Abstract
Fetal and infant rats can learn to avoid odors paired with illness before development of brain areas supporting this learning in adults, suggesting an alternate learning circuit. Here we begin to document the transition from the infant to adult neural circuit underlying odor-malaise avoidance learning using LiCl (0.3 M; 1% of body weight, ip) and a 30-min peppermint-odor exposure. Conditioning groups included: Paired odor-LiCl, Paired odor-LiCl-Nursing, LiCl, and odor-saline. Results showed that Paired LiCl-odor conditioning induced a learned odor aversion in postnatal day (PN) 7, 12, and 23 pups. Odor-LiCl Paired Nursing induced a learned odor preference in PN7 and PN12 pups but blocked learning in PN23 pups. 14C 2-deoxyglucose (2-DG) autoradiography indicated enhanced olfactory bulb activity in PN7 and PN12 pups with odor preference and avoidance learning. The odor aversion in weanling aged (PN23) pups resulted in enhanced amygdala activity in Paired odor-LiCl pups, but not if they were nursing. Thus, the neural circuit supporting malaise-induced aversions changes over development, indicating that similar infant and adult-learned behaviors may have distinct neural circuits.
Animals rapidly learn to avoid tastes, smells, and textures of foods associated with malaise, and this learning process produces conditioned taste and odor aversions (Garcia et al. 1966, 1974; Batsell and Best 1992, 1993). This learning has been demonstrated early in development, including both the embryonic day (ED) 18 rat and mouse fetuses, with retention time lasting weeks (Hennessy et al. 1976; Haroutunian and Campbell 1979; Smotherman 1982; Stickrod et al. 1982b; Rudy and Cheatle 1983; Smotherman and Robinson 1985, 1990; Alleva and Calamandrei 1986; Coopersmith et al. 1986; Molina et al. 1986; Hoffmann et al. 1987, 1990; Miller et al. 1990; Hunt et al. 1991, 1993; Abate et al. 2001; Richardson and McNally 2003; Gruest et al. 2004a). Taste/odor aversion acquisition shows some specific differences between pups and adults. First, nursing during acquisition disturbs taste-aversion learning, although this diminishes as pups approach weanling (Martin and Alberts 1979; Gubernick and Alberts 1984; Melcer et al. 1985; Kehoe and Blass 1986). Second, in contrast to the long temporal parameters permitted between the odor/taste (conditioned stimulus, CS) and illness (unconditioned stimulus, US) in adult taste aversion, the temporal constraints between the CS and the malaise producing US for pup learning are very limited (Rudy and Cheatle 1983; Kraemer et al. 1988, 1989; Hoffmann et al. 1990, 1991).
There is evidence that the neural basis of odor/taste aversion learning may change over development. In adults, the amygdala is thought to support taste-aversion learning, though there are discrepancies in the literature (Nachman and Ashe 1974; Burt and Smotherman 1980; Dunn and Everitt 1988; Yamamoto and Fujimoto 1991; Kesner et al. 1992; Yamamoto et al. 1994; Bures et al. 1998; Schafe et al. 1998; Sakai and Yamamoto 1999; Wilkins and Bernstein 2006). When the taste and smell components of the complex flavors in taste aversion are separated and just the olfactory components are assessed, the amygdala appears to have a more consistent role in learning, especially the basolateral complex of the amygdala (Bermudez-Rattoni et al. 1986; Touzani and Sclafani 2005). The delayed and protracted development of the amygdala suggests its participation may be minimal in early life learning (Berdel et al. 1997; Morys et al. 1999; Berdel and Morys 2000). Olfactory information is received by the amygdala in the early neonatal period (Schwob et al. 1986; Wilson et al. 2004), although the amygdala’s connections with other brain areas occur later in development (Nair and Gonzalez-Lima 1999; Bouwmeester et al. 2002a; Cunningham et al. 2002). Although brain areas may presumably become incorporated into different behavioral circuits at different ages, at least in odor-0.5mA shock fear conditioning, the amygdala does not appear to be incorporated into the learning until around PN10 (Sullivan et al. 2000; Roth and Sullivan 2005; Moriceau and Sullivan 2006; Moriceau et al. 2006). The developmental changes in pharmacological attenuation/potentiation of taste aversion also suggest that the underlying neural circuitry supporting taste/odor aversion may change (glutamate, Mickley et al. 2001) (opioids, Stickrod et al. 1982a). However, the molecular cascade of intracellular event associated with learning and memory appears rather consistent between pups and adults for both taste/odor aversion learning and odor learning in general (McLean et al. 1999; Gruest et al. 2004b).
With this study, we assessed the behavioral and neural development of odor-malaise learning using LiCl, which induces illness in young pups (Haroutunian and Campbell 1979; Spear and Rudy 1991). The ability of nursing to disrupt odor-LiCl conditioning was also explored. We used rats from an age of complete dependence on the mother through an age of emerging independence at weanling. We focused on two brain areas, the amygdala, which is implicated in adult odor-malaise learning (Bermudez-Rattoni et al. 1986; Batsell and Blankenship 2002; Holland and Gallagher 2004; Pickens et al. 2005; Touzani and Sclafani 2005) and the olfactory bulb, which has previously been implicated in infant odor aversion and preference learning (Coopersmith and Leon 1984, 1986; Coopersmith et al. 1986; Woo and Leon, 1987; Wilson and Leon 1988; Sullivan et al. 1989, 2000; Wilson and Sullivan 1990, 1991, 1992; Sullivan and Wilson 1991; McLean et al. 1999; Okutani et al. 1999; Yuan et al. 2002; Moriceau and Sullivan 2004).
Results
Behavior
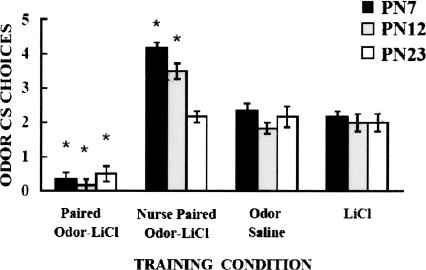
Odor in the presence of LiCl illness supports odor-aversion learning throughout development (Fig. 1), and these results replicate previous work on odor-malaise learning in pups (Hennessy et al. 1976; Haroutunian and Campbell 1979; Smotherman 1982; Stickrod et al. 1982b; Rudy and Cheatle 1983; Smotherman and Robinson 1985, 1990; Alleva and Calamandrei 1986; Coopersmith et al. 1986; Molina et al. 1986; Miller et al. 1990; Abate et al. 2001; Richardson and McNally 2003). Similar to previous work, nursing modifies odor-LiCl-induced aversion at all ages tested and produces an odor preference in pups 7–8- and 12–13-d old, but blocks the aversion learning in PN23–PN24 pups (Martin and Alberts 1979; Gubernick and Alberts 1984; Melcer et al. 1985).
Figure 1.
Mean number of choices toward conditioned odor in the Y-maze test following Paired odor-LiCl, Nursing Paired odor-LiCl and control odor-saline and LiCl groups by age. Asterisks represent significant differences (P < 0.05); bars represent standard error.
Figure 1 depicts performance in the Y-maze test and indicates that Paired odor-LiCl pups without the presence of a mother learned an odor aversion at all ages, and nursing pups learned an odor preference at the two youngest ages. No learning was seen in controls. ANOVA revealed a main effect of training condition (F(3,60) = 95.333, P < 0.0001), and a significant interaction between training condition and age (F(6,60) = 5.983, P < 0.0001). Post hoc Fisher tests for each age showed that each of the paired groups were significantly different from each of the control groups, except for the PN23–PN24 Paired nursing pups that did not differ from controls.
Olfactory bulb
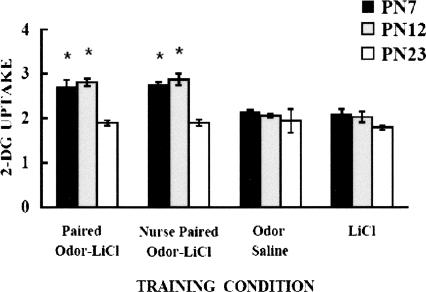
Odor-LiCl induces olfactory bulb learning changes in the two younger ages regardless of whether an odor preference or aversion was learned (Fig. 2) (Sullivan and Wilson 1991; Wilson et al. 1991). No olfactory bulb learning-induced changes were detected in weanling (PN23–PN24) aged pups, which replicates earlier work with LiCl on weanling rats (Coopersmith et al. 1986).
Figure 2.
Mean relative olfactory bulb 2-DG uptake during odor-LiCl conditioning by age. Asterisks represent significant differences (P < 0.05); bars represent standard error.
Figure 2 depicts olfactory bulb 2-DG uptake in PN7–PN8, PN12–PN13, and PN23–PN24 pups, and indicates that odor-LiCl conditioning induced enhanced 2DG uptake in the olfactory bulb of pups learning either an odor aversion (Paired) or an odor preference (Paired LiCl nursing pups) at the two younger age groups. No olfactory bulb changes were detected in controls or the older PN23–PN24 pups. ANOVA revealed a main effect of training condition (F(3,60) = 32.313, P < 0.0001), a main effect of age (F(2,60) = 56.436, P < 0.0001), and a significant interaction between training condition and age (F(6,60) = 7.344, P < 0.0001). Post hoc Fisher tests for the PN7–PN8 and PN12–PN13 showed that each of the paired groups were significantly different from each of the control groups.
Amygdala
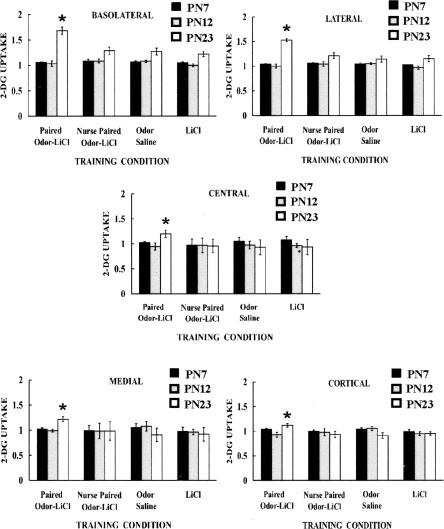
Odor-LiCl learning induced significant changes in the amygdala in weanling pups (Fig. 3). Figure 3 depicts odor-LiCl amygdala 2-DG uptake and indicates that odor-LiCl conditioning induced enhanced 2DG uptake in the amygdala of weanling aged rats (PN23–PN24) but not in the younger ages. PN23 pups receiving Paired odor-LiCl while nursing (failed to learn) showed amygdala activity similar to controls. No statistical differences were found for any amygdala nuclei at PN7–PN8 or PN12–PN13. Statistical analysis showed PN23–PN24 pups showed enhanced amygdala activity in the basolateral, lateral, cortical, medial, and central nuclei (Basolateral: ANOVA revealed a main effect of training condition [F(3,60) = 7.403, P < 0.0005], a main effect of age [F(2,60) = 62.151, P < 0.0001], and a significant interaction between training condition and age [F(6,60) = 7.990, P < 0.0001]; Lateral: ANOVA revealed a main effect of training condition [F(3,60) = 7.809, P < 0.0005], a main effect of age [F(2,60) = 46.900, P < 0.0001], and a significant interaction between training condition and age [F(6,60) = 7.862, P < 0.0001]; Cortical: ANOVA revealed a significant interaction between training condition and age [F(6,60) = 2.541, P < 0.05]; Medial: ANOVA revealed a main effect of training condition [F(3,60) = 4.707, P < 0.01] and a significant interaction between training condition and age [F(6,60) = 4.474, P < 0.0005]; Central: ANOVA revealed a main effect of training condition [F(3,60) = 3.094, P < 0.05] and a significant interaction between training condition and age [F(6,60) = 3.569, P < 0.005]). Post hoc Fisher tests showed the PN23–PN24 Paired odor-LiCl group was significantly different from each of the control groups and the Paired LiCl-odor nursing group for each amygdala nuclei.
Figure 3.
Mean relative amygdala nuclei 2-DG uptake during odor-LiCl conditioning by age. Asterisks represent significant differences (P < 0.05); bars represent standard error.
Discussion
Here we show odor-malaise aversion learning in rat pups throughout development, with the olfactory bulb supporting this learning in early life and switching to the amygdala in weaning-aged pups (PN23). Thus, the neural pathway supporting odor-malaise conditioning changes with development, suggesting that similar behaviors in development are not always supported by the same neural structures.
Olfactory bulb neural changes associated with infant rat odor preference and aversion learning
The olfactory bulb appears to code for odor learning, although it processes odors with positive and negative hedonic values similarly, acquired only during infancy and retained into adulthood (Coopersmith and Leon 1984, 1986; Coopersmith et al. 1986; Woo and Leon 1987; Wilson and Leon 1988; Sullivan et al. 1989, 2000; Wilson and Sullivan 1990, 1991, 1992; Sullivan and Wilson 1991; McLean et al. 1999; Okutani et al. 1999; Yuan et al. 2002; Moriceau and Sullivan 2004; Roth et al. 2006).
Piriform cortex may encode olfactory hedonic value
The olfactory bulb appears to process odors with positive and negative hedonic values similarly, suggesting that this information is encoded elsewhere. Our data with learned odors suggests that pre-weanling pups code the odor’s hedonic value in the piriform cortex, which is part of the olfactory cortex. Specifically, the anterior piriform is associated with odor preference and the posterior piriform is associated with aversion (Roth and Sullivan 2005; Moriceau et al. 2006; Roth et al. 2006). However, additional assessment of the piriform cortex’s role in pup olfactory valence is required.
In adults, the piriform codes for olfactory hedonic value, although valence is distributed throughout the olfactory pathway, as well as the amygdala, thalamus, prefrontal cortex, nucleus accumbens, and hippocampus (Price and Slotnick 1983; Staubli et al. 1984; Hamrick et al. 1993; Litaudon et al. 1997; Otto et al. 1997; Cousens and Otto 1998; Eichenbaum 1998; Wilson 1998, 2001, 2003; Saar et al. 1999; Ongur and Price 2000; Schoenbaum et al. 2000; Datiche et al. 2001; Linster and Hasselmo 2001; Mouly et al. 2001; Gottfried et al. 2002; Paschall and Davis 2002; Tronel and Sara 2002; Yuan et al. 2002; Cardinal et al. 2003; Holland and Gallagher 2004; Sevelinges et al. 2004; Wilson et al. 2004; de Araujo et al. 2005; Jones et al. 2005, 2006; Pickens et al. 2005). However, it is unclear whether pups have the same neural distribution of learned hedonic value as adults, since the brain is still undergoing considerable development in the age range assessed here. Specifically, while it is difficult to define an age at which a brain area becomes functional, especially since this age may vary based on the behavior assessed, some brain areas important in coding hedonic value may not be functional at all ages assessed here. For example, prefrontal cortex anatomical maturation occurs between PN8 and PN14, with amygdala connections increasing from PN7 to PN13 and transition to the “adult-like” bilaminar cellular organization by PN13–PN17 (Verwer et al. 1996; Bouwmeester et al. 2002a, b). At least based on extinction behavior, which involves the prefrontal cortex, this brain area emerges between PN12 and PN17 (Stanton et al. 1984; Morgan et al. 1993; Lilliquist et al. 1999; Nair and Gonzalez-Lima 1999; Nair et al. 2001; Milad and Quirk 2002). Hippocampus anatomical development is further delayed, and hippocampal-dependent learning emerges near weaning (Matthews et al. 1974; Nadler et al. 1974; Baudry et al. 1981; Lobaugh et al. 1989; Muller et al. 1989; Bekenstein and Lothman 1991; Lilliquist et al. 1993; DiScenna and Teyler 1994; Rudy 1994; Rudy and Morledge 1994; Nair and Gonzalez-Lima 1999; Kudryashov and Kudryashova 2001; Nair et al. 2001). Thus, based on anatomical maturation of brain areas associated with coding hedonic value, the anterior and posterior piriform may indeed code hedonic value in early life.
Amygdala neural changes associated with weanling rat odor aversion learning
The involvement of the amygdala has been implicated in odor/taste aversion conditioning (Nachman and Ashe 1974; Burt and Smotherman 1980; Bermudez-Rattoni et al. 1986; Dunn and Everitt 1988; Yamamoto and Fujimoto 1991;Kesner et al. 1992; Yamamoto et al. 1994; Schafe et al. 1998; Sakai and Yamamoto 1999; Bahar et al. 2004; Holland and Gallagher 2004; Touzani and Sclafani 2005; Wilkins and Bernstein 2006). The delayed incorporation of the amygdala into the malaise learning circuit is not unexpected. Amygdala development is protracted, with peak neurogenesis occurring during the first week of life (Berdel et al. 1997; Morys et al. 1999; Berdel and Morys 2000) and minimal connections with other brain areas (Nair and Gonzalez-Lima 1999; Bouwmeester et al. 2002a, b; Cunningham et al. 2002). It should also be noted that the amygdala output appears to have sequential development, such that components of the fear response become incorporated sequentially into behavior as pups mature (Richardson et al. 1995; Hunt and Campbell 1997; Hunt et al. 1997).
Amygdala in fear and malaise learning
Our data combined with the development of olfactory fear conditioning suggests that the amygdala becomes incorporated into different learning circuits at different ages. Compared with fear conditioning with odor-0.5mA shock, which incorporates the amygdala into the learning circuit by PN12, odor-LiCl amygdala involvement is delayed. Immaturity of LiCl afferents to the amygdala may contribute to this age difference in amygdala participation in learning, although other factors may also contribute, such as hormonal or neurotransmitter system development. To our knowledge, these anatomical development issues have not been explored. However, participation of the amygdala in infant fear conditioning appears dependent on levels of corticosterone (Moriceau and Sullivan 2006; Moriceau et al. 2006). Specifically, 0.5 mA fear conditioning can be induced to emerge precociously by 2 d if systemic or intra-amygdala corticosterone is provided during conditioning, or delayed by depleting pups’ corticosterone or delivering an intra-amygdala corticosterone receptor antagonist (Moriceau et al. 2006). Blocking opioids during consolidation can also precociously induce the amygdala to participate in fear conditioning (Roth et al. 2006). A similar process involving a hormonal or neurotransmitter system may be important for the inclusion of the amygdala in odor-malaise conditioning. LiCl does activate the hypothalamic-pituitary-adrenal axis (Sugawara et al. 1988; Spencer et al. 2005), which has been implicated in both adult and infant malaise conditioning (Hennessy et al. 1976; Smotherman et al. 1976; Smotherman 1985; Kent et al. 2002; Tenk et al. 2006). However, corticosterone does not appear necessary for odor/taste aversion (Smotherman et al. 1980; Smotherman 1985), although this requires further exploration.
Unique infant constraints on malaise learning
Although odor-malaise learning is robust, even in the fetus, nursing during conditioning can dramatically change this aversion learning to preference learning, or at least block learning (see Fig. 1) (Gubernick and Alberts 1984; Melcer et al. 1985). The mechanism for this is unclear, although its presumed significance is to transmit food odors/tastes from the mother’s diet to pups to enhance later food selection and consumption of presumably safe foods (Bilko et al. 1994; Galef Jr.1997; Mennella et al. 2001).
Other infant rat learning constraints have been shown suggesting that pups have an attenuated ability to learn odor aversions and inhibitory responses, thus preventing pups from learning to avoid/inhibit responses to the maternal odor. Indeed, this early malaise odor-avoidance learning is in sharp contrast to the attenuated olfactory fear/avoidance/inhibitory conditioning, as well as lack of fear expression to predator odors seen in neonatal rat pups (Collier and Mast 1979; Haroutunian and Campbell 1979; Blozovski and Cudennec 1980; Stehouwer and Campbell 1980; Bialik et al. 1984; Misanin et al. 1985; Camp and Rudy 1988; Takahashi 1992; Myslivecek 1997; Sullivan et al. 2000; Wiedenmayer and Barr 2001; Roth and Sullivan 2003, 2005; Moriceau and Sullivan 2004, 2005, 2006; Moriceau et al. 2004, 2006). The emergence of these learning abilities and fear to predator odor is coincident with the emergence of amygdala function.
Additional learning constraints become apparent when the mother is present. Specifically, maternal presence can dampen pups’ response to pain induced by shock as indicated by a decreased behavioral response (Richardson et al. 1989). Pain delivered while nursing increased latency to escape, which was not altered by opioids (Blass et al. 1995). Nursing or simple maternal presence dampens the release of the stress hormone corticosterone (Stanton et al. 1987; Moriceau and Sullivan 2006). The mother’s ability to block stress induced corticosterone release results in a block of fear conditioning (odor-0.5mA shock) and produces an odor preference in PN15. This maternal blockade of fear conditioning can be overridden with an intra-amygdala infusion of CORT (Moriceau and Sullivan 2006). The ability of social bonding related experiences to change an aversive stimulus to a preferred stimulus appears unusual, but is not uncommon (for review, see Insel and Young 2001). For example, sheep and rats show aversions to their offspring, which is overridden for expression of maternal behavior (Fleming et al. 1999; Keller et al. 2005).
Summary and implications
These data, along with previous research, suggest that the functional integration or dissociation of brain areas into a learning neural circuit can underlie maturational changes in learning. These data illustrate that the infant is not an immature version of the adult but has been molded through evolutionary pressures to be uniquely adapted for its particular stage of development (Rovee-Collier 1997; Hofer and Sullivan 2001).
Materials and Methods
Subjects
We used male and female pups born of Long-Evans rats (Harlan) in our animal vivarium. Pup ages were PN7–PN8 (pups are mostly confined to the nest), PN12–PN13 (pups are venturing outside the nest, but still require the mother for survival), and PN23–PN24 (weanlings, pups were still living with the mother). Mothers were housed in polypropylene cages with an abundant amount of aspen wood shavings for nest building, and kept in a 20°C environment with a 12-h light/12-h dark cycle. Food and water were available ad libitum. Litters were culled to 10–12 males and females on PN1 or PN2, with PN0 designated as the day of birth. The University of Oklahoma Institutional Animal Care and Use Committee, which follows guidelines from the National Institutes of Health, approved all animal care and experimental procedures.
LiCl conditioning
Training occurred in 27°C (weanling) to 30°C (infants) ± 0.1°C environment with temperature at the start of training maintained throughout conditioning. Pups were placed in the training chamber and given 10 min to recover from experimental handling. Malaise was confirmed by observing pups for defecation number, consistency, and color during and after (1 h) conditioning. Pups were returned to the nest until testing the next day.
The training chambers were (45.5 l × 30.5 w ×45 h cm) opaque Plexiglas boxes. Pups were randomly assigned to one of the following conditions: (1) Paired without mother and (2) Paired with mother. Both paired groups had LiCl (0.3 M) of 1% of body weight injected 5 min after the start of the 30-min odor presentation. (3) LiCl only: injected with LiCl 5 min after being placed in the training box. (4) Odor only: exposed to odor and injected with isotonic saline (1% body weight) 5 min after the start of the odor. The 30-min odor was presented on a Kim-wipe (25 μL of McCormick Pure Peppermint with alcohol removed just before use) and placed beneath the metal mesh floor. A new odor Kim wipe was replaced halfway through conditioning. Pups were injected with LiCl 5 min after the start of the odor. The mother (nonbiological) present during conditioning was anesthetized (1.5–3 mL urethane; 0.25 g/mL ip), which also prevents milk ejections. The mother was placed in the training box on her side to provide pups access to her nipples for nursing. To ensure nipple attachment, pups from all training conditions (including those not in the nursing training condition) were separated from the mother for 2–4 h before the experiment. All paired odor-LiCl nursing pups nursed during conditioning.
2-DG autoradiography
Half of the pups in each training condition were used to assess the neural circuitry associated with learning. Pups were injected with 14C 2-deoxyglucose (2-DG; 20 μCi/100g, sc) 5 min prior to conditioning; 45 min after the injection, pups were decapitated and their brains quickly removed, frozen in 2-methylbutane (−45°C), and stored in a −70°C freezer. For analysis, brains were sectioned (20 μm) in a −20°C cryostat, with every second section placed on a coverslip and exposed for 5 d along with standards (Carbon 14 standards 10 × 0.02 mCi, American Radiolabeled Chemicals, Inc.) to X-ray film (DiRocco and Hall 1981; Coopersmith and Leon 1986; Sullivan and Wilson 1995). The olfactory bulb does not require staining since anatomical landmarks are clearly visible with 2-DG, with 2-DG uptake in the peppermint specific area of the glomerular layer expressed relative to the bulb’s periventricular core (Greer et al. 1981). Specific amygdala nuclei were identified by counterstaining sections with cresyl violet and by making a template of that brain area for use with the autoradiographs. The 2-DG uptake was expressed relative to 2-DG uptake in the corpus callosum (which did not vary with conditioning group) to control for differences in section thickness and exposure (Sullivan et al. 2000). Brain areas examined were the basolateral, lateral, cortical, medial, and central amygdaloid nuclei.
Y-maze testing for odor aversion and preference
The Y-maze testing was conducted the day following training to assess odor learning, with each arm containing the conditioned odor vs. familiar clean-nest shavings. The Y-maze consisted of a start box (7 × 9 cm) separated by removable doors from the two alleys (22 × 9 cm) that extended at 45° angles. One arm contained the aspen wood odor used in the nest as bedding (20 mL of clean, aspen shavings in a petri dish), while the other arm contained the peppermint odor (25 μL of peppermint extract on a kimwipe placed in a ventilation hood for 5 min). Each pup was placed in the start box and given 5 sec before the alley doors were removed. Each subject had 60 sec to make a choice, which required the pup to enter one of the alleys. All pups tested made a choice within 60 sec. Each subject was given five sequential trials, and the floor was cleaned between each trial. Pup orientation was counterbalanced between trials. Observations of each pup were made blind to the training condition.
Acknowledgments
This work was funded by grants NICHD-HD33402, NSF IOB-0544406 and Oklahoma Center for Science and Technology OCAST to R.M.S. and NRSA DA06082 to T.L.R.
Footnotes
Article published online before print. Article and publication data are at http://www.learnmem.org/cgi/doi/10.1101/lm.316006
References
- Abate P., Spear N.E., Molina J.C. Fetal and infantile alcohol-mediated associated learning in the rat. Alcohol. Clin. Exp. Res. 2001;25:989–998. [PubMed] [Google Scholar]
- Alleva E., Calamandrei G. Odor-aversion learning and retention span in neonatal mouse pups. Behav. Neural Biol. 1986;46:348–357. doi: 10.1016/s0163-1047(86)90317-1. [DOI] [PubMed] [Google Scholar]
- Bahar A., Dorfman N., Dudai Y. Amygdala circuits required for either consolidation or extinction of taste aversion memory are not required for reconsolidation. Eur. J. Neurosci. 2004;19:1115–1118. doi: 10.1111/j.0953-816x.2004.03215.x. [DOI] [PubMed] [Google Scholar]
- Batsell W.R., Best M.R. Variations in the retention interval of taste aversions: Evidence for retrieval competition. Anim. Learn. Behav. 1992;20:146–159. [Google Scholar]
- Batsell W.R., Best M.R. One bottle too many? Method of testing determines the detection of overshadowing and retention of taste aversions. Learn. Behav. 1993;21:154–158. [Google Scholar]
- Batsell W.R., Blankenship A.G. Beyond potentiation: Synergistic conditioning in flavor-aversion learning. Brain Mind. 2002;3:383–408. [Google Scholar]
- Baudry M., Arst D., Oliver M., Lynch G. Development of glutamate binding sites and their regulation by calcium in rat hippocampus. Brain Res. 1981;227:37–48. doi: 10.1016/0165-3806(81)90092-4. [DOI] [PubMed] [Google Scholar]
- Bekenstein J.W., Lothman E.W. A comparison of the ontogeny of excitatory and inhibitory neurotransmission in the CA1 region and dentate gyrus of the rat hippocampal formation. Brain Res. Dev. Brain Res. 1991;63:237–243. doi: 10.1016/0165-3806(91)90083-u. [DOI] [PubMed] [Google Scholar]
- Berdel B., Morys J. Expression of calbindin-D28k and parvalbumin during development of rat’s basolateral amygdaloid complex. Int. J. Dev. Neurosci. 2000;18:501–513. doi: 10.1016/s0736-5748(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Berdel B., Morys J., Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int. J. Dev. Neurosci. 1997;15:755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F., Grijalva C.V., Kiefer S.W., Garcia J. Flavor-illness aversions: The role of the amygdala in the acquisition of taste-potentiated odor aversions. Physiol. Behav. 1986;38:503–508. doi: 10.1016/0031-9384(86)90417-8. [DOI] [PubMed] [Google Scholar]
- Bialik R.J., Pappas B.A., Roberts D.C. Deficits in conditioned avoidance responding following adrenalectomy and central norepinephrine depletion are dependent on postsurgical recovery period and phase of the diurnal cycle. Behav. Neurosci. 1984;98:847–857. doi: 10.1037//0735-7044.98.5.847. [DOI] [PubMed] [Google Scholar]
- Bilko A., Altbacker V., Hudson R. Transmission of food preference in the rabbit: The means of information transfer. Physiol. Behav. 1994;56:907–912. doi: 10.1016/0031-9384(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Blass E.M., Shide D.J., Zaw-Mon C., Sorrentino J. Mother as shield: Differential effects of contact and nursing on pain responsivity in infant rats—Evidence for nonopioid mediation. Behav. Neurosci. 1995;99:521–530. doi: 10.1037//0735-7044.109.2.342. [DOI] [PubMed] [Google Scholar]
- Blozovski D., Cudennec A. Passive avoidence learning in the young rat. Dev. Psychobiol. 1980;13:513–518. doi: 10.1002/dev.420130510. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Wolterink G., Van Ree J.M. Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J. Comp. Neurol. 2002a;450:239–249. doi: 10.1002/cne.10084. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Smits K., Van Ree J.M. Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J. Comp. Neurol. 2002b;450:241–255. doi: 10.1002/cne.10321. [DOI] [PubMed] [Google Scholar]
- Bures J., Fenton A.A., Kaminsky Y., Wesierska M., Zahalka A. Rodent navigation after dissociation of the allocentric and idiothetic representations of space. Neuropharmacology. 1998;37:689–699. doi: 10.1016/s0028-3908(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Burt G.S., Smotherman W.P. Amygdalectomy induced deficits in conditioned taste aversion: Possible pituitary-adrenal involvement. Physiol. Behav. 1980;24:651–655. doi: 10.1016/0031-9384(80)90391-1. [DOI] [PubMed] [Google Scholar]
- Camp L.L., Rudy J.W. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev. Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N., Parkinson J.A., Hall J., Everitt B.J. The contribution of the amygdala, nucleus accumbens and prefrontal cortex to emotion and motivated behavior. Int. Congr. Ser. 2003;1250:347–370. [Google Scholar]
- Collier A.C., Mast J. Alleviation of avoidance deficits by approach alternatives in 10-day old rats. Physiol. Behav. 1979;23:615–618. doi: 10.1016/0031-9384(79)90068-4. [DOI] [PubMed] [Google Scholar]
- Coopersmith R., Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith R., Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Res. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Coopersmith R., Lee S., Leon M. Olfactory bulb responses after odor aversion learning by young rats. Brain Res. 1986;389:271–277. doi: 10.1016/0165-3806(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Cousens G., Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav. Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Datiche F., Roullet F., Cattarelli M. Expression of Fos in the piriform cortex after acquisition of olfactory learning: An immunohistochemical study in the rat. Brain Res. Bull. 2001;55:95–99. doi: 10.1016/s0361-9230(01)00499-3. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E., Rolls E.T., Velazco M.I., Margot C., Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- DiRocco R.J., Hall W.G. Metabolic neural mapping in neonatal rats. J. Neurosci. Res. 1981;6:13–19. doi: 10.1002/jnr.490060103. [DOI] [PubMed] [Google Scholar]
- DiScenna P.G., Teyler T.J. Development of inhibitory and excitatory synaptic transmission in the rat dentate gyrus. Hippocampus. 1994;4:569–576. doi: 10.1002/hipo.450040506. [DOI] [PubMed] [Google Scholar]
- Dunn L.T., Everitt B.J. Double dissociations of the effects of amygdala and insular cortex lesions on conditioned taste aversion, passive avoidance, and neophobia in the rat using the excitotoxin ibotenic acid. Behav. Neurosci. 1988;102:3–23. doi: 10.1037//0735-7044.102.1.3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Using olfaction to study memory. Ann. N. Y. Acad. Sci. 1998;855:657–669. doi: 10.1111/j.1749-6632.1998.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Fleming A.S., O’Day D., Kraemer G. Neurobiology of mother–infant interaction experience and central nervous system plasticity across development. Neurosci. Biobehav. Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Galef B.G., Jr. Learning under the influence. Nat. Hist. 1997;106:47–49. [Google Scholar]
- Garcia J., Ervin F.R., Koelling R.A. Learning with prolonged delay of reinforcement. Psychon. Sci. 1966;5:121–122. [Google Scholar]
- Garcia J.D., Yang M.G., Wang J.H., Belo P.S. Translocation and fluxes of mercury in neonatal and maternal rats treated with methyl mercuric chloride during gestation. Proc. Soc. Exp. Biol. Med. 1974;147:224–231. doi: 10.3181/00379727-147-38315. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A., O’Doherty J., Dolan R.J. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J. Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C.A., Stewart W.B., Kauer J.S., Shepherd G.M. Topographical and laminar localization of 2-deoxyglucose uptake in rat olfactory bulb induced by electrical stimulation of olfactory nerves. Brain Res. 1981;217:279–293. doi: 10.1016/0006-8993(81)90004-4. [DOI] [PubMed] [Google Scholar]
- Gruest N., Richer P., Hars B. Emergence of long-term memory for conditioned aversion in the rat fetus. Dev. Psychobiol. 2004a;44:189–198. doi: 10.1002/dev.20004. [DOI] [PubMed] [Google Scholar]
- Gruest N., Richer P., Hars B. Memory consolidation and reconsolidation in the rat pup require protein synthesis. J. Neurosci. 2004b;24:10488–10492. doi: 10.1523/JNEUROSCI.2984-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick D.J., Alberts J.R. A specialization of taste aversion learning during suckling and its weaning-associated transformation. Dev. Psychobiol. 1984;17:613–628. doi: 10.1002/dev.420170605. [DOI] [PubMed] [Google Scholar]
- Hamrick W.D., Wilson D.A., Sullivan R.M. Neural correlates of memory for odor detection conditioning in adult rats. Neurosci. Lett. 1993;163:36–40. doi: 10.1016/0304-3940(93)90223-8. [DOI] [PubMed] [Google Scholar]
- Haroutunian V., Campbell B.A. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Hennessy J.W., Smotherman W.P., Levine S. Conditioned taste aversion and the pituitary-adrenal system. Behav. Biol. 1976;16:413–424. doi: 10.1016/s0091-6773(76)91571-6. [DOI] [PubMed] [Google Scholar]
- Hofer M.A., Sullivan R.M. 2001. Toward a neurobiology of attachment. In Handbook of developmental cognitive neuroscience (eds. C.A. Nelson and M. Luciana), pp. 599–616. MIT; Cambridge, MA [Google Scholar]
- Hoffmann H., Molina J.C., Kucharski D., Spear N.E. Further examination of ontogenetic limitations on conditioned taste aversion. Dev. Psychobiol. 1987;20:455–463. doi: 10.1002/dev.420200409. [DOI] [PubMed] [Google Scholar]
- Hoffmann H., Hunt P., Spear N.E. Ontogenetic differences in the association of gustatory and tactile cues with lithium chloride and footshock. Behav. Neural Biol. 1990;53:441–450. doi: 10.1016/0163-1047(90)90324-y. [DOI] [PubMed] [Google Scholar]
- Hoffmann H., Hunt P., Spear N.E. Ontogenetic differences in CS palatability following conditioned taste aversion. Learn. Motiv. 1991;22:329–352. [Google Scholar]
- Holland P.C., Gallagher M. Amygdala–frontal interactions and reward expectancy. Curr. Opin. Neurobiol. 2004;14:148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hunt P.S., Campbell B.A. Autonomic and behavioral correlates of appetitive conditioning in rats. Behav. Neurosci. 1997;111:494–502. doi: 10.1037//0735-7044.111.3.494. [DOI] [PubMed] [Google Scholar]
- Hunt P.S., Spear L.P., Spear N.E. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav. Neurosci. 1991;105:971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Hunt P.S., Molina J.C., Rajachandran L., Spear L.P., Spear N.E. Chronic administration of alcohol in the developing rat: Expression of functional tolerance and alcohol olfactory aversions. Behav. Neural Biol. 1993;59:87–99. doi: 10.1016/0163-1047(93)90795-j. [DOI] [PubMed] [Google Scholar]
- Hunt P.S., Richardson R., Hess M.F., Campbell B.A. Emergence of conditioned cardiac responses to an olfactory CS paired with an acoustic startle UCS during development: Form and autonomic origins. Dev. Psychobiol. 1997;30:151–163. [PubMed] [Google Scholar]
- Insel T.R., Young L.J. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Jones S.V., Heldt S.A., Davis M., Ressler K.J. Olfactory-mediated fear conditioning in mice: Simultaneous measurements of fear-potentiated startle and freezing. Behav. Neurosci. 2005;119:329–335. doi: 10.1037/0735-7044.119.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.V., Rattiner L.S., Ressler K.J. Functional anatomy of synaptic plasticity mediating olfactory learning. The Association for Chemoreception Sciences (ACHemS). Sarasota; FL: 2006. [Google Scholar]
- Kehoe P., Blass E.M. Conditioned aversions and their memories in 5-day-old rats during suckling. J. Exp. Psychol. Anim. Behav. Process. 1986;12:40–47. [PubMed] [Google Scholar]
- Keller M., Meurisse M., Levy F. Mapping of brain networks involved in consolidation of lamb recognition memory. Neuroscience. 2005;133:359–369. doi: 10.1016/j.neuroscience.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Kent W.D., Cross-Mellor S.K., Kavaliers M., Ossenkopp K.P. Acute effects of corticosterone on LiCl-induced rapid gustatory conditioning in rats: A microstructural analysis of licking patterns. Behav. Brain Res. 2002;136:143–150. doi: 10.1016/s0166-4328(02)00105-5. [DOI] [PubMed] [Google Scholar]
- Kesner R.P., Berman R.F., Tardif R. Place and taste aversion learning: Role of basal forebrain, parietal cortex, and amygdala. Brain Res. Bull. 1992;29:345–353. doi: 10.1016/0361-9230(92)90066-7. [DOI] [PubMed] [Google Scholar]
- Kraemer P.J., Lariviere N., Spear N.E. Expression of a taste aversion conditioned with an odor-taste compound: Overshadowing is relatively weak in weanlings and decreases over a retention interval in adults. Anim. Learn. Behav. 1988;16:185–190. [Google Scholar]
- Kraemer P.J., Kraemer E.L., Smoller D.E., Spear N.E. Enhancement of flavor aversion conditioning in weanling but not adult rats by prior conditioning to an odor. Psychobiology. 1989;17:34–42. [Google Scholar]
- Kudryashov I.E., Kudryashova I.V. Ontogeny of synaptic transmission in the rat hippocampus. Brain Res. 2001;892:263–268. doi: 10.1016/s0006-8993(00)03157-7. [DOI] [PubMed] [Google Scholar]
- Lilliquist M.W., Burkhalter E.C., Lobaugh N.J., Amsel A. Age-dependent effects of hippocampal muscarinic receptor blockade on memory-based learning in the developing rat. Behav. Brain Res. 1993;53:119–125. doi: 10.1016/s0166-4328(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Lilliquist M.W., Nair H.P., Gonzalez-Lima F., Amsel A. Extinction after regular and irregular reward schedules in the infant rat: Influence of age and training duration. Dev. Psychobiol. 1999;34:57–70. [PubMed] [Google Scholar]
- Linster C., Hasselmo M.H. Neuromodulation and the functional dynamics of piriform Cortex. Chem. Senses. 2001;26:585–594. doi: 10.1093/chemse/26.5.585. [DOI] [PubMed] [Google Scholar]
- Litaudon P., Mouly A., Sullivan R., Gervais R., Cattarelli M. Learning -induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur. J. Neurosci. 1997;9:1593–1602. doi: 10.1111/j.1460-9568.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Lobaugh N.J., Greene P.L., Grant M., Nick T., Amsel A. Patterned (single) alternation in infant rats after combined or separate lesions of hippocampus and amygdala. Behav. Neurosci. 1989;103:1159–1167. doi: 10.1037//0735-7044.103.6.1159. [DOI] [PubMed] [Google Scholar]
- Martin L.T., Alberts J.R. Taste aversions to mother’s milk: The age-related role of nursing in acquisition and expression of a learned association. J. Comp. Physiol. Psychol. 1979;93:430–445. doi: 10.1037/h0077568. [DOI] [PubMed] [Google Scholar]
- Matthews D.A., Nadler J.V., Lynch G.S., Cotman C.W. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev. Biol. 1974;36:130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- McLean J.H., Harley C.W., Darby-King A., Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn. Mem. 1999;6:608–618. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcer T., Alberts J.R., Gubernick D.J. Early weaning does not accelerate the expression of nursing-related taste aversions. Dev. Psychobiol. 1985;18:375–381. doi: 10.1002/dev.420180503. [DOI] [PubMed] [Google Scholar]
- Mennella J.A., Coren P., Jagnow C.P., Beauchamp G.K. Prenatal and postnatal flavor learning by human infants. Pediatrics. 2001;107:88–93. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley G.A., Remmers-Roeber D.R., Dengler C.M., Kenmuir C.L., Crouse C. Paradoxical effects of ketamine on the memory of fetuses of different ages. Brain Res. Dev. Brain Res. 2001;127:71–76. doi: 10.1016/s0165-3806(01)00119-5. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller J.S., Kelly K.S., Neisewander J.L., McCoy D.F., Bardo M.T. Conditioning of morphine-induced taste aversion and analgesia. Psychopharmacology. 1990;101:472–480. doi: 10.1007/BF02244224. [DOI] [PubMed] [Google Scholar]
- Misanin J.R., Turns L.E., Hinderliter C.F. Acquisition and retention of active avoidance behavior in previsual rats. Am. J. Psychol. 1985;98:485–501. [PubMed] [Google Scholar]
- Molina J.C., Hoffmann H., Spear N.E. Conditioning of aversion to alcohol orosensory cues in 5- and 10-day rats: Subsequent reduction in alcohol ingestion. Dev. Psychobiol. 1986;19:175–183. doi: 10.1002/dev.420190304. [DOI] [PubMed] [Google Scholar]
- Morgan M.A., Romanski L.M., LeDoux J.E. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav. Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Neurobiology of infant attachment. Dev. Psychobiol. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S , Sullivan R M . Nature Neurosci. 2006. Maternal presence serves as a switch between learning fear and attraction in infancy. doi:10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Roth T.L., Okotoghaide T., Sullivan R.M. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 2004;22:415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Corticosterone serves as a switch between love and hate in infancy: Dual circuitry for odor-shock conditioning during development. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morys J., Berdel B., Kowianski P., Majak K., Tarnawski M., Wisniewski H.M. Relationship of calcium-binding protein containing neurons and projection neurons in the rat basolateral amygdala. Neurosci. Lett. 1999;259:91–94. doi: 10.1016/s0304-3940(98)00908-2. [DOI] [PubMed] [Google Scholar]
- Mouly A.M., Fort A., Ben-Boutayab N., Gervais R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience. 2001;102:11–21. doi: 10.1016/s0306-4522(00)00476-0. [DOI] [PubMed] [Google Scholar]
- Muller W., Altermatt H.J., Arnold W. Immunoreactive somatostatin in the peripheral olfactory system and nasal mucosa of the human. Laryngorhinootologie. 1989;68:29–32. doi: 10.1055/s-2007-998280. [DOI] [PubMed] [Google Scholar]
- Myslivecek J. Inhibitory learning and memory in newborn rats. Prog. Neurobiol. 1997;53:399–430. doi: 10.1016/s0301-0082(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Nachman M., Ashe J.H. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J. Comp. Physiol. Psychol. 1974;87:622–643. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- Nadler J.V., Matthews D.A., Cotman C.W., Lynch G.S. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev. Biol. 1974;36:130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- Nair H.P., Gonzalez-Lima F. Extinction of behavior in infant rats: Development of functional coupling between septal, hippocampal, and ventral tegmental regions. J. Neurosci. 1999;19:8646–8655. doi: 10.1523/JNEUROSCI.19-19-08646.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H.P., Berndt J.D., Barrett D., Gonzalez-Lima F. Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Res. 2001;903:141–153. doi: 10.1016/s0006-8993(01)02469-6. [DOI] [PubMed] [Google Scholar]
- Okutani F., Yagi F., Kaba H. Gabaergic control of olfactory learning in young rats. Neuroscience. 1999;93:1297–1300. doi: 10.1016/s0306-4522(99)00224-9. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Otto T., Cousens G., Rajewski K. Odor-guided fear conditioning in rats: 1. Acquisition, retention, and latent inhibition. Behav. Neurosci. 1997;111:1257–1264. [PubMed] [Google Scholar]
- Paschall G.Y., Davis M. Olfactory-mediated fear-potentiated startle. Behav. Neurosci. 2002;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- Pickens C.L., Saddoris M.P., Gallagher M., Holland P.C. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav. Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Slotnick B.M. Dual olfactory representation in the rat thalamus: An anatomical and electrophysiological study. J. Comp. Neurol. 1983;215:63–77. doi: 10.1002/cne.902150106. [DOI] [PubMed] [Google Scholar]
- Richardson R., McNally G.P. Effects of an odor paired with illness on startle, freezing, and analgesia in rats. Physiol. Behav. 2003;78:213–219. doi: 10.1016/s0031-9384(02)00974-5. [DOI] [PubMed] [Google Scholar]
- Richardson R., Siegel M.A., Campbell B.A. Effect of maternal presence on the cardiac and behavioral responses to shock in rats as a function of age. Dev. Psychobiol. 1989;22:567–583. doi: 10.1002/dev.420220604. [DOI] [PubMed] [Google Scholar]
- Richardson R., Wang P., Campbell B.A. Delayed development of conditioned heart rate responses to auditory stimuli in the rat. Dev. Psychobiol. 1995;28:221–238. doi: 10.1002/dev.420280404. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol. Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Memory of early maltreatment:neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Moriceau S., Sullivan R.M. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn. Mem. 2006;13:590–598. doi: 10.1101/lm.301206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee-Collier C. Dissociations in infant memory: Rethinking the development of implicit and explicit memory. Psychol. Rev. 1997;104:467–498. doi: 10.1037/0033-295x.104.3.467. [DOI] [PubMed] [Google Scholar]
- Rudy J.W. Ontogeny of context-specific latent inhibition of conditioned fear: Implications for configural associations theory and hippocampal formation development. Dev. Psychobiol. 1994;27:367–379. doi: 10.1002/dev.420270605. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Cheatle M.D. Odor-aversion learning by rats following LiCl exposure: Ontogenetic influences. Dev. Psychobiol. 1983;16:13–22. doi: 10.1002/dev.420160103. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Morledge P. The ontogeny of contextual fear conditioning: Implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Saar D., Grossman Y., Barkai E. Reduced synaptic facilitation between pyramidal neurons in the piriform cortex after odor learning. J. Neurosci. 1999;19:8616–8622. doi: 10.1523/JNEUROSCI.19-19-08616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Yamamoto T. Possible routes of visceral information in the rat brain in formation of conditioned taste aversion. Neurosci. Res. 1999;35:53–61. doi: 10.1016/s0168-0102(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., Thiele T.E., Bernstein I.L. Conditioning method dramatically alters the role of amygdala in taste aversion learning. Erratum in. Learn. Mem. Learn. Mem. 1998;1999;56:481–492. [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Chiba A.A., Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J. Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J.E., Farber N.B., Gottlieb D.I. Neurons of the olfactory epithelium in adult rats contain vimentin. J. Neurosci. 1986;6:208–217. doi: 10.1523/JNEUROSCI.06-01-00208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y., Gervais R., Messaoudi B., Granjon L., Mouly A.M. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn. Mem. 2004;11:761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman W.P. Odor aversion learning by the rat fetus. Physiol. Behav. 1982;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P. Glucocorticoid and other hormonal substrates of conditioned taste aversion. Ann. N. Y. Acad. Sci. 1985;443:126–144. doi: 10.1111/j.1749-6632.1985.tb27068.x. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Robinson S.R. The rat fetus in its environment: Behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behav. Neurosci. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Robinson S.R. The prenatal origins of behavioral organization. Psychol. Sci. 1990;1:97–106. [Google Scholar]
- Smotherman W.P., Hennessy J.W., Levine S. Plasma corticosterone levels as an index of the strength of illness induced taste aversions. Physiol. Behav. 1976;17:903–908. doi: 10.1016/0031-9384(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Margolis A., Levine S. Flavor preexposures in a conditioned taste aversion situation: A dissociation of behavioral and endocrine effects in rats. J. Comp. Physiol. Psychol. 1980;94:25–35. doi: 10.1037/h0077643. [DOI] [PubMed] [Google Scholar]
- Spear N.E., Rudy J. Tests of learning and memory in the developing rat. In: Shair H.N., Barr G.A., Hofer M.A., editors. Developmental psychobiology: Current methodological and conceptual issues. Oxford University Press; New York: 1991. pp. 84–113. [Google Scholar]
- Spencer C.M., Jahng J.W., Ryu V., Houpt T.A. Lithium-induced gene expression of inducible cyclic adenosine monophosphate early repressor in rat adrenal gland. J. Neurosci. Res. 2005;82:273–282. doi: 10.1002/jnr.20617. [DOI] [PubMed] [Google Scholar]
- Stanton M., Lobaugh N., Amsel A. Age of first appearance of simultaneous and successive negative contrast in infant rats. J. Exp. Psychol. Anim. Behav. Process. 1984;10:376–389. [PubMed] [Google Scholar]
- Stanton M.E., Wallstrom J., Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev. Psychobiol. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- Staubli U., Ivy G., Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc. Natl. Acad. Sci. 1984;81:5885–5887. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer D.J., Campbell B.A. Ontogeny of passive avoidance: Role of task demands and development of species-typical behaviors. Dev. Psychobiol. 1980;113:385–398. doi: 10.1002/dev.420130405. [DOI] [PubMed] [Google Scholar]
- Stickrod G., Kimble D.P., Smotherman W.P. Met-enkephalin effects on associations formed in utero. Peptides. 1982a;3:881–883. doi: 10.1016/0196-9781(82)90054-7. [DOI] [PubMed] [Google Scholar]
- Stickrod G., Kimble D.P., Smotherman W.P. In utero taste/odor aversion conditioning in the rat. Physiol. Behav. 1982b;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Sugawara M., Hashimoto K., Hattori T., Takao T., Suemaru S., Ota Z. Effects of lithium on the hypothalamo-pituitary-adrenal axis. Endocrinol. Jpn. 1988;35:655–663. doi: 10.1507/endocrj1954.35.655. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. Neural correlates of conditioned odor avoidance in preweanling rats. Behav. Neurosci. 1991;105:307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. Dissociation of behavioral and neural correlates of early associative learning. Dev. Psychobiol. 1995;28:213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A., Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J. Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Landers M., Yeaman B., Wilson D.A. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi L.K. Developmental expression of defensive responses during exposure to conspecific adults in preweanling rats (Rattus norvegicus) J. Comp. Psychol. 1992;106:69–77. doi: 10.1037/0735-7036.106.1.69. [DOI] [PubMed] [Google Scholar]
- Tenk C.M., Kavaliers M., Ossenkopp K.P. The effects of acute corticosterone on lithium chloride-induced conditioned place aversion and locomotor activity in rats. Life Sci. 2006;79:1069–1080. doi: 10.1016/j.lfs.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Touzani K., Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur. J. Neurosci. 2005;22:1767–1774. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- Tronel S., Sara S.J. Mapping of olfactory memory circuits: Region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn. Mem. 2002;9:105–111. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer R.W., Van Vulpen E.H., Van Uum J.F. Prefrontal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J. Comp. Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer C.P., Barr G.A. Developmental changes in responsivity to threat are stimulus-specific in rats. Dev. Psychobiol. 2001;39:1–7. doi: 10.1002/dev.1022. [DOI] [PubMed] [Google Scholar]
- Wilkins E.E., Bernstein I.L. Conditioning method determines patterns of c-fos expression following novel taste-illness pairing. Behav. Brain Res. 2006;169:93–97. doi: 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. Habituation of odor responses in the rat anterior piriform cortex. J. Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn. Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.A. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J. Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J. Neurophysiol. 1988;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M. Olfactory associative conditioning in infant rats with brain stimulation as reward. I. Neurobehavioral consequences. Brain Res. Dev. Brain Res. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M. Olfactory associative conditioning in infant rats with brain stimulation as reward: II. Norepinephrine mediates a specific component of the bulb response to reward. Behav. Neurosci. 1991;105:843–849. doi: 10.1037//0735-7044.105.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M. Blockade of mitral/tufted cell habituation to odors by association with reward: A preliminary note. Brain Res. 1992;594:143–145. doi: 10.1016/0006-8993(92)91039-h. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M., Leon M. A search for neural correlates of postnatal olfactory conditioning. In: Shair H.N., Barr G.A., Hofer M.A., editors. Developmental psychobiology: Current methodological and conceptual issues. Oxford University Press; New York: 1991. pp. 287–300. [Google Scholar]
- Wilson D.A., Best A.R., Sullivan R.M. Plasticity in the olfactory system: Lessons for the neurobiology of memory. Neuroscientist. 2004;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.C., Leon M. Sensitive period for neural and behavioral response development to learned odors. Brain Res. 1987;433:309–313. doi: 10.1016/0165-3806(87)90038-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Res. Bull. 1991;27:403–406. doi: 10.1016/0361-9230(91)90133-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Shimura T., Sako N., Yasoshima Y., Sakai N. Some critical factors involved in formation of conditioned taste aversion to sodium chloride in rats. Chem. Senses. 1994;19:209–217. doi: 10.1093/chemse/19.3.209. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Harley C.W., McLean J.H., Knöpfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J. Neurophysiol. 2002;87:3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]