Abstract
Much recent research on mechanisms of learning and memory focuses on the role of heterosynaptic neuromodulatory signaling. Such neuromodulation appears to stabilize Hebbian synaptic changes underlying associative learning, thereby extending memory. Previous comparisons of three related sea-hares (Mollusca, Opisthobranchia) uncovered interspecific variation in neuromodulatory signaling: strong in Aplysia californica, immeasureable in Dolabrifera dolabrifera, and intermediate in Phyllaplysia taylori. The present study addressed whether this interspecific variation in neuromodulation is correlated with memory of associative (classical conditioning) learning. We differentially conditioned the tail-mantle withdrawal reflex of each of the three species: Mild touch to one side of the tail was paired with a noxious electrical stimulus to the neck. Mild touch to the other side served as an internal control. Post-training reflex amplitudes were tested 15–30 min after training and compared with pre-test amplitudes. All three species showed conditioning: training increased the paired reflex more than the unpaired reflex. However, the temporal pattern of conditioning varied between species. Aplysia showed modest conditioning that grew across the post-test period. Dolabrifera showed distinctly short-lived conditioning, present only on the first post-test. The time course of memory in Phyllaplysia was intermediate, although not statistically distinguishable from the other two species. Taken together, these experiments suggest that evolutionary changes in nonassociative heterosynaptic modulation may contribute to evolutionary changes in the stability of the memory of classical conditioning.
Contemporary models of learning and memory (Frey et al. 1990, 2001; Bailey et al. 2000a, b; Almaguer-Meliana et al. 2005) propose that heterosynaptic modulation during a learning event serves to stabilize Hebbian mechanisms of synaptic plasticity, thereby increasing the persistence of associative neural changes underlying several forms of learning and memory, including classical (Pavlovian) conditioning. This model suggests an evolutionary hypothesis: that evolutionary change in nonassociative heterosynaptic modulation should be associated with a concomitant change in the duration of associative memory.
A test of this hypothesis is afforded by the recent discovery of interspecific variation in nonassociative behavioral sensitization, and the heterosynaptic modulation that underlies it, in opisthobranch relatives of the model species, Aplysia californica. Modulation is produced by application of the modulatory transmitter, serotonin (Wright et al. 1996; Erixon et al. 1999), or by strong stimulation of peripheral nerves (Marinesco et al. 2003). Both of these nonassociative manipulations cause neuromodulatory changes in sensory-neuron firing properties of Aplysia, but their effects on two related species are much weaker. Specifically, in Dolabrifera dolabrifera, no serotonin-induced changes are observed, and a cladistic analysis (Wright et al. 1996) strongly suggests that this absence is due to a relatively recent evolutionary loss of response by sensory neurons to applied serotonin. Furthermore, stimulation of peripheral nerves has no effect on sensory neurons, and behavioral experiments (Wright 1998) confirm the complete lack of sensitization (short- or long-term) or dishabituation. In Phyllaplysia taylori, the modulatory effects of noxious stimulation of sensory neurons although significant, are weaker than those observed in Aplysia. This is likely due to inhibition by unknown mechanisms, because serotonin release (Marinesco et al. 2003) and the response of sensory neurons to serotonin (Wright et al. 1996), are indistinguishable from that of Aplysia. At the behavioral level, Phyllaplysia shows only very limited sensitization (Erixon et al. 1999).
If the above general model of the role of neuromodulation in classical conditioning is correct, then we would predict that the memory of classical conditioning should be of longer duration in Aplysia, whose neuromodulatory signal is strong, than in Dolabrifera, whose neuromodulatory signal is nil. Phyllaplysia, whose neuromodulatory signal and sensitization is weak, is expected to show associative memory intermediate between the other two species. The experiments presented here are consistent with these predicted patterns of associative memory and thereby support the evolutionary hypothesis that interspecific variation in nonassociative heterosynaptic modulation and sensitization has predictable consequences for the associative memory of classical conditioning.
Results
We classically conditioned the tail-mantle withdrawal reflex of all three species using a differential conditioning protocol: Mild touch to one side of the tail was paired with a noxious electrical stimulus to the neck. Mild touch to the other (unpaired) side of the tail served as an internal control for the pairing-independent effects of the stimuli. Thus, we could compare the reflex amplitudes of paired and unpaired reflexes, before and after training.
The tail-mantle withdrawal reflex before training was qualitatively and quantitatively similar in all three species (average withdrawal time, Aplysia: 11.4 ± 0.4 sec; Dolabrifera: 11.6 ± 0.6 sec; Phyllaplysia: 13.4 ± 0.9 sec) (see also Wright 1998; Erixon et al. 1999). Furthermore, all three species responded in the same way to the application of the noxious stimulus: strong withdrawal, release of defense secretions (ink and/or opaline), and rapid locomotion for 5–10 min after the shock. Furthermore, all three species showed significant associative learning in response to classical conditioning. However, the time-course of this associative memory showed evidence of heterogeneity across species.
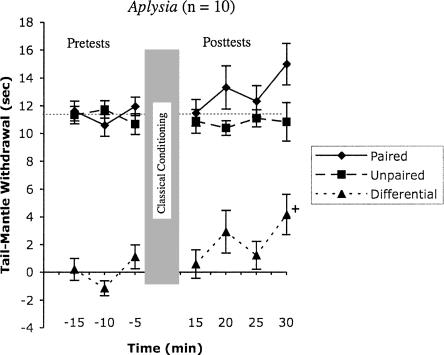
In Aplysia, differential conditioning enhanced the tail-mantle withdrawal reflex on the paired side of the tail, relative to the unpaired side (Fig. 1). In each of the four post-tests, the Paired side mean was higher than that of the Unpaired side. Inspection of Figure 1 suggests a gradual development of learning over the last three post-tests, but statistically, only the last test (30 min) trended above baseline (paired students t-tests with Bonferroni corrections for multiple comparisons; mean ± SEM, 4.2 ± 1.5; P ≤ 0.10). Nevertheless, the consistent difference across the post-tests resulted in a significant Training effect (F(1,101) = 6.3; P = 0.014) in a two-way (Trials, Training) repeated measures ANOVA. This significant learning did not change significantly across trials (Training X Trials interaction; F(6,101) = 1.1; P = 0.133). Limiting the ANOVA exclusively to post-test reflexes strengthened the Training effect somewhat (Training, F(1,51) = 8.0, P = 0.007), again without significant interaction (F(3,51) = 0.9; P = 0.459) effects. Thus, the ANOVA detected a strong effect of training integrated across all four post-tests, even though only one post-test (the last one) showed one-tailed significance. This suggests that the associative effects of classical conditioning throughout much of the post-test period contribute to the observed learning.
Figure 1.
Aplysia showed differential conditioning that was not expressed until 30 min after training. Shown are mean (± SEM) tail-mantle withdrawal durations before (negative time) and after (positive time) differential conditioning consisting of five pairings (20 min) of a tactile tail stimulus (paired) with noxious electric shock to the neck. Unpaired tactile stimuli were delivered exactly between the paired stimuli. The dashed line with triangles indicates the calculated difference between the reflex withdrawal of the paired and unpaired stimuli. + Indicates marginal statistical difference from pre-training baseline (repeated measures t-test with Bonferroni correction, two-tailed, P ≤ 0.05).
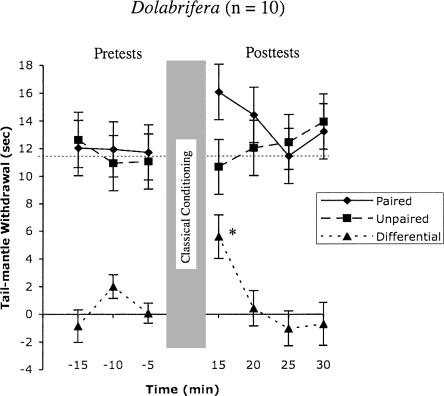
In sharp contrast to Aplysia, whose associative memory developed slowly and lasted relatively long, differential conditioning in Dolabrifera was expressed early and disappeared quickly (Fig. 2). Repeated measures ANOVA on the entire data set did not detect a significant Training effect (F(1,115) = 1.6; P = 0.2). This is likely due to an almost significant interaction of Training*Trials (F(6,11) = 2.035; P = 0.066). Repeating the analysis on the post-training reflexes, exclusively, confirmed a significant Trial*Training interaction (F(3,6) = 3.086; P = 0.034) with no main effect of Training (F(1,6) = 1.5; P = 0.2). Thus, the associative memory of classical conditioning in Dolabrifera changed significantly during the post-test period. Paired students t-tests (with Bonferroni correction) on the post-test data indicated significant classical conditioning at the 15-min test (Fig. 2; Paired—Unpaired = 5.3 ± 1.3 sec; t = 4.2; P ≤ 0.05), but not at any other tests. As in Aplysia, only the reflex on the paired side of the tail was elevated relative to pre-training, although not significantly (14.6 ± 1.6 sec vs. 9.0 ± 0.7 sec; P < 0.20) (Fig. 2). There appeared to be some delayed sensitization in both paired and unpaired reflexes in the last two post-tests, but these were not significantly above the pre-tests.
Figure 2.
Dolabrifera showed significant differential conditioning that lasted no more than 20 min after training. Only the first post-test showed a significant difference between the paired and unpaired reflexes. * Indicates statistical difference from pre-training baseline (repeated measures t-test with Bonferroni correction, two-tailed, P ≤ 0.05).
In order to compare statistically the behavioral responses of Dolabrifera to those of Aplysia, we performed a repeated measures ANOVA on the combined reflex amplitude data with Species as a between-group factor and Trials and Training as within-group (repeated measure) factors. Reflex amplitude (collapsed across Training and Species) varied significantly across Trials (F(6,218) = 3.2; P = 0.005), reflecting both associative and nonassociative changes in reflex amplitude. In addition, the clear evidence of training-induced separation between Paired and Unpaired reflexes in both species (Figs. 1, 2) was reflected in a significant effect of Training (F(1,217) = 6.2; P = 0.014). Finally, there was a significant three-way interaction (Species*Trial*Training; F(6,21) = 2.4; P = 0.028), indicating that differential conditioning across trials differed between Aplysia and Dolabrifera. To further clarify these effects, we ran the same three-way ANOVA exclusively on the post-test reflexes. This abolished the effect of Trials (F(3,114) = 1.4; NS), but both Training (F(1,113) = 7.0; P = 0.009) and the three-way interaction (F(3,113) = 3.0; P = 0.032) still showed significant effects (see Aplysia and Dolabrifera, Fig. 4, below), indicating that the training caused a significant memory of classical conditioning regardless of species, and that the time-course of this memory differed significantly between the two species.
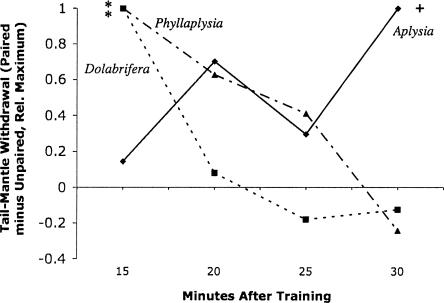
Figure 4.
Memory for classical conditioning was heterogenous across species: Aplysia (diamonds), Dolabrifera (squares), Phyllaplysia (triangles). Mean reflex differential (Paired minus Unpaired, standardized by mean maximum for each species). Data are derived from the difference scores in Figures 1, 2, 3 to facilitate comparison. See those figures to determine variation around each score. Dolabrifera and Phyllaplysia showed significant learning, but only 15 min after training. Aplysia also showed significant learning averaged over all four post-tests. Symbols indicate statistical difference from pre-training baseline (two-tailed probabilities: +P ≤ 0.10; **P ≤ 0.01) using a repeated measures t-test with Bonferroni correction.
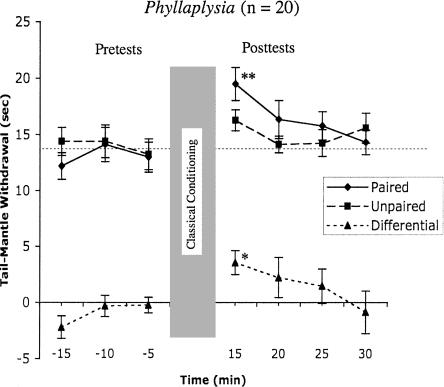
Aplysia and Dolabrifera represent two extremes in heterosynaptic neuromodulation and sensitization, with Aplysia showing strong evidence of both, and Dolabrifera showing no evidence of either (Wright et al. 1996; Wright 1998; Marinesco et al. 2003). We decided to test for classical conditioning in a third aplysiid, Phyllaplysia taylori, which was previously observed to have limited heterosynaptic modulation in response to nerve shock and only localized sensitization in response to noxious stimuli (Erixon et al. 1999; Marinesco et al. 2003). This species was thus expected to show intermediate duration of memory for classical conditioning. Consistent with this prediction, Phyllaplysia showed significant classical conditioning only during the first test, 15 min after training (Differential = 3.5 ± 1.1 sec; P ≤ 0.05) (Fig. 3). Unlike the other two species, both Paired and Unpaired reflexes in Phyllaplysia were at least somewhat elevated by training. However, only the Paired reflex was significantly greater than pre-test baseline (6.4 ± 1.7 sec; P ≤ 0.01). Because both Paired and Unpaired increased, repeated measures ANOVA produced a significant effect of Trials (F(6,229) = 3.8; P = 0.001), but no associative main effect (Training; F(1,229) = 0.7; NS). When we repeated the ANOVA without the pre-tests, the Trials effect was reduced, though still significant (F(4,121) = 2.7; P = 0.033), but now the effect of Training was significant (F(1,119) = 4.1; P = 0.046) as well. Note that in neither case did we see a significant interaction between Trial*Training such as we saw in Dolabrifera. These ANOVA results suggest that in Phyllaplysia, the memory for classical conditioning lies somewhere between that of Dolabrifera and Aplysia. Like Dolabrifera, the greatest learning occurred on the first post-test. Like Aplysia, temporal heterogeneity in learning (Training*Trial interaction) was reduced to nonsignificant levels.
Figure 3.
Phyllaplysia showed significant differential conditioning immediately after training that disappeared by 25–30 min after training. Only the first post-test showed a significant difference between the paired and unpaired reflexes. Asterisks indicate statistical difference from pre-training baseline (two-tailed probabilities: *P ≤ 0.05; **P ≤ 0.01) using a repeated measures t-test with Bonferroni correction.
We tested the ANOVA (post-tests only) of Phyllaplysia versus the other two species individually. Neither of these ANOVAs showed the three-way interaction of the Dolabrifera versus Aplysia ANOVA (versus Aplysia, F(3,170) = 0.9, NS; versus Dolabrifera, F(3,179) = 0.9, NS). These between-species ANOVAs indicate that, although the time course of memory in Phyllaplysia may lie between that of Dolabrifera and Aplysia (Fig. 4), the variation around that time course precludes the possibility of statistically distinguishing Phyllaplysia from either of the two other species.
Discussion
These experiments demonstrate significant classical conditioning in the tail-mantle withdrawal of three species of Opisthobranch mollusks. In addition, they also suggest heterogeneity in the time course of the associative memory for the conditioning.
In Aplysia, the consistently positive withdrawal difference scores (Fig. 1) and significant Training effect (repeated measures ANOVA, P = 0.007), without a significant Training X Trials interaction, suggests a persistent memory of classical conditioning across the four post-tests. There appears to be a gradual onset of conditioning, perhaps beginning as soon as the second (20-min) post-test. However, this interpretation must be tempered by the fact that only the last withdrawal reached one-tailed significance. Nevertheless, it is likely that some memory of the conditioning is expressed during the last three post-tests. We also think it likely that the conditioning persists long after the 30-min test. Previous studies in Aplysia, using the gill and siphon withdrawal reflex, show prolonged classical conditioning (to 24 h) (Carew et al. 1981, 1984; Hawkins et al. 1986) after the same five-trial protocol used in the present study (see further discussion below).
The apparent delay in expression of conditioning in Aplysia (Fig. 1) is similar to the gill withdrawal reflex (Carew et al. 1981), where expression of a conditioned response is delayed for at least 5 min. Recent differential conditioning experiments (also using five training trials) in the gill withdrawal of Aplysia (J.A. Shekib, D.L. Glanzman, and W.G. Wright, unpubl.) similarly suggest that classical conditioning is not expressed 5 min after training, and that full expression of classical conditioning is not realized until sometime after 15-min post-training. Finally, studies of sensitization in the siphon withdrawal reflex indicate delayed expression of sensitization until 20–30 min after a noxious stimulus (Carew et al. 1981; Mackey et al. 1987; Marcus et al. 1988). This is likely due to the recruitment of inhibitory processes that mask sensitization (Mackey et al. 1987; Wright and Carew 1995). In the present study, we observed a delay in expression of classical conditioning of 20–30 min, broadly consistent with these previous studies. It is worth noting that a neural analog of differential conditioning applied to the monosynaptic connections between sensory neurons and their followers mirrors classical conditioning, but without any delay (Hawkins et al. 1983; Walters and Byrne 1983; see also Eliot et al. 1994a), suggesting that the behavioral delay in expression depends on interneuronal loci rather than sensory neuron transmitter release. Other than one abstract (Ingram and Walters 1984), there are no previous publications of classical conditioning in the tail-mantle withdrawal reflex. Taken together, these results in Aplysia suggest that associative memory for classical conditioning after five training bouts may be inhibited for up to 15 min, and likely lasts substantially longer than our 30-min test period.
In Dolabrifera, conditioning was only observed at the 15-min post-test, dropping to baseline by 20 min after the end of training (Fig. 2). This short associative memory may relate to previous research on neuromodulation and nonassociative learning in Dolabrifera (Wright et al. 1996; Wright 1998). That research showed that, unlike Aplysia, tail-sensory neurons in Dolabrifera are completely unresponsive to applied serotonin, and a cladistic analysis of the phylogenetic distribution of that response in related species strongly suggests that unresponsiveness to be a derived trait, that is, a trait lost from a recent ancestor that possessed it (Wright et al. 1996). Consistent with this loss, strong stimulation of peripheral nerves in Dolabrifera induces no change in sensory neuron firing properties (Marinesco et al. 2003). In contrast, such stimulation causes robust changes to sensory-neuron firing properties in Aplysia (Walters et al. 1983; Marinesco et al. 2003). These neurobiological differences in modulatory phenotype extend to the behavioral level as well: Dolabrifera shows no evidence of dishabituation or short- or long-term sensitization following noxious stimuli, which routinely causes both forms of learning in Aplysia (Wright 1998). Combining previous and present observations, we hypothesize that the evolutionary loss of the neuromodulatory response of sensory neurons to serotonin in the lineage leading to Dolabrifera not only blocked the expression of sensitization in this species, but also sharply reduced the associative memory for classical conditioning. Clearly, analyzing the associative memory of Dolabrifera at synaptic and cellular levels is a critical next step in order to further test this hypothesis.
Although the evidence from Phyllaplysia is less compelling, it suggests an intermediate pattern. We observed significant learning in Phyllaplysia, and the temporal pattern of that learning (Fig. 4) suggests a slightly longer memory than observed in Dolabrifera, yet shorter than that of Aplysia. However, direct statistical comparisons of this time course between Phyllaplysia and the other two species (repeated measures ANOVA) revealed no significant species differences for either comparison. Thus, the evidence for intermediate memory is relatively weak. Nevertheless, if the memory is indeed intermediate, it is consistent with the intermediate level of serotonin signaling and sensitization observed in Phyllaplysia (Erixon et al. 1999; Marinesco et al. 2003).
Our experiments used a common five-trial protocol to induce short-term memory of classical conditioning in Aplysia, a protocol that produces memory lasting for many hours in the siphon and gill withdrawal reflexes (Carew et al. 1981, 1983). This same protocol, applied to Dolabrifera and Phyllaplysia enhanced the reflex on the paired side of the tail for only 15 min in Dolabrifera, and perhaps a little longer in Phyllaplysia. However, because we did not test the reflex beyond 30 min, there is a possibility that an associative memory in these two species would be observable at later times. Although such a biphasic memory has been observed in associative memory of other invertebrates (Greggers and Menzel 1993; Menzel 1999), and even in Aplysia after sensitization training (Carew and Sutton 2001; Sutton et al. 2001, 2004), there is little evidence of this in classical conditioning studies in Aplysia. Furthermore, sensitization experiments in Dolabrifera show no evidence of either short-term (1 h) or long-term (24 h) memory (Wright 1998). This is particularly interesting in light of the apparent trend toward sensitization (both paired and unpaired reflexes above pre-test levels) in Figure 2. Although these reflexes never rise to even the level of one-tailed significance, the pattern suggests that the possibility of late learning is not altogether excluded. To conclude, the fact that we did not test beyond 30 min leaves open the possibility for a biphasic associative memory that future studies will need to address.
A related issue is that we do not know how other protocols might affect memory in the tail-mantle withdrawal circuit, either for Aplysia, or for the other two species. The idea that neuromodulation by serotonin can stabilize the synapses that support the memory of classical conditioning (Bailey et al. 2000a, b; see below), suggests that increasing the number of trials would lengthen the duration of the associative memory. Indeed, experiments in the SWR and GWR in Aplysia showed that tripling the number of training trials creates a long-term memory that lasts much longer (many days). Such long-term classical conditioning would be of considerable interest, not only in the tail-mantle withdrawal reflex of Aplysia, but also of the other two species as well. The general model of serotonin as a stabilizer of the synaptic underpinnings of associative memory for classical conditioning would predict that tripling the number of trials would cause little or no memory change in Dolabrifera, in which sensory neurons are not modulated by serotonin, a slight increase in memory in Phyllaplysia, in which serotonin signaling is muted, and the same profound increase observed in other reflexes in Aplysia, a species with robust serotonin modulation of reflex circuitry. Clearly such long-term studies of classical conditioning are of primary interest.
Even without the extensive parametric study of classical conditioning in Dolabrifera and Phyllaplysia required to address the above caveats, the results of the present study are consistent with recent models of associative learning. Such models propose that associative synaptic plasticity is stabilized into a lasting memory by heterosynaptic modulation (Bailey et al. 2000a; Frey et al. 2001). They stem from observations in vertebrates as well as invertebrates. For example, a widely used mammalian model of associative synaptic plasticity, long-term potentiation (LTP), produces much longer-lasting changes in synaptic strength in the presence of heterosynaptic modulation by dopamine or norepinephrine than when such modulation is blocked (Frey et al. 1990; Almaguer-Meliana et al. 2005). Similarly, stimulation of the modulatory amygdalar region is necessary for long-term associative memory of fear conditioning (McGaugh 2000). In Aplysia, potentiation of a sensorimotor synapse after tetanus of the sensory neuron is prolonged when co-occurring with release of the modulatory transmitter, serotonin (Eliot et al. 1994a; Bailey et al. 2000a, b). Thus, in a wide variety of systems, heterosynaptic modulation appears to “stabilize” synaptic changes induced by associative (Hebbian) protocols. A reasonable extension of these models predicts that the evolutionary loss of modulation by serotonin in Dolabrifera (Wright 1998), and the relatively lower level of modulation caused by noxious stimuli in Phyllaplysia (Marinesco et al. 2003), should limit the stability of associative memory in these species relative to Aplysia, whose heterosynaptic modulation and behavioral sensitization in response to noxious stimuli is robust (e.g., Walters et al. 1983; Wright 1998; Marinesco et al. 2003; Bristol et al. 2004; for review, see Barbas et al. 2003). The results of the present study are consistent with that prediction, thereby strengthening the idea that evolutionary variation in nonassociative learning and neuromodulation is related to concomitant change in the persistence of associative memory. It is important to note that in neither Phyllaplysia nor Dolabrifera do we see any evidence of a diminution of the sensory detection of the US. Both species showed similar behavioral thresholds to electrical stimulation as observed in Aplysia, and their responses to the noxious stimulus were fully comparable, including strong withdrawal, balling up, and the release of ink and/or opaline. Thus, the neuromodulatory response appears to be selectively reduced or eliminated, without obvious concomitant reductions in other responses to the noxious stimulus.
Not all mechanistic models predict robust classical conditioning with a shorter memory in a species that is missing nonassociative neuromodulatory signaling. An older synaptic model of classical conditioning in Aplysia (Kandel et al. 1983) views classical conditioning as a variation of sensitization-related heterosynaptic facilitation following noxious stimulation, a modulation that causes a general nonassociative enhancement or sensitization of reflex strength. Associative memory is formed when such facilitation is amplified in any particular reflex circuit that is activated just before a noxious stimulation, as would occur during classical conditioning. This model strongly predicts that a species lacking heterosynaptic facilitation, even if all other reactions to noxious stimulation remain intact, should show no classical conditioning, since the very basis of that conditioning is removed. That prediction is not supported by the present study, which showed clear classical conditioning in Dolabrifera, a species lacking neuromodulation of sensory neurons by serotonin or noxious stimuli (Wright et al. 1996). In contrast, an extension of the more recently articulated Hebbian view of classical conditioning in Aplysia (Bailey et al. 2000a, b) would predict that a species like Dolabrifera should show short-lasting conditioning, reflecting Hebbian synaptic plasticity without stabilization by serotonergic heterosynaptic modulation (Eliot et al. 1994a, b). This prediction is fully consistent with the present results.
The idea that associative memory is more stable when it is formed in the context of increased modulation does not explicitly specify what process might degrade that memory. Thus, we do not know whether the rapid decrease in associative memory observed in Dolabrifera (Fig. 2) is due to increased susceptibility of that memory to the passage of time, or its susceptibility to extinction due to the repeated unreinforced presentations of the CS+ during the post-training period. Clearly, future experiments will need to include a control that receives training but delivers only a test at 30 min. If time is the only issue, such a group should show no learning. If extinction is the only issue, that group would be expected to retain the separation between Paired and Unpaired reflexes shown at the 15-min test in Figure 2. Until these important experiments can be performed, we can only conclude that associative memory in Dolabrifera is less stable, either due to the passage of time or the decrementing effect of unreinforced stimuli.
At an adaptive level, these results raise the possibility that the interspecific variation in associative memory identified here reflects differences in each species’ ecology. Because we have little, if any, idea of the adaptive significance of differential conditioning for sea hares in nature, it is premature to propose an adaptive hypothesis. Alternatively, we can hypothesize that the stability of associative memory is a pleiotropic effect of heterosynaptic modulation, the most important function of which may relate to nonassociative behavioral sensitization. Indeed, the fact that sensitization is reduced in Phyllaplysia (Erixon et al. 1999) and unmeasureable in Dolabrifera (Wright 1998) leaves open the possibility that sensitization is the evolutionarily important phenotype, rather than classical conditioning. The adaptive value of sensitization in sea hares is scarcely better understood than that of classical conditioning. However, it is reasonable to assume that sensitization, a generalized increase in reflex withdrawal following a noxious stimulus, protects individuals from repeated damage by some ecological agent, such as a predator (Pennings 1990; Nolen et al. 1995; Rogers et al. 2002). Preliminary observations (Ross et al. 2006; Thomas et al. 2006) in my lab demonstrated that sublethal attack by two natural predators of Aplysia causes significant sensitization in tail-mantle and head withdrawal, but whether such behavior change protects individual Aplysia from subsequent attack remains to be seen.
Interestingly, the habitats of the other two species with compromised sensitization are relatively free of predators. In particular, Phyllaplysia in the northeastern Pacific lives exclusively in thick eelgrass beds at the sea surface (Ricketts et al. 1985), safe from any benthic predators such as crabs and benthic fish. Similarly, Dolabrifera in Hawaii lives in the narrow space under boulders resting on shallow coral gravel (Kay 1979; W. Wright pers. obs.). Individuals only appear on boulder tops during the night, when visual predators are absent. Thus, because their habitats are already relatively predator free, reduction or loss of sensitization may offer these two species considerable savings in energy and/or time. If this view is correct, the shorter associative memory observed in the present study in these two species could be a relatively harmless nonadaptive consequence of selection against sensitization. Clearly, a better understanding of the behavioral ecology of these three species will help resolve these possibilities.
Materials and Methods
Animals and preparation
Wild-caught Aplysia californica, Phyllaplysia taylori, and Dolabrifera dolabrifera of similar size (3–4 cm) were used. Aplysia and Phyllaplysia were collected in temperate waters (Alacrity Marine, Redondo Beach, CA; David Duggins, Friday Harbor, WA), while Dolabrifera was collected from Hawaii (Karen Maruska, Kaheole, HI). Specimens were held in small aquaria at 15°C (Aplysia and Phyllaplysia) or 25°C (Dolabrifera). Animals were utilized within 7 d and were not fed during this time.
A Teflon-coated platinum electrode wire (0.002″ diameter, A-M Systems, Inc.) was inserted through the neck of each subject, 24 h prior to experimentation, and specimens were placed in an experimental chamber 10–15 min before the experiment. Filtered oxygenated water (15°C for Aplysia and Phyllaplysia, 25°C for Dolabrifera) was circulated through the experimental chamber throughout the experiment.
Experimental procedure
Animals were given weak tactile stimuli to either side of the tail, and noxious electrical stimuli through a neck-inserted electrode. The tactile stimuli were administered with a blunt rod (2-mm diameter), attached to a spring-loaded syringe. This apparatus was pneumatically powered through a Picospritzer (General Valve Corporation) and a digital stimulator (S8800; Grass Electronics) to give a short barrage (500 msec; 20 Hz) of tactile stimulation. The air pressure was set to the level that caused a clearly observable tail-mantle withdrawal reflex. The noxious stimulus was delivered through the electrodes inserted in the neck (500 msec; 20 Hz). Shock intensity was set for three times the threshold (6–10 V, individually tested for each animal) for an observable response.
During pre-training, the tail-mantle reflex was elicited with tactile stimuli to each side of the tail (three pre-test stimuli, 2.5 min. between each side). Training (differential conditioning) commenced 2.5 min after the last pre-test. Training consisted of five bouts of paired stimulation, in which mild tactile stimulation of one of the pre-test sites (the paired side) was paired with the noxious neck stimulus. The unpaired pre-test site was similarly stimulated five times, but without being paired to the noxious stimulus. Paired and unpaired training trials were spaced at 2.5-min intervals. After training, each individual received four post-tests, tactile stimulation on both CS+ and CS− sites, at 15, 20, 25, and 30 min after training.
All tail-mantle reflexes were videotaped, and the analysis of reflex amplitude was later performed by an observer who was ignorant of which CS was paired with the US. This observer scored the time of the tail-mantle withdrawal reflex from its onset until it had relaxed to >70% of its original position (Wright 1998).
Statistics
We tested for learning in each species by performing repeated-measures ANOVA using the MIXED-LINEAR analysis in the Statistics Package for Social Sciences (SPSS). This approach to repeated measures is particularly good for experiments like ours because it does not exclude subjects that have one or two missing observations (ca 30% of subjects in our experiments). For each species, we began by performing a two-way ANOVA on the entire set of reflex responses (pre- and post-training response durations) to detect significant main effects of Training (Paired vs. Unpaired; within-subject effect) and Trials (1–7; within-subject effect). A significant interaction between Training and Trials indicates either a particularly strong acquisition of learning or a rapid change in the strength of the memory after training. To focus on the latter quality, we then performed the same two-way ANOVA, but only on the reflex withdrawals after training. In order to test the significance of individual reflexes, we performed repeated measures t-tests on the four post-training reflexes relative to their mean pre-training reflex. We also used a repeated measures t-test to test whether the Paired and Unpaired reflexes were significantly different from each other. For all individual t-tests, we adjusted the critical α level using the Bonferroni correction for multiple (in our case four) comparisons.
We also compared the memory of each pair of sea hares by performing a repeated measures three-way ANOVA with Trials and Training as within subject (repeated) measures, and Species as a between-subject measure. As with the single species ANOVAs, we did two analyses, one on all of the data, and one on the post-training reflexes alone.
Acknowledgments
We thank the National Science Foundation (IBN- IBN-9632069, IBN-0131743, IBN-0304983) for their support.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.284006
References
- Almaguer-Meliana W., Rojas-Reyesa Y., Alvarea A., Rosilloa J.C., Frey J.U., Bergadoa J.A. Long-term potentiation in the dentate gyrus in freely moving rats is reinforced by intraventricular application of norepinephrine, but not oxotremorine. Neurobiol. Learn. Mem. 2005;83:72–78. doi: 10.1016/j.nlm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bailey C.H., Giustetto M., Huang Y.-Y., Hawkins R.D., Kandel E.R. Is heterosynaptic modulation essential for stabilizing hebbian plasticity and memory? Nat. Rev. Neurosci. 2000a;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Bailey C.H., Giustetto M., Zhu H., Chen M., Kandel E.R. A novel function for serotonin-mediated short-term facilitation in Aplysia: Conversion of a transient cell-wide homosynaptic Hebbian plasticity into a persistent protein synthesis independent synapse-specific enhancement. Proc. Natl. Acad. Sci. 2000b;97:11581–11586. doi: 10.1073/pnas.97.21.11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D., DesGroseillers L., Castellucci V.F., Carew T.J., Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in Aplysia: Evidence of diverse serotonin subtypes. Learn. Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol A.S., Sutton M.A., Carew T.J. Neural circuit of tail-elicited siphon withdrawal in Aplysia. I. Differential lateralization of sensitization and dishabituation. J. Neurophysiol. 2004;91:666–677. doi: 10.1152/jn.00666.2003. [DOI] [PubMed] [Google Scholar]
- Carew T.J., Sutton M.A. Molecular stepping stones in memory consolidation. Nat. Neurosci. 2001;4:769–771. doi: 10.1038/90458. [DOI] [PubMed] [Google Scholar]
- Carew T.J., Walters E.T., Kandel E.R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J. Neurosci. 1981;1:1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T.J., Hawkins R.D., Kandel E.R. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983;219:397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Carew T.J., Hawkins R.D., Abrams T.W., Kandel E.R. A test of Hebb’s postulate at identified synapses which mediate classical conditioning in Aplysia. J. Neurosci. 1984;4:1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L.S., Hawkins R.D., Kandel E.R., Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J. Neurosci. 1994a;14:368–383. doi: 10.1523/JNEUROSCI.14-01-00368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot L.S., Kandel E.R., Hawkins R.D. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J. Neurosci. 1994b;14:3280–3292. doi: 10.1523/JNEUROSCI.14-05-03280.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon N.J., DeMartini L.J., Wright W.G. Dissociation between sensitization and learning-related neuromodulation in an aplysiid species. J. Comp. Neurol. 1999;408:506–514. doi: 10.1002/(sici)1096-9861(19990614)408:4<506::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Frey U., Schroeder H., Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Frey S., Bergado-Rosado J., Seidenbecher T., Pape H.-C., Frey J.U. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J. Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggers U., Menzel R. Memory dynamics and foraging strategies of honeybees. Behav. Ecol. Sociobiol. 1993;32:17–29. [Google Scholar]
- Hawkins R.D., Abrams T.W., Carew T.J., Kandel E.R. A cellular mechanism of classical conditioning in Aplysia: Activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R.D., Carew T.J., Kandel E.R. Effects of interstimulus interval and contingency on classical conditioning of the Aplysia siphon withdrawal reflex. J. Neurosci. 1986;6:1695–1701. doi: 10.1523/JNEUROSCI.06-06-01695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D.A., Walters E.T. Differential classical conditioning of tail and siphon withdrawal in Aplysia. Soc. Neurosci. 1984;10:270. [Google Scholar]
- Kandel E.R., Abrams T., Bernier L., Carew T.J., Hawkins R.D., Schwartz J.H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb. Symp. Quant. Biol. 1983;48:821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kay A. Hawaiian marine shells. Bernice Pauahi Bishop Museum; Honolulu, HI: 1979. [Google Scholar]
- Mackey S.L., Glanzman D.L., Small S.A., Dyke A.M., Kandel E.R., Hawkins R.D. Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: Possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc. Natl. Acad. Sci. 1987;84:8730–8734. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus E.A., Nolen T.G., Rankin C.H., Carew T.J. Behavioral dissociation of dishabituation, sensitization, and inhibition in Aplysia. Science. 1988;241:210–213. doi: 10.1126/science.3388032. [DOI] [PubMed] [Google Scholar]
- Marinesco S., Duran K.L.D., Wright W.G. Evolution of learning in three aplysiid species: Differences in heterosynaptic plasticity contrast with conservation in serotonergic pathways. J. Physiol. 2003;550:241–253. doi: 10.1113/jphysiol.2003.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J. Neuroscience—Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. [A] 1999;185:323–340. [Google Scholar]
- Nolen T.G., Johnson P.M., Kicklighter C.E., Capo T. Ink secretion by the marine snail Aplysia californica enhances its ability to escape from a natural predator. J. Comp. Physiol. [A] 1995;176:239–254. [Google Scholar]
- Pennings S. Predator-prey interactions in opisthobranch gastropods: Effects of prey body size and habitat complexity. Mar. Ecol. Prog. Ser. 1990;62:95–101. [Google Scholar]
- Ricketts E.F., Calvin J., Hedgpeth J.W., Phillips D.W. Between pacific tides, 5th ed. Stanford University Press.; Stanford, CA: 1985. [Google Scholar]
- Rogers C.N., de Nys R., Steinberg P.D. Effects of algal diet on the performance and susceptibility to predation of the sea hare Aplysia parvula. Mar. Ecol. Prog. Ser. 2002;236:241–254. [Google Scholar]
- Ross F., Wilder L., Tillet S.L., Wright W.G. Sub-lethal attack by Panulirus interuptus (Crustacea) produces sensitization in Aplysia californica. 2006. Abstract Viewer/Itinerary Planner, Program No. 813.12. Society for Neuroscience, Washington, D.C.
- Sutton M.A., Masters S.E., Bagnall M.W., Carew T.J. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Bagnall M.W., Sharma S.K., Shobe J., Carew T.J. Intermediate-term memory for site-specific sensitization in Aplysia is maintained by persistent activation of protein kinase. J. Neurosci. 2004;24:3600–3609. doi: 10.1523/JNEUROSCI.1134-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.S., Rodriguez A., Tillett S.L., Wright W.G. Sublethal attack by Navanax inermis (Phylum, Mollusca) produces sensitization in Aplysia californica. 2006. Abstract Viewer/Itinerary Planner, Program No. 813.11. Society for Neuroscience, Washington, D.C.
- Walters E.T., Byrne J.H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983b;219:405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E.T., Byrne J.H., Carew T.J., Kandel E.R. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimuli. J. Neurophysiol. 1983;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Wright W.G. Behavioral analysis of a “phylogenetic lesion”. Neurobiol. Learn. Mem. 1998;69:326–337. doi: 10.1006/nlme.1998.3829. [DOI] [PubMed] [Google Scholar]
- Wright W.G., Carew T.J. A single identified interneuron gates tail-shock induced-inhibition in the siphon withdrawal reflex of Aplysia. J. Neurosci. 1995;15:790–797. doi: 10.1523/JNEUROSCI.15-01-00790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W.G., Kirschman D., Rozen D., Maynard B. Phylogenetic analysis of learning-related neuromodulation in molluscan mechanosensory neurons. Evolution Int. J. Org. Evolution. 1996;50:2248–2263. doi: 10.1111/j.1558-5646.1996.tb03614.x. [DOI] [PubMed] [Google Scholar]