Abstract
Differentiation of Trypanosoma cruzi trypomastigotes to amastigotes inside myoblasts or in vitro, at low extracellular pH, in the presence of [3H]palmitic acid or [3H]inositol revealed differential labeling of inositolphosphoceramide and phosphatidylinositol, suggesting that a remodeling process takes place in both lipids. Using 3H-labeled inositolphosphoceramide and phosphatidylinositol as substrates, we demonstrated the association of at least five enzymatic activities with the membranes of amastigotes and trypomastigotes. These included phospholipase A1, phospholipase A2, inositolphosphoceramide-fatty acid hydrolase, acyltransferase, and a phospholipase C releasing either ceramide or a glycerolipid from the inositolphospholipids. These enzymes may be acting in remodeling reactions leading to the anchor of mature glycoproteins or glycoinositolphospholipids and helping in the transformation of the plasma membrane, a necessary step in the differentiation of slender trypomastigotes to round amastigotes. Synthesis of inositolphosphoceramide and particularly of glycoinositolphospholipids was inhibited by aureobasidin A, a known inhibitor of fungal inositolphosphoceramide synthases. The antibiotic impaired the differentiation of trypomastigotes at acidic pH, as indicated by an increased appearance of intermediate forms and a decreased expression of the Ssp4 glycoprotein, a characteristic marker of amastigote forms. Aureobasidin A was also toxic to differentiating trypomastigotes at acidic pH but not to trypomastigotes maintained at neutral pH. Our data suggest that inositolphosphoceramide is implicated in T. cruzi differentiation and that its metabolism could provide important targets for the development of antiparasitic therapies.
Trypanosoma cruzi is the etiologic agent of Chagas' disease or American trypanosomiasis. T. cruzi has been recognized as a significant cause of morbidity and mortality from Mexico to South America (4). Chagas' disease remains a problem because of limited therapeutic choices and adverse reactions to the two drugs available, nifurtimox and benznidazole (4, 14). Therefore, it is important to identify enzymes and metabolic pathways in T. cruzi that might be potential targets for drug development. T. cruzi has three main developmental stages: the epimastigote, which is found in the insect vector and can be grown in axenic culture; the amastigote or intracellular form, which lives in the cytosol of nucleated cells; and the trypomastigote, which is the terminal differentiation stage in the vector (metacyclic form) or is found in the bloodstream of mammalian hosts (bloodstream form).
All life cycle stages of T. cruzi possess on their cell surface glycoinositolphospholipid (GIPL)-anchored glycoproteins as well as free (non-protein-linked) GIPLs (24). The inositolphospholipids (IPLs) of these anchors can possess either a glycerolipid or a ceramide. In contrast, only acylglycerophospholipids have been identified in the anchor of T. brucei glycoproteins (17, 18), while diacyl- or alkylacylglycerolipids are part of the anchors of mammalian glycoproteins (5, 25, 30).
Several important glycoproteins of T. cruzi are attached to the cell surface via a ceramide-containing anchor linked to the C terminus of the protein (24), such as the Ssp4 antigen of amastigote forms (3, 7), the unique trans-sialidase glycoprotein (2), and the mucins of metacyclic trypomastigotes (1). Interestingly, the Ssp4 of amastigotes (3) and the trans-sialidase of trypomastigotes (2) are released to the medium by an endogenous phosphatidylinositol-phospholipase C (PI-PLC). In the free GIPLs of log-phase epimastigotes, both an alkylacylglycerol (AAG), 1-O-hexadecyl-2-O-palmitoylglycerol, and ceramide have been identified (11), whereas only ceramide was found in the related lipopeptide phosphoglycan of stationary-phase epimastigotes (12). The two major ceramides in the lipopeptide phosphoglycan are palmitoylsphinganine and lignoceroylsphinganine. However, no lignoceric acid is present in the radioactive precursor inositolphosphoceramides (IPCs), nor in the cold samples obtained from T. cruzi (8).
The biosynthetic pathway for the introduction of ceramide in the anchor of glycoproteins of T. cruzi is not known, but this lipid was also described as an anchor of glycoproteins of Saccharomyces cerevisiae (10, 16). In this organism, ceramide is introduced by a remodeling reaction (28, 32, 33) that replaces the glycerolipid with a newly synthesized ceramide (29). The variations found in the lipid moiety of T. cruzi GIPLs suggest that a remodeling mechanism may occur here, too. This is supported by the finding that IPC is not a substrate for the first step of the biosynthesis of GIPLs (L. E. Bertello et al., unpublished results). A detailed analysis of the IPLs and of their possible use as substrates of T. cruzi phospholipases is a necessary step for understanding the remodeling processes.
The composition of the IPLs from epimastigote (8) and trypomastigote (37) forms has been previously reported. When the lipids were labeled by incorporation of [3H]palmitic acid, IPCs accounted for 80 to 85% of the label of the total IPLs in epimastigotes and they contributed to 58% of the labeled IPLs in infective trypomastigotes.
The transformation of trypomastigotes into amastigotes occurs after invasion of mammalian cells but can also be induced in vitro by low extracellular pH (36). The glycosylphosphatidylinositol (GPI)-anchored surface glycoprotein Ssp4 was found to be a marker for the differentiation process, since it is detected with monoclonal antibodies only in recently differentiated amastigotes (3). This antigen is then progressively shed, by the action of an endogenous PI-PLC (3). The lipid moiety of the anchor of Ssp4 is the ceramide palmitoyldihydrosphingosine (PDHS), which reaches maximal concentration in the cells at 18 to 24 h of trypomastigote-to-amastigote transformation, coinciding with Ssp4 shedding (7). During this differentiation process the expression of a PI-PLC, which is able to hydrolyze either PI or IPC (31), is induced, and the enzyme associates with the plasma membrane and increases its catalytic activity (19). Taken together, these results suggest that profound changes occur in the composition of the plasma membrane during the differentiation of trypomastigotes to amastigotes. Interestingly, phosphoinositol-containing sphingolipids, such as IPC, are present in fungi and protozoa but absent in humans, and their metabolism is therefore likely to offer unique targets for antifungal (27) and antiparasitic drugs. In addition, sphingolipids such as ceramide have been proposed as intracellular mediators in various cellular responses (21, 22) and could potentially be involved in the differentiation process or in the pathogenic mechanisms of T. cruzi.
We report here that the differentiation of trypomastigotes to amastigotes in vitro or inside infected myoblasts is accompanied by the production and remodeling of IPC and PI. Evidence is provided that membranes of trypomastigotes and amastigotes possess the enzymes required for this remodeling process. We also show that aureobasidin A, a known inhibitor of yeast IPC synthase (27), inhibited the biosynthesis of IPC and GIPLs during in vitro differentiation of trypomastigotes. The antibiotic also impaired differentiation, as shown by the large number of intermediate forms produced and by the lack of expression of the amastigote marker Ssp4.
MATERIALS AND METHODS
Cultures and metabolic labeling of T. cruzi.
T. cruzi amastigotes and trypomastigotes (Y strain) were obtained from the culture medium of L6E9 myoblasts as described previously (19). T. cruzi epimastigotes from the Y strain were grown at 28°C in liver infusion tryptose medium (9) supplemented with 5% newborn calf serum. Trypomastigotes were induced to transform into amastigotes axenically as described previously (19, 36). Briefly, trypomastigotes were washed twice with medium 199 supplemented with 3.7 g of NaHCO3/liter and 0.4% bovine serum albumin (fraction V), pH 7.5. The cells were resuspended at a concentration of 107 cells/ml in fresh medium, at either pH 7.5 or 5.0, containing [3H]palmitic acid or 2-[3H]myo-inositol and 0.4% bovine serum albumin. The medium was buffered with 20 mM 2-[N-morpholino]ethanesulfonic acid (MES; pH 5.0) or 20 mM HEPES (pH 7.5). For experiments without radiolabeled compounds (Fig. 9), the differentiation was induced in Dulbecco's modified Eagle's medium under the same conditions described for the use of medium 199. Parasites were incubated at 35°C for different times, washed twice with Dulbecco's phosphate-buffered saline, and extracted with chloroform-methanol-water (10:10:3). The extracts were dried in a Speed-Vac evaporator and further purified by partition in water-1-butanol (1:1, vol/vol). The organic phase was dried and kept at −20°C for further analysis. Aureobasidin A was dissolved in ethanol and added to the cultures in the amounts indicated in the figure legends. At a final concentration of 0.05 to 0.2%, ethanol had no significant effects on differentiation. However, concentrations of ethanol of 1% or above resulted in an increase in intermediate forms.
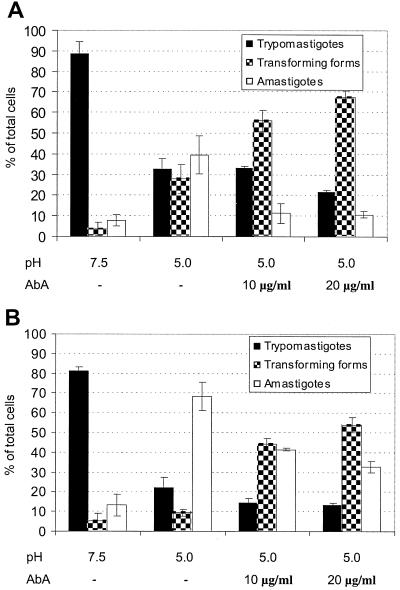
FIG. 9.
Aureobasidin A increases the number of intermediate forms in an in vitro differentiation system. Trypomastigotes were induced to differentiate (2 and 5 h, panels A and B, respectively) as described under Materials and Methods. Aureobasidin A was added at the concentrations indicated. Results shown are the averages of three separate experiments.
Chemicals.
Dulbecco's modified Eagle's medium, Dulbecco's phosphate-buffered saline, Hanks medium, cold fish gelatin, fetal and newborn calf sera, LiCl, bee venom phospholipase A2 (PLA2) from Apis mellifera (1,200 U/mg of solid, 1,350 U/mg of protein), and MES were purchased from Sigma Chemical Co (St. Louis, Mo.). [3H]palmitic acid (39 Ci/mmol), 2-[3H]myo-inositol (21,000 Ci/mmol), and En3Hance were from NEN Life Science Products (Boston, Mass.). Medium 199 was from Invitrogen, GIBCO-BRL Products (Grand Island, N.Y.). Aureobasidin A was from Takara Biomedicals (Shiga, Japan). PI-PLC from Bacillus thuringiensis was from Oxford GlycoSciences (Abingdon, Oxford, United Kingdom). All other reagents were analytical grade.
Immunofluorescence microscopy.
Parasites fixed with 4% freshly prepared formaldehyde were made to adhere to coverslips previously coated with poly-l-lysine, permeabilized with 0.5% Triton X-100 for 5 min, blocked with 0.45% cold fish gelatin in Dulbecco's phosphate-buffered saline, and prepared for immunofluorescence by using a 1:50 dilution of monoclonal antibody 2C2 (anti-Ssp4; a kind gift of Norma W. Andrews, Yale University) and a rhodamine-conjugated goat anti-mouse immunoglobulin G secondary antibody (1:100). Immunofluorescence images were obtained with an Olympus BX-60 fluorescence microscope.
Labeling of myoblasts and infection with trypomastigotes.
L6E9 myoblasts (107) were incubated with [3H]inositol (10 μCi/ml) or [3H]palmitic acid (5 μCi/ml) for 24 h in medium 199 containing 20% dialyzed fetal calf serum (Atlanta Biologics). Cells were washed three times with 4 ml of Hanks medium to remove excess radioactive compounds and were then infected with 5 × 108 trypomastigotes. After 3 h the trypomastigotes in the supernatant were collected. Less than 1% of the added radioactivity could be detected in the collected parasites. The infected myoblasts were cultured for 6, 12, 18, 24, and 48 h. Control uninfected myoblasts were prepared in the same way. Three hours before the end of the cultivation period, 15 mM LiCl, a phosphatase inhibitor, was added to the culture medium. After incubation for the indicated times, 0.5 ml of Hanks medium and 1 ml of 0.5 M HClO4 were added and the culture was kept at 0°C for 20 min. After centrifugation for 10 min at 1,500 × g the pellet was separated and the lipids were extracted with chloroform-methanol-concentrated hydrochloric acid (100:100:1, by volume) and analyzed by thin-layer chromatography (TLC) with solvent system A (see below).
Preparation of membrane and supernatant fractions.
Amastigotes and trypomastigotes were lysed by suspension in hypotonic medium containing 0.5 mM NaHCO3, 10 mM HEPES (pH 7.4), 1 mM EGTA, 1 mM dithiothreitol, and 10 μg of leupeptin/ml. The suspension was mixed in a vortex for 30 s, left for 15 min at 0°C, and further homogenized with a Teflon tissue homogenizer. The homogenate was then centrifuged at 37,000 × g for 12 min at 4°C. The supernatant and the pellet were stored separately at −70°C in aliquots corresponding to 108 cells.
Extraction and purification of IPLs from epimastigotes.
Epimastigotes at the logarithmic growth stage (2 days of culture; 2 × 108 cells) were labeled with [3H]palmitic acid for 18 h as reported previously (8). Cells were freeze-dried and extracted with 2:1 (vol/vol; 20 ml) and 1:1 (vol/vol; 20 ml) mixtures of chloroform-methanol. The extracts were dried, and the inositolphosphate (IP)-containing lipids were separated from the neutral lipids by DEAE-Sephadex A25 (acetate form) column chromatography (23). Neutral lipids were eluted with 50 ml of methanol-chloroform-water (30:15:4, by volume), and acidic lipids were eluted with 50 ml of methanol-chloroform-0.8 M sodium acetate (30:15:4, by volume). The last fraction was evaporated, and salts were removed by passing them through a C18 cleanup cartridge (Accubond II; Agilent Technologies, Palo Alto, Calif.). Salts were removed with water, and IPLs were eluted with methanol. Further purification was achieved by column chromatography on silica gel 60 equilibrated with chloroform, with elution with a 4:1 (vol/vol) and then a 1:1 (vol/vol) mixture of chloroform-methanol.
Analytical methods.
TLC was performed using Silica Gel 60 plates (Merck) with the following solvent systems and procedures. Chromatography with chloroform-methanol-acetone-acetic acid-water (90:30:18:27:17, by volume; solvent A) was performed on oxalate-EDTA-impregnated plates. The plates were prepared by immersion in a solution containing 1.3% potassium oxalate and 2 mM EDTA in methanol-water (2:3, vol/vol) for 30 min at room temperature. Then plates were allowed to dry overnight and heated at 110°C for 30 min (15). Other solvent systems were chloroform-methanol-2.5 M NH4OH (15:10:2, by volume; solvent B), chloroform-methanol (38:3, vol/vol; solvent C), hexane-ethyl acetate (4:1, vol/vol; solvent D), and chloroform-methanol-2.5 M NH4OH (40:10:1, by volume; solvent E). Reverse-phase TLC was performed on RP-18 F254s plates (Merck) with the solvent chloroform-methanol-water (40:100:3, by volume; solvent F). When necessary, IPLs were eluted from the silica gel with chloroform-methanol-water (10:10:3, by volume). Radiolabeled lipids were detected by fluorography. The TLC plates were sprayed with En3Hance and were exposed to X-Omat AR-5 films (Kodak, Rochester, N.Y.) or blue-sensitive X-ray film (Midwest Scientific, St. Louis, Mo.) at −70°C. The nonradioactive standards were detected by spraying the plates with a solution containing 5% H2SO4 in ethanol.
Assay of phospholipase activities.
Radioactive PI and IPC isolated as described above were used as substrates for the subsequent experiments. The standard incubation mixture consisted of the radioactive substrate (250,000 cpm) and 108 cell equivalents of T. cruzi membrane fraction in buffer A (50 mM HEPES [pH 7.0], 0.1% deoxycholate, 3 mM MgCl2, 10 μM CaCl2). The substrate dissolved in chloroform-methanol (1:1, vol/vol) was dried in the incubation tubes under a N2 stream, and the membrane fraction, suspended in 100 μl of buffer, was added. After incubation in a shaker at 30°C for different times (5, 15, 60, and 120 min and 18 h), the reaction was stopped by addition of 660 μl of chloroform-methanol (1:1, vol/vol). A control incubation of the substrate without membranes was also done. An overnight incubation with a supernatant corresponding to 108 cells was performed to test the activity of the soluble fraction. After the incubation, the membranes were extracted twice with chloroform-methanol-water (10:10:3, by volume). The organic extracts were dried in a Speed-Vac evaporator and subjected to 1-butanol-water partition. The radiolabeled lipids were recovered in the 1-butanol phase, which was washed with water, dried, and analyzed by TLC in solvent B.
Effect of aureobasidin A on in vitro differentiation of trypomastigotes.
Trypomastigotes (107 cells/ml) were incubated with 10 μCi of [3H]inositol/ml or 10 μCi of [3H]palmitic acid/ml in the presence of 5 to 20 μg of aureobasidin A/ml, for different times. Differentiation in vitro was induced as described above. After incubation, lipids were extracted and analyzed by TLC.
HPLC analysis of IPs.
IPs and inositol were analyzed by high-pressure liquid chromatography (HPLC) with a ternary gradient system (ISCO) and an anionic-exchange column, Partisil Sax (0.46 by 25 cm by 10 μm; Alltech). A gradient of ammonium formiate (pH 3.8, adjusted with phosphoric acid) was used as eluent, as described previously (15). Radioactivity derived from the 3H-labeled samples was detected with a Hewlett-Packard radio detector.
Enzymatic treatments.
Samples of the IPLs were resuspended in 100 μl of 50 mM Tris-HCl buffer, pH 7.2, containing 0.1% deoxycholate and incubated with 0.35 U of PI-PLC from B. thuringiensis for 90 min at 37°C. The lipids were extracted with 1-butanol (three times with 0.5 ml each) and analyzed by TLC in solvent C or D. The PLA2 reaction was performed in the same buffer with 2.5 mM CaCl2 added. The samples were incubated with 25 U of bee venom PLA2 from A. mellifera for 2 to 4 h at 37°C. After the reaction the lipids were extracted with 1-butanol and analyzed by TLC in solvent B.
Chemical degradations.
Radioactive IPC was hydrolyzed for 18 h at 70 to 78°C with HCl-methanol-water (3:29:4, by volume), and the hydrolysate was extracted with diethyl ether. The long-chain bases were analyzed by TLC in solvent E. The original lipids or the lipids isolated after PI-PLC treatment were hydrolyzed with 0.1 M NaOH in 90% methanol for 1 h at 37°C. After neutralization with 10% (vol/vol) glacial acetic acid, the lipids were extracted with 1-butanol and analyzed by TLC.
Electron microscopy.
Trypomastigotes were induced to differentiate at pH 5.0, as described above. Controls remained at pH 7.5. After 5 h, parasites were washed with buffers at the corresponding pH and fixed in Karnovsky's solution. Fixed samples were washed with 0.1 M cacodylate buffer, pH 7.2, and postfixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide at room temperature in the same buffer. After washing in 10% ethanol, samples were dehydrated by incubations with increasing ethanol concentrations (25, 50, 75, and 100%) at room temperature. Infiltration and embedding were done in Epon, and blocks were polymerized at 60°C. Ultrathin sections were collected on copper grids, stained with uranyl acetate and lead citrate, and observed by transmission electron microscopy.
RESULTS
Synthesis of IPC during trypomastigote-to-amastigote differentiation in infected myoblasts.
In order to investigate the synthesis of IPC during the trypomastigote-to-amastigote differentiation inside mammalian cells, we labeled L6E9 myoblasts with either [3H]palmitic acid or [3H]inositol for 24 h. The myoblasts were washed to eliminate nonincorporated radioactivity and infected with trypomastigotes for 3 h. After that time, noninvading parasites were removed and IPL analysis was performed at different times postinfection.
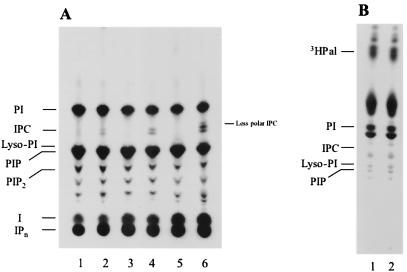
Figure 1A shows the TLC analysis of the 3H-IPLs extracted from the control (lanes 1, 3, and 5) and infected (lanes 2, 4, and 6) cells after different incubation times (18, 24, and 48 h). All the compounds with higher mobility than that of inositol were sensitive to bacterial PI-PLC treatment, indicating their IPL nature (data not shown). Compounds with somewhat lower mobility than that of PI, corresponding to IPC, were extracted from the infected cells but not from control cells after 18 h of incubation (Fig. 1A, lane 2), and their amount increased with time (Fig. 1A, lanes 4 and 6). Analysis of samples taken after 6 and 12 h of incubation did not show any difference between infected and control cells (data not shown). When these experiments were repeated using myoblasts labeled with [3H]palmitic acid instead of [3H]inositol, no radioactive IPC could be detected in either control or infected cells (Fig. 1B). Taken together these results suggest that the inositol in the IPC is coming from [3H]inositol incorporated by the myoblasts, while the ceramide is probably of parasite origin.
FIG. 1.
Analysis of IPLs in myoblasts infected with T. cruzi trypomastigotes. (A) Myoblasts (107) were labeled with [3H]inositol for 24 h and infected with 5 × 108 trypomastigotes for 3 h. Trypomastigotes were then washed out, and the myoblasts were incubated for different times in fresh medium (18, 24, and 48 h in lanes 2, 4, and 6, respectively). Control noninfected cells were prepared in the same way (lanes 1, 3, and 5). (B) Myoblasts labeled with [3H]palmitic acid and infected as described for panel A were incubated for 24 h (lane 2). A noninfected control is shown in lane 1. After incubation the radiolabeled lipids were extracted and analyzed by TLC with solvent system A. The positions of standards are indicated on the left. PIP2, phosphatidylinositolbisphosphate; I, inositol; IPn, IPs.
Synthesis of IPC during in vitro differentiation of trypomastigotes to amastigotes.
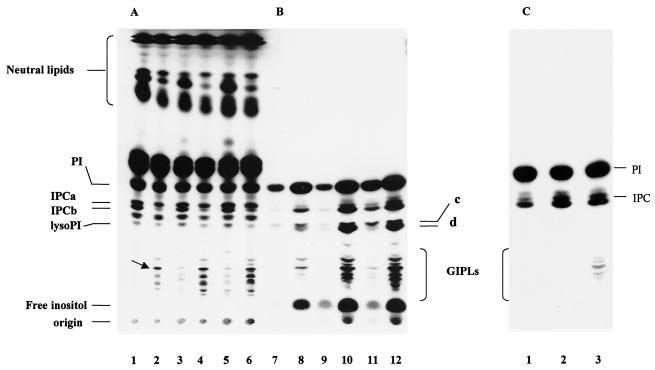
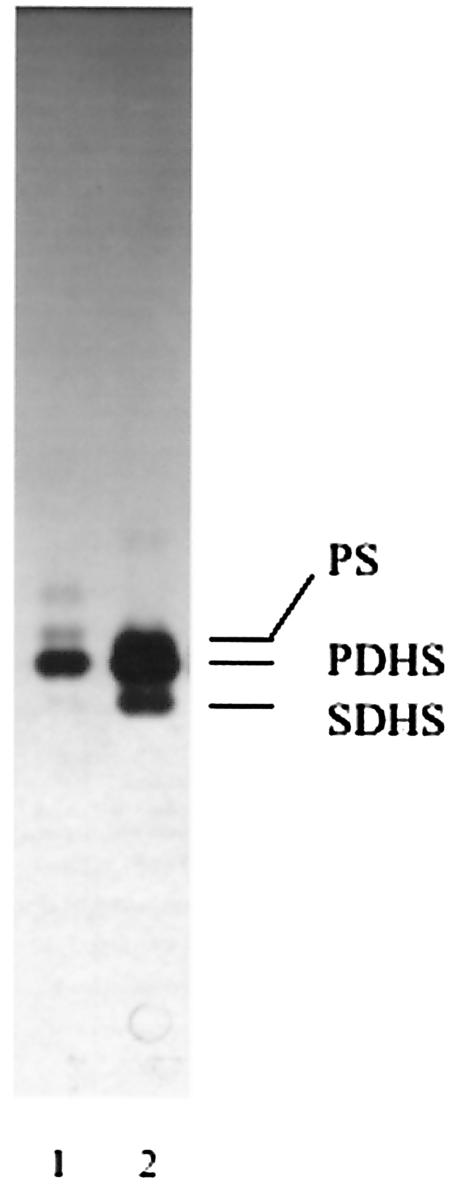
We also investigated the synthesis of IPC during the in vitro differentiation of trypomastigote to amastigote stages induced by low extracellular pH (36). As described before (19, 36), ∼70% of the cells appeared rounded, as amastigotes, after 5 h at pH 5.0, while trypomastigotes were morphologically unchanged at pH 7.5. Both [3H]palmitic acid (Fig. 2A) and [3H]inositol (Fig. 2B) were incorporated into PI and IPC. Two bands corresponding to IPC (IPCa and IPCb) were detected that were previously identified in trypomastigotes (37), the ceramide corresponding mainly to PDHS in IPCa and palmitoylsphingosine (PS) in IPCb. Their structures were confirmed by elution of the bands from the plate shown in Fig. 2A, treatment with bacterial PI-PLC, and identification of the ceramides released by reverse-phase TLC in solvent F, as described in Materials and Methods (Fig. 3, lane 1). The ceramides released by similar treatment of epimastigote IPCs (8) were also analyzed for comparison (Fig. 3, lane 2). The major ceramide present in differentiating trypomastigotes was PDHS (Fig. 3, lane 1), as occurs in epimastigotes (Fig. 3, lane 2), with a minor amount of PS. The structure of the base was confirmed by acid methanolysis and TLC in solvent E. Dihydrosphingosine was the main base, with a minor amount of sphingosine (data not shown).
FIG. 2.
Analysis of IPLs in differentiating trypomastigotes in vitro. (A and B) T. cruzi trypomastigotes obtained from the culture medium of myoblasts were incubated with [3H]palmitic acid (A) at pH 7.5 for 2, 4, and 6 h (lanes 1, 3, and 5, respectively) or at pH 5 for the same times (lanes 2, 4, and 6, respectively) or with [3H]inositol (B) at pH 7.5 for 2, 4, and 6 h (lanes 7, 9, and 11, respectively) or at pH 5 for the same times (lanes 8, 10, and 12). (C) Trypomastigotes were incubated with [3H]inositol for 5 h. After that time, 108 cells were extracted (lane 1) or were incubated for 5 additional h at either pH 7.5 (lane 2) or pH 5.0 (lane 3). The lipids were extracted and analyzed by TLC in solvent F. The arrow shows the main GIPL labeled with [3H]palmitic acid. c and d show bands corresponding to the lyso-PIs.
FIG. 3.
Analysis of the ceramide in the IPCs of amastigotes. The IPCs were eluted from the plate shown in Fig. 2A (lanes 2, 4, and 6) and treated with PI-PLC from B. thuringiensis. The lipids were extracted and analyzed by reverse-phase TLC in solvent G (lane 1). The ceramides obtained from the IPCs of epimastigotes are shown for comparison (lane 2). SDHS, stearoyldihydrosphingosine.
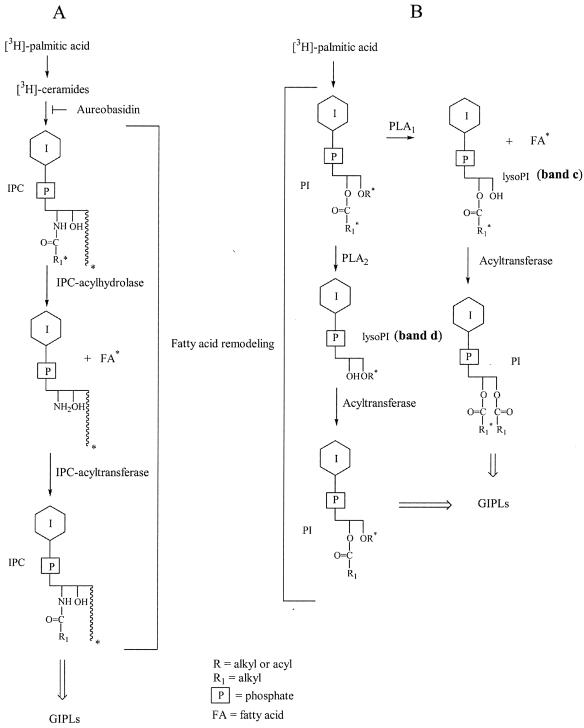
IPCb was more abundant in the [3H]inositol-labeled samples after 2 to 6 h of incubation (Fig. 2B, lanes 8, 10, and 12), whereas it was the minor component when cells were labeled with [3H]palmitic acid (Fig. 2A, lanes 2, 4, and 6). This suggests that a remodeling process is taking place in IPCb, introducing either a nonradioactive ceramide or a nonradioactive fatty acid, which could be cold palmitic acid or another fatty acid that does not affect the mobility of IPCb. The pathway proposed for the remodeling, involving the function of an IPC-acylhydrolase and an IPC-acyltransferase, is shown in Fig. 4A.
FIG. 4.
Fatty acid remodeling of the IPC (A) and PI (B) of T. cruzi. Inhibition of the biosynthesis of IPC and GIPLs by aureobasidin A is shown in panel A.
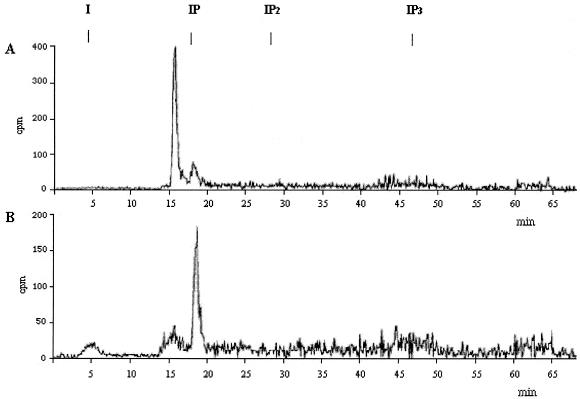
The compound labeled with [3H]inositol with the mobility of either lysophosphatidylinositol (lyso-PI) or phosphatidylinositolmonophosphate (PIP) (indistinguishable in the plates, double bands c and d, in Fig. 2B) was eluted from the plate and treated with bacterial PI-PLC. Only IP, not IP2, could be detected by HPLC (Fig. 5), ruling out the presence of PIP.
FIG. 5.
HPLC analysis of the [3H]inositol-labeled compounds c and d (Fig. 2) treated with PI-PLC. Compounds c and d (Fig. 2B) were eluted from the plate with chloroform-methanol-water (10:10:3), treated with PI-PLC (B. thuringiensis, 0.35 U at 37°C for 2 h), and analyzed by HPLC (A), with use of the nucleotides AMP, ADP, and GTP as internal standards, as previously described (15). (B) Same treatment as for panel A but with an additional acid treatment (HCl, 0.1 N; 1 h at room temperature; neutralization with 0.2 N NaOH) in order to open the cyclic phosphate formed after PI-PLC hydrolysis. Elution times of the standards inositol (I), IP, inositol 1,4-bisphosphate (IP2), and inositol 1,4,5-trisphosphate (IP3) are shown on the top of the chromatogram.
PLA2 treatment of the lyso-PIs (c plus d), eluted from the plate shown in Fig. 2B, showed that only band c was affected, indicating that it was the 2-O-acyl derivative, formed by the action of an endogenous PLA1 on PI. When band d was subjected to saponification and butanol-water partition, the radioactivity was distributed between the two phases. The radioactivity in the aqueous phase corresponded to the 1-O-acyl-lyso-PI and that in the butanol phase corresponded to the 1-O-alkyl-lyso-PI, both originally formed by an endogenous PLA2 (Fig. 4B shows the possible pathway). A similar result was previously shown for epimastigotes (6).
The lyso-PIs are strongly labeled with [3H]inositol and only faintly labeled with [3H]palmitic acid, suggesting that remodeling of fatty acids is taking place in the PIs (Fig. 2A and B). Both precursors were also incorporated into the more polar GIPLs (Fig. 2A and B). Their nature was confirmed by their susceptibility to bacterial PI-PLC and analysis by TLC (data not shown). When [3H]inositol was used as the precursor, an increase in PI labeling occurred upon differentiation (Fig. 2B, lanes 8, 10, and 12), whereas there was no significant increase in labeling when [3H]palmitic acid was used, again indicating remodeling of the fatty acid in the PI, by the action of endogenous PLA and acyltransferase activities (Fig. 4B). The less polar lipids were labeled only with [3H]palmitic acid and corresponded to neutral lipids (Fig. 2A). The presence of free inositol (Fig. 2B) was confirmed by HPLC. The larger amount detected upon differentiation suggests either the action of a more active PI-PLC releasing IPs that are subsequently hydrolyzed by inositolphosphatases or a higher incorporation of inositol at low pH. To test if increased labeling of IPC and GIPLs in differentiating parasites was the result of a higher incorporation of inositol at pH 5, a control experiment was performed, in which the cells were labeled before their incubation at different pHs (Fig. 2C). Trypomastigotes were labeled with [3H]inositol for 4 h (lane 1, Fig. 2C), washed, and incubated for a further 5 h at either pH 7.5 (Fig. 2C, lane 2), or pH 5 (Fig. 2C, lane 3). No increase in labeling of IPCs above the increase observed when the cells were incubated at pH 7.5 (Fig. 2C, lane 2) was observed when cells were incubated at pH 5.0 (Fig. 2C, lane 3). In fact, a lower labeling of IPC was observed (Fig. 2C, lane 3), suggesting that the IPCs were used for the biosynthesis of GIPLs and that the differences shown in Fig. 2A and B were not due to increased incorporation of inositol at acidic pH.
Phospholipases in amastigotes and trypomastigotes active on PI.
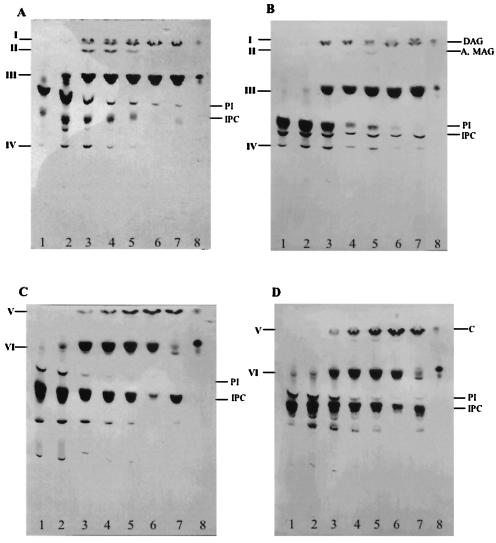
In order to confirm the presence of PI-PLC and PLA activities in membrane and soluble fractions of amastigote and trypomastigote forms of T. cruzi, we used radioactive IPLs as exogenous substrates. Thus, the IPLs from epimastigote forms of T. cruzi, metabolically labeled with [3H]palmitic acid, were separated into PI and IPC subfractions (8) and used to investigate these phospholipase activities. Figure 6 shows the results of incubation of [3H]PI with membranes of amastigotes (Fig. 6A, lanes 2 to 6) and trypomastigotes (Fig. 6B, lanes 2 to 6) for different times (5, 15, 60, and 120 min and 18 h). Also, the activity in the supernatant of the membrane preparation was analyzed in an overnight incubation (Fig. 6, lanes 7). After the indicated times, the lipids were extracted and analyzed by TLC with solvent B. The formation of less polar lipids than the IPLs was detected in all lanes except those corresponding to the controls, which were incubated in the buffer without membranes (Fig. 6A and B, lanes 1). The compounds of higher mobility (I, Fig. 6A and B) were eluted from the plates and rechromatographed in solvent D (Fig. 7). Labeled 1,2-AAG and 1,2-di-O-palmitoylglycerol (DAG), produced by an endogenous PI-PLC, together with 1,3-AAG (see below), were detected after short incubation times (15 to 60 min, Fig. 7A, lane 1). The absence of radioactive 1,2-DAG after the 2-h incubation (Fig. 7A, lane 2) suggests that an acyltransferase activity introduced nonradioactive fatty acids after the action of a PLA1 and a PLA2 on the PI.
FIG. 6.
Phospholipases in T. cruzi amastigote and trypomastigote forms, active on PI and IPC. [3H]palmitic acid-labeled PI (A and B) and IPC (C and D) purified from T. cruzi epimastigotes were incubated for different times (5 min, lane 2; 15 min, lane 3; 1 h, lane 4; 2 h, lane 5; 18 h, lane 6) with a membrane fraction of amastigotes (A and C) or trypomastigotes (B and D). A supernatant of the membrane preparations was incubated for 18 h with the radioactive substrates (lane 7). A control consisting of the substrate incubated overnight with the buffer alone is shown in lane 1. A standard of [3H]palmitic acid is shown in lane 8. Incubations were performed with 250,000 cpm of substrate. After incubation the lipids were extracted as described under Materials and Methods and analyzed by TLC with solvent system B. The position of standards is indicated on the right. A, alkylglycerol; MAG, monoacylglycerol; C, ceramide. Characterization of compounds I to VI is described in Results.
FIG. 7.
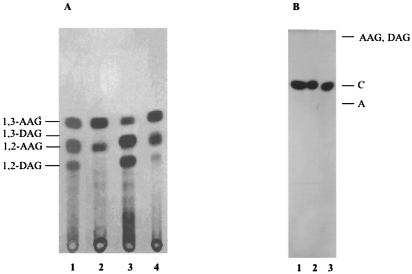
Analysis of the lipids released during the incubation of PI (A) or IPC (B) with membranes of T. cruzi amastigotes and trypomastigotes. (A) Compound I was eluted from the plate shown in Fig. 6A and B and rechromatographed in solvent D. Lane 1, compound I (lanes 3 and 4, Fig. 6A); lane 2, compound I (lanes 5 and 6, Fig. 6A); lane 3, compound I (lanes 3 and 4, Fig. 6B); lane 4, compound I (lanes 5 and 6, Fig. 6B). (B) Compound V was eluted from the plate shown in Fig. 6C and D and rechromatographed in solvent C. Lane 1, ceramide obtained from epimastigotes (6); lane 2, compound V (Fig. 6C) from amastigotes; lane 3, compound V (Fig. 6D) from trypomastigotes. C, ceramide; A, alkylglycerol; AAG, alkylacylglycerol. Compounds I to VI are described in the text.
Compound II was monoacylglycerol, as confirmed by saponification. The released radioactivity corresponded to fatty acid (data not shown). Monoacylglycerol (compound II, Fig. 6A) is probably formed by hydrolysis of a lyso-PI by the PI-PLC. This suggests that a PLA from amastigotes, probably PLA1, is acting faster than the PI-PLC on the DAG of the labeled PI. This activity is reduced in trypomastigotes (Fig. 6B), as less monoacylglycerol (compound II) is formed during this incubation. The PI containing AAG is not a substrate for PLA1. In fact, the total radioactivity of AAG does not change significantly with time (Fig. 7A). The intramolecular acyl migration observed in AAG (Fig. 7A) from position 2 to the more stable 1,3-isomer has been observed before (34).
The main degradation product in the incubation of radioactive PI with membranes of amastigotes and trypomastigotes was the fatty acid (compound III, Fig. 6A and B). The release of fatty acid was faster in amastigotes (5 min). After 15 min, most of the radioactive PI was degraded by amastigote membranes, but a considerable amount of PI still remained when it was incubated with equivalent amounts of trypomastigote membranes. Compound IV has the mobility of a lyso-PI generated by the endogenous PLA activities. For the identification of lipid IV (Fig. 6A and B) the samples eluted from the plate were treated with PLA2 from A. mellifera. The release of fatty acid, as detected by TLC (data not shown), confirmed that a 2-O-acyl-glycerophosphoinositol is part of the lyso-PI and a substrate for the commercial PLA2. The 2-O-acyl derivative originated from the action of an endogenous PLA1 on the original DAG of the PI. Part of the lyso-PIs was not degraded by the PLA2 treatment, suggesting that it was formed by an endogenous PLA2 during incubation of PI with the membranes. In agreement with these results, treatment of [3H]inositol-labeled lyso-PI, eluted from the plate shown in Fig. 2B (bands c and d), showed that only the upper band was a substrate for the commercial PLA2 and confirmed the action of an endogenous PLA1 on PI (Fig. 4B). The lysolipid, which was resistant to the commercial PLA2, should be the product of the endogenous enzyme (data not shown). We also found that PI-PLC and PLA activities are present in a soluble fraction from amastigotes and trypomastigotes (Fig. 6A and B, lanes 7). In this regard, a lysosomal PLA1 activity has been detected in T. cruzi with phosphatidylcholine as substrate (38), and PLA1 and PLA2 activities in epimastigote membranes have been previously reported (6).
Phospholipases in amastigotes and trypomastigotes active on IPC.
To investigate IPC-phospholipase and fatty acid hydrolase activities, radioactive IPC was incubated with membrane and soluble fractions of amastigotes (Fig. 6C) and trypomastigotes (Fig. 6D). The compound of higher mobility (compound V; Fig. 6C and D) was identified as ceramide by elution and further analysis by TLC (Fig. 7B, lanes 2 and 3, respectively). This lipid is formed by an endogenous PI-PLC. The lipid released by incubation with a membrane fraction from epimastigotes is shown for comparison (Fig. 7B, lane 1). Fatty acid was also released by incubation of IPC with the membranes of amastigotes and trypomastigotes (band VI). However, the IPC-acylhydrolase activity was not soluble (Fig. 6C and D, lanes 7). The low amount of fatty acid detected after 18 h of incubation with the soluble fraction could originate from the contaminating PI present in the original sample of IPC.
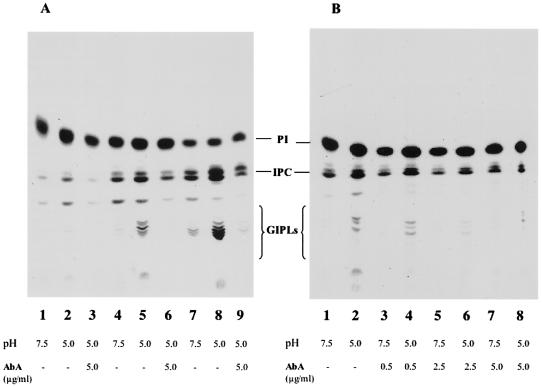
Effect of aureobasidin A on IPC and GIPL synthesis in differentiating trypomastigotes.
We also investigated whether the T. cruzi IPC synthesis was sensitive to aureobasidin A inhibition. Aureobasidin A inhibits the IPC synthase of S. cerevisiae (27), and it has been used to demonstrate that ceramides, which are introduced during remodeling of the GPI anchors, are not generated by the breakdown of IPCs but are newly synthesized (29). Differentiation of trypomastigotes in vitro was induced as described above in the presence of 5 μg of aureobasidin A/ml and [3H]inositol (Fig. 8). Incubation in the presence of aureobasidin A substantially decreased labeling of IPC (Fig. 8, lanes 3, 6, and 9), while labeling of GIPLs was completely inhibited. In the absence of antibiotic there was a time-dependent increase in the labeling of the more polar glycolipids, which were all sensitive to bacterial PI-PLC treatment. The main GIPL labeled with [3H]palmitic acid for 2 h (arrow, Fig. 2A, lane 2) released AAG, whereas the more polar GIPLs, which increased with the incubation time, mainly showed ceramide as the lipid anchor (data not shown).
FIG. 8.
Aureobasidin A inhibits the biosynthesis of IPC and GIPLs during differentiation of trypomastigotes in vitro. (A) Differentiation in vitro was induced as described for Fig. 2. Trypomastigotes were incubated with [3H]inositol at pH 7.5 (lanes 1, 4, and 7) or at pH 5.0 (lanes 2, 3, 5, 6, 8, and 9). Lanes 3, 6, and 9 correspond to cells treated with 5 μg of aureobasidin A/ml during the differentiation. The same numbers of cells were extracted at different times: 2 h (lanes 1 to 3), 5 h (lanes 4 to 6), and 18 h (lanes 7 to 9). (B) Concentration-dependent inhibition of IPC and GIPL biosynthesis by aureobasidin A. Differentiation in vitro was induced as described for panel A. Trypomastigotes were incubated with [3H]inositol at either pH 7.5 (lanes 1, 3, 5, and 7) or pH 5.0 (lanes 2, 4, 6, and 8) in the presence of 0.5 (lanes 3 and 4), 2.5 (lanes 5 and 6), or 5.0 (lanes 7 and 8) μg of aureobasidin A/ml.
GIPL and IPC biosyntheses were inhibited, in a dose-dependent manner (Fig. 8B), by aureobasidin A. This inhibition was almost total for GIPLs at 5 μg/ml, whereas 65% inhibition was observed for IPC, as indicated by radioactive scanning of the TLCs.
Inhibition of trypomastigote differentiation by aureobasidin A.
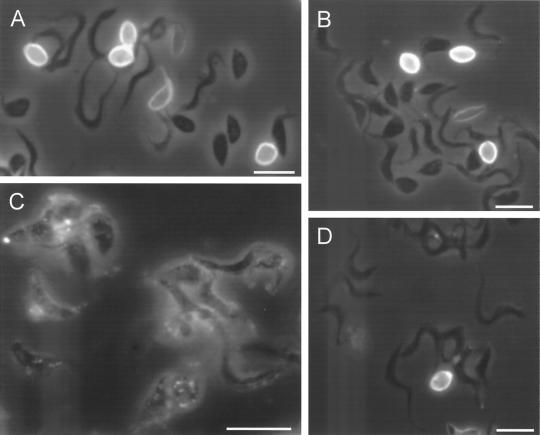
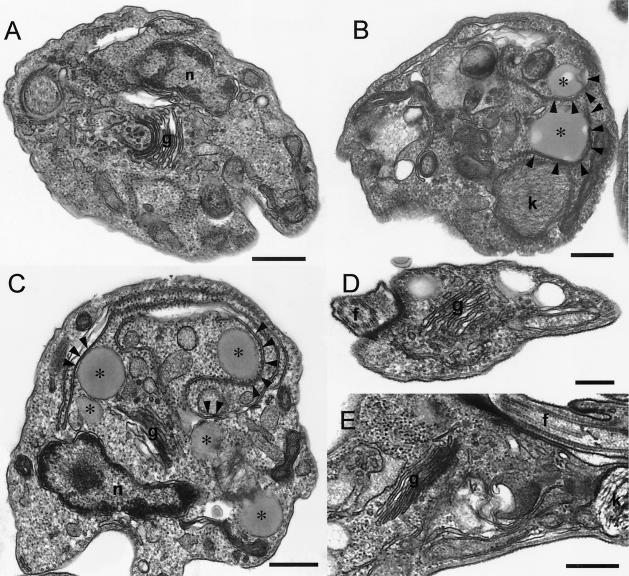
Aureobasidin A also inhibited the differentiation of trypomastigotes in vitro. Figure 9 shows that after 2 (Fig. 9A) and 5 (Fig. 9B) h of incubation of trypomastigotes at pH 5.0 around 40 and 70% of the cells, respectively, had morphological characteristics of amastigotes (rounded and without a visible flagellum). Aureobasidin A decreased the percentage of amastigotes and increased the percentage of intermediate forms (“fat trypomastigotes”) in a dose- and time-dependent manner (Fig. 9). Immunofluorescence staining for the Ssp4 epitope (Fig. 10) revealed fewer cells expressing Ssp4 and more intermediate forms in aureobasidin A-treated preparations at acidic pH (Fig. 10B and C) compared with controls (Fig. 10A). When aureobasidin A was used at high concentrations, parasites maintained at acidic pH showed signs of toxicity (Fig. 10C) while those maintained at neutral pH appeared normal (Fig. 10D). This is better illustrated in the electron micrographs of Fig. 11. Aureobasidin A-treated differentiating parasites showed alterations in the Golgi apparatus as well as large lipidic vacuoles in close contact with the endoplasmic reticulum (Fig. 11B and C, arrowheads), while those maintained at pH 7.5 in the presence of aureobasidin A appeared normal (Fig. 11D and E). Most of the cells treated with aureobasidin A at pH 5.0 corresponded to intermediate forms; this was judged by the proximity of the kinetoplast to the nucleus, and the “basket-like” appearance of the kinetoplast in most cases, which is typical of trypomastigotes (13).
FIG. 10.
Effect of aureobasidin A on the morphology and expression of stage-specific epitope Ssp4. Trypomastigotes were induced to differentiate as described for Fig. 2. After 5 h, parasites were fixed and stained by immunofluorescence with monoclonal antibodies to Ssp4. (A) Control trypomastigotes incubated at pH 5.0 for 5 h; (B) trypomastigotes incubated at pH 5.0 with 10 μg of aureobasidin A/ml; (C) trypomastigotes incubated at pH 5.0 with 20 μg of aureobasidin A/ml. (D) trypomastigotes incubated at pH 7.5 with 20 μg of aureobasidin A/ml. Bars, 5 μm.
FIG. 11.
Effect of aureobasidin A on the ultrastructure of differentiating trypomastigotes. Trypomastigotes were induced to differentiate as described for Fig. 2. After 5 h, parasites were prepared for electron microscopy as described in Materials and Methods. (A) Control trypomastigotes incubated at pH 5.0 for 5 h; (B and C) trypomastigotes incubated at pH 5.0 with 10 μg of aureobasidin A/ml; (D and E) trypomastigotes incubated at pH 7.5 with 10 μg of aureobasidin A/ml. Abbreviations: n, nucleus; k, kinetoplast; g, Golgi apparatus; f, flagellum. Asterisks indicate lipid inclusions. Arrowheads show points of apparent contact between the lipid inclusions and the endoplasmic reticulum. Bars, 0.4 (A and B), 0.5 (C), 0.25 (D), and 0.6 (E) μm.
DISCUSSION
Differentiation of T. cruzi trypomastigotes to amastigotes inside myoblasts was accompanied by the formation of IPC, as revealed by the increased labeling of IPC detected in extracts from [3H]inositol-labeled myoblasts infected with trypomastigotes (Fig. 1A). When the differentiation occurred inside myoblasts labeled with [3H]palmitic acid, no radioactive IPC was detected (Fig. 1B). This result suggested that the ceramide in the IPC was not originating from the myoblasts but was probably of parasite origin. The inositol, however, was probably coming from the labeled pool of PI in the myoblasts, if synthesis in T. cruzi occurs as described for S. cerevisiae (29). In S. cerevisiae IPC is formed from PI by the action of an IPC synthase, which catalyzes the transfer of phosphorylinositol to ceramide (29). De novo synthesis of phosphorylinositol by the parasites with use of the free [3H]inositol available in the infected myoblasts cannot be ruled out, although it is not probable, since the amount of free inositol increased, rather than decreased, during differentiation (Fig. 1A). The Ssp4 surface glycoprotein that appears upon differentiation of trypomastigotes to amastigotes is known to be linked to a ceramide-GPI (7). Since a PI-PLC from T. cruzi is activated during differentiation (19) and is capable of cleaving IPC (31), it is possible that this ceramide could be used to synthesize the IPC that appears in the myoblasts after 18 h postinfection (Fig. 1A, lane 2). An IPC synthase has not been described and the biosynthetic pathway of IPC has not been elucidated in T. cruzi. No sequences with homology to S. cerevisiae IPC synthase have been found in searches of the currently available T. cruzi database (http://TcruziDB.org/) (data not shown).
The biosynthesis of IPC was also monitored during differentiation of trypomastigotes in vitro at low extracellular pH. In this case a different pattern was obtained when cells were labeled with [3H]palmitic acid or [3H]inositol. Two main IPCs were detected in differentiating trypomastigotes. The chemical nature of the IPCs in trypomastigotes was previously studied (37). When cells were labeled with [3H]inositol, it was evident that the levels of radioactive IPCs increased significantly during 6 h, in particular the less mobile IPC containing PS (Fig. 2B). However, when differentiating trypomastigotes were labeled with [3H]palmitic acid, labeling of IPCb greatly decreased with time (Fig. 2A, lanes 2, 4, and 6) while remaining at the same level in nondifferentiating trypomastigotes (Fig. 2A, lanes 1, 3, and 5). These results indicate that a remodeling step on the ceramide is taking place, with replacement of the N-linked radioactive fatty acid, particularly in the IPCb.
Evidence was found for the presence of an IPC-acylhydrolase in membranes of amastigotes and trypomastigotes (Fig. 6). It is interesting that the IPC-acylhydrolase activity was not soluble. As neither lysosphingolipid nor free sphingosine was formed, an IPC-acyltransferase introducing endogenous fatty acid should also be acting (Fig. 4). One enzyme could be responsible for both activities. In this regard, an enzyme which catalyzes the hydrolysis of ceramide, and also its synthesis in an acyl coenzyme A-independent manner, has been cloned from the mouse (35).
Remodeling of the fatty acids in the PIs, upon differentiation, was also evident (Fig. 2A and B) and in agreement with the presence of the enzyme activities detected in membranes and soluble fractions of both stages of the parasites (Fig. 6). The following results suggest that PLA activities remove radioactive fatty acids and are followed by PI acyltransferase activities that catalyze the transfer of cold fatty acids, thus leading to remodeled PI and lyso-PI (Fig. 4). (i) Labeling with [3H]inositol of PI and lyso-PI increased with time of differentiation, whereas there was no increase in labeling of PI with [3H]palmitic acid (Fig. 2A and B). (ii) Incubation of [3H]palmitic acid-labeled PI with membranes of both stages of the parasite (Fig. 6A and B) showed much more release of radioactive fatty acid than of radioactive lyso-PI. It was previously shown that alkylglycerol, present in the major component of PI, is strongly labeled (6). Therefore, the lyso-PI formed after fatty acid removal should have been labeled to the same extent as the fatty acid unless transfer of a cold fatty acid had occurred. (iii) Analysis of the glycerolipids released by the endogenous PI-PLC after short incubation times (15 to 60 min) showed 1,2-AAG and 1,2-DAG (Fig. 7A, lane 1). However, no labeled 1,2-DAG was detected after 2 h (Fig. 7A, lane 2).
PI-PLC, as well as IPC-PLC, activities were found in the membrane and soluble fractions of amastigotes and trypomastigotes (Fig. 6). The PI-PLC previously cloned (19) could be responsible for these activities.
Cellular events regulated by ceramide have received growing attention in recent years (21, 22), among them induction of cell differentiation (22), a crucial step in T. cruzi infection. In vitro differentiation of trypomastigotes by external PI-PLC treatment has been reported (26). Our results suggest that IPC could fulfill a function similar to that of sphingolipids in mammalian cells by generating second messengers.
Activation of GIPL biosynthesis upon differentiation to amastigotes was demonstrated (Fig. 2 and 8). The findings that glycerolipids were the main lipids in the GIPLs detected in the early stages of differentiation, that ceramides were the major lipids after longer times of incubation, and that only ceramide has been found in the anchor of Ssp4 (7) points to a remodeling step for introduction of ceramide. In S. cerevisiae, by using aureobasidin A, an inhibitor of IPC synthase, it was shown that the ceramide necessary for remodeling the GIPLs linked to the protein is not generated by the breakdown of IPC (29). In the case of T. cruzi we also found inhibition of IPC synthesis by aureobasidin A, but in contrast to what occurs in yeast, the drug also inhibited the biosynthesis of GIPLs, suggesting that the ceramide used for remodeling is generated by the breakdown of IPC. On the other hand, in yeast ceramide is introduced only in the GIPLs already linked to the protein (28).
Aureobasidin A inhibited trypomastigote differentiation to amastigotes, leading to the increased appearance of intermediate forms and decreased expression of Ssp4. It has been reported previously (20) that heterologous expression of a T. brucei GPI-PLC in T. cruzi results in limited proliferation and inhibition of differentiation of amastigotes to trypomastigotes. It was suggested that GPI-anchored proteins or free GIPLs of the parasites act as mediators for signals necessary for amastigote development (26). Taking into account our results, this could be related to the presence of ceramide in the GPI anchor of amastigote-specific glycoproteins. It is interesting that only trypomastigotes that were differentiating, not trypomastigotes maintained at neutral pH, showed signs of toxicity upon treatment with high concentrations of aureobasidin A. This would suggest that, like the PI-PLC (19), an IPC-synthesizing enzyme is induced upon differentiation. Inhibition of this enzyme in differentiating parasites would lead to accumulation of precursors such as ceramide that could be toxic to the cells. The IPC synthase, or an equivalent pathway, which is absent in mammalian cells, could be therefore a good target for chemotherapy.
Acknowledgments
We thank Norma W. Andrews for the monoclonal antibody to Ssp4, David A. Scott for reading the manuscript, and Linda Brown for technical assistance.
This work was supported in part by grants from the American Heart Association, Midwest Affiliate, and the National Institutes of Health (AI-48039) (to S.N.J.M.) and from the University of Buenos Aires and Agencia Nacional de Promoción Científica y Tecnológica (PICT 06-05133) (to R.M.D.).
REFERENCES
- 1.Acosta Serrano, A., S. Schenkman, N. Yoshida, A. Mehlert, J. Richardson, and M. A. J. Ferguson. 1995. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J. Biol. Chem. 270:27244-27253. [DOI] [PubMed] [Google Scholar]
- 2.Agusti, R., A. S. Couto, O. E. Campetella, A. C. C. Frasch, and R. M. de Lederkremer. 1997. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology 7:731-735. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, N. W., E. S. Robbins, V. Ley, K. S. Hong, and V. Nussenzweig. 1988. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J. Exp. Med. 167:300-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 1997. Thirteenth programme report. UNDP/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases, p. 112-123. World Health Organization, Geneva, Switzerland.
- 5.Armesto, J., E. Hannappel, K. Leopold, W. Fischer, R. Bublitz, L. Langer, G. A. Cumme, and A. Horn. 1996. Microheterogeneity of the hydrophobic and hydrophilic part of the glycosylphosphatidylinositol anchor of alkaline phosphatase from calf intestine. Eur. J. Biochem. 238:259-269. [DOI] [PubMed] [Google Scholar]
- 6.Bertello, L. E., M. J. M. Alves, W. Colli, and R. M. de Lederkremer. 2000. Evidence for phospholipases from Trypanosoma cruzi active on phosphatidylinositol and inositolphosphoceramide. Biochem. J. 345:77-84. [PMC free article] [PubMed] [Google Scholar]
- 7.Bertello, L. E., N. W. Andrews, and R. M. de Lederkremer. 1996. Developmentally regulated expression of ceramide in Trypanosoma cruzi. Mol. Biochem. Parasitol. 79:143-151. [DOI] [PubMed] [Google Scholar]
- 8.Bertello, L. E., M. F. Gonçalves, W. Colli, and R. M. de Lederkremer. 1995. Structural analysis of inositol phospholipids from Trypanosoma cruzi epimastigote forms. Biochem. J. 310:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone, G. J., and M. Steinert. 1956. Isotopes incorporate in the nucleic acid of Trypanosoma mega. Nature 178:308-309. [DOI] [PubMed] [Google Scholar]
- 10.Conzelmann, A., A. Puoti, R. L. Lester, and C. Desponds. 1992. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 11:457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lederkremer, R. M., C. Lima, M. I. Ramirez, M. F. Gonçalves, and W. Colli. 1993. Hexadecylpalmitoylglycerol or ceramide is linked to similar glycophosphoinositol anchor-like structures in Trypanosoma cruzi. Eur. J. Biochem. 218:929-936. [DOI] [PubMed] [Google Scholar]
- 12.de Lederkremer, R. M., C. Lima, M. I. Ramirez, and O. L. Casal. 1990. Structural features of the lipopeptidophosphoglycan from Trypanosoma cruzi common with the glycophosphatidylinositol anchors. Eur. J. Biochem. 192:337-345. [DOI] [PubMed] [Google Scholar]
- 13.De Souza, W. 1984. Cell biology of Trypanosoma cruzi. Int. Rev. Cytol. 86:197-283. [DOI] [PubMed] [Google Scholar]
- 14.Docampo, R. 2001. Recent developments in the chemotherapy of Chagas disease. Curr. Pharm. Des. 7:1157-1164. [DOI] [PubMed] [Google Scholar]
- 15.Docampo, R., and O. P. Pignataro. 1991. The inositol phosphate/diacylglycerol signaling pathway in Trypanosoma cruzi. Biochem. J. 275:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fankhauser, C., S. W. Homans, J. E. Thomas-Oates, M. J. McConville, C. Desponds, A. Conzelmann, and M. A. J. Ferguson. 1993. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J. Biol. Chem. 268:26365-26374. [PubMed] [Google Scholar]
- 17.Ferguson, M. A. J., M. G. Low, and G. A. M. Cross. 1985. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J. Biol. Chem. 260:14547-14555. [PubMed] [Google Scholar]
- 18.Field, M. C., A. K. Menon, and G. A. M. Cross. 1991. A glycosylphosphatidylinositol protein anchor from procyclic stage Trypanosoma brucei: lipid structure and biosynthesis. EMBO J. 10:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuya, T., C. Kashuba, R. Docampo, and S. N. J. Moreno. 2000. A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation. J. Biol. Chem. 275:6428-6438. [DOI] [PubMed] [Google Scholar]
- 20.Garg, N., M. Postan, K. Mensa-Wilmot, and R. L. Tarleton. 1997. Glycosylphosphatidylinositols are required for the development of Trypanosoma cruzi amastigotes. Infect. Immun. 65:4055-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh, S., J. C. Strum, and R. M. Bell. 1997. Lipid biochemistry: functions of glycerolipids and sphingolipids in cellular signaling. FASEB J. 11:45-50. [DOI] [PubMed] [Google Scholar]
- 22.Hannun, Y. A. 1996. Functions of ceramide in coordinating cellular responses to stress. Science 274:1855-1859. [DOI] [PubMed] [Google Scholar]
- 23.Ledeen, R. W., and R. K. Yu. 1982. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 83:139-191. [DOI] [PubMed] [Google Scholar]
- 24.Lederkremer, R. M., and L. E. Bertello. 2001. Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr. Pharm. Des. 7:1165-1179. [DOI] [PubMed] [Google Scholar]
- 25.Medof, M. E., E. I. Walter, W. L. Roberts, R. Haas, and T. L. Rosenberry. 1986. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry 25:6740-6747. [DOI] [PubMed] [Google Scholar]
- 26.Mortara, R. A., L. M. S. Minelli, F. Vandekerckhove, V. Nussenzweig, V. Nussenzweig, and F. J. Ramalho-Pinto. 2001. Phosphatidylinositol-specific phospholipase C (PI-PLC) cleavage of GPI-anchored surface molecules of Trypanosoma cruzi triggers in vitro morphological reorganization of trypomastigotes. J. Eukaryot. Microbiol. 48:27-37. [DOI] [PubMed] [Google Scholar]
- 27.Nagiec, M. M., E. E. Nagiec, J. A. Baltisberger, G. B. Wells, R. L. Lester, and R. C. Dickson. 1997. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 272:9809-9817. [DOI] [PubMed] [Google Scholar]
- 28.Reggiori, F., E. Canivenc-Gansel, and A. Conzelmann. 1997. Lipid remodeling leads to the introduction and exchange of defined ceramides on GPI proteins in the ER and Golgi of Saccharomyces cerevisiae. EMBO J. 16:3506-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reggiori, F., and A. Conzelmann. 1998. Biosynthesis of inositol phosphoceramides and remodeling of glycosylphosphatidylinositol anchors in Saccharomyces cerevisiae are mediated by different enzymes. J. Biol. Chem. 273:30550-30559. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, W. L., B. H. Kim, and T. L. Rosenberry. 1987. Differences in the glycolipid membrane anchors of bovine and human erythrocyte acetylcholinesterases. Proc. Natl. Acad. Sci. USA 57:608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salto, M. L., T. Furuya, S. N. J. Moreno, R. Docampo, and R. M. de Lederkremer. 2002. The phosphatidylinositol-phospholipase C from Trypanosoma cruzi is active on inositolphosphoceramide. Mol. Biochem. Parasitol. 119:131-133. [DOI] [PubMed] [Google Scholar]
- 32.Sipos, G., A. Puoti, and A. Conzelmann. 1994. Glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae: absence of ceramides from complete precursor glycolipids. EMBO J. 13:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sipos, G., F. Reggiori, C. Vionnet, and A. Conzelmann. 1997. Alternative lipid remodeling pathways for glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae. EMBO J. 16:3494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sjursnes, B. J., and T. Anthonsen. 1994. Acyl migration in 1,2-dibutyrin, dependence on solvent and water activity. Biocatalysis 9:285-297. [Google Scholar]
- 35.Tani, M., N. Okino, S. Mitsutake, T. Tanigawa, H. Izu, and M. Ito. 2000. Purification and characterization of a neutral ceramidase from mouse liver. A single protein catalyzes the reversible reaction in which ceramide is both hydrolyzed and synthesized. J. Biol. Chem. 275:3462-3468. [DOI] [PubMed] [Google Scholar]
- 36.Tomlinson, S., F. VanDekerckhove, U. Frevert, and V. Nussenzweig. 1995. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitology 110:547-554. [DOI] [PubMed] [Google Scholar]
- 37.Uhrig, M. L., A. S. Couto, W. Colli, and R. M. de Lederkremer. 1996. Characterization of inositolphospholipids in Trypanosoma cruzi trypomastigote forms. Biochim. Biophys. Acta 1300:233-239. [DOI] [PubMed] [Google Scholar]
- 38.Wainszelbaum, M., E. Isola, S. Wilkowsky, J. J. B. Cannata, J. Florin-Christensen, and M. Florin-Christensen. 2001. Lysosomal phospholipase A1 in Trypanosoma cruzi: an enzyme with a possible role in the pathogenesis of Chagas' disease. Biochem. J. 355:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]