Abstract
The Barnes maze is a spatial memory task that requires subjects to learn the position of a hole that can be used to escape the brightly lit, open surface of the maze. Two experiments assessed the relative importance of spatial (extra-maze) versus proximal visible cues in solving the maze. In Experiment 1, four groups of mice were trained either with or without a discrete visible cue marking the location of the escape hole, which was either in a fixed or variable location across trials. In Experiment 2, all mice were trained with the discrete visible cue marking the target hole location. Two groups were identical to the cued-target groups from Experiment 1, with either fixed or variable escape locations. For these mice, the discrete cue either was the sole predictor of the target location or was perfectly confounded with the spatial extra-maze cues. The third group also used a cued variable target, but a curtain was drawn around the maze to prevent the use of spatial cues to guide navigation. Probe trials with all escape holes blocked were conducted to dissociate the use of spatial and discrete proximal cues. We conclude that the Barnes maze can be solved efficiently using spatial, visual cue, or serial-search strategies. However, mice showed a strong preference for using the distal room cues, even when a discrete visible cue clearly marked the escape location. Importantly, these data show that the cued-target control version of the Barnes maze as typically conducted does not dissociate spatial from nonspatial abilities.
The Barnes maze (Barnes 1979) consists of a flat, circular disk with a number of holes around its perimeter that permit the subject to exit the maze into an escape box. The task relies on the innate preference of rodents for dark, enclosed spaces over open areas. Subjects are presumed to learn the location of an escape hole using spatial reference points that either are fixed in relation to the maze (extra-maze cues) or are on the maze itself fixed in relation to the escape hole (proximal cues). The Barnes maze has been used to assess spatial learning in mice (Fox et al. 1998; Paylor et al. 2001; Holmes et al. 2002; Koopmans et al. 2003; Seeger et al. 2004) as an alternative to the Morris water maze, the most commonly used test of spatial learning in rodents (Morris 1984; Frick et al. 2000). The Barnes maze does not involve swimming and is therefore considered to be less anxiogenic than the Morris water maze (Pompl et al. 1999; Miyakawa et al. 2001; Deacon and Rawlins 2002; Holmes et al. 2002). However, we know of no data specifically demonstrating this difference.
Barnes maze studies using a number of different methods support the notion of a spatial component involved in success on the maze (see Pompl et al. 1999; Koopmans et al. 2003). For example, mice tested with intra- and extra-maze cues present exhibited much better performance than did mice trained with no spatial cues present (Pompl et al. 1999). Moreover, correlations between hippocampal damage and performance provide support for the spatial nature of the Barnes maze task (Fox et al. 1998; Paylor et al. 2001; Deacon and Rawlins 2002; Raber et al. 2004). Nevertheless, both the Barnes maze and Morris water maze may also be influenced by a number of noncognitive factors such as anxiety, thigmotaxis, immobility, or exploratory activity (Wolfer et al. 1998; Miyakawa et al. 2001; Holmes et al. 2002; Bernardo et al. 2006; Reiserer et al. 2006). Such noncognitive factors are important to consider when interpreting the results of a spatial memory task.
The Morris water maze has been used in a large number of studies to assess spatial learning and memory in mutant mouse lines (see Lijam et al. 1997; Dumont et al. 2004; Wright et al. 2004; Bernardo et al. 2006). In contrast, the Barnes maze has not been used extensively with mice, and this, combined with the variety of methods and versions of the maze itself, makes it difficult to compare published results. Given the advantages of the Barnes maze described above, and that there is clearly a spatial component, this task could provide an excellent complement to the Morris water maze in a comprehensive test battery. Nevertheless, in Barnes maze experiments in which it appears that mice are using distal cues and spatial abilities to navigate the maze, they may in fact not be using spatial abilities or may be using a combination of spatial and nonspatial abilities. Although a visible platform version of the water maze is typically conducted to partial out spatial, hippocampally-mediated learning from nonspatial, hippocampal-independent processes, this dissociation has been shown to be less than perfect in mice. Specifically, inbred wild-type mice with excitotoxic or seizure-induced lesions of the hippocampus or entorhinal cortex are often impaired on both in the hidden- and visible-platform versions of the water maze (Hardman et al. 1997; Logue et al. 1997; Lipp and Wolfer 1998; Mohajeri et al. 2003, 2004; Lipp et al. 2004). It is not clear from these studies whether hippocampal insults impair the ability to make the association between the escape platform and the discrete cue, or whether mice attempt to use a spatial strategy to solve the cued-platform version and are unable because of the lesion (Lipp and Wolfer 1998; Hauben et al. 1999; Janus 2004). Some deficits following large lesions may result from damage to association areas within the hippocampal formation (Lipp and Wolfer 1998). It has also been suggested that rats swimming toward a cued platform in the water maze make use of both extra-maze cues and proximal target cues (Hamilton et al. 2004). A number of studies have used a cued-target version of the Barnes maze (Paylor et al. 2001; Zhang et al. 2002; Raber et al. 2004; Rizk et al. 2004; Reiserer et al. 2006). However, none of these studies specifically examined the extent to which spatial and nonspatial abilities contributed to the Barnes maze impairments.
We have reported previously that mice trained successively on both the cued- and hidden-target versions of the Barnes maze performed better in the second condition than the first, regardless of which condition was conducted first (Reiserer et al. 2006). This led us to question the importance of the hidden-target/spatial versus cued-target/nonspatial parameters, compared with a general knowledge of the context of the maze and the process of maze learning. In Experiment 1, four groups of mice were used, each of which had the opportunity to learn the context of the Barnes maze during the test sessions along with the rule that a single escape hole is located somewhere on the maze. The four conditions differed in the rules regarding the location of the escape hole (fixed or variable location) and whether or not the hole had a discrete visible cue marking it (cued or hidden target). We hypothesized that mice in cued-target groups would make fewer errors than would mice in hidden-target groups, and that mice for which the target was both fixed in location and marked with a visible proximal cue would commit the fewest errors. In Experiment 2, three groups of mice were trained on the cued-target version of the Barnes maze. In this experiment, the discrete proximal cue was perfectly confounded with distal spatial cues, was randomly moved to prevent reliable association with distal cues, or was made the sole predictive cue by drawing a featureless curtain around the maze. We expected that all mice would learn to solve the Barnes maze proficiently but that the curtain drawn around the maze would make the discrete cue more salient, and thus, this group would make the fewest errors.
Results
Experiment 1
Barnes maze acquisition
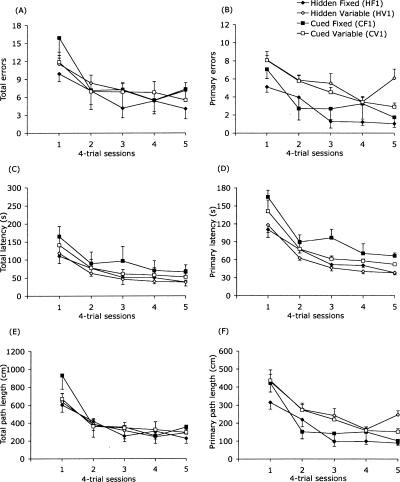
As shown in Figure 1, learning was apparent across all groups as measured by each of the six acquisition measures. Consistent with these observations, there was a significant main effect of session for each of the six variables (df = 4, 112; total errors: ε = 0.752, F = 25.14, adjusted P < 0.0001; primary errors: ε = 0.866, F = 26.10, adjusted P < 0.0001; total latency: ε = 0.633, F = 17.95, adjusted P < 0.0001; primary latency: ε = 0.495, F = 40.75, adjusted P < 0.0001; total path length: ε = 0.733, F = 25.15, adjusted P < 0.0001; primary path length: ε = 0.671, F = 50.48, adjusted P < 0.0001). There were no significant interactions involving the session factor (F(4,112) < 2.06; adjusted P > 0.60).
Figure 1.
Barnes maze acquisition in Experiment 1 (mean ± SEM of four trials per day). Performance improved significantly in all groups over the course of training. There were no significant group differences on the measures of total errors (A). In contrast, mice trained with a fixed target location (HF1 and CF1 groups) made significantly fewer primary errors (B) and had shorter primary path lengths (F) than did mice trained with a variable target location (HV1 and CV1 groups) during training trials. Experimental groups did not differ on total latency, primary latency, or total path length (C, D, E, respectively).
There was a significant effect of target location (fixed or variable) on both primary errors (F(1,28) = 14.93, adjusted P < 0.001) and primary path length, (F(1,28) = 14.48, adjusted P < 0.001), reflecting the fact that mice in the fixed-target groups made fewer primary errors and had shorter path lengths during acquisition (Fig. 1). There were no significant effects of target location on any of the other four acquisition variables (F(1,28) < 1.20; adjusted P > 0.95). In addition, there were no significant main effects of cue condition (hidden or cued) or cue condition × target location interactions on any of the six variables (F(1,28) < 2.30, adjusted P > 0.85), indicating that mice learned to find the hole equally well whether its location was predicted by distal extra-maze cues or a discrete proximal cue. Mice moved more quickly on the maze when the target location was fixed than when it varied across trials (F(1,28) = 5.41, P < 0.05) (data not shown). There was no effect of cue condition on moving speed, indicating that speeds were similar whether or not the hole location was identified with a discrete proximal cue (F(1,28) = 1.80, P = 0.191). The cue condition × target location interaction was not significant with respect to moving speed (F(1,28) = 0.48, P = 0.490).
Search strategy analysis
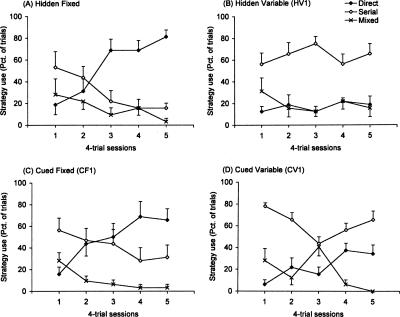
The low number of mixed/random trials in session 5 demonstrates that by the final day of training all mice had learned to locate the escape hole using either direct or serial-search strategies on the majority of trials (Fig. 2). At the beginning of training, all groups displayed similar search patterns; serial searches were used on a little more than half of the trials, and the remaining trials were evenly split between direct and mixed/random strategies. Over the course of training, the use of mixed/random strategies dropped to negligible levels in all groups. However, striking differences emerged among the groups with respect to the use of direct and serial-search strategies. Target location (fixed or variable) had the greatest effect on search strategy (target location × strategy: F(2,56) = 17.86, P < 0.001; target location × strategy × session: F(8,224) = 3.35, P < 0.001). Cue condition (hidden or cued) was also important in determining strategy use, albeit through its interaction with target location (cue condition × target location × strategy × session: F(8,224) = 2.11, P < 0.05). By the final acquisition session, both fixed-target groups displayed a preference for direct over serial searches (HF1: F(1,28) = 16.08, P < 0.001; CF1: F(1,28) = 4.41, P < 0.05) (Fig. 2A,C). Inversely, serial searches were used with greater frequency than direct searches by the variable-target groups (HV1: F(1,28) = 8.19, P = 0.01; CV1: F(1,28) = 3.64, P = 0.06) (Fig. 2B,D).
Figure 2.
Search strategies used during Barnes maze training in Experiment 1. Mice in all groups started training with a preference for using a serial search; direct searches on the first day of training were at chance levels. Over the course of training, mice trained with a fixed target location (HF1 and VF1 groups) completed more trials with direct than serial-search strategies (A, C). Inversely, mice trained with a variable target location (HV1 and CV1 groups) showed a clear preference for serial over direct search strategies (B, D). Data represent the mean (±SEM) percentage of trials on which each strategy type was used.
Probe trial data
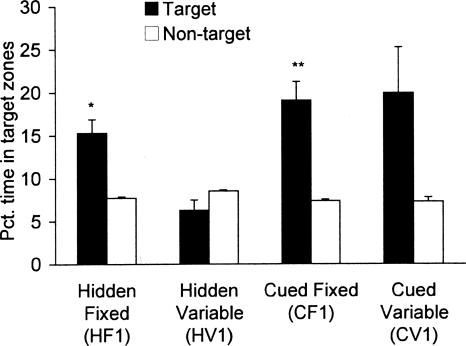
Selective search is a significant preference for the former location of the escape hole during the probe trial and indicates good memory for that location. On the probe trial, mice in the HF1 and CF1 groups showed selective search. (HF1: F(1,7) = 19.95, P < 0.01; CF1: F(1,7) = 25.03, P < 0.01) (Fig. 3). The CV1 group also spent more time at the target location than nontarget locations, although this difference was not significant (F(1,7) = 4.74, P = 0.066). As expected, the HV1 group did not show any preference for the target location (F(1,7) = 2.95, P = 0.13).
Figure 3.
Mean (±SEM) percentage of time spent in target and nontarget zones on the 5-min probe trial in Experiment 1. All groups except HV1 spent a greater proportion of time at the target location compared with the average time spent at other hole locations, demonstrating good memory for the location of the former escape hole. For the HV1 group, the escape hole was unmarked and varied randomly from trial to trial during training. Thus for this group, the target from the final training trial was assigned as the target hole for the probe trial and compared with the other 11 holes. Predictably, this group did not show a preference for any of the holes. Asterisks represent significant difference from mean of time spent at nontarget locations: *P < 0.05, **P < 0.01.
Discussion
The data from the current experiment highlight several points. First is that mice are able to solve the maze efficiently by using information inherent to the maze, i.e., that an escape box is located somewhere in the periphery under the surface of the maze. When the spatial room cues were made irrelevant, mice in the HV1 group were able to find the escape hole quickly by adopting a serial-search strategy. Second is that mice with fixed target locations tended to use a direct search strategy to find the escape hole, whereas mice in the variable target groups searched serially. Probe trial data confirmed that mice readily associated both distal room cues and the discrete proximal cue with the escape hole, when such cues were available. Third, in addition to learning about how mice perform under different cue and target position conditions, we observed that some measures in the Barnes maze are more informative than are others. Specifically, errors to the first encounter with the escape hole (primary errors) revealed group differences that total errors did not. Similarly, primary path length was better able to differentiate among experimental groups than was total path length. Neither of the latency measures differed across groups.
Perhaps the most unexpected observation from Experiment 1 is that mice in the CV1 group persisted in using a serial-search strategy despite the presence of a clearly-visible beacon marking the location of the escape hole (Fig. 2D). In contrast, the CF1 group showed a preference for direct escape paths, running straight to the same discrete cue on most trials (Fig. 2C). In fact, the performance of the CF1 group was more similar to that of the HF1 group (Fig. 2A) than to the CV1 group, suggesting that having a fixed target location was more useful than having a discrete cue, when learning how to solve the maze. This raises the possibility that mice in the CF1 group may have been using the static, distal room cues to navigate the maze instead of, or in addition to, the discrete cue. If this is the case, it is possible that futile attempts by mice in the CV1 group to use the spatial cues may have been responsible for their slower learning (Fig. 1B) and persistence in using serial-search strategies (Fig. 2D). Unfortunately, we cannot derive this information from the data collected in Experiment 1. Thus, a second experiment was conducted to determine whether mice were in fact using distal spatial cues to navigate the maze and whether they used spatial cues preferentially when a proximal discrete cue was also available.
Experiment 2 was conducted with three groups of naive mice, all of which were trained on the Barnes maze using a discrete cue marking the location of the escape hole, as described above. Two of the groups were treated identically to the CF1 and CV1 groups from Experiment 1 for the first five training sessions. The third group was identical to the CV1 except that a white, featureless plastic curtain was drawn around the maze during training to prevent use of the spatial cues to guide navigation. Additional manipulations were made following training to determine how the mice were solving the maze.
Materials and Methods
Experiment 1
Subjects
Sixteen male and 16 female B6C3F1/J mice were obtained at 6 wk of age from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in single-sex cages in groups of four in a light- and temperature-controlled environment on a 12-h light/12-h dark schedule with free access to food and water for the duration of the experiment. Testing began when mice were aged 7–8 wk, and all animals were tested over the course of 2 wk. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Apparatus
The Barnes maze consisted of a white, acrylic, circular disk 90 cm in diameter with 12 equally spaced holes (5-cm diameter) located 5 cm from the edge, as previously described (Reiserer et al. 2006). Each of the holes could be opened or closed by means of a sliding, white acrylic door that fit snugly under the hole. A black acrylic escape box (8 × 8 × 8 cm), to which the mice could gain access by way of a white acrylic ramp, could be fitted under any of the holes in place of the door. From the center of the maze, the white acrylic ramp looked identical to the white acrylic sliding doors used to block the other 11 holes. Thus the mice could not visually discriminate the escape hole location from the other holes from most points on the maze. However, when mice were situated adjacent to the escape hole, they could discriminate the escape from nonescape locations either tactilely or visually by looking down the tunnel into the black escape box. The maze was raised 56 cm from the floor and rested on a pedestal that enabled it to be rotated 360° on a horizontal plane. The black acrylic start box was a 13 × 13 × 13 cm bottomless cube with a hinged lid and a handle for easy lifting. Trials were recorded by using a CCD camera connected to a Macintosh G4 computer and were analyzed by using the public domain NIH Image program (rsb.info.nih.gov/nih-image), using a macro written specifically for the Barnes maze (Miyakawa et al. 2001). This software allows automated tracking and analysis of escape paths. The target zone was defined as the escape hole and 1 cm around it.
Procedures
Each trial began with the start box positioned in the center of the maze and the mouse placed inside it. The mouse remained in the start box for 30 sec with the hinged lid closed, providing a standard starting context for each trial and ensuring that initial orientation of the mouse on the maze varied randomly from trial to trial. On each training trial, 11 of the 12 holes were blocked. The remaining hole provided access to the escape box, which was positioned on the underside of the maze. The ramp leading to the escape box was the same color and texture as the doors blocking the holes, so that from the center of the maze it could not be distinguished visually from the other 11 holes. Each mouse was permitted to explore the maze freely. After the mouse entered the escape box, the hole was covered to prevent it from resurfacing onto the maze. The mouse was left in the escape box for 30 sec before being returned to its home cage. If the mouse did not enter the escape box within 300 sec, it was gently picked up by the experimenter and placed over the target hole and allowed to enter the escape box. The hole was then covered for 30 sec before the subject was returned to its home cage. We did not use fans, loud noise, exceptionally bright lights, or any other of the aversive methods sometimes used to motivate escape in the Barnes maze (Pompl et al. 1999; Paylor et al. 2001; Bredy et al. 2004; Seeger et al. 2004; Komater et al. 2005). We have found that the sole motivator of escape from the brightly lit maze surface, along with gentle handling and allowing the mice to remain in the start and escape boxes for a brief period, results in relatively short escape latencies and asymptotic learning in just a few sessions (Reiserer et al. 2006).
The maze and escape box were cleaned carefully with a 10% alcohol solution to dissipate odor cues and provide a standard olfactory context for each trial, and the maze was rotated between trials to eliminate the use of intra-maze cues. Five training sessions consisting of four trials each were run on subsequent days. Superior learning in the Barnes maze is reflected by fewer errors during training trials, as well as requiring fewer trials to reach asymptotic performance. A 300-sec probe trial, conducted 1 h after the final training trial on the fifth day, was identical to the training trials except that all 12 holes were blocked. The mouse was therefore unable to escape the maze during the probe trial. Good memory on probe trials in tasks such as the Barnes maze and water maze is defined as selective search for the former location of the target, compared with equivalent locations in other zones (Miyakawa et al. 2001; Bernardo et al. 2006; Bolding and Rudy 2006; Reiserer et al. 2006). Selective search in the present study was defined as time spent within the target zone, which comprised the blocked hole and 1 cm surrounding it, compared with the average of the time spent in the 11 nontarget zones.
Groups
Mice were allocated randomly to one of four groups, which determined the rules governing the location of the escape hole. Each group comprised four male and four female mice. For the hidden-target fixed-location (HF1) group, the hole was always located in the same position relative to distal extra-maze cues in the test room, even though the maze itself was rotated between trials. This is the standard method used to assess spatial learning in the Barnes maze. In the hidden-target variable-location (HV1) group, the position of the escape hole was varied such that on any given trial the escape box was never at the same location, or a location adjacent to that position in any daily, four-trial session. The cued-target fixed-location (CF1) group was equivalent to the HF1 group except that the location of the escape hole was also marked by a conspicuous polystyrene cone attached to the perimeter of the maze next to the target hole (15 cm high, 7.5 cm diameter at top, 2.5 cm from the proximal side of the cone to the edge of the maze). The cued-target variable-location (CV1) group followed the same rules as the HV1 group, but the escape hole was marked by the polystyrene cone. Previous work in our laboratory has shown that mice quickly learn to navigate to the cone under the same conditions used in the present experiments (Reiserer et al. 2006).
Acquisition measures
Traditionally, learning in the Barnes maze is assessed by the total number of errors committed before entering the escape hole, as well as the escape latency and path length required to enter the escape box. However, we found previously that mice sometimes navigated directly to the escape hole at the beginning of the trial, but then left the target zone without entering the escape box (Reiserer et al. 2006). As a result, despite having learned the association between the spatial room cues and the escape location, the mice continued to make errors by further exploring the maze. Thus the measure of total errors during a trial may be inflated by exploratory tendencies and not accurately reflect what the mouse has learned. To control for this, we calculated the number of errors to the first encounter of the escape hole, termed primary errors. This measure may provide a better indication of whether the mouse has learned the location of the escape hole at any given point in training. Similarly, we recorded the latency and path length to the first encounter with the escape hole, termed primary latency and primary path length, respectively, as well as the total escape latency and path length for the entire duration of the trial.
Data analysis
An experienced observer classified the search strategies for each mouse on every trial as either “direct” (moving either directly to the target hole or to an adjacent hole before visiting the target) or “serial” (the first visit to the target hole was preceded by visits to at least two of the adjacent holes in a serial manner). The majority of trials could be assigned unambiguously to either the direct or serial strategy categories; however, a small number of trials could not be classified in this way and were designated “mixed/random” searches. Such trials represented an apparently unordered or random search of the maze. Strategies were assigned according to the initial visit to the target hole and did not take into account any subsequent activity on the maze. This ensured that only target-location strategies were assessed, and minimized the effect of other motivational or exploration-related behaviors. An overall frequency was calculated for each type of search strategy for each mouse, and these scores were averaged to obtain a group mean for each search type for a session. It is important to note that a mouse will sometimes run directly to the escape hole or an adjacent hole by chance (a one in 12 chance; 0.083). Each time a mouse runs to one of the two target-adjacent holes, there is a 50% likelihood of then turning toward the hole and having the path scored as direct (two in 12 [hole location] × one in two [correct direction]; 0.083). Thus a mouse that uses a serial strategy 100% of the time will by chance use a path that appears direct 16.67% of the time.
Statistical analyses
For each of the six acquisition measures (primary and total errors, latency, and path length) tracked during acquisition, the average of the four daily trials was calculated. Three-way (cue condition × target location × session) mixed-model ANOVAs were performed on the six acquisition variables using the SAS/STAT software procedure PROC GLM, version 9 of the SAS System for Windows. Cue condition (hidden or cued) and target location (fixed or variable) were between-subjects factors, and session was the repeated measure. To correct for potential violations of sphericity, degrees of freedom for all repeated-measures effects were adjusted by using the Greenhouse-Geisser estimate of epsilon (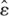 ). For simplicity, we report the unadjusted degrees of freedom and the value of
). For simplicity, we report the unadjusted degrees of freedom and the value of 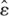 in the results section.
in the results section.
Because analyses were conducted on six dependent variables, we used a step-down Bonferroni (sometimes known as Holm) procedure to generate adjusted P-values (Holm 1979; Westfall et al. 1999). This procedure limits family-wise type I error rates to the desired α = 0.05 and is more powerful than the nonsequential Bonferroni technique. For a given effect (e.g., main effect of target location), the unadjusted P-values of the six measures were first ranked in ascending order (i.e., smallest P-value ranked first). Then the critical α level for the variable with the smallest P-value was set at 0.05/6 = 0.0083. If the null hypothesis was not rejected, the procedure stopped and no effects were declared statistically significant. If the null hypothesis was rejected, then the variable with the second-smallest P-value was tested with a critical α of 0.05/5 = 0.01. If the null hypothesis was not rejected, the sequence stopped at this point and no additional effects on this variable or the remaining four variables were declared significant. If the null hypothesis was rejected, then the variable with the third-smallest P-value was tested at α = 0.05/4–0.0125, and so on. In the text below, we report the adjusted P-values for each effect, calculated using the SAS/STAT software procedure PROC MULTTEST, version 9 of the SAS System for Windows. These are computed as the raw (i.e., unadjusted) P-value times the total number of variables remaining in the set of possible comparisons at each step (i.e., K = 6, 5, 4 . . . . 1). We also corrected all adjusted P-values for possible violations of monotonicity across pairs of variables (i.e., one variable with a larger unadjusted P-value associated with a smaller adjusted P-value) by substituting the larger of the two adjusted P-values. Because the family-wise type I error rate was limited to 0.05, adjusted P-values ≤ 0.05 were considered statistically significant (see Westfall et al. 1999).
All other analyses were conducted by using SPSS 13.0 for Windows. GLM RMANOVA model was used to analyze search paths, with search strategy as the repeated measure. Time spent at each zone during the probe trials was calculated as a percentage of total time spent within all possible target zones. To assess whether each group showed selective search for the target location over nontarget holes, a separate repeated-measures analysis was run for each group individually, with hole location (target or nontarget) as the repeated measure. The target location for the HV1 group was chosen as the target location from the final training trial. This target was chosen in order to maximize the probability of showing a preference for it. Initially, data were analyzed with gender as a blocking variable. However, there were no significant gender effects, so groups were pooled. Unless stated otherwise, all groups were included in all analyses. The α level for statistical significance was set at 0.05.
Results
Experiment 2
Barnes maze acquisition
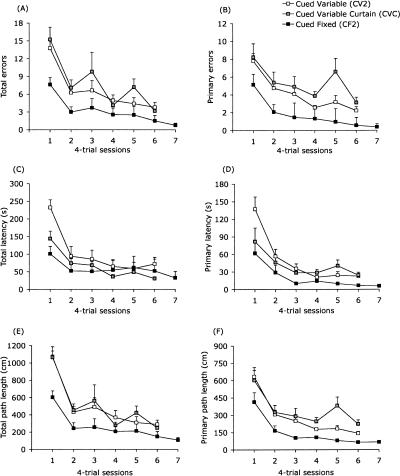
Repeated-measures group × session ANOVAs that included data from the first six sessions indicated a significant effect of session that reflected the general decreases in errors, escape latencies, and path lengths across sessions (dfs = 5,105; total errors: ε = 0.510, F = 26.11, adjusted P < 0.0001; primary errors: ε = 0.731, F = 17.72, adjusted P < 0.0001; total latency: ε = 0.538, F = 32.05, adjusted P < 0.0001; primary latency: ε = 0.364, F = 29.43, adjusted P < 0.0001; total path length: ε = 0.554, F = 45.85, adjusted P < 0.0001; primary path length: ε = 0.524, F = 34.05, adjusted P < 0.0001) (Fig. 4). There was a significant effect of group on primary errors (F(2,21) = 11.64, adjusted P < 0.005) and primary path length (F(2,21) = 10.27, adjusted P < 0.005) but not on the other four dependent measures (F(2,21) < 5.33, adjusted P > 0.053). Consistent with the pattern of group differences evident in Figure 4, pairwise comparisons showed that the CF2 group committed significantly fewer primary errors than did both the CV2 (F(1,21) = 9.19, adjusted P < 0.025) and CVC groups (F(1,21) = 22.72, adjusted P < 0.001). Similarly, primary path lengths were significantly shorter in the CF2 group compared with the other two groups (CV2: F(1,21) = 8.86, adjusted P < 0.025; CVC: F(1,21) = 19.79, adjusted P < 0.001). There were no significant differences between the CV2 and CVC groups on either measure (adjusted P > 0.25). The only other effect yielded by these analyses was a significant group × session interaction on total latency, (ε = 0.538; F(10,105) = 3.95, adjusted P < 0.02). Follow-up analysis indicated that this effect was primarily attributable to the longer escape latencies on the part of the CV2 group relative to the other groups during the first session (omnibus F(2,21) = 9.86, adjusted P < 0.01; CV2 vs. CF2 Fisher LSD P < 0.001; CV2 vs. CVC Fisher LSD P < 0.01). There were no significant between-group differences in total latency in subsequent sessions (P > 0.40). For all other comparisons, the effects of group, and group × session interactions were not statistically significant during acquisition (F(10,105) < 2.60; adjusted P > 0.30). Moving speed did not differ among the three groups (F(2,21) = 0.79, P = 0.47) (data not shown).
Figure 4.
Acquisition curves from Experiment 2. Consistent with the results of Experiment 1, the fixed-target group (CF2) performed better than did the variable-target groups. Mice in the CF2 group made significantly fewer primary errors (B) and had shorter primary path lengths (F) than did CV2 and CVC groups. There were no significant group differences in total errors (A), total path length (E), total latency (C), or primary latency (D). Data represent mean (±SEM) of four trials per day.
The CF2 group continued to perform well during the uncued block of four trials during the seventh training session (Fig. 4). None of the acquisition measures differed significantly between the seventh and sixth training sessions (total errors: F(1,7) = 1.85, P = 0.22; primary errors: F(1,7) = 2.74, P = 0.14; total latency: F(1,7) = 1.38, P = 0.28; primary latency: F(1,7) = 1.04, P = 0.34; total path length: F(1,7) = 1.60, P = 0.25; primary path length: F(1,7) = 0.18, P = 0.68), indicating that mice had not been using solely the discrete cue to navigate the maze.
Search strategy analysis
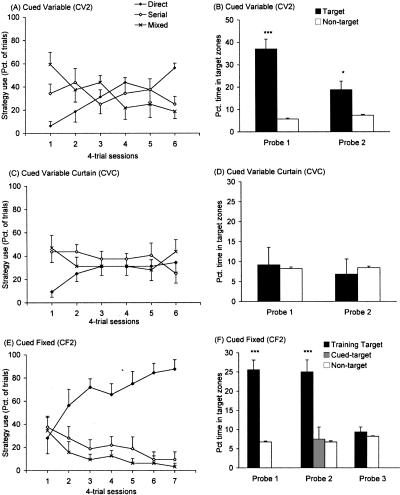
Analysis of the search strategies used across acquisition trials revealed significant strategy × group (F(4,42) = 9.90, P < 0.001) and strategy × session (F(10,210) = 5.84, P < 0.001) interactions (Fig. 5). Follow-up analyses were conducted on each four-trial acquisition session that preceded a probe trial. Mice in the CF2 group exhibited a strong preference for a direct-search strategy compared with serial searches on sessions 5 (F(1,21) = 8.40, P < 0.001) and 6 (F(1,21) = 40.46, P < 0.001). During the final acquisition session, when the visible target had been removed, the CF2 group continued to make more direct than serial searches (F(1,7) = 28.97, P < 0.001). This result is consistent with the primary error measure reported during acquisition trials, and provides additional evidence that escape strategies had been guided by extra-maze spatial cues and not solely by the discrete beacon marking the escape hole. The CV2 group showed no preference for direct versus serial searches during the fifth acquisition session (F(1,21) = 0.00, P = 1.0). However, mice in the CV2 group used significantly more direct than serial searches on the sixth and final test session (F(1,21) = 7.02, P < 0.05), suggesting that they were learning to use the discrete cue to navigate the maze but at a slower rate than the CF2 group. The use of serial and direct searches did not differ in the CVC group on either session 5 (F(1,21) = 0.23, P = 0.63) or session 6 (F(1,21) = 8.4, P = 0.44).
Figure 5.
Strategy use and time spent at target locations during probe trials in Experiment 2. Neither CV2 nor CVC groups showed a preference for any particular search strategy (A, C). Mice in the CF2 group had a strong preference for direct- over serial- or mixed/random-search strategies (E). The CF2 group continued to use direct searches on day 7, when training trials were conducted in the absence of the discrete cue. During the probe trials, mice in the CV2 group showed a distinct preference for the target location marked with a discrete visible cue (B). Mice run under identical conditions but with a curtain drawn around the maze (CVC) failed to show a preference for the cued location over nontarget locations during probe trials (D). The CF2 group, for which spatial and discrete cues were confounded and always marked the location of the escape hole during training, spent more time near the target during the first probe trial (F). On the second probe trial, when the discrete cue was moved to the opposite side of the maze, mice in the CF2 group ignored the cue and lingered near the target zone associated with the spatial room cues. On the third probe trial, a white curtain was drawn around the maze to obscure the room cues. Under these conditions, the CF2 group did not show a preference for the target location over the other 11 locations. Asterisks represent significant difference from mean time spent at nontarget locations: *P < 0.05, ***P < 0.001.
Probe trial data
The CV2 group demonstrated a significant preference for the cued target location over nontarget locations on both probe trials, despite the fact that the discrete cue moved from trial to trial during both training and probe trials (probe 1: F(1,7) = 43.76, P < 0.001; probe 2: F(1,7) = 7.66, P < 0.05) (Fig. 5B). In contrast, the CVC group failed to show a preference for the target over nontarget locations on either probe trial, even though the target was clearly marked on all training and probe trials with the same discrete cue used with the CV2 group (probe 1: F(1,7) = 0.22, P = 0.65; probe 2: F(1,7) = 1.05, P = 0.34) (Fig. 5D). Three probe trials were conducted with the CF2 group. The first was under conditions identical to those during training, i.e., the distal spatial and proximal discrete cues both marked the location of the escape hole. On this probe trial, the CF2 group showed a significant preference for target over nontarget locations (F(1,7) = 45.35, P < 0.001) (Fig. 5F). In the second probe trial, the discrete cue was moved 180° to the opposite side of the maze, but the spatial cues were still available and remained exactly as they had been during acquisition trials. Under these conditions, mice in the CF2 group showed selective search for the spatial cue–associated target location (F(2,14) = 12.44, P < 0.001) (Fig. 5F). More time was spent at this target than ether the cued-target or nontarget zones (pairwise comparisons P < 0.05). In contrast, mice spent no more time in the zone associated with the discrete cue than they did in the other 10 nontarget zones (pairwise comparisons P = 0.83). During the third probe trial, in which the room cues were obscured by the featureless curtain and the visual cue was not present, mice in the CF2 group showed no preference for the target location over the nontarget locations (F(1,7) = 0.66, P = 0.44).
Discussion
In Experiment 2, we again demonstrate that mice make spatial associations between the distal room cues and the location of the escape hole on the Barnes maze. Mice in the CF2 group, trained with both spatial and discrete cues marking the location of the escape hole, were unable to navigate the maze when the spatial cues were obscured. In addition, they ran directly to the escape hole associated with the spatial cues when the discrete cue was removed and showed preference only for the spatial cues during a probe trial in which the discrete cue was moved to another location. Taken together, these results illustrate that mice not only use spatial cues to learn the location of the escape hole in the Barnes maze but also use spatial cues preferentially when both spatial and discrete proximal cues are both available.
In Experiment 1, we predicted superior performance when a discrete visible cue indicated the target location, and that the presence of both spatial (extra-maze) and proximal cues would lead to the greatest learning about the target location. However, mice in all groups improved across training sessions, indicating that significant learning occurred during the acquisition phase regardless of the rules that governed target location. Mice are capable of circling the perimeter of the maze so quickly that use of serial-search strategies can lead to location of the escape hole almost as quickly as can use of direct spatial strategies based on extra-maze cues (see Fig. 1). This conclusion is supported by examination of the search strategies from the last session of acquisition in the hidden-target groups (Fig. 2). Because the hidden target moved from trial to trial, mice in the HV1 group relied on a serial search on nearly every trial; in contrast, mice in the HF1 group had a fixed target location and used primarily direct search strategies. Despite the HV1 group making nearly twice as many total errors as the HF1 group on the fifth session, the escape latencies of the two groups were identical. However, examination of the overall latencies and total number of errors may not be the best source of information about what the mouse has learned. When primary errors were analyzed, group differences emerged that were not evident when looking at total errors (Fig. 1B). Specifically, groups trained with a fixed target location made significantly fewer primary errors and had shorter path lengths than did those trained with a variable target location. A similar pattern was evident in Experiment 2. These differences between total and primary measures reflect the fact that mice sometimes run directly to the escape hole using the distal and proximal cues or navigational rules available to them but, instead of entering the hole immediately, leave to explore the maze further before returning at a later point to effect their escape. It is not clear why the mice first run to the escape hole rather than simply exploring the maze immediately after the start of the trial. It is possible that the mice want the assurance that an option for escape is available before exploring further. Being in a brightly lit, open space is considered stressful for mice, and verification of the location of the escape hole may reduce stress sufficiently to allow them to explore other parts of the maze. Regardless of the reason, the low number of primary errors suggests that mice in the fixed-target groups knew precisely the location of the escape hole.
To get a clearer idea of how the mice were solving the maze, we examined escape strategy. The fixed-target groups (HF1, CF1, and CF2) showed a strong preference for a spatial, direct search over a serial-search strategy. The inverse relationship was observed in the variable-target groups in Experiment 1 (HV1 and CV1), but in Experiment 2 the variable-target groups (CV2 and CVC) did not favor a particular strategy. This pattern suggests that when the target was spatially fixed, mice chose a spatial navigation technique; however, when the target location varied across trials there was no predominant search strategy. This is perhaps surprising in the case of the CV1 and CV2 groups, which did not show a preference for using a direct strategy despite the presence of a large and clearly visible beacon marking the target location. The preference of the CV1 and CV2 groups for the beacon-marked target location during the probe trials clearly demonstrates that the mice perceived the cue and made the association between it and the escape hole. Thus, as with distal spatial cues, mice may use a discrete proximal cue to solve the Barnes maze on some trials, but they do not rely solely upon this marker. The greater use of direct searches among the fixed-target groups provides a clear explanation for their superior performance with respect to primary errors and primary path length. By definition, a direct search strategy can include a maximum of one primary error, whereas with a serial-search strategy this number can vary from two to 11. Thus an analysis of search strategies is required to determine whether the increase in primary errors is attributable to spatial learning impairments.
Performance on the probe trial largely reflected the group differences in primary errors on the fifth day of acquisition. A preference for the target over nontarget locations in each of the three rule-governed groups in Experiment 1 (HF1, CF1, and CV1) during the probe trial clearly showed that these mice had learned how to locate the position of the escape box by methods other than just a serial-search strategy (which could not be successful in the case of the probe trial, during which all holes were blocked). The CV1 group, which persisted in using a serial-search strategy during acquisition trials, spent a large portion of time near the cue during the probe trial. The average number of primary errors committed by the CV1 and CV2 groups on the fifth day of acquisition was between two and three; two is the minimal number of errors a mouse can make using a serial search. This suggests that mice in the CV1 and CV2 groups were more likely to run to a location close to the discrete cue on the probe trial, as opposed to a location on the opposite side of the maze. Thus they appear to have made some association between the proximal cue and the escape hole despite their use of serial or random searches on 44%–66% of the trials. Importantly, the use of direct search strategies continued to increase over the course of training sessions in the CV1 and CV2 groups, suggesting that with additional training they may have reached direct search proportions similar to those observed in the fixed-target groups. Taken together, these data suggest slower learning about the discrete cue in the CV1 and CV2 groups, rather than differences in strategy use per se.
One reason for slower learning in the CV1 group may lie with the cue itself. Unlike visible-platform trials in the water maze, in which the cue is located within the pool, the discrete cue in the Barnes maze is located slightly outside the maze and thus may have appeared as a landscape cue for a mouse located in the center of the maze. Configural learning theories propose that an entire environment or set of stimuli (such as the test room cues and the polystyrene cone) may be represented together and form a single association with a reinforcer (in this case leaving the maze through the escape hole), rather than each individual element of the environment forming separate associations (Rudy and Sutherland 1989; Sutherland et al. 1989; Wilson and Pearce 1989; Pearce and Wilson 1990; Pearce et al. 1992, Pearce 2002; Rudy 1994). Thus the distal room cues and the discrete cue may have been perceived by the mice as a single compound element. Because the environmental configuration provided no information as to the location of the escape hole for the CV1 and CV2 groups, perception of the proximal cue as part of the landscape would have inhibited its predictive strength. If the discrete cue was in fact a weak predictor, it is not surprising that mice in the CV1 and CV2 groups did not rely on it to escape the maze but instead were slower to change from using primarily serial- to primarily direct-search patterns.
If mice in the CV1 and CV2 groups readily made configural associations among landscape cues that included the discrete proximal cue, their slower learning may reflect a learned irrelevance. Learned irrelevance is a process whereby prior uncorrelated exposure to a stimulus and reinforcer will retard subsequent learning about that stimulus when it does have consequences (Mackintosh 1973). Although the discrete cue and escape hole were perfectly correlated throughout training in the CV1 and CV2 groups, the perception of the cone as part of a configuration may have impeded mice from learning that it is a distinct predictive cue. Our data demonstrate that mice initially learned to solve the maze using a serial-search strategy. With repeated training, the mice learned that the polystyrene cone was the only cue that reliably predicted the escape hole location, and performance improved. Similar learning impairments resulting from a bias toward distal spatial cues over proximal cues have been reported on other tasks, such as the eight-arm radial maze, Morris water maze, and a food-finding task in a large arena (Kraemer et al. 1983; Chamizo et al. 1985; March et al. 1992; McDonald and White 1994; Gibson and Shettleworth 2003). A potential problem with this interpretation is that one would predict more rapid learning in the CVC group, given the absence of distal room cues and the apparent increased salience of the discrete proximal cue. Instead, acquisition was slower in this group, and they showed no preference for the cued target location during the probe trial. This would seem to suggest that they had not formed an association between the discrete cue and the escape hole during training. However, an examination of the change in search strategies shows that use of direct searches increased over training to levels twice that expected by chance. This suggests that these mice were learning the association with the discrete cue, albeit at a slower rate than the CF2 group. Nevertheless, an interpretation of learned irrelevance in the case of the CVC group is untenable given the lack of cue competition and the perfect correlation between the cue and escape hole. A more plausible explanation comes from the work of Biegler and Morris (1996). They showed that learning was superior when two cues marked the location of the reinforcer, compared to a single cue, in a food-finding task in a large arena. This was true regardless of whether the location of the cues was fixed or variable and despite the fact that the location of the reinforcer was predictable from the position of a single cue under every condition. In a follow-up experiment using similar methods, Biegler and Morris (1999) demonstrated that the spatial arrangement of the cues was more important to finding the reinforcer than the physical features of the cues themselves. Performance was unimpaired when some of the cues were rearranged, as long as the spatial arrangement remained intact and continued to predict the location of the reinforcer. This is consistent with considerable evidence showing that rodents use the geometric arrangement of cues in a room to navigate to a goal and only use proximal cues as a secondary mechanism when distal cues are unavailable (Cheng 1986; Collett et al. 1986; Biegler and Morris 1993; Brown and Terrinoni 1996; Hayward et al. 2004; Vlasak 2006). In Experiment 2, the proximal and distal cues provided more information to the CF2 and CV2 groups than the discrete cue alone provided to the CVC group, despite the fact that the discrete cue was perfectly correlated with the escape hole in all groups and was the only information that mice needed to solve the maze. The Barnes maze in the present study was located approximately equidistant from the four walls. Thus the CV2 group could have used the spatial and proximal cues to generate vectors that provided information about the path length and direction to the escape hole, despite the fact that the physical features of the distal cues changed (relative to the proximal cue) from trial to trial. Because this information was unavailable to the CVC group, their poorer performance may be attributable to futile attempts to generate geometric relationships among the nonexistent spatial cues and the polystyrene beacon. The amount of information may also have been a factor in the slower learning of the CV1 and CV2 groups compared with the CF1 and CF2 groups, but this does not preclude the simultaneous operation of a learned irrelevance process. Additional experiments are required to determine the relative contribution of the amount and type of information and inhibitory processes such as learned irrelevance, under these training conditions. Regardless of the processes involved, we can state with confidence that the cued version of the Barnes-maze task as typically conducted is not simply a perceptual control for the visuo-spatial abilities required to solve the hidden-target version.
The above results demonstrate that mice can use either distal spatial cues or a proximal beacon to solve the Barnes maze, or may use a serial-search strategy that need not involve either spatial or discrete cues. When both types of information are available, mice preferentially use distal spatial information and may ignore proximal cues. However, it is important to note that even mice searching for a hidden target in a fixed location sometimes used a serial-search strategy and sometimes run directly to the escape hole. Thus we contend that primary errors, primary path length and escape strategy are more informative than total errors, total path length, or escape latency when attempting to determine whether Barnes-maze performance can be attributed to spatial abilities. Latency measures especially may lack sensitivity in the Barnes maze, given the speed at which a serial search can be performed. Finally, we show that the cued-target version of the Barnes-maze task involves more than simple reference memory for a visual cue. Mice readily form configural associations with environmental cues, and the proximal discrete cue may become part of this compound cue. The inclusion of the discrete cue in a configuration with distal spatial cues may serve to slow formation of associations between the cue and the escape hole. Thus the cued-target task as typically conducted may not be able to dissociate spatial and nonspatial abilities in the Barnes maze.
Materials and Methods
Experiment 2
Subjects
Three groups of eight naive mice (four male and four female per group) were used in Experiment 2, of the same strain and age as those used in Experiment 1. Housing and experimental handling conditions were identical.
Apparatus
The same Barnes maze from Experiment 1 was used in Experiment 2, and room cues remained the same. The only difference was the inclusion of a white curtain surrounding the maze on some of the trials. The curtain was made of white plastic and was suspended from a circular metal frame (diameter, 150 cm) attached to the ceiling above the maze. The curtain extended from ∼5 cm from the ceiling to the floor and, when hung vertically, did not contain any folds, creases, or other distinguishing features with the exception of a slight overlap in the two edges of the curtain. The curtain was used for training for one of the groups and on a probe trial for another, and was removed when not needed for the trial.
Procedures
All three groups received 5 d of training in four-trial blocks followed 1 h later by a probe trial, as in Experiment 1. Two groups were identical to the CV1 and CF1 groups from Experiment 1, through the first probe trial, and are similarly designated for this experiment (CV2 and CF2). The third group was treated identically to the CV1 group, except that the plastic curtain was drawn around the maze on all the training trials and the probe trials. This group is designated CVC. The day after the first probe trial, all three groups received a sixth session of four training trials followed 1 h later by a second probe trial. In groups CV2 and CVC, the second probe trial was conducted in an identical manner to the first, i.e., with the cue in the same position as during training. In group CF2, the discrete cue was moved 180° on the maze for the second probe to allow a comparison between preference for the discrete visible cue and the distal room cues. If the mice used the discrete visible cue for navigation, they would spend more time near the cue during the probe trial. However, if they used spatial cues to locate the escape hole, the position of the discrete cue would be irrelevant, and most of their time would be spent near the hole that had previously afforded access to the escape tunnel. Finally, a seventh day of four training trials was conducted in the CF2 group only, without the discrete cue present. These trials were conducted to provide further indication of the extent to which mice in the CF2 group used the discrete versus spatial cues when locating the escape hole. If they used only the discrete visible cue, performance on these training trials would be significantly disrupted; if they used the spatial room cues for navigation, removing the cone would not affect performance. The final probe trial for this group was conducted 1 h following the last trial, with the white curtain surrounding the maze and hiding the room cues in order to demonstrate clearly whether the mice were using room cues in order to solve the maze.
Statistical analyses
We used a data-analytic strategy in Experiment 2 that paralleled that of Experiment 1. Group × session ANOVAs were performed on each of the six primary dependent variables (primary and total errors, path length, and latency). Step-down Bonferroni adjustments were used to adjust P-values for multiple analyses. A Fisher LSD approach was used to conduct pairwise comparisons among the three groups. Specifically, pairwise comparisons were conducted if the omnibus effect for group was significant according to the step-down Bonferroni analyses. In this regard, we should note that when the number of groups equals three (as in the present case), the Fisher LSD approach provides strong family-wise control of type 1 errors and is typically more powerful than are alternative procedures (Levin et al. 1994; Seaman et al. 1991). The raw P-values for a given pairwise comparison (e.g., CF2 vs. CV2) were step-down Bonferroni adjusted for the number of dependent variables on which comparisons were performed. Probe trial data were analyzed as in Experiment 1. Additional two-session repeated-measures ANOVAs were conducted for each measure for the CF2 group for sessions 6 and 7 because this was the only group to receive a seventh day of training trials.
Acknowledgments
Support was provided by the National Institute of Aging (AG022439, AG023138) and National Institute of Child Health and Development (HD015052).
Footnotes
Article published online before print. Article and publication date are at http://www.learnmem.org/cgi/doi/10.1101/lm.334306
References
- Barnes C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bernardo A., McCord M., Troen A.M., Allison J.D., McDonald M.P. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.035. (in press) [DOI] [PubMed] [Google Scholar]
- Biegler R., Morris R.G. Landmark stability is a prerequisite for spatial but not discrimination learning. Nature. 1993;361:631–633. doi: 10.1038/361631a0. [DOI] [PubMed] [Google Scholar]
- Biegler R., Morris R.G. Landmark stability: Further studies pointing to a role in spatial learning. Q. J. Exp. Psychol. B. 1996;49:307–345. doi: 10.1080/713932636. [DOI] [PubMed] [Google Scholar]
- Biegler R., Morris R.G. Blocking in the spatial domain with arrays of discrete landmarks. J. Exp. Psychol. Anim. Behav. Process. 1999;25:334–351. [PubMed] [Google Scholar]
- Bolding K., Rudy J.W. Place learning in the Morris water task: Making the memory stick. Learn. Mem. 2006;13:278–286. doi: 10.1101/lm.146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy T.W., Lee A.W., Meaney M.J., Brown R.E. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm. Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Brown M.F., Terrinoni M. Control of choice by the spatial configuration of goals. J. Exp. Psychol. Anim. Behav. Process. 1996;22:438–446. doi: 10.1037//0097-7403.22.4.438. [DOI] [PubMed] [Google Scholar]
- Chamizo V.D., Sterio D., Mackintosh N.J. Blocking and overshadowing between intra-maze and extra-maze cues: A test of the independence of locale and guidance learning. Q. J. Exp. Psychol. B. 1985;37:235–253. [Google Scholar]
- Cheng K. A purely geometric module in the rat’s spatial representation. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Collett T.S., Cartwright B.A., Smith B.A. Landmark learning and visuo-spatial memories in gerbils. J. Comp. Physiol. [A] 1986;158:835–851. doi: 10.1007/BF01324825. [DOI] [PubMed] [Google Scholar]
- Deacon R.M., Rawlins J.N. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav. Neurosci. 2002;116:472–478. doi: 10.1037//0735-7044.116.3.472. [DOI] [PubMed] [Google Scholar]
- Dumont M., Strazielle C., Staufenbiel M., Lalonde R. Spatial learning and exploration of environmental stimuli in 24-month-old female APP23 transgenic mice with the Swedish mutation. Brain Res. 2004;1024:113–121. doi: 10.1016/j.brainres.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Fox G.B., Fan L., LeVasseur R.A., Fadden A.I. Effect of traumatic brain injury on mouse spatial and non spatial learning in the Barnes circular maze. J. Neurotrauma. 1998;15:1037–1046. doi: 10.1089/neu.1998.15.1037. [DOI] [PubMed] [Google Scholar]
- Frick K.M., Stillner E.T., Berger-Sweeney J. Mice are not little rats: Species differences in a one-day water maze task. Learn. Mem. 2000;11:3461–3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Gibson B.M., Shettleworth S.J. Competition among spatial cues in a naturalistic food-carrying task. Learn. Behav. 2003;31:143–159. doi: 10.3758/bf03195977. [DOI] [PubMed] [Google Scholar]
- Hamilton D.A., Rosenfelt C.S., Whishaw I.Q. Sequential control of navigation by locale and taxon cues in the Morris water task. Behav. Brain Res. 2004;154:385–397. doi: 10.1016/j.bbr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hardman R., Evans D.J., Fellows L., Hayes B., Rupniak H.T., Barnes J.C., Higgins G.A. Evidence for recovery of spatial learning following entorhinal cortex lesions in mice. Brain Res. 1997;758:187–200. doi: 10.1016/s0006-8993(97)00223-0. [DOI] [PubMed] [Google Scholar]
- Hauben U., D’Hooge R., Soetens E., De Deyn P.P. Effects of oral administration of the competitive N-methyl-d-aspartate antagonist, CGP 40116, on passive avoidance, spatial learning, and neuro-motor abilities in mice. Brain Res. Bull. 1999;48:333–341. doi: 10.1016/s0361-9230(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Hayward A., Good M.A., Pearce J.M. Failure of a landmark to restrict spatial learning based on the shape of the environment. Q. J. Exp. Psychol. B. 2004;57:289–314. doi: 10.1080/02724990344000150. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Holmes A., Wrenn C.C., Harris A.P., Thayer K.E., Crawley J.N. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn. Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komater V.A., Buckley M.J., Browman K.E., Pan J.B., Hancock A.A., Decker M.W., Fox G.B. Effects of histamine H3 receptor antagonists in two models of spatial learning. Behav. Brain Res. 2005;159:295–300. doi: 10.1016/j.bbr.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Koopmans G., Blokland A., van Nieuwenhuijzen P., Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol. Behav. 2003;79:683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Kraemer P.J., Gilbert M.E., Innis N.K. The influence of cue type and configuration upon radial-maze performance in the rat. Anim. Learn. Behav. 1983;11:373–380. [Google Scholar]
- Levin J.R., Serlin R.C., Seaman M.A. A controlled, powerful multiple-comparison strategy for several situations. Psychol. Bull. 1994;115:153–159. [Google Scholar]
- Lijam N., Paylor R., McDonald M.P., Crawley J.N., Deng C.X., Herrup K., Stevens K.E., Maccaferri G., McBain C.J., Sussman D.J., et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Lipp H.P., Wolfer D.P. Genetically modified mice and cognition. Curr. Opin. Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- Lipp H.P., Deacon R.M.J., Ben Abdallah N., Galsworthy M.J., Serkov A., Dell’Omo G., Amrein I., Rawlins N.J.P., Wolfer D.P. Abstract Viewer/Itinerary Planner, Program No. 83.5. Society for Neuroscience; 2004. Hippocampal lesions alter spatial behavior in the water-maze, naturalistic environments, and intellicage. [Google Scholar]
- Logue S.F., Paylor R., Wehner J.M. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Mackintosh N.J. 1973. Stimulus selection: Learning to ignore stimuli that predict no change in reinforcement. In Constraints on learning (eds. R.A. Hinde and J.S. Hinde) pp. 75–96. Academic Press, London [Google Scholar]
- March J., Chamizo V.D., Mackintosh N.J. Reciprocal overshadowing between intra- maze and extra-maze cues. Q. J. Exp. Psychol. B. 1992;45:49–63. [PubMed] [Google Scholar]
- McDonald R.J., White N.M. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav. Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Yared E., Pak J.H., Huang F.L., Huang K.P., Crawley J.N. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–775. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- Mohajeri M.H., Saini K., Li H., Crameri A., Lipp H.P., Wolfer D.P., Nitsch R.M. Intact spatial memory in mice with seizure-induced partial loss of hippocampal pyramidal neurons. Neurobiol. Dis. 2003;12:174–181. doi: 10.1016/s0969-9961(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Mohajeri M.H., Madani R., Saini K., Lipp H.P., Nitsch R.M., Wolfer D.P. The impact of genetic background on neurodegeneration and behavior in seizured mice. Genes Brain Behav. 2004;3:228–239. doi: 10.1111/j.1601-1848.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Paylor R., Zhao Y., Libbey M., Westphal H., Crawley J.N. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol. Behav. 2001;73:781–792. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Pearce J.M. Evaluation and development of a connectionist theory of configural learning. Anim. Learn. Behav. 2002;30:73–95. doi: 10.3758/bf03192911. [DOI] [PubMed] [Google Scholar]
- Pearce J.M., Wilson P.N. Configural associations in discrimination learning. J. Exp. Psychol. Anim. Behav. Process. 1990;16:250–261. [PubMed] [Google Scholar]
- Pearce J.M., Adam J., Wilson P.N., Darby R.J. Effects of discrimination training on responding during a compound conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1992;18:379–386. doi: 10.1037//0097-7403.18.4.379. [DOI] [PubMed] [Google Scholar]
- Pompl P.N., Mullan M.J., Bjugstad K., Arendash G.W. Adaptation of the circular platform spatial memory task for mice: Use in detecting cognitive impairment in the APPSW transgenic mouse model for Alzheimer’s disease. J. Neurosci. Methods. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- Raber J., Rola R., LeFevour A., Morhardt D., Curley J., Mizumatsu S., VandenBerg S.R., Fike J.R. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat. Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Reiserer R.S., Harrison F.E., Syverud D.C., McDonald M.P. Impaired spatial learning in the APPSwe + PSEN1ΔE9 bigenic mouse model of Alzheimer’s disease. Genes Brain Behav. 2006 doi: 10.1111/j.1601-183X.2006.00221.x. (in press) [DOI] [PubMed] [Google Scholar]
- Rizk A., Curley J., Robertson J., Raber J. Anxiety and cognition in histamine H3 receptor−/− mice. Eur. J. Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- Rudy J.W. Ontogeny of context-specific latent inhibition of conditioned fear: Implications for configural associations theory and hippocampal formation development. Dev. Psychobiol. 1994;27:367–379. doi: 10.1002/dev.420270605. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Sutherland R.J. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav. Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. [DOI] [PubMed] [Google Scholar]
- Seaman M.A., Levin J.R., Serlin R.C. New developments in pairwise multiple comparisons: Some powerful and practicable procedures. Psychol. Bull. 1991;110:577–586. [Google Scholar]
- Seeger T., Fedorova I., Zheng F., Miyakawa T., Koustova E., Gomeza J., Basile A.S., Alzheimer C., Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J. Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R.J., McDonald R.J., Hill C.R., Rudy J.W. Damage to the hippocampal formation in rats selectively impairs the ability to learn cue relationships. Behav. Neural Biol. 1989;52:331–356. doi: 10.1016/s0163-1047(89)90457-3. [DOI] [PubMed] [Google Scholar]
- Vlasak A.N. Global and local spatial landmarks: Their role during foraging by Columbian ground squirrels (Spermophilus columbianus) Anim. Cogn. 2006;9:71–80. doi: 10.1007/s10071-005-0006-3. [DOI] [PubMed] [Google Scholar]
- Westfall P.H., Tobias R.D., Rom D., Wolfinger R.D., Hochberg Y. Multiple comparisons and multiple tests using the SAS system. SAS Institute; Cary, N.C: 1999. [Google Scholar]
- Wilson P.N., Pearce J.M. A role for stimulus generalization in conditional discrimination learning. Q. J. Exp. Psychol. B. 1989;41:243–273. [PubMed] [Google Scholar]
- Wolfer D.P., Stagljar-Bozicevic M., Errington M.L., Lipp H.-P. Spatial memory and learning in transgenic mice: Fact or artifact? News Physiol. Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- Wright J.W., Alt J.A., Turner G.D., Krueger J.M. Differences in spatial learning comparing transgenic p75 knockout, New Zealand Black, C57BL/6, and Swiss Webster mice. Behav. Brain Res. 2004;153:453–458. doi: 10.1016/j.bbr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Zhang J., McQuade J.M., Vorhees C.V., Xu M. Hippocampal expression of c-fos is not essential for spatial learning. Synapse. 2002;46:91–99. doi: 10.1002/syn.10115. [DOI] [PubMed] [Google Scholar]