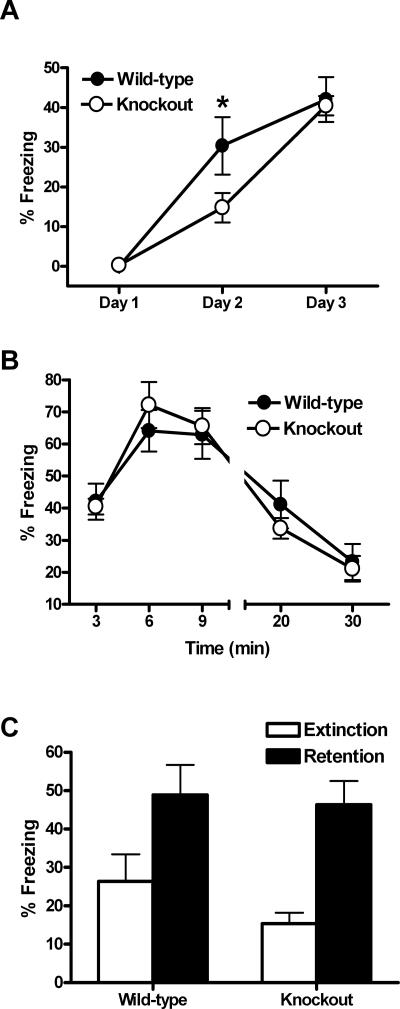
Our preliminary experiments suggested that the impairment in consolidation of contextually conditioned fear exhibited by the CaV1.3 KO mice in the F2 hybrid background could be overcome by additional training. Thus, we used a separate group of mice to test extinction of contextually conditioned fear (Fig. 3). For this experiment, CaV1.3 KO mice (n = 17) and WT mice (n = 20) were conditioned as before, but an additional day of training was administered so that extinction learning could be examined (see Materials and Methods). On the following day, approximately one-half of the mice (nine KO mice and 12 WT mice) were returned to the conditioning chambers for a 30-min extinction session and contextually conditioned fear was assessed by measuring freezing throughout the session. The remaining mice (eight KO mice and eight WT mice) were not re-exposed to the context (retention control groups). Neither CaV1.3 KO nor WT littermates froze significantly during the 3 min prior to the shock on day 1 (Fig. 3A). Consistent with our previous results, CaV1.3 KO exhibited significantly less freezing than WT littermates on day 2, 24 h following a single training trial (F(1,35) = 4.3, P < 0.05; Fig. 3A). However, CaV1.3 KO and WT littermates exhibited similar levels of freezing during the first 3 min of context exposure on day 3, 24 h after a second training trial (F(1,19) = 0.05, P > 0.05; Fig. 3A). This confirmed that the additional training was sufficient for CaV1.3 KO mice to overcome their impaired ability to consolidate contextually conditioned fear after a single training trial. Across the 30-min extinction session, both the CaV1.3 KO and WT mice exhibited significant extinction of contextually conditioned fear (F(1,19) = 53.2, P < 0.05 for the effect of CS exposure) with the CaV1.3 KO mice freezing on average 21.1% ± 4.0% during the last 10 min of CS exposure, while the WT mice similarly froze on average 23.3% ± 5.6% during the same time interval (Fig. 3B). This observation, coupled with the fact that there was no significant difference between genotypes during the session (F(1,19) = 0.51, P > 0.05), demonstrates that the CaV1.3 L-type calcium channel isoform is not required for short-term extinction of conditioned fear. Twenty-four hours later, all of the mice were returned to the conditioning chambers for a 5-min exposure to the context (Fig. 3C). CaV1.3 KO mice in the retention control group exhibited robust freezing (46.4%) as did their WT littermates (48.9%). In contrast, all mice in the extinction group froze significantly less with the CaV1.3 KO mice freezing only 15.4% of the time, while the WT mice froze slightly more at 26.4%. A two-way ANOVA revealed a significant effect of group (F(1,33) = 16.9, P < 0.05) with no significant effect of genotype (F(1,33) = 1.1, P > 0.05). Taken collectively, these data demonstrate that CaV1.3 is not involved in short- or long-term extinction of conditioned fear.
Figure 3.
CaV1.3 knockout mice exhibit normal short- and long-term extinction. (A) CaV1.3 knockout freeze to a similar degree as their wild-type littermates at the beginning of testing on day 3, overcoming the impairment exhibited on day 2. *P < 0.05 for post hoc comparisons between genotypes. (B) Short-term extinction in CaV1.3 knockout mice was not significantly different from wild-type mice, with both groups freezing significantly less by the end of the 30-min exposure to the context (10-min bins). (C) Twenty-four hours after the extinction training, mice were re-exposed to the same context for 5 min to measure long-term extinction. There was no difference between CaV1.3 knockout and wild-type mice in levels of freezing (P > 0.1) after extinction. However, both groups (knockouts and wild types) showed significant reductions in freezing when compared with mice of the same genotype that had not been re-exposed to the context (P < 0.05 and P < 0.005 for wild-type and knockouts vs. retention control group). All data are presented as mean ±SEM.
Cav1.3 KO mice are not impaired on the hippocampus-dependent version of the Morris water maze
There are numerous reports demonstrating that the dorsal hippocampus is critically involved in contextually conditioned fear (e.g., Kim and Fanselow 1992; Daumas et al. 2005), whereas cued conditioning (where the CS is a tone, for example) is thought to be critically dependent on the amygdala, but less dependent on the hippocampus (for recent review, see Fanselow and Poulos 2005). Because the CaV1.3 KO mice are deaf (Platzer et al. 2000), tone-cued fear conditioning could not be used as a means to dissociate which anatomical location was impacted by the deletion of CaV1.3. Instead, we sought to assess hippocampal function in the CaV1.3 KO mice by examining their ability to encode spatial information in the Morris water maze (MWM).
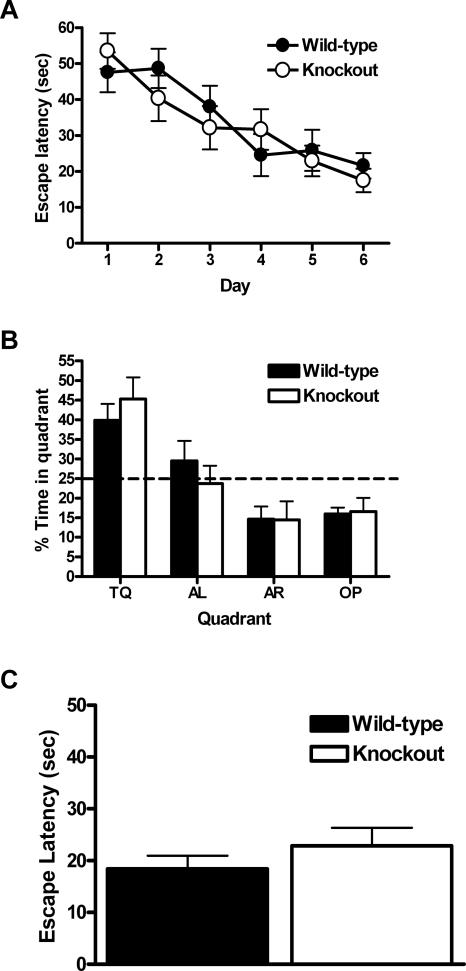
Both CaV1.3 KO mice (n = 7) and WT littermates (n = 9) were trained two trials a day for 6 d on the hidden-platform version of the MWM. Acquisition data is shown in Figure 4A. The latency to reach the platform in both groups decreased as training progressed, reaching an average escape latency of 17.5 ± 3.2 sec for the CaV1.3 KO mice and 21.6 ± 3.5 sec for the WTs. There was a main effect of training day on the latency to find the hidden platform (F(5,70) = 10.9, P < 0.05); however, there was no main effect of genotype on latency (F(1,14) = 0.13, P > 0.05) and no interaction between training day and genotype (F(5,70) = 0.77, P > 0.05). These data suggest that CaV1.3 KO and WT mice acquire the hidden-platform version of the MWM equally well.
Figure 4.
CaV1.3 knockout mice are not impaired in the Morris water maze. (A) Mice were trained for two trials a day for 6 d. The time to reach the hidden platform (escape latency) was not significantly different for CaV1.3 knockout mice when compared with wild-type littermate control mice. (B) A 60-sec probe trial completed 24 h after the last training trail (trial 12; day 6) reveals that both CaV1.3 knockout and wild-type mice spend a significant amount of time during the trial searching in the quadrant where the platform was previously located (TQ; training quadrant), but there was no significant difference between the genotypes. The dashed line (25%) represents random or “chance” performance. (AR) Adjacent right; (AL) adjacent left; (OP) opposite. (C) Average escape latencies for CaV1.3 knockout mice recorded during the visible platform version of the Morris water maze were not significantly different when compared with wild-type littermate controls. All data are presented as mean ±SEM.
In addition to measuring latency to platform during the training trails, a probe trial was conducted 24 h after completion of the last training trial on day 6. Probe trial data are shown in Figure 4B. During the probe trial, the CaV1.3 KO mice spent significantly more time (45% ± 5.5%) in the quadrant where the platform was previously located (training quadrant TQ in Fig. 4B) than would be predicted by chance (t(8) = 3.7; P < 0.05 single group t-test compared with 25%). Similarly, the WT mice spent the majority of the probe trial (39.9% ± 4.1%) selectively searching in the training quadrant (t(8) = 3.6; P < 0.05 single group t-test compared with 25%). However, there was no significant difference in the amount of time that the CaV1.3 KO mice spent in the training quadrant compared with their WT littermates (t(14) = 0.8; P > 0.05 unpaired t-test). In addition, these same mice were tested in a nonspatial, hippocampal-independent version of the MWM, in which the escape platform was clearly marked with a small flag. When the platform was marked in this manner, both groups found the platform with minimal latencies and exhibited comparable swim speeds (Fig. 4C,D). These results demonstrate that the hippocampal function in the CaV1.3 KO mice is not overtly disrupted, and therefore suggest that the deficits observed in the fear-conditioning experiments are likely the result of impaired functioning in the amygdala.
Discussion
The principal finding of the present study is that Cav1.3 KO mice are impaired in their ability to consolidate contextually conditioned fear. In addition, we find that deletion of the CaV1.3 gene does not alter extinction of contextually conditioned fear in these mice. These results indicate that the L-type calcium channel Cav1.3 is critical for normal consolidation, but not extinction, of contextually conditioned fear. These results are to our knowledge the first demonstration of an isoform-specific role for L-type calcium channels in Pavlovian conditioned fear. In our hands these mice appear to have normal weight gain and normal performance on the rota-rod and in the open field. In addition, we found no significant difference between CaV1.3 KO mice and their WT littermates in the visible platform version of the MWM. Taken collectively, these data are consistent with a previous study (Clark et al. 2003), which found that the CaV1.3 KO mice were neurologically normal and strongly suggest that the deficit in consolidation of contextually conditioned fear is not the result of gross neurological impairment.
Consistent with the results of the present study is the demonstration in rats that blockade of LVGCCs in the lateral amygdala impairs acquisition of long-term auditory conditioned fear (Bauer et al. 2002). The results of this study, however, are inconsistent with those from a study in which systemic blockade of LVGCCs did not block consolidation of conditioned fear (Cain et al. 2002). There are a few issues that may account for the inconsistency. First, it is difficult to know the degree to which LVGCC activity in general, and Cav1.3 activity in particular, were blocked by the various LVGCC antagonists used in Cain et al. (2002). In heterologous expression systems, dihydropyridine antagonists like nifedipine and nimodipine are significantly less efficient at blocking Cav1.3 currents than Cav1.2 currents (Koschak et al. 2001; Xu and Lipscombe 2001). It is possible that the residual activity resulting from incomplete blockade of Cav1.3 would be sufficient to allow for consolidation of conditioned fear. Second, differences in training protocol may account for these apparent inconsistencies. The training protocol used by Cain and colleagues (Cain et al. 2002) involved one session in which five shocks were administered, and thus, was quite intense. This is in contrast to the single shock used in the present study. Our conditioning protocol produces a more gradual learning curve and may be more sensitive in revealing subtle changes in learning produced by Cav1.3 deletion. Others have used similar conditioning protocols to examine subtle effects on acquisition of conditioned fear (Young and Fanselow 1992). The number of conditioning trials seems particularly important in light of the fact that, in our experiments, additional training trials are sufficient to overcome the impairments in acquisition of conditioned fear observed after a single training trial. Further supporting the importance of training trials is the observation that overtraining can mitigate the detrimental effects of basolateral amygdala lesions on acquisition of contextual fear conditioning in rats (Maren 1998, 1999).
Acquisition of contextually conditioned fear is thought to be dependent on both the amygdala and hippocampus (Blanchard et al. 1970; Kim and Fanselow 1992; Phillips and LeDoux 1992; Maren et al. 1997). Because the knockout mice used in the present experiments lack CaV1.3 in both of these brain regions, it is not possible at this point to definitively know whether the hippocampus, amygdala, or both areas are impacted by the loss of CaV1.3. However, it is widely thought that during context conditioning, the hippocampus acts to integrate the many elements of the context into a functional representation (Rudy et al. 2002). Thus, the observation that the CaV1.3 KO mice exhibited no impairments in the hidden platform version of the MWM leads us to conclude that the CaV1.3 KO mice can, in fact, form a spatial representation and that hippocampus function is intact in these mice. Therefore, it seems likely that the deficits observed in the CaV1.3 KO mice are the result of a disruption of neuronal function within the amygdala proper. This is of particular interest given that LTP, a form of synaptic plasticity thought to be a cellular substrate of learning and memory, is L-type calcium channel dependent in the amygdala (Bauer et al. 2002). Impaired consolidation of contextually conditioned fear in CaV1.3 KO mice may represent a disruption of this putative cellular substrate of learning and memory in the amygdala and thus serve as an excellent model for studying the relationship between LTP in the amygdala and contextual fear conditioning. Interestingly, it was recently reported that LTP of synapses in the lateral amygdala, whose presynaptic neurons arise in the cortex and hippocampus, can be specifically blocked by the L-type calcium channel antagonist nifedipine (Drephal et al. 2006). This is important, as it demonstrates an L-type calcium channel-dependent communication between two brain structures known to be critical for contextually conditioned fear.
Despite impaired consolidation of contextually conditioned fear, CaV1.3 KO mice extinguish contextually conditioned fear normally. In light of previous experiments demonstrating that systemic injections of nifedipine/nimodipine can block extinction of context fear (Cain et al. 2002), our results would suggest that CaV1.3 is not the key LVGCC isoform involved in this process and instead point to CaV1.2 as the likely candidate. Indeed, it has recently been reported that CaV1.2 is found in abundance within the basolateral amygdala and is primarily found on the soma and dendrites of pyramidal neurons, but is occasionally found on inhibitory interneurons (Pinard et al. 2005). Although similar immunohistochemical data regarding the cellular and subcellular distribution for CaV1.3 within the amygdala is lacking, these data open up the intriguing possibility that two different isoforms of the same calcium channel subtype might subserve two fundamentally different forms of learning. These data also suggest that any therapeutic interventions designed to treat emotional disorders in humans by pharmacologically manipulating LVGCCs will likely need to achieve sufficient specificity to distinguish the CaV1.2 and CaV1.3 isoforms.
Materials and Methods
Mice
For all experiments, both male and female Cav1.3 knockout (KO) mice and their wild-type littermates (WT) were used. All mice were between 2 and 4 mo old at the time of testing. In the KO mice, the gene for the pore-forming subunit of the Cav1.3 calcium channel has been deleted by insertion of a neomycin cassette into exon 2, which results in a complete null mutation (Platzer et al. 2000). Mice were maintained on a C57BL/6 background by successively crossing heterozygous offspring with C57BL/6 WT mice purchased from Taconic Farms. The majority of the experiments were conducted on mice on a C57BL/6:129Sve F2 hybrid background. We also did a small subset of fear-conditioning experiments using mice on a C57BL/6 genetic background as noted in the results section.
Open field
The open field chamber was a white acrylic box (71 × 71 × 30 cm) in a room lit by indirect white light. Mice were placed singly in the center of the chamber and allowed to explore for 5 min. The open field was divided into a center zone and a peripheral zone. Total distance traveled and center distance: total distance ratio were calculated.
Rotarod
Mice were placed on the rotating drum of an accelerating rotarod (UGO Basile Accelerating Rotarod), and the time each mouse was able to walk on top of the drum was measured. The speed of the rotarod accelerated from 4 to 40 rpm over a 5-min period. Mice were given 1 trial/day for 5 d with a maximum time of 300 sec (5 min).
Pavlovian fear conditioning
Each of the four conditioning chambers was equipped with a stainless-steel grid floor designed for mice (Med Associates). The grid floor was positioned over a stainless-steel drop-pan, which was lightly cleaned with 95% ethyl alcohol to provide a background odor. The front, top, and back of the chamber were made of clear acrylic and the two sides made of modular aluminum. The conditioning chambers were arranged in a 2 × 2 configuration on a steel rack. The rack was positioned in an isolated room lit by overhead fluorescent lighting. Each chamber was connected to a solid-state shock scrambler and each scrambler was connected to an electronic constant-current shock source that was controlled via an interface connected to a Dell Windows XP computer running Actimetrics FreezeFrame software. Four cameras were mounted (one above each chamber) to the steel rack, and video signals were sent to the same computer. Freezing was assessed using the Actimetrics FreezeFrame software, which digitizes the video signal at 4 Hz and compares movement frame by frame to determine the amount of freezing.
Contextual fear conditioning
Mice were transferred from their home cages into the conditioning chambers individually in groups of four at one time. During conditioning, mice were placed in the chamber for 3 min prior to the onset of the US (2-sec 0.50 mA footshock). Thirty seconds after the footshock, mice were removed from the chambers and returned to their home cages. Contextual conditioning was assessed in different groups of mice 1, 6, and 24 h after conditioning by returning mice to the same chambers and measuring freezing during a 3-min shock-free test session.
Extinction of contextually conditioned fear
Behavioral training proceeded in three phases, i.e., fear acquisition, fear extinction, and testing. During the acquisition phase, mice were conditioned as described above; however, because CaV1.3 KO mice exhibited impairments with respect to contextually conditioned fear following a single training trial (see Results), an additional training trial was administered by delivering a single shock immediately following the 3-min shock-free test session on the second day. As with the first training trial, mice were removed from the chambers 30 sec after the shock. The following day (the third day), mice were returned to the same chambers and contextual conditioning was again assessed by measuring freezing during a 3-min shock-free test session. Because contextually conditioned fear does not differ significantly between KO and WT mice on the third day, following two training trials, mice were left in the chambers for an additional 27 min following the 3-min test session on the third day (30 min total) to extinguish contextually conditioned fear. An additional control group of CaV1.3 KO and WT mice (the retention control group) was included in this experiment. These mice received the exact same training as described above, but were not given the 30-min extinction trail. On the following day, all mice were returned to the same chambers for a 5-min test session.
Morris water maze
The Morris water maze (MWM) used in these experiments consisted of a 1.2-meter diameter pool filled with water that was made opaque with white nontoxic paint. Water temperature was maintained at 25 ± 2 C° throughout the experiment.
Every training trial began with the mouse on the platform for 15 sec. The mouse was then placed into the water facing the wall of the pool and allowed to search for the platform. The trial ended either when the mouse climbed onto the platform or when 60 sec had elapsed. At the end of each trial, the mouse was allowed to rest on the platform for 15 sec. Mice were given two trials per day for 6 d, with the starting position chosen pseudorandomly among six start positions. Probe trials were conducted 24 h after the last training trail. During the probe trial, the escape platform was removed and mice were placed in the pool at the start location directly opposite of the platform and allowed to swim for 60 sec. Mice were run in the visible-platform version of the water maze 24 h following the probe trial. The visible-platform version consisted of a single day of training with six trials, during which the platform was moved to a new quadrant (excluding the target quadrant from the hidden-platform version) every two trials and marked with a distinct local cue.
Data analysis
Statistical comparisons for single measures across genotype were made using a two-tailed unpaired student’s t-test. In addition, a single group t-test was used within genotypes to compare MWM probe trial performance with respect to chance (25%). All other comparisons were made using analysis of variance (ANOVA) with post hoc comparisons being made using Fisher’s PLSD.
Acknowledgments
We thank Dr. Stephen Maren for his helpful comments on this manuscript. This work was supported by grants from The National Institute of General Medical Sciences 5T32GM008322 (to B.C.M.) and The National Institute on Aging R21AG025471 (to G.G.M.) and the University of Michigan Nathan Shock Center for the Basic Biology of Aging (5P30AG013283).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.279006
References
- Barad M. Fear extinction in rodents: Basic insight to clinical promise. Curr. Opin. Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bauer E.P., Schafe G.E., LeDoux J.E. NMDA receptors and l-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J. Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R.J., Blanchard D.C., Fial R.A. Hippocampal lesions in rats and their effect on activity, avoidance, and aggression. J. Comp. Physiol. Psychol. 1970;71:92–101. doi: 10.1037/h0028958. [DOI] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J. Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark N.C., Nagano N., Kuenzi F.M., Jarolimek W., Huber I., Walter D., Wietzorrek G., Boyce S., Kullmann D.M., Striessnig J., et al. Neurological phenotype and synaptic function in mice lacking the CaV1.3 α subunit of neuronal L-type voltage-dependent Ca2+ channels. Neuroscience. 2003;120:435–442. doi: 10.1016/s0306-4522(03)00329-4. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Daumas S., Halley H., Frances B., Lassalle J.-M. Encoding, consolidation, and retrieval of contextual memory: Differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learn. Mem. 2005;12:375–382. doi: 10.1101/lm.81905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drephal C., Schubert M., Albrecht D. Input-specific long-term potentiation in the rat lateral amygdala of horizontal slices. Neurobiol. Learn. Mem. 2006;85:272–282. doi: 10.1016/j.nlm.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Poulos A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Koschak A., Reimer D., Huber I., Grabner M., Glossmann H., Engel J., Striessnig J. α 1D (CaV1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J. Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann. N.Y. Acad. Sci. 2003;985:106–113. doi: 10.1111/j.1749-6632.2003.tb07075.x. [DOI] [PubMed] [Google Scholar]
- Maren S., Aharonov G., Fanselow M.S. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pinard C.R., Mascagni F., McDonald A.J. Neuronal localization of cav1.2 L-type calcium channels in the rat basolateral amygdala. Brain Res. 2005;1064:52–55. doi: 10.1016/j.brainres.2005.09.066. [DOI] [PubMed] [Google Scholar]
- Platzer J., Engel J., Schrott-Fischer A., Stephan K., Bova S., Chen H., Zheng H., Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class d L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Barrientos R.M., O’Reilly R.C. Hippocampal formation supports conditioning to memory of a context. Behav. Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf M.G., Bauer E.P., LeDoux J.E. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J. Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Lipscombe D. Neuronal CaV1.3 α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S.L., Fanselow M.S. Associative regulation of Pavlovian fear conditioning: Unconditional stimulus intensity, incentive shifts, and latent inhibition. J. Exp. Psychol. Anim. Behav. Process. 1992;18:400–413. doi: 10.1037//0097-7403.18.4.400. [DOI] [PubMed] [Google Scholar]