Abstract
The anterior cingulate cortex (ACC) has been implicated in encoding whether or not an action is worth performing in view of the expected benefit and the cost of performing the action. Dopamine input to the ACC may be critical for this form of effort-based decision making; however, the role of distinct ACC dopamine receptors is yet unknown. Therefore, we examined in rats the effects of an intra-ACC D1 and D2 receptor blockade on effort-based decision making tested in a T-maze cost-benefit task. In this task, subjects could either choose to climb a barrier to obtain a high reward in one arm or a low reward in the other arm without a barrier. Unlike vehicle-treated rats, rats with intra-ACC infusion of the D1 receptor antagonist SCH23390 exhibited a reduced preference for the high-cost– high-reward response option when having the choice to obtain a low reward with little effort. In contrast, in rats with intra-ACC infusion of the D2 receptor antagonist eticlopride, the preference for the high-cost–high-reward response option was not altered relative to vehicle-treated rats. These data provide the first evidence that D1 receptors in the ACC regulate effort-based decision making.
In order to make adaptive decisions, subjects have to analyze costs and benefits of the available response options. A number of studies indicate that the anterior cingulate cortex (ACC), a major subregion of the prefrontal cortex, is involved in these evaluative processes and might serve to encode whether or not an action is worth performing in view of the expected benefit and the cost of performing the action (Rushworth et al. 2004). For instance, after excitotoxic ACC lesions, rats no longer selected the high-cost–high-reward option in a cost-benefit T-maze task if having the choice between climbing a barrier to obtain a large reward in one arm or to run for a low reward into the other arm with no barrier present (Walton et al. 2003).
There is a large body of evidence to suggest that mesolimbic dopamine (DA) fibers projecting to the nucleus accumbens are critical for enabling an organism to overcome response costs to gain access to greater reward (Salamone et al. 1997). Recent studies indicate that mesocortical DA fibers projecting to the ACC (Berger et al. 1991) may be important in effort-based decision making as well. Like rats with excitotoxic lesions, rats with DA depletion of the ACC no longer chose effortful but high-reward action in a T-maze cost-benefit task (Schweimer et al. 2005). However, using the same task Walton et al. (2005) reported that DA depletion of the ACC had no effect on effort-based decision making. As Schweimer et al. (2005) infused a markedly higher dose of the catecholaminergic neurotoxin 6-hydroxydopamine into the ACC than Walton et al. (2005), different magnitudes of DA depletions may largely account for the discrepant results. Together, these two studies suggest that a substantial loss of DA in the ACC may be necessary to impair effort-based decision making.
Prefrontal DA plays an essential role in cognitive processes and regulates aspects of working memory and attention through actions on D1 receptors (e.g., Granon et al. 2000; Seamans and Yang 2004) or set-shifting through actions on both D1 and D2 receptors (Ragozzino 2002; Floresco et al. 2006). However, the role of D1 and D2 receptors of the ACC in effort-based decision making is yet unknown.
Here we examined the effects of a selective intra-ACC blockade of D1 and D2 receptors on response selection in a cost-benefit T-maze task that is sensitive to ACC dysfunction in the rat (Walton et al. 2003; Schweimer and Hauber 2005; Schweimer et al. 2005). In this task, subjects could either choose to climb a barrier (30 cm) to obtain a high reward (four pellets) in one arm or a low reward (two pellets) in the other arm without a barrier.
Results
Histology
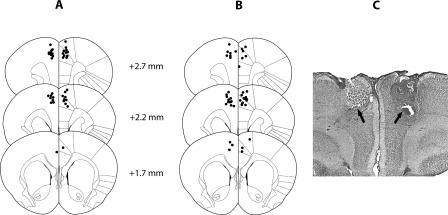
The location of the injection cannulae tips within the ACC from animals in Experiments 1 and 2 is shown in Figure 1.
Figure 1.
Cannulae placements in the ACC. The schematics depict the location of the injection cannulae tips (●) in the ACC for rats in Experiment 1 (A) and Experiment 2 (B). Plates are adaptations used with permission from Elsevier © 1986, Paxinos and Watson 1986. Numbers beside each plate correspond to millimeters anterior to bregma. (C) Nissl stain of a coronal section indicating the cannulae tip placements.
Experiment 1: Blockade of ACC D1 receptorsand effort-related decision making
Twenty-one animals were divided into two groups according to their preoperative performance in block A (Fig. 2). The groups were chosen so that there was no significant difference detectable before surgery (F < 1). In postoperative blocks B and C, one group received intra-ACC microinjections of SCH23390 (1 μg in 0.5 μL per side; n = 10), the other group received vehicle microinjections (0.5 μL per side, n = 11).
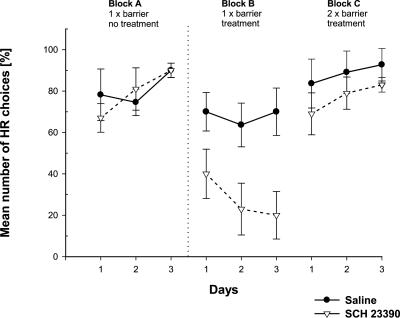
Figure 2.
Effects of an intra-ACC D1 receptor blockade on effort-based decision making in a T-maze cost-benefit task. Mean (±SEM) percentage of high-rewarded arm choices (HR) per day is given. Testing block A was preoperatively without treatment, blocks B and C post-operatively after pre-trial infusions of SCH23390 (1 μg in 0.5 μL per side; n = 10; ▿) or saline (0.5 μL; n = 11; ●) into the ACC. Each block consisted of three consecutive test days; on blocks A and B, a 30-cm barrier was placed in the HR; on block C, identical 30-cm barriers were placed into each goal arm, respectively.
In the preoperative testing block, all animals exhibited a strong preference to surmount the barrier to obtain the large reward with an average of more than 75% of the choices for the HR arm. This preference was reduced after intra-ACC microinjections of the D1 receptor antagonist SCH23390 if tested in the one-barrier condition (block B), but not if tested in the two-barrier condition (block C).
Repeated measures three-way ANOVA with two within-subject factors (blocks, three; days, three) and one between-subject factor (treatment, SCH23390 vs. saline) revealed no significant main effect of treatment (F(1,19) = 3.57; P = 0.07), but a significant main effect of testing blocks (F(1.94,36.92) = 34.90; P < 0.001). Furthermore, there was a treatment × block interaction (F(1.94,36.92) = 9.42; P < 0.001) and a block × day interaction (F(3.02,57.38) = 3.84; P < 0.05).
For additional analysis, blocks A and B were compared by a separate ANOVA. There was significant main effect of treatment (F(1,19) = 4.46; P < 0.05), blocks (F(1,19) = 46.83; P < 0.001) and a treatment × block interaction (F(1,19) = 16.94; P < 0.001), and a block × day interaction (F(1.79,33.93) = 5.81; P < 0.01). A comparison of blocks B and C revealed a significant main effect of treatment (F(1,19) = 5.92; P < 0.05), blocks (F(1,19) = 49.72; P < 0.001) as well as a treatment × block interaction (F(1,19) = 8.73; P < 0.01) and a block × day interaction (F(1.52,28.97) = 4.01; P < 0.05). A comparison of simple contrasts revealed a significant effect of treatment in block B (F(1,19) = 9.85, P < 0.01), but not in block C (F(1,19) = 1.14; P = 0.30).
Experiment 2: Blockade of ACC D2 receptorsand effort-related decision making
Twenty-two animals were divided into two groups according to their preoperative performance as shown in Figure 3. In the preoperative block A, no difference between groups was detectable (F < 1). Both groups preferred to climb the barrier to obtain the higher reward in more than 80% of the trials. When tested after recovery, one group received intra-ACC microinjections of the D2 receptor antagonist eticlopride (1 μg in 0.5 μL per side; n = 10), the other group vehicle infusions (0.5 μL per side, n = 12). This preference was not reduced after intra-ACC microinjections of the D2 receptor antagonist eticlopride, if tested in the one-barrier (block B) and two-barrier condition (block C).
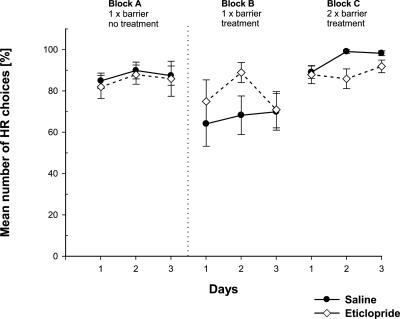
Figure 3.
Effects of an intra-ACC D2 receptor blockade on effort-based decision making in a T-maze cost-benefit task. Mean (±SEM) percentage of high-rewarded arm choices (HR) per day is given. Testing block A was preoperatively without treatment, blocks B and C postoperatively after pre-trial infusions of eticlopride (1 μg in 0.5 μL per side; n = 10; ◇) or saline (0.5 μL; n = 12; ●) into the ACC. Each block consisted of three consecutive test days; on blocks A and B, a 30-cm barrier was placed in the HR; on block C, identical 30-cm barriers were placed into each goal arm, respectively.
Repeated measures three-way ANOVA with two within-subject factors (blocks, three; days, three) and one between-subject factor (treatment, eticlopride vs. saline) demonstrated no significant main effect of treatment (F(1,20) = 0.013; n.s.), but a significant main effect of testing blocks (F(1.63,32.59) = 12.78; P < 0.001). In addition, there was a near-significant main effect for days (F(1.95,39.02) = 3.26; P = 0.0502).
A separate ANOVA to compare blocks A and B demonstrated a significant main effect of blocks (F(1,20) = 12.24; P < 0.01), but no block × treatment interaction (F(1.63,32.59) = 2.77, n.s.). Likewise, a comparison of blocks B and C revealed a significant main effect of blocks (F(1,20) = 17.03; P < 0.001). There was a trend for a significant treatment × block interaction (F(1,20) = 3.63; P = 0.07). A comparison of simple contrasts revealed no significant effect of treatment in block B (F(1,20) = 1.19, P = 0.29), but there was a significant effect in block C (F(1,20) = 4.52, P = 0.046). This latter effect was caused by a very high HR arm preference of vehicle-treated animals, rather than a reduced HR preference of eticlopride-treated animals.
Discussion
The results from the present experiments reveal that intra-ACC microinjection of the D1 receptor antagonist SCH23390 reduced the preference for the high-cost–high-reward response option when having the choice to obtain a low reward with little effort. SCH23390-induced effects cannot be attributed to changes in primary food motivation or appetite, to motor or spatial impairments, or to problems in reward magnitude discrimination, because after introduction of a barrier into the LR arm, SCH23390-treated rats selected the high-cost–high-reward option. These findings suggest that DA input to the ACC acting on D1 receptors is critical for effort-based decision making. In contrast, intra-ACC microinjection of the D2 antagonist eticlopride did not significantly alter the preference for the high-cost–high-reward response option in the one-barrier condition. Responding for high reward was enhanced on day 2 in the one-barrier condition; however, this is unlikely to reflect an effect of eticlopride. If treatment effects on effort-based decision making in this task occurred in previous studies, they were observed on all three days in the one-barrier condition (Walton et al. 2002, 2003; Schweimer and Hauber 2005, Schweimer et al. 2005).
The behavioral effects observed here are likely to be caused by drug actions within the ACC. Microinjections of radiolabeled SCH23390 into prefrontal subregions such as the prelimbic cortex exhibited relatively little spread into adjacent subregions (Granon et al. 2000). Also, a spread of drug rostrally into the prelimbic–infralimbic cortex would not affect behavior, as these prefrontal subregions do not play a role in the form of decision making examined here: Walton et al. (2003) demonstrated that excitotoxic lesions to the prelimbic–infralimbic cortex had no effect on rats’ ability to make effort-based decisions. In addition, SCH23390 (Bischoff et al. 1988) and eticlopride (Seeman and Ulpian 1988) are highly selective and potent antagonists at D1 or D2 receptors, respectively. Although SCH23390 displays affinity to 5-HT2 receptors as well (Bischoff et al. 1988), actions on 5-HT2 receptors might not account for the SCH23390-induced behavioral effects. A recent study revealed that a systemic blockade of 5-HT synthesis by para-chlorophenyl-alanine did not alter effort-based decision making as tested here (Denk et al. 2005). Furthermore, our data provide no evidence for reduced efficacy with repeated microinjections of SCH23390. Similarly, Baldwin and colleagues demonstrated that repeated daily prefrontal injections of SCH23390 impaired instrumental learning without loss of efficacy (Baldwin et al. 2002). The relative densities of prefrontal D1 and D2 receptors are different (Lidow et al. 1991; Goldman-Rakic et al. 1992; Gaspar et al. 1995; Sesack et al. 1995) and we cannot rule out that the doses of SCH23390 and eticlopride (1 μg, respectively) used here were not fully equipotent in behavioral terms. The doses of SCH23390 and eticlopride were based on pilot studies and data reported in the literature (Seamans et al. 1998; Ragozzino 2002; Sun and Rebec 2005; Floresco et al. 2006). For instance, this dose, but not a lower dose, of SCH23390 (Ragozzino 2002) or eticlopride (Floresco et al. 2006) microinjected into the medial prefrontal cortex impaired set-shifting of rats in a maze task. Thus, the failure of intra-ACC eticlopride to affect cost-benefit decision making is unlikely to reflect inadequate drug dosing.
ACC dopamine and effort-based decision making
Recent studies demonstrated that the ACC has a fundamental role in effort-based decision making (Walton et al. 2003; Schweimer and Hauber 2005), though not in all categories of decisions requiring an assessment of costs and benefits (Schweimer and Hauber 2005). As ACC DA depletion reduced the preference of rats for the high-cost–high-reward option in a similar manner as excitotoxic ACC lesions or nucleus accumbens DA depletion, we proposed that DA inputs to the ACC might be essential for effort-based decision making (Schweimer et al. 2005). In line with this notion, our present results reveal that effort-based decision making critically depends on the activity of ACC D1 receptors.
The cognitive processes regulated by ACC D1 receptors subserving response selection are difficult to assess. In the ACC, DA has been implicated to convey prediction error signals (Holroyd and Coles 2002) and to modulate the amplitude of reinforcement-related brain potentials generated in the ACC (Nieuwenhuis et al. 2004). Therefore, it is conceivable that a blockade of ACC D1 receptors could produce abnormal reinforcement learning signals, thereby compromising the sensitivity to benefits. However, such an effect should impair effort-related decisions both in the one- and two-barrier conditions, which has not been observed. Notably, rats with an intra-ACC D1 receptor blockade were not insensitive to costs and benefits. Similar to rats with excitotoxic ACC lesions (Walton et al. 2002, 2003; Schweimer and Hauber 2005) or 6-OHDA lesions (Schweimer et al. 2005), they seemed to operate according to a cost-benefit analysis in the two-barrier condition and preferred the high-cost–high-reward response option when having the choice to obtain a low reward with high costs. Therefore, the ability to evaluate and integrate the costs and benefits of response options appeared to be intact in rats with an intra-ACC D1 receptor blockade. Furthermore, as DA dysfunction can cause impulsive choice (e.g., Cole and Robbins 1989; Wade et al. 2000) one could argue that in the one-barrier condition an intra-ACC D1 receptor blockade increased the tendency to make impulsive choices. However, this explanation is not plausible. Though not explicitly measured here, the delay to obtain large reward due to surmounting the barrier is short (<5 sec), and delay discounting was not impaired by excitotoxic ACC lesions (Cardinal et al. 2002b).
Moreover, the ACC has been proposed to monitor conflict as a function of task difficulty (Botvinick et al. 2001, 2004). It is therefore possible that an intra-ACC D1 receptor blockade compromised conflict monitoring, which could, in part, account for impaired decision making. For instance, the relative values of response options should be less disparate and more difficult to discriminate in the one-barrier condition compared with the two-barrier condition. Thus, relative to the two-barrier condition, response selection in the one-barrier condition may be viewed as a situation in which conflict is more likely to arise in the form of competition among the available responses. Hence, the SCH23390-induced effects on decision making in the one-barrier condition could reflect disturbed conflict monitoring during performance of a more complex condition of the task. Taken together, though the exact contribution to effort-related decisions remains elusive, it is reasonable to propose that D1 receptors of the ACC regulate response selection based on the relative values associated with different actions.
Prefrontal DA neurotransmission not only plays a critical role in the form of decision making tested here, but also in working memory or behavioral flexibility (Robbins 2005). Notably, prefrontal D1 receptor activity is critically involved in mediating working memory (Sawaguchi and Goldman-Rakic 1991), while both D1 and D2 receptors act in a cooperative manner to facilitate behavioral flexibility (Ragozzino 2002; Floresco et al. 2006). Furthermore, prefrontal D1, D2, and D4 receptors contribute to a form of decision making that requires rats to direct instrumental responding to minimize conditioned punishment (Floresco and Magyar 2006), while our results suggest that effort-related decision making in a T-maze task requires prefrontal D1, not D2, receptor activity. Working memory, behavioral flexibility, and different forms of decision making may involve distinct cognitive functions as well as distinct neural circuits. Thus, mesocortical DA modulation of different types of cognitive and executive functions appears to be mediated by dissociable patterns of prefrontal DA receptor activity (for review, see Floresco and Magyar 2006).
Furthermore, the receptor mechanisms by which mesocortical and mesolimbic DA contribute to effort-based decision making could be distinct. Previous studies (Salamone et al. 1994) indicate that DA depletion of the nucleus accumbens caused similar impairments of effort-based decision making in a T-maze task as seen here. Nowend et al. (2001) further demonstrated that D1 and D2 receptors within the nucleus accumbens mediate the ability of rats to overcome work-related response costs in order to get food, while our results suggest that in the ACC DA activity only at D1, not at D2, receptors seem to be critical. However, a concurrent lever pressing/choice paradigm has been used by Nowend et al. (2001), and it’s questionable whether effort-based decisions in this task that require a decision between lever pressing for preferred food or consumption of less-preferred lab chow is mediated by the ACC (Schweimer and Hauber 2005). Thus, the specific accumbens DA receptor subtypes regulating the form of effort-based decision making examined here remain to be assessed.
Taken together, our present and previous findings (Schweimer et al. 2005) indicate the mesocortical DA projection to the ACC is critical for effort-based decision making as tested here, in addition to the mesolimbic DA projection to the nucleus accumbens as previously shown (Salamone et al. 1994). Recent studies elegantly demonstrate that response selection depending on a cost-benefit analysis requires serial transfer of information between basolateral amygdala and ACC (Floresco and Ghods-Sharifi 2006). In view of the connectivity of basolateral amygdala, ACC, and nucleus accumbens (e.g., Cardinal et al. 2002a), it is tempting to speculate that the ACC integrates reward-related information from the BLA and, perhaps, other sources to select a particular response in view of the relative values of the available options and to mediate response execution through downstream projections to the nucleus accumbens. This circuit regulating effort-based decision making is modulated by DA input to the ACC acting on D1 receptors and by DA input to the nucleus accumbens.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River) were used, weighing between 200 and 300 g at the time of surgery. They were housed in groups of four to six animals in a 12-h light/12-h dark cycle (lights on at 7.00 a.m.) with ad libitum access to water; food was restricted to 15 g per animal and day (standard maintenance chow; Altromin). Temperature (20 ± 2°C) and humidity (50 ± 10%) were kept constant in the animal house. Experiments were performed according to the German Law on Animal Protection and approved by the proper authorities in Stuttgart, Germany.
Surgery
For stereotaxic surgery, animals were anesthetized with a mixture of ketamine (80 mg/kg i.m.; Bela-Pharm) and xylazine HCl (9 mg/kg i.m.; Bayer) and secured in a stereotaxic apparatus with atraumatic ear bars (David Kopf Instruments). Bilateral stainless-steel guide cannulae (0.8 mm outer diameter) aimed at the Cg1 and Cg2 regions of the ACC were implanted by standard stereotaxic procedures. The coordinates with reference to the atlas of Paxinos and Watson (1986) were: 2.2 mm anterior to bregma, ±1.6 mm lateral and 2.5 mm ventral to bregma with the guide cannulae positioned in an angle of 20° from the midline (tooth bar 3.3 mm below the interaural line). The guide cannulae were occluded by stainless-steel stylets. Animals were allowed to recover for at least 7 d.
Apparatus
A T-maze task involving effort-based decision making was used (Salamone et al. 1994; Walton et al. 2002). The elevated T-maze consisted of a start arm and two goal arms (each 17-cm wide and 70-cm long) made of laminated wood; the walls were 30-cm high. A food well was placed at the end of each goal arm. On “forced” trials, a solid block was used to prevent entering one goal arm. The barriers that the animals had to surmount were made of wire mesh in the shape of a three-dimensional right-angled triangle. The rats had to climb the vertical side of the triangle and descend down the slope to attain the reward. The height of the barriers was increased during training from 15 cm at the beginning to a final height of 30 cm (final steepness ∼50°).
Training and experimental procedure
The experimental procedure corresponds to the schedule described by Walton et al. (2002). One training or test session was given per day throughout the experiment. In brief, after habituation to the maze on the first day, animals learned to discriminate a high-rewarded goal arm (HR) containing four food pellets (45 mg dustless precision pellets, Bioserv) from a low-rewarded goal arm (LR) containing two food pellets. For one-half of the group the HR was on the right, for the other half on the left. Animals were trained until choosing the HR in ≥80% of trials (i.e., 5 d), before a 15-cm barrier was introduced into the HR. After 3 d, animals selected the HR in the majority of trials (≥70%). Then, the height of the barrier was increased to 20 cm and, thereafter, to 25 cm for 3 d, respectively. On the first two trials, the animals were forced to run in opposite directions, followed by 10 choice trials each day. After a total of 12 training days, the experiment consisting of three testing blocks of three consecutive testing days each was started. During the pre-treatment block (block A) as well as the first treatment block (block B), there was a 30-cm barrier in the HR only (one-barrier condition). During the second treatment block (block C), identical 30-cm barriers were present in each goal arm (two-barrier condition) to assess whether the infusions of the drugs caused any motor or spatial deficits, or an inability to remember the reward size in the goal arms. Stereotaxic surgery was done between block A and block B.
Drug treatment
Schweimer et al. (2005) observed that intra-ACC DA depletion caused impairments in effort-based decision making in the one-barrier condition, which were least on the first testing day and became markedly stronger on the subsequent two testing days. To capture such within-block treatment effects, animals received drug or vehicle microinfusions prior to each of three testing days in the one-barrier condition (block B) and the two-barrier condition (block C) as well. In Experiment 1, animals received six bilateral intra-ACC microinjections of the D1 receptor antagonist SCH-23390 (Research Biochemicals International); in Experiment 2, six bilateral intra-ACC microinjections of the D2 receptor antagonist eticlopride hydrochloride (Sigma-Aldrich). Microinfusions were given prior to each testing day. Drugs were dissolved in physiological saline (0.9%) and injected at a dose of 1 μg in 0.5 μL. Control subjects received saline injections of 0.5 μL.
All injections were delivered through injection cannulae (0.45 mm outer diameter; Braun) over a 50-sec interval; injection cannulae were left in position for a further minute to allow for diffusion. Injection cannulae protruded 1 mm beyond the guide cannulae. After injection, each animal remained in its home cage for an additional 5 min before being placed in the T-maze.
Histology
After completion of the experiment, animals were euthanized with isoflurane (IsoFlo, Abbott) so that the brains could be removed for histological verification of correct cannulae placements. Brains were removed and placed in 10% formalin saline overnight and stored in 30% sucrose. Coronal brain sections (50 μm) were collected. Every second section was mounted on coated slides, stained with cresyl violet, and coverslipped using DePeX (Serva).
Animals were only included if their cannulae tip placements deviated less than ∼0.5 mm from target coordinates in the ACC. Four animals (Experiment 1: one vehicle-, one SCH23390-treated rat; Experiment 2: two eticlopride-treated rats) were excluded from analysis as cannulae tips were misplaced in the anterior direction, i.e., within the rostral ACC/prelimbic cortex about +3.2 mm relative to bregma.
Data analysis and statistics
Choices of the HR arm on each testing day were counted and given as percentage means ±SEM. The data were subjected to repeated measures analysis of variances (ANOVA) with two within-subject factors (blocks of testing, testing days) and one between-subject factor (treatment, saline vs. SCH23390 or eticlopride). The results given were calculated using a Greenhouse-Geisser correction to compensate for problems caused by violations of the sphericity assumption when using an F-test. All statistical computations were carried out with STATISTIC (version 7.1, StatSoft, Inc.). The level of statistical significance (α-level) was set at P < 0.05.
Acknowledgments
We thank P. Rommel for help with animal training and A. Murschall and S. Sommer for assistance with intracranial microinfusions. This research was supported by the Deutsche Forschungsgemeinschaft (Ha2340/5-1).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.409306
References
- Baldwin A.E., Sadeghian K., Kelley A.E. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J. Neurosci. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B., Gaspar P., Verney C. Dopaminergic innervation of the cerebral cortex: Unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Bischoff S., Heinrich M., Krauss J., Sills M.A., Williams M., Vassout A. Interaction of the D1 receptor antagonist SCH 23390 with the central 5-HT system: Radioligand binding studies, measurements of biochemical parameters and effects on L-5-HTP syndrome. J. Recept. Res. 1988;8:107–120. doi: 10.3109/10799898809048981. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002a;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Lachenal G., Halkerston K.M., Rudarakanchana N., Hall J., Morrison C.H., Howes S.R., Robbins T.W., Everitt B.J. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav. Neurosci. 2002b;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Cole B.J., Robbins T.W. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: Implications for theories of selective attention and arousal. Behav. Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Denk F., Walton M.E., Jennings K.A., Sharp T., Rushworth M.F., Bannerman D.M. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Floresco S.B., Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb. Cortex. 2006 doi: 10.1093/cercor/bhj143. doi:10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco S.B., Magyar O. Mesocortical dopamine modulation of executive functions: Beyond working memory. Psychopharmacology (Berl). 2006 doi: 10.1007/s00213-006-0404-5. doi:10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco S.B., Magyar O., Ghods-Sharifi S., Vexelman C., Tse M.T. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Gaspar P., Bloch B., Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: Cellular localization in different classes of efferent neurons. Eur. J. Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Lidow M.S., Smiley J.F., Williams M.S. The anatomy of dopamine in monkey and human prefrontal cortex. J. Neural Transm. Gen. Sect. 1992;36:163. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Granon S., Passetti F., Thomas K.L., Dalley J.W., Everitt B.J., Robbins T.W. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J. Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Lidow M.S., Goldman-Rakic P.S., Gallager D.W., Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Holroyd C.B., Mol N., Coles M.G. Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neurosci. Biobehav. Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nowend K.L., Arizzi M., Carlson B.B., Salamone J.D. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol. Biochem. Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, New York: 1986. [Google Scholar]
- Ragozzino M.E. The effects of dopamine D(1) receptor blockade in the prelimbic-infralimbic areas on behavioral flexibility. Learn. Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. J. Comp. Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Walton M.E., Kennerley S.W., Bannerman D.M. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Salamone J.D., Cousins M.S., Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav. Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone J.D., Cousins M.S., Snyder B.J. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neurosci. Biobehav. Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schweimer J., Hauber W. Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn. Mem. 2005;12:334–342. doi: 10.1101/lm.90605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J., Saft S., Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav. Neurosci. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans J.K., Floresco S.B., Phillips A.G. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Ulpian C. Dopamine D1 and D2 receptor selectivities of agonists and antagonists. Adv. Exp. Med. Biol. 1988;235:55–63. doi: 10.1007/978-1-4899-2723-1_5. [DOI] [PubMed] [Google Scholar]
- Sesack S.R., Snyder C.L., Lewis D.A. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J. Comp. Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Sun W., Rebec G.V. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Wade T.R., de Wit H., Richards J.B. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology. 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Walton M.E., Bannerman D.M., Rushworth M.F. The role of rat medial frontal cortex in effort-based decision making. J. Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M.E., Bannerman D.M., Alterescu K., Rushworth M.F. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J. Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M.E., Croxson P.L., Rushworth M.F., Bannerman D.M. The mesocortical dopamine projection to anterior cingulate cortex plays no role in guiding effort-related decisions. Behav. Neurosci. 2005;119:323–328. doi: 10.1037/0735-7044.119.1.323. [DOI] [PubMed] [Google Scholar]