Abstract
The present study used event-related potentials (ERPs) to investigate the underlying neural mechanisms of visual affective priming. Eighteen young native English-speakers (6 males, 12 females) participated in the study. Two sets of 720 prime-target pairs (240 affectively congruent, 240 affectively incongruent, and 240 neutral) used either words or pictures as primes and only words as targets. ERPs were recorded from 64 scalp electrodes while participants pressed either “Happy” or “Sad” buttons to indicate target pleasantness. The response time (RT) results confirmed an affective priming effect, with faster responses to affectively congruent trials (659 ms) than affectively incongruent trials (690 ms). Affectively incongruent trials had larger and more negative N200 activation than those to neutral trials. Importantly, a delayed N400 for word prime-target pairs matched the RT results with larger negative amplitudes for incongruent than congruent pairs. This finding suggests that the N400 component is not only sensitive to semantic mismatches, but is also sensitive to affective mismatches for word prime-target pairs.
Keywords: Valence, Semantic, EEG/ERP, Visual evaluative task, N200, N400
1. Introduction
Priming refers to the phenomenon that previous experience with a given stimulus (e.g., word or picture) biases or alters subsequent behavior (e.g., faster response time, improved accuracy, or biased response to the same or similar stimulus). Affective priming occurs when the processing of a positive stimulus (e.g., love) is faster and more accurate when it is preceded by a positive stimulus (e.g., sunshine) rather than a negative stimulus (e.g., death). The time needed to evaluate a target item as either “positive” or “negative” is significantly shorter when its prime shares the same valence (i.e., positive-positive or negative-negative; affectively congruent) in comparison to a prime and target of opposite valence (i.e., positive-negative or negative-positive; affectively incongruent).
Unlike semantic priming and repetition priming which can last for minutes or days, affective priming is short-lived in time [18,24,27,31]. Thus, the timing between prime and target stimuli seems to be more critical for obtaining affective priming than for other forms of priming. In typical affective priming studies (e.g., [18]), a positive or negative prime is presented for 200 msec and then followed by a positive or negative target after an inter-stimulus interval (i.e., ISI) of 100 msec, resulting in a stimulus onset asynchrony (SOA, the interval between the onset of the prime and the onset of the target) of 300 msec. Short SOAs at or below 300 msec have been shown to be an important parameter for obtaining robust affective priming effects [30].
In recent years, various experimental tasks including evaluative categorization [15,24,26,31,49], naming [2,16,20,23,24], and lexical decision tasks [25,26,52,53] have been utilized to elicit affective priming effects. However, some failures to find affective priming have been reported with pronunciation responses [29,51] and when using a semantic categorization task [15]. Since affective priming is task-dependent and the original evaluative categorization task reliably elicits this priming effect, we chose to use such a task for the current investigation.
Most research on affective priming was done by measuring response time (RT). So far, however, relatively little is known about the neural mechanisms of this priming phenomenon. The high temporal resolution of event-related potentials (ERPs) allows for a more detailed analysis of the time course of neural mechanisms that constitute affective priming. Previous studies (e.g., [12,28,35]) have shown that ERPs are effective at revealing neural responses associated with semantic priming effects. They reported that semantically related target words evoked a smaller N400 (i.e., a negative component observed in ERPs around 400 msec after the target onset) than non-related targets, termed the N400 priming effect. The source of the N400 is thought to be localized to the superior temporal lobes, which are related to semantic processing [34]. The underlying mechanisms for this N400 priming effect have been interpreted in terms of spreading of activation within a semantic network (e.g., [13,14]) or integration of semantic information (e.g., [7,8,12]).
Recently Schirmer and colleagues [45] used ERPs to investigate the influence of emotional prosody context on written word processing (i.e., cross-model affective priming effect) with a lexical decision task. The written emotional words were either congruous or incongruous with the emotional prosody of a preceding spoken sentence. Their results showed that incongruous words elicited a larger N400 than congruous words. The authors suggested that emotional prosody modulates word processing in a similar way as semantic sentence context. In a follow-up study [44], the effect of emotional prosody was replicated in the prosody-word interference task with spoken words that were either congruous or incongruous with the emotional prosody of the speaker (e.g., the word “succeeded” spoken with angry or happy prosody). There was a larger N400 to words that were spoken with incongruous compared to congruous prosody. Similar findings have also been reported by Bostanov and Kotchoubey [3] using auditory emotion name - exclamation pairs. They found a negative wave, the N300, in ERPs to contextually incongruous exclamations, and interpreted this component as analogous to the well-known N400 response to semantically inappropriate words.
Although the discussed ERP studies used auditory prime stimuli to investigate affective priming, whether such results could be expanded to visual prime stimuli including written words and pictures is currently unknown. The objective of the present study is to examine the electrophysiological correlates of the affective priming effect using visual primes.
Spruyt et al. [50] found that replicable affective priming of naming responses can be obtained when pictures are used as primes but not when words are used as primes. Several other researchers, however, used words as primes and found affective priming effects (e.g., [2, 23]). Thus, the current study included both picture-as-prime and word-as-prime conditions. We hypothesized that differential ERPs would be associated with picture and word affective prime targets.
Additionally, a large portion of affective priming studies considered the affective priming effect to constitute differences between affectively congruent trials and affectively incongruent trials, although studies examining other priming effects (e.g., semantic priming effect) typically compared congruent (or related) trials with neutral (or unrelated) trials. Several studies (e.g., [18, 22, 24]), indeed, utilized a neutral control condition to assess the influence of facilitation and inhibition processes in affective priming, and demonstrated facilitation for affectively congruent trials and/or inhibition for affectively incongruent trials. The present study also examined ERPs evoked by neutral and affectively congruent and incongruent trials by applying neutral primes. We hypothesized that affective priming and semantic priming would share a similar mechanism, i.e., an N400-like component indexing affective priming.
2. Method
2.1. Participants
Eighteen right-handed volunteers (6 males, 12 females; M age = 22.39) from the University of Kentucky participated in this experiment. Inclusion criteria were normal or corrected to normal vision, a score of 10 or below on the BECK depression inventory, no history of mental disorders (e.g., clinical anxiety), and English as their first language. All subjects received monetary compensation for participating in the study.
2.2. Stimulus Material & Experimental Design
The stimulus material consisted of 1440 prime-target pairs, which were divided into two groups: 720 prime-target pairs using words as primes and 720 prime-target pairs using pictures as primes. Only words were used as target items. Each group were subdivided into 240 affectively congruent prime-target pairs (120 positive-positive, 120 negative-negative), 240 affectively incongruent prime-target pairs (120 positive-negative, 120 negative-positive), and 240 neutral prime-target pairs (120 neutral-negative, 120 neutral-positive). No prime-target pairs had a semantic or associative relationship beyond affect type.
Prime stimuli consisted of 120 positive, 120 negative, and 240 neutral words from the Affective Norms for English Words (ANEW) [4], as well as 120 positive, 120 negative, and 80 neutral pictures from the International Affective Picture System (IAPS) [33]. The mean valence at a 1-9 point scale (with 9 being the most positive in valence dimension) was 2.38 for negative words, 7.61 for positive words, 5.09 for neutral words, 2.52 for negative pictures, 7.49 for positive pictures, and 4.98 for neutral pictures, respectively. In order to make 1440 prime-target pairs, each positive and negative prime was used in two separate pairs, each neutral prime word was used in only one pair, and each neutral prime picture was used in three separate pairs.
Word targets consisted of 240 words selected from ANEW [4] and equally divided in valence (120 positive words, M = 7.64; 120 negative words, M = 2.44; 1-9 point scale with 9 being the most positive in valence dimension). In order to make 1440 prime-target pairs, each target was used in six separate pairs.
To reduce repetition times of primes and targets, 1440 prime-target pairs were assigned to three lists. Each list contained 240 prime-target pairs using words as primes (80 affectively congruent, 80 affectively incongruent, and 80 neutral) and 240 prime-target pairs using pictures as primes (80 affectively congruent, 80 affectively incongruent, and 80 neutral). In each list, only targets were repeatedly presented twice (i.e., targets in the picture-as-prime condition were the same as those in the word-as-prime condition). Each participant received a list. Six participants received the first list, six participants received the second list, and the remaining six participants received the third list.
Figure 1 shows the timing of a trial. Each trial began with a fixation point presented for 300 msec. Following the fixation, a prime stimulus was displayed on the screen for 100 msec (for words) or 200 msec (for pictures),1 and was replaced by the fixation point for 100 msec. After disappearance of the fixation point, a target stimulus was presented for 300 msec, followed by a 2500∼2700 msec presentation of another fixation point. All words and pictures were presented against a black background on a computer screen. To avoid possible order effects, presentation order of picture and word conditions was counterbalanced across subjects.
Figure 1.
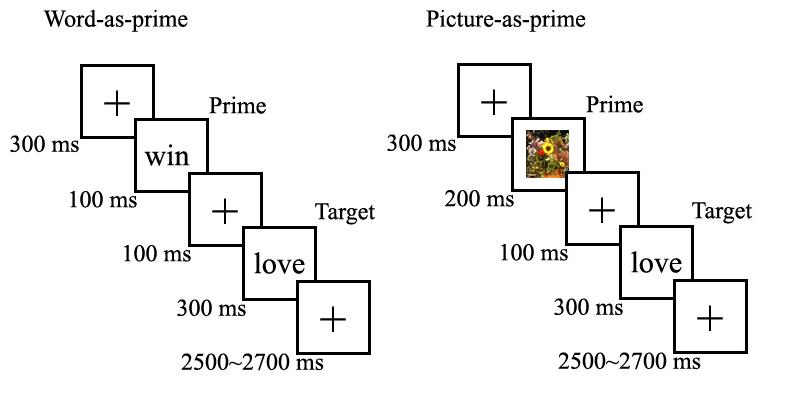
The affective priming task and timing of visual stimuli. The left panel shows a trial using a word as a prime. The right illustrates a trial using a picture as a prime.
2.3. Evaluative Categorization Task
Participants were told that a word or picture would be briefly presented on the screen and be immediately followed by a word. Participants were instructed to observe the first item (i.e., prime) but only respond to the second word (i.e., target). Responses were based on whether the target word was pleasant or unpleasant. A keypad with a happy face and sad face indicated to participants which of the two buttons to press. Participants were also instructed to respond as quickly and accurately as possible and to refrain from head movements and eye blinking. A 2-minute break after each 60-trial block was provided to help offset excessive movements. Prior to testing, each participant performed 60 practice trials to ensure that the testing procedure was well understood.
2.4. ERP recordings
Electroencephalogram (EEG) was recorded from 64 scalp electrodes (Figure 2) using an electrode cap with Ag/AgCl inserts. All scalp electrodes were referenced to an electrode placed between Cz and CPz. Vertical electrooculogram (VEOG) and horizontal electrooculogram (HEOG) were recorded with two pairs of electrodes, one pair placed above and below left eye, and another pair placed beside the two eyes. EEG signals were filtered with a bandpass of 0.1∼40 Hz and sampled at a rate of 500 Hz. Impedance was less than 5KΩ. Average ERPs were formed offline from correct-response trials free of ocular and movement artifacts (>±75μV). Each averaging epoch lasted 1200 msec (for the word-as-prime condition) or 1300 msec (for the picture-as-prime condition) with an additional 100 msec recorded prior to prime onset to allow for baseline correction. Each scalp site resulted in six separate ERPs. The mean number of individual trials per waveform was 58.
Figure 2.
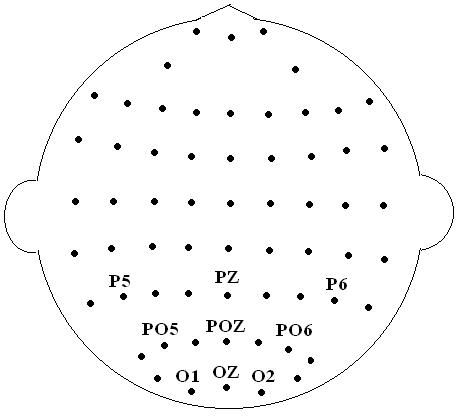
The approximate locations of the electrode sites.
2.5. ERP Statistical Analyses
A visual inspection of the waveforms and topographies were initially conducted to detect obvious differences among three affective congruency conditions. Additionally, explorative analyses involving 2 (prime type) X 3 (affective congruency) ANOVAs were applied to each electrode site at 50 msec consecutive samples in order to reveal consistent differences among the three affective congruency conditions during the time course of the recording epoch. The significant affective congruency effects were found mainly at parieto-occipital electrodes and at 180-280 and 480-680 msec time windows post target onset.
Based on these explorative analyses, mean amplitudes were computed at time windows 180-280 and 480-680 msec for each subject and condition type. The amplitude measurements were referenced to pre-prime baseline activity. Mean amplitude data for each time window was subjected to a 2 prime type (word, picture) X 3 affective congruency (congruent, incongruent, neutral) X 9 electrode site (P5, Pz, P6, PO5, POz, PO6, O1, Oz, O2) repeated measures analysis of variance (ANOVA) test. Significance levels were set at 0.05 and Greenhouse-Geisser corrections were reported with all effects having two or more degrees of freedom in the numerator. All significant ANOVA effects were supplemented with Bonferroni pairwise comparisons or simple main effects comparisons when appropriate.
3. Results
3.1. Behavioral results
Table 1 presents the mean response time to target stimuli (RT)2 and response consistency (i.e., % of responses consistent with ANEW [for words] or IAPS [for pictures] valence scores) data for each stimulus type and affective condition [4, 33].
Table 1.
Mean response time (msec) and percentage of responses that were consistent with norms for each condition
| affectively congruent |
neutral | affectively incongruent |
|
|---|---|---|---|
| Words as primes | 655(92%) | 666(92%) | 681(88%) |
| Pictures as primes | 664(94%) | 666(92%) | 699(89%) |
A 2 prime stimulus type (word, picture) X 3 affective congruency (congruent, incongruent, neutral) repeated measures ANOVA was performed for RT and response consistency data. Analysis of RT revealed a significant main effect for affective congruency, F(2,34) = 22.26, p < 0.001. Pairwise comparisons showed that responses to affectively incongruent trials were slower than neutral trials (p < 0.005) and affectively congruent trials (p < 0.001). No interaction effects for RT data nor any significant effects for response consistency data were found.
3.2. ERP results
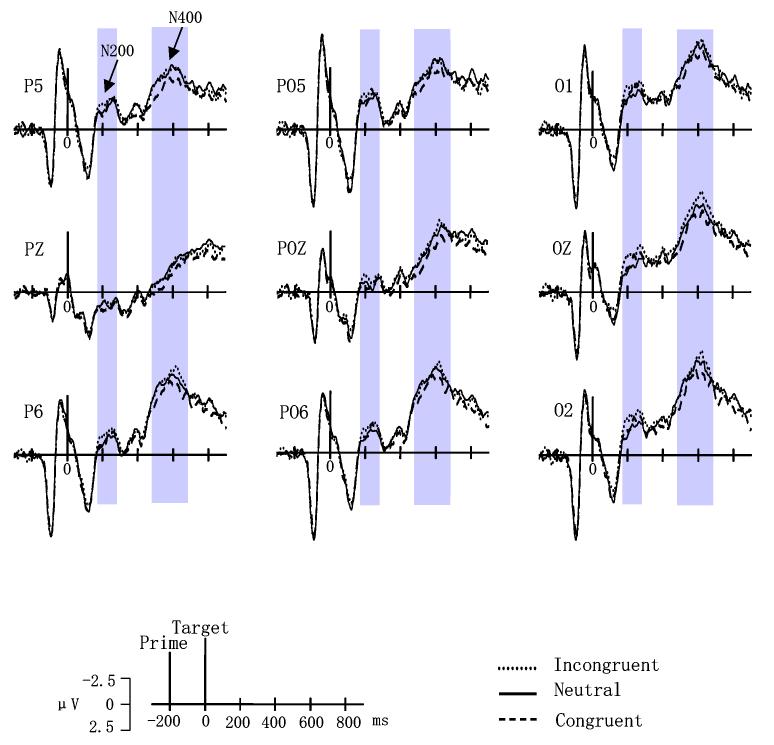
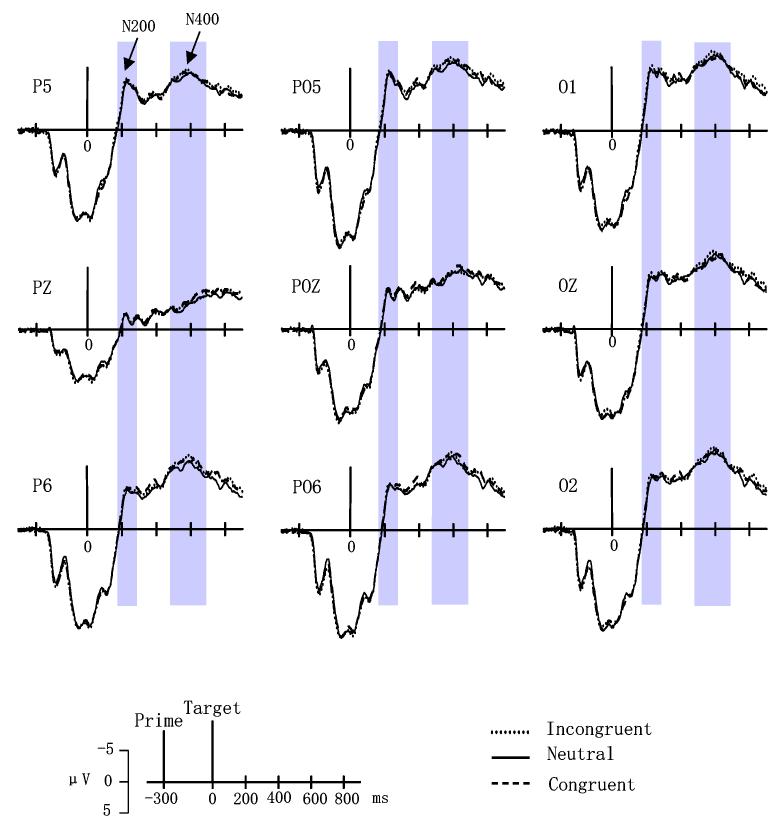
The grand mean ERPs elicited by affectively congruent, affectively incongruent, and neutral trials in the word-as-prime and picture-as-prime conditions are shown in Figures 3 and 4, respectively.
Figure 3.
Grand mean event-related brain potentials (ERPs) from P5, PZ, P6, PO5, POZ, PO6, O1, OZ and O2 to affectively congruent, incongruent, and neutral trials in the word-as-prime condition.
Figure 4.
Grand mean ERPs from P5, PZ, P6, PO5, POZ, PO6, O1, OZ and O2 to affectively congruent, incongruent, and neutral trials in the picture-as-prime condition.
180—280 msec
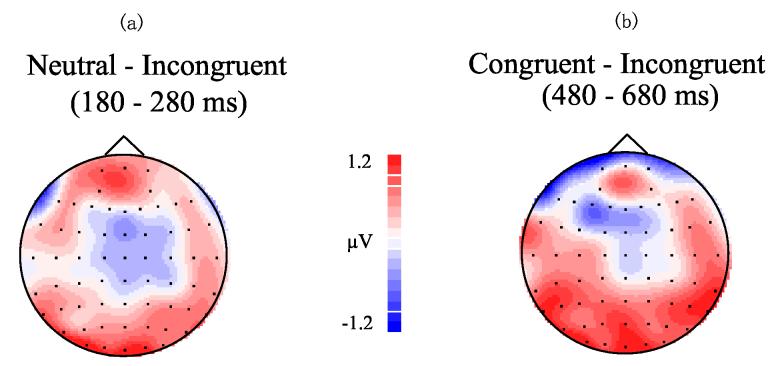
This time interval corresponded to a negative-going wave (N200) that peaked around 250 msec at parietal and occipital sites. A significant main effect of prime stimulus type, F(1,17) = 40.602, p < 0.0005, indicated that trials with pictures as primes had greater negativity than word as prime trials. The interaction between electrode site and affective congruency also reached significance, F(16,272) = 2.629, p < 0.05. Tests for simple effects indicated no differences in affective congruency at any electrode site for the picture-as-prime condition. In the word-as-prime condition, however, voltages to affectively incongruent trials were more negative than those to neutral trials at sites Oz and O2 (all p < 0.05). Figure 5(a) shows the topography of difference waves (subtracting incongruent trials from neutral trials) in this time window.
Figure 5.
Topographies of difference waves in the word-as-prime condition. (a) subtracting incongruent trials from neutral trials in 180-280 msec after target onset; (b) subtracting incongruent trials from congruent trials in 480-680 msec after target onset.
480—680 msec
This time interval encompassed a negative-going wave (N400) peaking around 600 msec. A significant main effect of prime stimulus type, F(1,17) = 73.582, p < 0.0005, indicated that trials with picture primes had larger overall negative amplitudes than word prime trials. The interaction between affective congruency and prime stimulus type also reached significance, F(2,34) = 3.453, p < 0.05. Simple effects indicated no significant affective congruency effects in the picture-as-prime condition (Figure 4). For the word-as-prime condition, however, voltages to affectively incongruent trials were more negative than those to affectively congruent trials (p < 0.05). Fig. 5(b) illustrates the topography of difference waves (subtracting incongruent trials from congruent trials) in this time window.
4. Discussion
The current study applied behavioral and ERP measures to compare affective priming when pictures or words were used as primes. Previous behavioral studies have reported the affective priming effect using pictures [20,23,50] or words [18,24,31] as primes. Behaviorally, the present study replicated the affective priming effects, i.e., responses to affectively congruent trials were faster than those to affectively incongruent trials using both word and picture primes. In order to assess the influence of facilitation and inhibition processes in affective priming, the present study also used neutral primes. Inconsistent with some studies [22,24], our RT results showed that responses to neutral trials were significantly faster than affectively incongruent trials, but the difference between neutral and affectively congruent trials failed to reach significance. These findings suggest that affective priming may result from inhibition during the processing of affectively incongruent primes. Incongruent primes disrupted these affective processes while congruent primes had no effect. RT results alone, however, can not determine whether inhibition is linked to semantic or decision-making processes. Our ERP data showed that an N200 component distinguished incongruent prime-target pairs from neutral pairs, although incongruent pairs were not clearly distinguishable from affectively congruent pairs. For word primes, however, a N400 component indexed affective priming with larger negative amplitudes occurring for incongruent than congruent pairs.
For both the N200 and N400 components, consistently larger negative amplitudes were found when pictures were used as primes than when words were used as primes. This effect could simply be related to cortical processes specific to stimulus type (i.e., pictures versus words), or may reflect stimulus type differences/similarities between the prime and target. Since only words were used as target items, the use of pictures as primes may have evoked greater negativity by virtue of their stimulus mismatch between the prime and target. The latter interpretation seems most appropriate since this component is sensitive to changes in stimulus features and has a similar amplitude distribution (i.e., maximal at occipital / temporal sites) [17].
Affective priming was indexed by a N400 component with incongruent trials having larger negative amplitudes than congruent trials in the word as prime condition. This result parallels several previous studies [3,45], which investigated affective priming effects using auditory stimuli (e.g., emotional prosody, auditory emotion name) as primes and using written emotional words or auditory exclamations as targets. Schirmer et al. [45] found that incongruous words elicited a larger N400 than congruous words and suggested that emotional prosody context operates in a similar way as semantic sentence context. Also, Bostanov and Kotchoubey [3] found a negative wave, the N300, that indexed contextually incongruous exclamations, and interpreted this component as analogous to the well-known N400 response to semantically inappropriate words. Because semantically unrelated word pairs were found to produce more negative N400 than the related word pairs in many studies [12,28,34,35], our results also suggest that this previously known index (N400) of semantic priming should be extended to affective priming. Furthermore, a recent brain imaging study (fMRI) has shown that affective incongruity activates similar brain region as semantic incongruity [46]. N400 has been previously shown to be sensitive to semantic features (e.g., [43]), which supports the semantic account for affective priming.
Like semantic priming, repetition priming also elicits a much larger reduction in N400 amplitude [38,48]. In addition, a study on phonological priming [41] showed that the amplitude of a negative component, N400 peaking around 450 msec, was larger for non-priming than for priming words. Radeau et al. [39] examined semantic, phonological, and repetition priming for auditory words using both behavioral RTs and ERPs measures. They found that both phonological and semantic priming seemed to evoke the same ERP component, namely the N400. In the present study, we carefully normed each prime-target pair in the attempt to eliminate any semantic relationship other than valence. Based on these studies and the present results, we postulate that N400 components are not only associated with semantic and phonological mismatches, but are also elicited in response to affective mismatches for word pairs. Of course, its latency was modulated by the onset of the prime prior to the target. That is, the negativity peaking around 600 msec in our study is a delayed N400.
During the 480-680 msec interval, ERP mean amplitudes reflected the order of the behavioral RT results, but ERP amplitudes to neutral trials was very close to ERP amplitudes to incongruent trials and was larger than ERP amplitudes to congruent trials (see Fig.3). The fact that voltages to neutral and affectively incongruent trials were very close and they all were more negative than the voltages to affectively congruent trials may indicate that primarily primes facilitate the semantic processing of affectively congruent targets, but less inhibit the semantic processing of affectively incongruent targets. However, during 180-280 msec after target onset, ERPs to neutral trials was more positive than those to affectively incongruent trials. In other words, this early component may indicate an emotional process, i.e., emotional arousal (positive or negative, especially negative) versus neural stimuli. Some studies also obtain similar results that an early negative ERP component, N200 [47] or N300 [9] has been associated with reactions to the valence of visual stimuli.
Although our behavioral results showed clear affective priming effects for both word-as-prime and picture-as-prime conditions, the ERP indices of affective priming only reached significance in the word-as-prime condition. One possible interpretation for our data can be that RTs and ERPs might be sensitive to different cognitive operations, and affective priming is a phenomenon that can be induced by several mechanisms [15]. Some researchers reported inconsistencies between RT studies and ERP studies (e.g., [32, 54]). For example, Kounios and Holcomb showed that RTs and N400 amplitudes were not correlated in a sentence verification task [32]. They argued that RTs and ERPs may not necessarily tap into the same set of underlying cognitive operations. Consistent with their opinion, we also assume that RT might be much more sensitive to participants' decision-making processes and task-dependent strategies than N400 amplitude, but N400 was sensitive primarily to semantic processing rather than changes in the decision-making process. Based on such inconsistencies and our results, we speculated that affective priming could arise from the semantic processing and decision-making processes in the word-as-prime condition. In the picture-as-prime condition, however, affective priming could arise only from the decision-making processes because of stimulus type or SOA differences between the prime and target So the present study showed only affective priming behaviorally in the picture-as-prime condition.
In fact, some researchers found evidence for different or dual semantic systems that process pictures and words independently (e.g., [11, 36]). Yet, others studies suggested that an overlapping semantic system underlies the processing of meaning represented by words and pictures (e.g., [19,21,40]). Although our results indicate that words and pictures show affective priming behaviorally, the neural processes underlying processing of emotional pictures and words may be different. This could explain why we obtained significant ERP results using words but not pictures as primes.
Current explanations of affective priming (for a review, see[30]) are mostly adopted from semantic processing or response selecting without much consideration for emotional processes in the brain. One future direction is to examine the potential interplay between neuroanatomical sources of the classic N400 and the brain structures that are involved in emotional processes has been clearly established in both humans and monkeys. Specifically, the amygdala is an important mediator of emotional influences on perception. Research has revealed anatomical and functional associations between the amygdala and visual cortex, where amygdala receives visual inputs from ventral visual pathways and sends feedback projections to processing stages within this pathway [1,5,6,10,37,42]. Further research should examine the role and potential interplay between the amygdala and the ventral temporal visual cortex on affective priming processes.
Conclusions
The present study found both RT and ERP indices of affective priming using an evaluative categorization task. The delayed N400 effect found with word primes suggests that this negative deflection indexes affective mismatches between primes and targets. Our findings are consistent with the semantic account of affective priming. Future examination of the neural mechanisms underlying affective priming will further our understanding of the processes related to semantic versus affective mismatches.
Acknowledgments
This work was presented at the 34th Annual Meeting of SfN, 2004. This work was supported by NIH Grant AG00986 and a pilot grant as part of NIH grant P50 AG05144-21to Y. J., Chinese Ministry of Education grant 20040028001 and National Natural Science Foundation of China (No.30570603) to C. Y., and Beijing grant 20041D0501607 to Q. Z..
Footnotes
Pilot testing indicated that word primes were accurately perceived with a stimulus duration of 100 msec while picture stimuli required 200 msec for comparable accuracy scores.
Any trial having a response inconsistent with its previously determined norm or having a RT value outside of a predetermined 300 - 1500 msec range was excluded from mean RT calculations (1.6% to all trials).
References
- 1.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 2.Bargh JA, Chaiken S, Raymond P, Hymes C. The automatic evaluation effect: Unconditional automatic activation with a pronunciation task. J. Exp. Social Psychol. 1996;32:104–128. [Google Scholar]
- 3.Bostanov V, Kotchoubey B. Recognition of affective prosody: Continuous wavelet measures of event-related brain potentials to emotional exclamations. Psychophysiology. 2004;41:259–268. doi: 10.1111/j.1469-8986.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradley MM, Lang PJ. Affective norms for English words (ANEW) The NIMH Center for the Study of Emotion and Attention, University of Florida; Gainesville, FL: 1999. [Google Scholar]
- 5.Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King WK, Desai P. Activation of the visual cortex in motivated attention. Behav. Neurosci. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- 6.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 7.Brown C, Hagoort P. The processing nature of the N400: evidence from masked priming. J. Cogn. Neurosci. 1993;5:34–44. doi: 10.1162/jocn.1993.5.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Brown C, Hagoort P, Chwilla D. An event-related brain potential analysis of visual word-priming effects. Brain Lang. 2000;72:158–190. doi: 10.1006/brln.1999.2284. [DOI] [PubMed] [Google Scholar]
- 9.Carretie L, Iglesias J, Garcia T. A study on the emotional processing of visual stimuli through event-related potentials. Brain Cogn. 1997;34:207–217. doi: 10.1006/brcg.1997.0895. [DOI] [PubMed] [Google Scholar]
- 10.Catani M, Jones DK, Donato R, ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 11.Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat. Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 12.Chwilla DJ, Brown CM, Hagoort P. The N400 as a function of the level of processing. Psychophysiology. 1995;32:274–285. doi: 10.1111/j.1469-8986.1995.tb02956.x. [DOI] [PubMed] [Google Scholar]
- 13.Deacon D, Uhm TJ, Ritter W, Hewitt S, Dynowska A. The lifetime of automatic semantic priming effects may exceed two seconds. Cogn. Brain Res. 1999;7:465–472. doi: 10.1016/s0926-6410(98)00034-2. [DOI] [PubMed] [Google Scholar]
- 14.Deacon D, Hewitt S, Yang CH, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: evidence that the N400 does not reflect a post-lexical process. Cogn. Brain Res. 2000;9:137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 15.De Houwer J, Hermans D, Rothermund K, Wentura D. Affective priming of semantic categorization responses. Cogn. Emot. 2002;16:643–666. [Google Scholar]
- 16.De Houwer J, Hermans D, Spruyt A. Affective priming of pronunciation responses: Effects of target degradation. J. Exp. Social Psychol. 2001;37:85–91. [Google Scholar]
- 17.Fabiani M, Gratton G, Coles MGH. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd ed. Cambridge Univ. Press; Cambridge: 2000. pp. 53–84. [Google Scholar]
- 18.Fazio RH, Sanbonmatsu DM, Powell MC, Kardes FR. On the automatic activation of attitudes. J. Pers. Social Psychol. 1986;50:229–238. doi: 10.1037//0022-3514.50.2.229. [DOI] [PubMed] [Google Scholar]
- 19.Federmeier KD, Kutas M. Meaning and modality: influences of context, semantic memory organization, and perceptual predictability on picture processing. J. Exp. Psychol. Learn. Mem. Cogn. 2001;27:202–224. [PubMed] [Google Scholar]
- 20.Giner-Sorolla R, Garcia MT, Bargh JA. The automatic evaluation of pictures. Social Cogn. 1999;17:76–96. [Google Scholar]
- 21.Glaser WR. Picture naming. Special issue: Lexical access in speech production. Cognition. 1992;42:61–105. doi: 10.1016/0010-0277(92)90040-o. [DOI] [PubMed] [Google Scholar]
- 22.Hermans D, Crombez G, Eelen P. Automatic attitude activation and efficiency: The fourth horseman of automaticity. Psychologica Belgica. 2000;40:3–22. [Google Scholar]
- 23.Hermans D, De Houwer J, Eelen P. The affective priming effect: Automatic activation of evaluative information in memory. Cogn. Emot. 1994;8:515–533. [Google Scholar]
- 24.Hermans D, De Houwer J, Eelen P. A time course analysis of the affective priming effect. Cogn. Emot. 2001;15:143–165. [Google Scholar]
- 25.Hermans D, De Houwer J, Smeesters D, Van den Broeck A. Affective priming with associatively unrelated primes and targets; Paper presented at the 6th Tagung der Fachgruppe Sozialpsychologie; Konstanz, Germany. 1997, June. [Google Scholar]
- 26.Hermans D, Smeesters D, De Houwer J, Eelen P. Affective priming for associatively unrelated primes and targets. Psychologica Belgica. 2002;42:191–212. [Google Scholar]
- 27.Hermans D, Spruyt A, Eelen P. Automatic affective priming of recently acquired stimulus valence: Priming at SOA 300 but not at SOA 1000. Cogn. Emot. 2003;17:83–99. doi: 10.1080/02699930302276. [DOI] [PubMed] [Google Scholar]
- 28.Holcomb PJ, Neville HJ. Auditory and visual semantic priming in lexical decision: a comparison using event-related potentials. Lang. Cogn. Processes. 1990;5:281–312. [Google Scholar]
- 29.Klauer KC, Musch J. Does sunshine prime loyal? Affective priming in the naming task. Quarterly J. Exp. Psychol. 2001;54A:727–751. doi: 10.1080/713755986. [DOI] [PubMed] [Google Scholar]
- 30.Klauer KC, Musch J. Affective priming: Findings and theories. In: Musch J, Klauer KC, editors. The psychology of evaluation: Affective processes in cognition and emotion. Lawrence Erlbaum; Mahwah, NJ: 2003. pp. 7–49. [Google Scholar]
- 31.Klauer KC, Rossnagel C, Musch J. List-context effects in evaluative priming. J. Exp. Psychol. Learn. Mem. Cogn. 1997;23:246–255. doi: 10.1037//0278-7393.23.1.246. [DOI] [PubMed] [Google Scholar]
- 32.Kounios J, Holcomb PJ. Structure and process in semantic memory: Evidence from event-related brain potentials and reaction times. J. Exp. Psychol. General. 1992;121:459–479. doi: 10.1037//0096-3445.121.4.459. [DOI] [PubMed] [Google Scholar]
- 33.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. The Center for Research in Psychophysiology; Gainesville, Florida: 1999. (Technical Report A-4). [Google Scholar]
- 34.Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N. Linking semantic priming effect in functional MRI and event-related potentials. NeuroImage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Nobre AC, McCarthy G. Language-related ERPs: scalp distributions and modulation by word type and semantic priming. J. cogn. Neurosci. 1994;6:233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- 36.Paivio A. Dual coding theory: retrospect and current status. Can. J. Psychol. 1991;45:255–287. [Google Scholar]
- 37.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc. Natl. Acad. Sci. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickering EC, Schweinberger SR. N200, N200 r, and N400 event-related brain potentials reveal three loci of repetition priming for familiar names. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29:1298–1311. doi: 10.1037/0278-7393.29.6.1298. [DOI] [PubMed] [Google Scholar]
- 39.Radeau M, Besson M, Elisabeth Fonteneau E, Castro SL. Semantic, repetition and rime priming between spoken words: behavioral and electrophysiological evidence. Biological Psychol. 1998;48:183–204. doi: 10.1016/s0301-0511(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 40.Riddoch MJ, Humphreys GW, Coltheart M, Funnell E. Semantic systems or system? Neuropsychological evidence re-examined. Cogn. Neuropsychol. 1988;5:3–25. [Google Scholar]
- 41.Rugg M. Event-related potentials and the phonological processing of words and nonwords. Brain Lang. 1984;23:225–240. doi: 10.1016/0093-934x(84)90065-8. [DOI] [PubMed] [Google Scholar]
- 42.Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Sartori G, Mameli F, Polezzi D, Lombardi L. An ERP study of low and high relevance semantic features. Brain Res. Bull. 2006;69:182–186. doi: 10.1016/j.brainresbull.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Schirmer A, Kotz SA. ERP evidence for a sex-specific Stroop effect in emotional speech. J. Cogn. Neurosci. 2003;15:1135–1148. doi: 10.1162/089892903322598102. [DOI] [PubMed] [Google Scholar]
- 45.Schirmer A, Kotz SA, Friederici AD. Sex differentiates the role of emotional prosody during word processing. Cogn. Brain Res. 2002;14:228–233. doi: 10.1016/s0926-6410(02)00108-8. [DOI] [PubMed] [Google Scholar]
- 46.Schirmer A, Zysset S, Kotz SA, von Cramon DY. Gender differences in the activation of inferior frontal cortex during emotional speech perception. NeuroImage. 2004;21:1114–1123. doi: 10.1016/j.neuroimage.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 47.Schupp HT, Junghofer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: A ERP analysis. Psychophysiology. 2004;41:451–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 48.Schweinberger SR, Pfütze EM, Sommer W. Repetition priming and associative priming of face recognition: Evidence from event-related potentials. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21:722–736. [Google Scholar]
- 49.Sollberger B, Reber R, Eckstein D. Musical chords as affective priming context in a word-evaluation task. Music Perception. 2003;20:263–282. [Google Scholar]
- 50.Spruyt A, Hermans D, De Houwer J, Eelen P. On the nature of the affective priming effect: Affective priming of naming responses. Social Cogn. 2002;20:227–256. [Google Scholar]
- 51.Spruyt A, Hermans D, Pandelaere M, De Houwer J, Eelen P. On the replicability of the affective priming effect in the pronunciation task. Exp. Psychol. 2004;51:109–115. doi: 10.1027/1618-3169.51.2.109. [DOI] [PubMed] [Google Scholar]
- 52.Wentura D. Affektives Priming in der Wortentscheidungsaufgabe: Evidenz fur post-lexikalische Urteilstendenzen [Affective priming in the lexical decision task: Evidence for post-lexical judgement tendencies] Sprache und Kognition. 1998;17:125–137. [Google Scholar]
- 53.Wentura D. Dissociative affective and associative priming effects in the lexical decision task: Responding with “yes” vs. “no” to word targets reveals evaluative judgment tendencies. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:456–469. doi: 10.1037//0278-7393.26.2.456. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, Guo CY, Ding JH, Wang ZY. Concreteness effects in the processing of Chinese words. Brain Lang. 2006;96:59–68. doi: 10.1016/j.bandl.2005.04.004. [DOI] [PubMed] [Google Scholar]