Abstract
The working-memory functions of the prefrontal cortex (PFC) are improved by stimulation of postsynaptic, α2A-adrenoceptors, especially in aged animals with PFC cognitive deficits. Thus, the α2A-adrenoceptor agonist, guanfacine, greatly improves working-memory performance in monkeys and rats following systemic administration or intra-PFC infusion. α2A-adrenoceptors are generally coupled to Gi, which can inhibit adenylyl cyclases and reduce the production of cAMP. However, no study has directly examined whether the working-memory enhancement observed with guanfacine or other α2A-adrenoceptor agonists results from cAMP inhibition. The current study confirmed this hypothesis in both rats and monkeys, showing that treatments that increase cAMP-mediated signaling block guanfacine’s beneficial effects. In aged rats, guanfacine was infused directly into the prelimbic PFC and was challenged with co-infusions of the cAMP analog, Sp-cAMPS. In aging monkeys, systemically administered guanfacine was challenged with the phosphodiesterase 4 inhibitor, rolipram, using intramuscular doses known to have no effect on their own. In both studies, agents that mimicked the actions of cAMP (rats) or increased endogenous cAMP (monkeys) completely blocked the enhancing effects of guanfacine on working-memory performance. These results are consistent with α2A-adrenoceptor stimulation enhancing PFC working-memory function via inhibition of cAMP-mediated signaling.
The prefrontal cortex (PFC) mediates working memory, the ability to transiently hold task-relevant information “on line” in order to guide behavior in the absence of environmental cues. The information held in working-memory buffers is constantly updated and is thought to rely on reverberating networks of PFC neurons with shared mnemonic characteristics. In contrast, other memory processes, such as memory consolidation, require the long-term fixation of information through architectural changes in brain circuitry and/or activation of translational mechanisms. Not surprisingly, working memory and long-term memory consolidation appear to be modulated in very different ways. The varying influences of norepinephrine (NE) and cAMP on working memory vs. long-term memory consolidation are especially striking. Whereas memory consolidation is strengthened by stimulation of β-adrenoceptors and the production of cAMP, working memory appears to be enhanced by stimulation of α2A-adrenoceptors and the inhibition of cAMP signaling.
NE enhances PFC function via α2A-adrenoceptor stimulation in a number of different species. α2A-adrenoceptor agonists enhance PFC cognitive functions in mice (Franowicz et al. 2002), rats (Tanila et al. 1996), monkeys (Arnsten and Goldman-Rakic 1985; Arnsten et al. 1988; Arnsten and Cai 1993), and humans (Jakala et al. 1999a, b), including patients with PFC cognitive disorders (Hunt et al. 1995; Scahill et al. 2001; Taylor and Russo 2001). Conversely, blockade of these receptors in the monkey PFC impairs spatial working memory (Li and Mei 1994). In contrast to the PFC, stimulation of α2A-adrenoceptors impairs or has no effect on hippocampal memory functions (Tanila 2001). Thus, the beneficial effects of α2A-adrenoceptor stimulation are specific for the working-memory functions of the PFC, since their activation improves spatial working-memory performance (Arnsten et al. 1988; Carlson et al. 1992; Coull et al. 1995; Rama et al. 1996), but have a small effect or even impair tasks dependent on posterior cortices or hippocampus (for review, see Arnsten 1998). Similarly, activation of a cAMP-dependent cascade impairs working-memory functions (Taylor et al. 1999; Ramos et al. 2003; Arnsten et al. 2005), but improves memory consolidation and long-term potentiation (LTP; late phase) (Frey et al. 1993; Abel et al. 1997; Bernabeu et al. 1997; Bourtchouladze et al. 1998; Huang and Kandel 1998; Barros et al. 2000; Huang et al. 2000; Rotenberg et al. 2000; Schafe and LeDoux 2000).
Most research has focused on presynaptic α2A-adrenoceptors, which inhibit NE release and NE cell firing (Cedarbaum and Aghajanian 1977; Engberg and Eriksson 1991). It is noteworthy that guanfacine is 10 times less potent than clonidine at these presynaptic sites (Engberg and Eriksson 1991). However, the majority of α2A-adrenoceptors in brain are actually postsynaptic to NE neurons (U’Prichard et al. 1979) and electron-micrographic studies of monkey PFC have documented both presynaptic receptors (likely on NE axons), and postsynaptic α2A-adrenoceptors on the dendritic spines of PFC pyramidal cells (Aoki et al. 1998). Previous research indicates that the working-memory enhancing effects of α2-adrenoceptor agonists result from stimulation of postsynaptic receptors, as drugs become more, rather than less potent when the presynaptic element is destroyed with 6-OHDA in the PFC of monkeys (Arnsten and Goldman-Rakic 1985), or when NE is depleted in the PFC of both monkeys (Cai et al. 1993) and humans (McEntee and Mair 1990). Previous research has also shown that the α2A-receptor subtype likely underlies guanfacine’s beneficial effects on PFC functioning (Arnsten et al. 1988), as α2 agonists lose efficacy in mice with a functional knockout of the α2A-adrenoceptor subtype, but remain effective in α2C-adrenoceptor knockout mice (Tanila et al. 1999; Franowicz et al. 2002). Finally, low doses of guanfacine are able to improve working memory without inducing hypotension or sedation (Arnsten et al. 1988). Despite the extensive research on α2A-adrenoceptors and the use of guanfacine in the treatment of PFC disorders, no study until now has examined which intracellular pathway mediates the enhancing effects of guanfacine.
Previous research in the lab has shown that activation of the cAMP pathway impaired PFC function in both rats and monkeys, particularly in aged animals (Taylor et al. 1999; Ramos et al. 2003; Arnsten et al. 2005). Since α2-adrenoceptors are generally coupled to Gi proteins, guanfacine’s enhancing effects may be due to inhibition of cAMP production. We tested this hypothesis in aging rats and monkeys with a pronounced response to guanfacine. In rats we examined whether intra-PFC infusions of the cAMP analog, Sp-cAMPS could reverse the beneficial effects of the α2A-adrenergic agonist, guanfacine. We also performed a parallel study in monkeys to determine whether the indirect activator of the cAMP-pathway, rolipram, could reverse the beneficial effects of guanfacine. Rolipram inhibits the cAMP catabolic enzyme, phosphodiesterase type 4 (PDE4), and thus increases endogenous levels of cAMP. To our knowledge, rolipram is the only PDE4 inhibitor that can be administered systemically and penetrate into the brain. Working memory was assessed by a spatial delayed alternation task in a T-maze (rats) or by a spatial delayed response task (monkeys). The results from this study suggest that guanfacine’s beneficial effects result from inhibition of cAMP-dependent signaling.
Results
Rats
Aged rats (∼24 mo when tested) were pre-trained on the spatial delayed alternation task in a T-maze. They were surgically implanted with guide cannula directed above the medial PFC (Fig. 1), and adapted to infusion procedures. After stable baseline cognitive performance of 60%–70% was established, they were infused with either saline, guanfacine (0.0001 μg/0.5 μL), Sp-cAMPS (0.21 or 0.021 nmol), or guanfacine + Sp-cAMPS, 30 min prior to testing. The 17 rats in the current study were randomly divided into two groups: those that received the 0.21 nmol dose of Sp-cAMPS (n = 9, group 1 in Fig. 2) and those that received the 0.021 nmol dose of Sp-cAMPS (n = 8, group 2 in Fig. 3). It should be noted that the 0.21-nmol dose of Sp-cAMPS is 10 times lower than the dose needed to impair working-memory performance in aged animals.
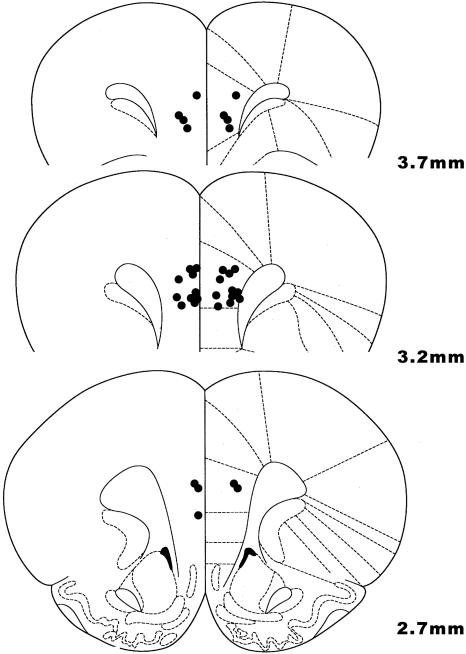
Figure 1.
Location of cannula tips in the rat medial PFC (prelimbic cortex). All bilateral infusions of 0.5 μL occurred at 4.5 mm DV. Coronal slices indicate distance (mm) anterior from bregma.
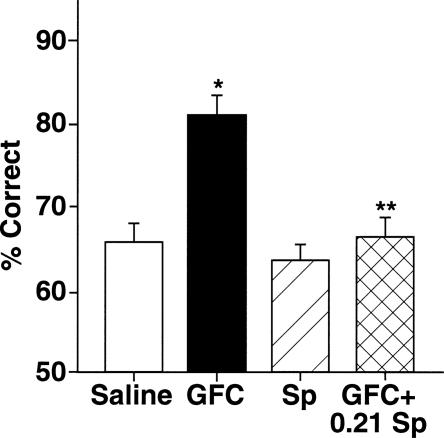
Figure 2.
Guanfacine’s beneficial effects on working-memory performance of rats are via inhibition of cAMP actions. Guanfacine (0.0001 μg/0.5 μL) improves rats’ performance in the delayed alternation task in a T-maze when compared with performance following a vehicle treatment. Activation of cAMP signaling, with a dose of 0.21 nmol of Sp-cAMPS that has no effect on its own blocks guanfacine’s beneficial effects. Results represent mean ± SEM percent correct, n = 9. *P = 0.0004 compared with vehicle; **P = 0.00007 compared with guanfacine alone.
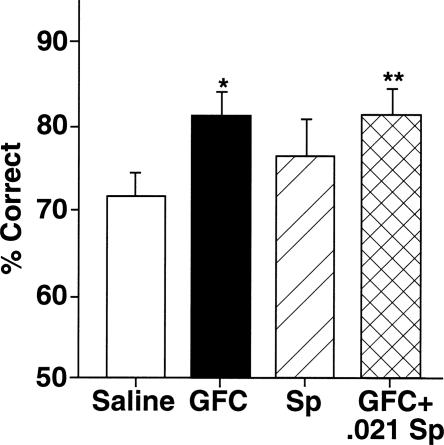
Figure 3.
Pharmacological specificity of Sp-cAMPS ability to reverse guanfacine’s beneficial effects. A lower dose of Sp-cAMPS (0.021 nmol), that has no effects on its own, fails to block guanfacine’s beneficial effect on working-memory performance. Results represent mean ± SEM percent correct, n = 8. *P = 0.02 compared with vehicle; **P = 0.003 compared with guanfacine alone.
The 0.21-nmol dose of Sp-cAMPS reversed the enhancing effects of guanfacine on working memory (Fig. 2). 2-ANOVA-R analysis demonstrated a significant main effect of guanfacine infusion, (F(1,9) = 17.54, P = 0.002), a significant main effect of 0.21-nmol Sp-cAMPS, (F(1,9) = 33.13, P = 0.0002), and a significant interaction between guanfacine and Sp-cAMPS infusions, (F(1,9) = 14.7, P = 0.004). Planned comparisons (test of effects) showed that infusion of guanfacine alone significantly improved accuracy of responding compared with saline control (81.25 ± 1.88% correct after guanfacine treatment vs. 66.00 ± 2.33% correct after saline: F(1,9) = 30.89, P = 0.0004). Planned comparisons also showed that Sp-cAMPS infusion had no effect on its own (64.29 ± 1.45% after Sp-cAMPS vs. 66.00 ± 2.33% after saline: F(1,9) = 0.60, P = 0.46), but significantly reversed the enhancing effects of guanfacine (81.25 ± 1.88% after guanfacine treatment vs. 67.14 ± 2.06% after guanfacine +0.21 nmol Sp-cAMPS: F(1,9) = 48.0, P = 0.00007; guanfacine +0.21 nmol Sp-cAMPS not significantly different from saline: F(1,9) = 0.14, P = 0.72).
A lower dose of Sp-cAMPS was tested in the second experiment to examine a dose/response for Sp-cAMPS (Fig. 3). For group 2 rats, there again was a significant main effect of guanfacine (F(1,8) = 8.34, P = 0.02). However, there was no main effect of Sp-cAMPS treatment (F(1,8) = 0.94, P = 0.36) and no significant interaction between Sp-cAMPS and guanfacine infusions (F(1,8) = 0.95, 0.36). Planned comparisons showed that guanfacine significantly improved working-memory performance for the group 2 rats compared with their own performance on saline (F(1,8) = 9.59, P = 0.02). The 0.021 nmol Sp-cAMPS had no effect on its own (77.14 ± 3.84% after 0.021 nmol Sp-cAMPS vs. 71.67 ± 3.06% after saline: F(1,8) = 1.96, P = 0.2). This very low dose of Sp-cAMPS did not reverse guanfacine’s beneficial effects (81.43 ± 2.75% after guanfacine vs. 81.43 ± 3.47% after guanfacine + 0.021 nmol Sp-cAMPS: F(1,8) = 0, P = 1; guanfacine + 0.021 nmol Sp-cAMPS significantly different from saline: F(1,8) = 17.15, P = 0.003). Thus, the 0.21-nmol dose, but not the 0.021-nmol dose, reversed guanfacine’s enhancing effects on the delayed alternation task.
Monkeys
Sp-cAMPS does not cross the blood brain barrier, and thus cannot be administered systemically. However, the PDE4 inhibitor, rolipram, is capable of penetrating the brain following systemic administration. Thus, rolipram was administered to monkeys, with or without guanfacine, 2 h prior to cognitive testing on the spatial delayed response task. Within each test session, performance was assessed across a variety of delay lengths, ranging between a “0” sec delay control condition and the delay where each monkey performed at chance (50%). Three intermediate delay intervals were also used so that drug effects could be examined across the span of each monkey’s abilities. A wide guanfacine dose range was examined (0.0001–0.5 mg/kg) to determine the most effective dose to enhance working-memory performance. For each monkey, a rolipram dose between 0.001 and 0.05 μg/kg that had no effect on its own was selected based on previous work in the lab (Ramos et al. 2003). That rolipram dose was then readministered to verify that it did not have any cognitive effect in the current study, and was then coinjected with a dose of guanfacine that previously improved working-memory performance. Treatments were administered in random order with at least 1-wk washout between doses.
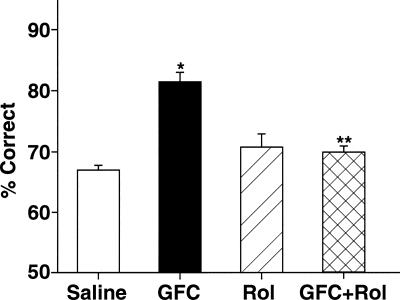
Rolipram significantly reversed the enhancing effects of guanfacine in eight monkeys (Fig. 4). Analysis using 2-ANOVA-R revealed a significant main effect of guanfacine injection, (F(1,7) = 9.03, P = 0.02), a significant main effect of rolipram, (F(1,7) = 6.83, P = 0.04), and a significant interaction between guanfacine and rolipram injections, (F(1,7) = 93.41, P < 0.001). Planned comparisons (test of effects) showed that guanfacine significantly improved accuracy of responding compared with saline control (81.67 ± 1.65% correct after guanfacine treatment vs. 67.63 ± 0.76% correct after saline: F(1,7) = 46.34, P < 0.001). Rolipram had no effect on performance on its own (70.42 ± 2.25% after rolipram vs. 67.63 ± 0.76% after saline: F(1,7) = 3.23, P = 0.17), but reversed the enhancing effects of guan- facine (81.67 ± 1.65% correct after guanfacine treatment vs. 69.88 ± 1.11% after guanfacine + rolipram: F(1,7) = 51.3, P = 0.0002; no significant difference between saline treatment and guanfacine + rolipram: F(1,7) = 3.57, P = 0.101). An analysis of guanfacine’s effects on performance at each delay length confirmed the previous statistical analyses. There was a significant main effect of guanfacine treatment (F(1,7) = 25.46, P = 0.002) and a significant main effect of delay (F(4,28) = 6.48, P = 0.001). However, there was no significant interaction between guanfacine and delay, because guanfacine improved performance at every delay length. There was no evidence of side effects with any of the treatments used in these studies (median sedation score of “0”, normal behavior), suggesting that effects seen in the current study arose from altered cognitive ability rather than from nonspecific effects on performance. Thus, similar to rat studies, guanfacine’s enhancement of PFC function is consistent with inhibition of cAMP signaling.
Figure 4.
Inhibition of the cAMP pathway mediates the beneficial effects of systemic administration of guanfacine on the working-memory performance of monkeys. Systemic administration of guanfacine (0.0001–0.5 mg/kg) improves the monkeys’ performance in the delayed response task when compared with performance following a vehicle treatment. Similar to the rat studies, increasing cAMP actions with rolipram at a dose that has no effects on its own, blocks guanfacine’s beneficial effects on working-memory performance. Results represent mean ± SEM percent correct, n = 8. *P < 0.001 compared with saline vehicle; **P = 0.0002 compared with guanfacine alone.
Discussion
The current study examined the hypothesis that α2A-adrenoceptor stimulation improves PFC cognitive function by inhibiting cAMP signaling. The results showed that infusion of the α2A-adrenoceptor agonist, guanfacine (0.0001 μg/0.5 μL), into prelimbic PFC-enhanced delayed alternation performance in aged rats. This improvement in PFC cognitive function was completely blocked by coinfusion of the cAMP analog, Sp-cAMPS in a dose-related manner. As Sp-cAMPS mimics the effects of cAMP, these data indicate that guanfacine improves working-memory functions in PFC by inhibiting cAMP actions. Results in monkeys also supported this hypothesis, as the improvement observed with systemic guanfacine was blocked by coadministration of rolipram, a PDE4 inhibitor that increases endogenous cAMP levels. Importantly, the reversal of guanfacine’s beneficial effects on working memory by Sp-cAMPS and rolipram could not be explained by additive effects of the two treatments, because the latter drugs were given at doses that had no significant effect on their own. Guanfacine may be particularly efficacious in aging animals by inhibiting excessive cAMP signaling in the aged PFC (Ramos et al. 2003).
The fact that cAMP activation has different effects on working memory compared with long-term memory processes should not be surprising, since working memory requires the continuous and dynamic updating of memory buffers, whereas long-term memory consolidation involves changes that are long-lasting and less dynamic. Thus, hippocampal memory functions are impaired by stimulation of α2-adrenoceptors (Tanila 2001) and enhanced by activation of the cAMP-dependent protein kinase A (PKA) pathway. For example, memory consolidation and LTP (late phase) depend on activation of cAMP/PKA signaling (Frey et al. 1993; Abel et al. 1997; Bernabeu et al. 1997; Bourtchouladze et al. 1998; Huang and Kandel 1998; Barros et al. 2000; Huang et al. 2000; Rotenberg et al. 2000; Schafe and LeDoux 2000). In contrast, PFC working-memory functions are impaired by activation of the cAMP pathway in both young (Taylor et al. 1999) and aged (Ramos et al. 2003) animals. However, it is possible that cAMP/PKA activation in the PFC may be necessary in paradigms using long delays that require hippocampal interactions with the PFC or that involve encoding mechanisms similar to those used in the hippocampal studies mentioned above. Consistent with this hypothesis, a study by Beninger and colleagues has suggested that cAMP/PKA activation in the PFC may be necessary in paradigms using long delays that require hippocampal interactions with the PFC (Aujla and Beninger 2001). Thus, inhibition of cAMP/PKA signaling in the PFC immediately before testing impaired working-memory performance when long delays (30 min) were used (Aujla and Beninger 2001). Moreover, work from Seamans and colleagues has shown that dopamine D1 receptors, which couple to Gs and can increase cAMP levels, selectively modulate hippocampal afferents to the PFC during the performance of a long-delay spatial win-shift task, but not during performance of a nondelayed task (Seamans et al. 1998). Thus, the ability to use previously acquired spatial information to guide responding 30 min later on a radial arm maze requires D1 receptor activation in the PFC and D1 receptor modulation of hippocampal inputs to the PFC. Finally, a more recent study found that cAMP/PKA activation within the PFC impaired information storage for seconds, but not for minutes (Runyan and Dash 2005). Taken together, these data suggest that cAMP/PKA activation may be beneficial for PFC cognitive functions under conditions that require hippocampal-PFC interactions or long-term changes in PFC architecture. In contrast, transient working-memory operations relying on existing PFC networks would be strengthened by inhibition of cAMP signaling.
It is not yet clear whether the beneficial effects of cAMP inhibition are due to direct actions of cAMP itself, cAMP activation of PKA, or cAMP actions on other intracellular signaling pathways (e.g., EPAC). If the beneficial effects of reducing cAMP levels are via PKA inhibition, then it is possible that a cell-permeable, direct inhibitor of PKA would mimic the effects of guanfacine or Rp-cAMPS and reverse the impairing effects of Sp-cAMPS or rolipram. Conversely, cAMP could be improving working memory via its direct modulatory actions on other molecules. Our research in progress indicates that cAMP may influence PFC function through direct actions on Hyperpolarization-activated Cyclic Nucleotide-gated cation (HCN) channels, but it is likely that other downstream targets are altered as well. Either way, cAMP actions appear to be very rapid, and likely do not involve traditional translational processing.
The current study cannot fully rule out a presynaptic effect of guanfacine, since the presynaptic terminals are intact and α2A-adrenoceptors are localized pre- as well as postsynaptically in PFC. There is also some controversy as to whether cAMP mechanisms contribute to the regulation of NE release from NE terminals (Hertting et al. 1990; Schwartz 1997; Tsuda et al. 2003). However, as described above, the cognitive enhancing effects of guanfacine are likely occurring at postsynaptic receptors on PFC neurons that receive NE innervation.
In summary, guanfacine enhances PFC function in both aged rats and monkeys. The data suggests that this effect is due, at least in part, to the α2A-adrenoceptor’s ability to decrease cAMP-mediated signaling in the PFC. The current study suggests that levels of cAMP signaling could be altered with guanfacine treatment to benefit individuals with advancing age or with PFC disorders such as Attention Deficit Hyperactivity Disorder.
Materials and Methods
All procedures were approved by the Yale Institutional Animal Care & Use Committee. Care of the rats and monkeys followed the guidelines in “Guide for the Care and Use of Laboratory Animals.”
Rat studies
Subjects
Aged (20 mo), retired breeder Sprague Dawley rats from Harlan were single-housed in filter frame cages. Aged rats were ∼24 mo old upon initiation of pharmacological testing. Given the fragile health of the aged rats, only a total of 17 of over 30 rats were healthy enough to complete training, surgical implantation of cannula, and drug infusions. Animals were kept on a 12-h light/12-h dark cycle, and experiments were conducted during the light phase. Rats were slowly habituated to a restricted diet (16 gm/day per rat) of autoclaved Purina rat chow during the first 2 wk. Food was given immediately after behavioral testing and water was available ad libitum. Rats were weighed weekly to confirm that they were not undergoing irregular weight loss due to regulated diet. Food rewards during cognitive testing were highly palatable miniature chocolate chips. Rats were assigned a single experimenter who handled them extensively before behavioral testing.
Delayed alternation in T-maze
Rats were habituated to a T-maze (dimensions, 90 × 65 cm) until they were readily eating chocolate chips placed at the end of each arm and were acclimated to handling. After habituation, rats were trained on the delayed alternation task. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 10 trials per session, rats were rewarded only if they entered the maze arm that was not previously chosen. Between trials the choice point was wiped with alcohol to remove any olfactory clues. The delay between trials started at “0” sec (i.e., about 1.5 sec, minimum possible for delayed alternation) and was subsequently raised in 5-sec intervals as needed to maintain performance at 60%–70% correct.
Cannulae implantation
After training on the delayed alternation task, animals underwent stereotaxic implantation of chronic guide cannulae as described previously (Taylor et al. 1999). Guide cannulae (Plastics One; 2.8 mm) with stylettes were aimed dorsal to the medial PFC (see Fig. 1; prelimbic PFC; stereotaxic coordinates- anterioposterior: +3.2 mm; mediolateral: ±0.75 mm; dorsoventral: stylette reaching to −4.2 mm). Due to the rats’ age and fragility, surgery was performed under low doses of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) injected (i.p.) prior to the start of the procedure. These agents were supplemented with gas anesthesia (isoflurane) administered during surgery via nose cone. Sterile stylettes were inserted in the cannula to maintain patency. Great care was taken to minimize pain and infection postoperatively to decrease stress to the animal. The region surrounding the cemented guide cannula was treated with triple antibiotic and cleaned daily if needed for a period of about a week. Animals were also acutely treated with Buprenex (0.01 mg/kg) to decrease pain.
Drug infusions
Rats were initially adapted to a mock infusion protocol to minimize any stress associated with the procedure. Rats were gently restrained while the stylets were removed and replaced with 30-gauge sterile infusion needles that extended to 4.5 mm dorsoventral below the skull. Bilateral infusions were driven by a Harvard Apparatus syringe pump set at a flow rate of 0.25 μL/min using 25 μL Hamilton syringes for an infusion time of 2 min. Needles remained inserted in place for 2 min after completion of the infusion. Stylettes were inserted back into the cannulae, and behavioral testing was performed 30 min after the infusion procedure. Drug treatments and vehicle were administered in a counterbalanced order with at least 1 wk between each infusion. Counterbalancing ensured that rats received drug infusions both early in the study when delays were short as well as later in the study when delays were longer. Animals were required to exhibit stable baseline performance (two consecutive test sessions of 60%–70% correct) prior to drug administration.
Sp-cAMPS was purchased from Sigma RBI. Sp-cAMPS was dissolved in sterile PBS as described previously (Punch et al. 1997) to a dose of either 0.21 or 0.021 nmol. Guanfacine was generously provided by Shire Pharmaceuticals. Guanfacine was dissolved in sterile saline solution to the appropriate dose (0.0001 μg/0.5μL). The dose presented in the current study was based on a pilot study that identified this dose as the most effective. The experimenter testing the animal was unaware of drug treatment conditions. Note: due to the fragility of the aged rats’ health and limited number of infusions that can be done after surgery, we performed the lower and higher Sp-cAMPS doses in separate groups of aged rats and were unable to get a dose response curve for guanfacine in these animals.
Histology
Rats were placed in a knock jar that contained a gauze that was moistened with a large dose of isoflurane and sacrificed by decapitation. Brains were removed, stored in formalin, sectioned, and analyzed for histological verification of cannulae placement. All rats had correctly placed cannulae within the prelimbic or infralimbic regions of the rat cortex (Fig. 1).
Data analysis
The guanfacine vs Sp-cAMPS data (both 0.21- and 0.0021-nmol doses) were analyzed using a two-way analysis of variance with repeated measures (2-ANOVA-R) with within-subject factors of guanfacine treatment and Sp-cAMPS treatment. Planned comparisons (user defined contrasts) were performed to examine whether (1) vehicle/saline significantly differed from guanfacine/saline, (2) vehicle/saline differed from Sp-cAMPS/saline, (3) guanfacine/saline differed from guanfacine/Sp-cAMPS, (4) vehicle/saline differed from guanfacine/Sp-cAMPS. A paired t-test was also used to analyze the data from all of the rats in the study that had been treated with guanfacine alone.
Monkey studies
Subjects
The animals used in this study were rhesus monkeys (Macaca mulatta, n = 8) ranging in age from 13 yr (middle-aged) to over 30 yr. Two of eight monkeys were between 13 and 16 yr old; the rest were 18 yr or older. However, actual birthdates were not available for several of the aged animals who were wild caught. Ages were estimated by the veterinarians based on health records, teeth, and known history; several had been in the Yale colony for more than 15 yr. These animals were selected for inclusion in the current study as all had previously shown robust improvement with guanfacine treatment. The monkeys were individually housed and maintained on a diet of Purina monkey chow supplemented with fruit. Animals were always tested at the same time of day immediately prior to feeding. Highly palatable food rewards (e.g., peanuts, raisins, or chocolate chips) were utilized during testing to minimize the need for dietary regulation.
Delayed response testing
Cognitive testing occurred in a Wisconsin General Testing Apparatus (WGTA) situated in a sound-attenuating room. Background masking noise (60 dB, wideband) was also used to minimize auditory distractions. Each monkey was assigned to a single experimenter who knew the animal well, but was unaware of the drug treatment conditions. The animals were tested twice a week with 3–4 d separating each test session (e.g., Monday and Thursday). The monkeys had been previously trained on the spatial delayed response task as described (Arnsten et al. 1988). During delayed response, the animal watched as the experimenter baited one of several foodwells with a food reward. The number of foodwells varied from two to four wells depending on the monkey’s performance level and experience with the task. Care was taken by the experimenter to ensure that the animal attended the baiting procedure. The foodwells were then covered with identical cardboard plaques, and an opaque screen was lowered between the animal and the foodwells for a specified delay. At the end of the delay, the screen was raised and the animal was allowed to choose. Reward was quasirandomly distributed between the left and right wells over the 30 trials that made up a daily test session. Five different delay lengths (referred to as delays A through E) were quasirandomly distributed over these 30 trials. The shortest of these delays was <1 sec (the “0”-sec A delay). A transparent screen was lowered for the “0”-sec delay condition. However, as many monkeys are distracted by this event, this “0”-sec delay condition likely requires PFC function for successful performance. The remaining delays were in the range which, for each individual monkey, yielded baseline performance of about 70% across all delays (i.e., 18–22 trials correct of the possible 30 trials). For example, the delays for one animal might be A = 0, B = 5, C = 10, D = 15, and E = 20 sec.
Sedation assessment
During each cognitive testing session, the experimenter rated the animals’ level of sedation/agitation and aggression on rating scales. Sedation and agitation were rated using a 9-point scale where −4 = too agitated to test, −3 = agitation that interferes with testing, −2 = slight agitation that does not interfere with testing, −1 = more alert than usual, 0 = normal level of arousal, 1 = quieter than usual, 2 = sedated (drooping eyelids, slowed movements), 3 = intermittent sleeping, and 4 = too sedated to test. Aggression was rated using a similar scale, where −3 = dramatically more aggressive, −2 = moderately more aggressive, −1 = mildly more aggressive, 0 = normal, 1 = mildly less aggressive, 2 = moderately less aggressive, 3 = dramatically less aggressive.
Drug administration
Guanfacine was generously provided by Shire Pharmaceuticals. Guanfacine was dissolved in saline to the appropriate dose. Rolipram (purchased from Sigma RBI) was dissolved in 0.2 mL 100% ethanol and 0.8 mL sterile saline and diluted with saline to the appropriate concentration, based on a previous study (Ramos et al. 2003). We first determined which dose of guanfacine was the most effective in enhancing working-memory performance in the monkeys that participated in the study. The effective dose of guanfacine varied between monkeys (0.0001–0.01 mg/kg; with the exception of one agitated older monkey who was most improved by 0.5 mg/kg). The rolipram dose also varied between monkeys (0.001–0.05 μg/kg). Please note that a fixed dose of both guanfacine and rolipram was used for each individual monkey; however, these doses varied between animals. Once an effective dose of guanfacine was found that reliably improved working-memory performance, the guanfacine vs. rolipram study was started. Drug solutions were made up fresh each day under sterile conditions. The drug treatments saline, guanfacine, rolipram, and guanfacine/rolipram were administered in random order with at least 1-wk washout between treatments, and the experimenter testing the animal was unaware of the treatment condition. A dose of rolipram that had no effect on its own was injected with or without guanfacine 2 h before testing. A washout period of at least 10 d occurred between drug treatments. Monkeys were required to return to stable baseline performance for two consecutive testing days prior to new drug treatment. Given these prolonged washout conditions, the research took ∼10 mo to complete. Note: no significant correlation was found for drug treatment vs. estimated age of monkeys (all r < 0.3) due to the fact that all monkeys used in the study were selected based on a pronounced response to guanfacine, and all monkeys were middle aged or older. However, a significant correlation between age and guanfacine efficacy would likely have been seen if younger monkeys (under 10 yr old) were included in the study as well (Arnsten 1999).
Data analysis
The data was analyzed using a two-way analysis of variance with repeated measures (2-ANOVA-R) with factors of systemic drug administration (vehicle, guanfacine or rolipram). Planned comparisons (user defined contrasts) were performed to examine whether (1) vehicle/saline significantly differed from guanfacine/saline; (2) vehicle/saline differed from rolipram/saline; (3) vehicle/saline differed from guanfacine/rolipram; (4) guanfacine/saline differed from guanfacine/rolipram. A detailed analysis of the effects of guanfacine at each delay interval was also performed on the data (2-ANOVA-R; within subjects factors of guanfacine and delay interval).
Acknowledgments
We are grateful to Tracy Sadlon, Lisa Ciavarella, Sam Johnson, and Jessica Thomas for their technical expertise in carrying out this research. This research was supported by MERIT Award AG06036 to A.F.T.A., by a Ford Foundation Predoctoral Fellowship to B.P.R., and by a research grant from Shire Pharmaceuticals. A.F.T.A. and Yale University have a license agreement with Shire Pharmaceuticals for the development of guanfacine for the treatment of PFC disorders.
Footnotes
Article published online before print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.298006
References
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Aoki C., Venkatesan C., Go C.-G., Forman R., Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb. Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn. Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Age-related cognitive deficits and neurotransmitters: The role of catecholamine mechanisms in prefrontal cortical cognitive decline. In: Peters A., Morrison J., editors. Cerebral cortex: Neurodegenerative and age-related changes in structure and function of cerebral cortex. Plenum Press; New York: 1999. pp. 89–110. [Google Scholar]
- Arnsten A.F.T., Cai J.X. Post-synaptic α-2 receptor stimulation improves working memory in aged monkeys: Indirect effects of yohimbine vs. direct effects of clonidine. Neurobiol. Aging. 1993;14:597–603. doi: 10.1016/0197-4580(93)90044-c. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T., Goldman-Rakic P.S. α-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T., Cai J.X., Goldman-Rakic P.S. The α-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects. J. Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T., Ramos B., Birnbaum S.B., Taylor J.R. Protein kinase A as a therapeutic target for memory disorders: Rationale and challenges. Trends Mol. Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Aujla H., Beninger R.J. Hippocampal-prefrontocortical circuits: PKA inhibition in the prefrontal cortex impairs delayed nonmatching in the radial maze in rats. Behav. Neurosci. 2001;115:1204–1211. [PubMed] [Google Scholar]
- Barros D.M., Izquierdo L.A., Mello e Souza T., Ardenghi P.G., Pereira P., Medina J.H., Izquierdo I. Molecular signaling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav. Brain Res. 2000;114:183–192. doi: 10.1016/s0166-4328(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Bernabeu R., Bevilaqua L., Ardenghi P., Bromberg E., Schmitz P., Bianchin M., Izquierdo I., Medina J.H. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R., Abel T., Berman N., Gordon R., Lapidus K., Kandel E. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn. Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Cai J.X., Ma Y., Xu L., Hu X. Reserpine impairs spatial working memory performance in monkeys: Reversal by the α-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- Carlson S., Tanila H., Rama P., Mecke E., Pertovaara A. Effects of medetomidine, an α-2 adrenoceptor agonist, and atipamezole, an α-2 antagonist, on spatial memory performance in adult and aged rats. Behav. Neural Biol. 1992;58:113–119. doi: 10.1016/0163-1047(92)90327-z. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Aghajanian G.K. Catecholamine receptors on locus coeruleus neurons: Pharmacological characterization. Eur. J. Pharmacol. 1977;44:375–385. doi: 10.1016/0014-2999(77)90312-0. [DOI] [PubMed] [Google Scholar]
- Coull J.T., Middleton H.C., Robbins T.W., Sahakian B.J. Contrasting effects of clonidine and diazepam on tests of working memory and planning. Psychopharmacology. 1995;120:311–321. doi: 10.1007/BF02311179. [DOI] [PubMed] [Google Scholar]
- Engberg G., Eriksson E. Effects of α-2-adrenoceptor agonists on locus coeruleus firing rate and brain noradrenaline turnover in EEDQ-treated rats. Naunyn Schmiedebergs Arch. Pharmacol. 1991;343:472–477. doi: 10.1007/BF00169548. [DOI] [PubMed] [Google Scholar]
- Franowicz J.S., Kessler L., Dailey-Borja C.M., Kobilka B.K., Limbird L.E., Arnsten A.F.T. Mutation of the α2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U., Huang Y.-Y., Kandel E.R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Hertting G., Wurster S., Allgaier C. Regulatory proteins in presynaptic function. Ann. N. Y. Acad. Sci. 1990;604:289–304. doi: 10.1111/j.1749-6632.1990.tb32001.x. [DOI] [PubMed] [Google Scholar]
- Huang Y.-Y., Kandel E.R. Post-synaptic induction of PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Huang Y.Y., Martin K.C., Kandel E.R. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J. Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R.D., Arnsten A.F.T., Asbell M.D. An open trial of guanfacine in the treatment of attention deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1995;34:50–54. doi: 10.1097/00004583-199501000-00013. [DOI] [PubMed] [Google Scholar]
- Jakala P., Riekkinen M., Sirvio J., Koivisto E., Kejonen K., Vanhanen M., Riekkinen P.J. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999a;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- Jakala P., Sirvio J., Riekkinen M., Koivisto E., Kejonen K., Vanhanen M., Riekkinen P.J. Guanfacine and clonidine, α-2 agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology. 1999b;20:119–130. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Li B.-M., Mei Z.-T. Delayed response deficit induced by local injection of the α-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- McEntee W.J., Mair R.G. The Korsakoff syndrome: A neurochemical perspective. Trends Neurosci. 1990;13:340–344. doi: 10.1016/0166-2236(90)90146-2. [DOI] [PubMed] [Google Scholar]
- Punch L.J., Self D.W., Nestler E.J., Taylor J.R. Opposite modulation of opiate withdrawal behaviors on microinfusion of protein kinase A inhibitor vs. activator into the locus coeruleus or periaqueductal gray. J. Neurosci. 1997;17:8520–8527. doi: 10.1523/JNEUROSCI.17-21-08520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P., Linnankoski I., Tanila H., Pertovaara A., Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol. Biochem. Behav. 1996;54:1–7. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Ramos B., Birnbaum S.B., Lindenmayer I., Newton S.S., Duman R., Arnsten A.F.T. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Rotenberg A., Abel T., Hawkins R.D., Kandel E.R., Muller R.U. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. J. Neurosci. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan J.D., Dash P.K. Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes. Learn. Mem. 2005;12:232–238. doi: 10.1101/lm.92405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L., Chappell P.B., Kim Y.S., Schultz R.T., Katsovich L., Shepherd E., Arnsten A.F.T., Cohen D.J., Leckman J.F. Guanfacine in the treatment of children with tic disorders and ADHD: A placebo-controlled study. Am. J. Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., LeDoux J.E. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-18-j0003.2000. RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.D. Activation of α-2 adrenergic receptors inhibits norepinephrine release by a pertussis toxin-insensitive pathway independent of changes in cytosolic calcium in cultured rat sympathetic neurons. J. Pharmacol. Exp. Ther. 1997;282:248–255. [PubMed] [Google Scholar]
- Seamans J.K., Floresco S.B., Phillips A.G. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanila H. Noradrenergic regulation of hippocampal place cells. Hippocampus. 2001;11:793–808. doi: 10.1002/hipo.1095. [DOI] [PubMed] [Google Scholar]
- Tanila H., Rama P., Carlson S. The effects of prefrontal intracortical microinjections of an α-2 agonist, α-2 antagonist and lidocaine on the delayed alternation performance of aged rats. Brain Res. Bull. 1996;40:117–119. doi: 10.1016/0361-9230(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Tanila H., Mustonen K., Sallinen J., Scheinin M., Riekkinen P. Role of α-2C-adrenoceptor subtype in spatial working memoryas revealed by mice with targeted disruption of the α-2C-adrenoceptor gene. Eur. J. Neurosci. 1999;11:599–603. doi: 10.1046/j.1460-9568.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- Taylor F.B., Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult Attention Deficit-Hyperactivity Disorder. J. Clin. Psychopharmcol. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- Taylor J.R., Birnbaum S.G., Ubriani R., Arnsten A.F.T. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. (Online) 1999;19 doi: 10.1523/JNEUROSCI.19-18-j0001.1999. RC23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Tsuda S., Nishio I. Role of α2-adrenergic receptors and cyclic adenosine monophosphate-dependent protein kinase in the regulation of norepinephrine release in the central nervous system of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2003;42:S81–S85. doi: 10.1097/00005344-200312001-00018. [DOI] [PubMed] [Google Scholar]
- U’Prichard D.C., Bechtel W.D., Rouot B.M., Snyder S.H. Multiple apparent α-noradrenergic receptor binding sites in rat brain: Effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]