Abstract
Background
The incidence of type 2 diabetes increases with age. It is unknown whether interventions to prevent diabetes are as effective in elderly persons as in younger adults.
Methods
The Diabetes Prevention Program (DPP) demonstrated that an intensive lifestyle intervention (ILS) or metformin could prevent or delay diabetes. A predefined secondary outcome of DPP was to determine if treatment effects varied by age.
Results
At baseline, participants aged 60–85 years were leaner and had the best insulin sensitivity and lowest insulin secretion compared to younger age groups. Diabetes incidence rates did not differ by age in the placebo group, but ILS was more effective with increasing age (6.3, 4.9, and 3.3 cases per 100 person-years, in the 25–44, 45–59, and 60–85 year age groups, respectively; ptrend = .007). Participants aged 60–85 years had the most weight loss and metabolic equivalent (MET)-hours of physical activity. The metformin group showed a trend toward higher diabetes incidence among older participants (6.7, 7.7, and 9.3 cases per 100 person-years in the 25–44, 45–59, and 60–85 year age groups, respectively; ptrend = .07); and diabetes risk increased with age (hazard ratio [age 60–85 vs 25–44] 1.63, p .02), after adjusting for the greater weight loss in the 60–85 year age group.
Conclusions
Lifestyle modification was exceptionally effective in preventing diabetes in older individuals; this finding was largely explained by greater weight loss and physical activity. The limited effectiveness of metformin in older persons may reflect age-related differences in insulin action and secretion. A lifestyle modification program can be recommended for older individuals at high risk for type 2 diabetes.
THE prevalence and incidence of type 2 diabetes increase dramatically as a function of age, affecting more than 30% of U.S. adults 60 years old and older (2). An additional 25% have milder forms of hyperglycemia, including impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), which increase the risk of diabetes (3). The most serious consequence of incident diabetes among elderly persons is a 2-fold increased risk of coronary heart disease, although microvascular complications may also cause significant morbidity in this age group (3-5). Furthermore, there is evidence that different patterns of hyperglycemia (fasting vs postchallenge) may occur at different ages, which may affect diagnosis and treatment (6-8). Therefore, recognition of abnormal glucose metabolism in elderly persons and development of age-appropriate preventive and therapeutic strategies assume major clinical importance.
The Diabetes Prevention Program (DPP), a multicenter, randomized, controlled trial, demonstrated that medication (metformin) or lifestyle modification could prevent or delay the development of diabetes in a high-risk population who had IGT and an elevated fasting glucose. Metformin reduced the development of diabetes by 30%, and lifestyle modification did so by 55% in the cohort as a whole (1,9). Recruitment into the DPP was designed to represent varied ethnic and racial groups, both sexes, and both younger and older individuals to reflect the U.S. population at risk for diabetes. The prevention or delay of diabetes by metformin or lifestyle modification was the primary outcome in DPP, but a predefined secondary outcome was to determine whether treatment effects varied by age, sex, or race. The metabolic defects underlying the development of diabetes and the ability to adhere to lifestyle modification may vary by age and could influence the effectiveness of diabetes prevention in older individuals. This report describes the age differences in response to DPP interventions.
Methods
Design
The eligibility criteria, design, and methods of the DPP have been reported elsewhere (10,11), and the DPP protocol is available at www.bsc.gwu.edu/dpp. Briefly, eligibility criteria included: age ≥25 years, body mass index of ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), and fasting plasma glucose levels between 95 and 125 mg/dL in addition to IGT (2-hour postload glucose of 140–199 mg/dL). Persons were excluded if they had any conditions or medications that would impair their ability to participate. These conditions included recent stroke or cardiovascular hospitalization, congestive heart failure (≥American Heart Association class 3), or chronic pulmonary disease requiring daily treatment or home oxygen therapy. Additionally, participants had to be able to walk 0.25 miles in 10 minutes. All participants provided written informed consent and signed documents approved by the Institutional Review Board at each center. Eligible participants received standard advice on healthy diet and physical activity and were randomized to one of three additional interventions: (a) intensive lifestyle intervention (ILS) with a goal of ≥ 150 min/wk of activity and loss of ≥7% of body weight; (b) metformin 850 mg bid; or (c) matching placebo. The ILS stressed low- to moderate-level aerobic exercise, such as brisk walking, swimming, or bicycling, although participants were permitted to include resistance exercise for up to 20% of the prescribed weekly hours. The dietary recommendations stressed a low-fat, reduced-calorie diet. ILS interventions were delivered via a structured 16-week core curriculum in individual sessions with a lifestyle coach (who usually was a dietician or registered nurse) and was followed by at least semimonthly visits for the duration of the study. ILS goals and procedures were uniform across all age groups.
Outcomes
Development of diabetes was determined by an annual oral glucose tolerance test and semiannual fasting plasma glucose tests, and required confirmation by a second test, using the criteria of the American Diabetes Association and the World Health Organization (12,13). The amount of physical activity was assessed using two standardized and validated questionnaires, the Low-level Physical Activity Recall (LoPAR) and the Modified Activity Questionnaire (MAQ), which were completed annually (14). Physical activity level was calculated as the product of duration and frequency of each activity, weighted by an estimate of the metabolic equivalent (MET) of the activity, with results expressed as average MET-hours per week. Dietary intake was assessed at baseline and year 1 by using a modified version of the Block Food Frequency Questionnaire (15). Medication adherence was determined by pill count at each quarterly visit. Anthropometrics (height, weight, waist circumference) were measured annually using standard techniques. All analytical measurements were performed at the Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle).
Data Analysis
This analysis is based on data collected from the start of DPP (June 1996) through July 31, 2001, after which the study results and treatment assignment were unmasked. Participants were followed for an average of 3.2 years. For these analyses, participants were divided into 3 age groups: 25–44 years, 44–59 years, and 60–85 years, representing young, middle-aged, and older age individuals.
The Kruskal–Wallis test (16) was used to compare the three age groups for baseline characteristics because these characteristics were not normally distributed. Medians and interquartile ranges are reported. Diabetes incidence rates were calculated as the number of participants diagnosed per 100 person-years. For analyses of change during DPP, continuous data are presented as mean (standard error), and change is from baseline, averaged throughout the study using mixed effect models (17). Percentage of participants achieving exercise and weight loss goals were assessed semiannually and modeled using generalized estimation equations (18). Caloric intake was measured at baseline and year 1, and change was modeled using a regular linear model. To correct for some imbalance among the three age groups at baseline, the above models were adjusted for baseline values when applicable. Cox proportional hazards models (19) were used to assess time to diabetes diagnosis, and linear trend was tested among the 3 groups. Time-dependent proportional hazards analyses were performed to adjust for weight change and physical activity change during the study. Pearson's chi-square test was used to compare diabetes conversion patterns by age among the treatment groups. Values of p for pair-wise comparison between any two age groups were adjusted for multiple comparisons by using the step-down Bonferroni method (20). The SAS system was used for all analyses (version 8.2; SAS Institute Inc., Cary, NC).
Results
Baseline characteristics of the DPP cohort by age category are shown in Table 1. Twenty percent of participants were 60 years old or older at the beginning of the study, with a mean age of 66.4 years. The mean ages of the young and middle-aged groups were 38.8 and 51.6 years, respectively. Compared to young and middle-aged participants, older participants were more likely to be male (21% vs 31% vs 49%, p < .0001) and Caucasian (47% vs 55% vs 66%, p < .0001). The 60–85 year age group also had the lowest baseline waist circumference, weight, body mass index, insulin secretion, and insulin resistance. Baseline fasting plasma glucose and 2-hour glucose were similar in all 3 age groups.
Table 1.
Metabolic Characteristics at Baseline According to Age and Treatment Group
| Treatment Group | Measures | 25–44 Years | 45–59 Years | 60–85 Years | p Value* |
|---|---|---|---|---|---|
| Lifestyle | N | 358 | 488 | 233 | |
| Weight, kg | 94.2 (30.4) | 91.0 (24.0) | 85.4 (21.9) | <.001 | |
| Body mass index | 34.4 (9.7) | 32.4 (7.9) | 30.5 (6.6) | <.001 | |
| Waist circumference, cm | 105.7 (22.9) | 102.6 (16.0) | 102.7 (17.3) | .02 | |
| Fasting glucose, mg/dL | 104.0 (11.0) | 105.0 (12.0) | 106.0 (12.0) | .12 | |
| 2-hour glucose, mg/dL | 163.0 (27.0) | 162.0 (28.0) | 161.0 (26.0) | .43 | |
| Insulin resistance (HOMA-IR) | 7.3 (5.2) | 5.7 (4.7) | 5.5 (4.1) | <.001 | |
| Corrected insulin response (CIR30) | 0.6 (0.5) | 0.5 (0.4) | 0.5 (0.4) | <.001 | |
| Metformin | N | 318 | 541 | 214 | |
| Weight, kg | 95.0 (28.0) | 92.2 (26.6) | 86.4 (19.0) | <.001 | |
| Body mass index | 34.4 (9.2) | 33.0 (8.9) | 30.3 (5.4) | <.001 | |
| Waist circumference, cm | 104.5 (20.5) | 104.0 (19.7) | 103.7 (14.1) | .37 | |
| Fasting glucose, mg/dL | 104.0 (12.0) | 105.0 (11.0) | 105.5 (12.0) | .05 | |
| 2-hour glucose, mg/dL | 162.0 (29.0) | 163.0 (29.0) | 165.5 (29.0) | .25 | |
| Insulin resistance (HOMA-IR) | 7.6 (5.6) | 6.2 (4.7) | 5.2 (3.2) | <.001 | |
| Corrected insulin response (CIR30) | 0.6 (0.5) | 0.5 (0.4) | 0.4 (0.4) | <.001 | |
| Placebo | N | 324 | 557 | 201 | |
| Weight, kg | 95.5 (29.3) | 91.5 (27.1) | 87.8 (21.8) | <.001 | |
| Body mass index | 35.3 (10.0) | 32.9 (8.5) | 30.8 (7.6) | <.001 | |
| Waist circumference, cm | 105.7 (21.1) | 103.5 (19.6) | 103.0 (17.8) | .01 | |
| Fasting glucose, mg/dL | 105.0 (13.0) | 106.0 (10.0) | 105.0 (10.0) | .59 | |
| 2-hour glucose, mg/dL | 160.0 (27.5) | 162.0 (27.0) | 166.0 (34.0) | .11 | |
| Insulin resistance (HOMA-IR) | 7.1 (5.2) | 6.2 (4.6) | 5.5 (4.1) | <.001 | |
| Corrected insulin response (CIR30) | 0.6 (0.5) | 0.5 (0.5) | 0.4 (0.5) | <.001 |
Notes: Nonparametric Kruskal–Wallis test was used to test for differences among age groups. Median and interquartile range (IQR) are reported.
HOMA = Homeostasis model assessment.
Figure 1 shows the diabetes incidence rates by age in the 3 treatment arms. Diabetes incidence did not differ by age in the placebo group (11.0, 10.8, and 10.3 cases per 100 person-years in young, middle-aged, and older groups, respectively; p = .71). However, there were significant age differences in the response to metformin and ILS. Although ILS was effective in all age groups, diabetes incidence rates fell with increasing age (6.3 vs 4.9 vs 3.3 cases per 100 person-years in the 25–44, 45–59, and 60–85 year age groups, respectively; Ptrend = .007). In contrast, diabetes incidence in the metformin group was lowest among the youngest participants (6.7 vs 7.7 vs 9.3 cases per 100 person-years; Ptrend = .07), a trend that was of borderline significance. ILS became significantly more effective than metformin with increasing age (p = .005).
Figure 1.
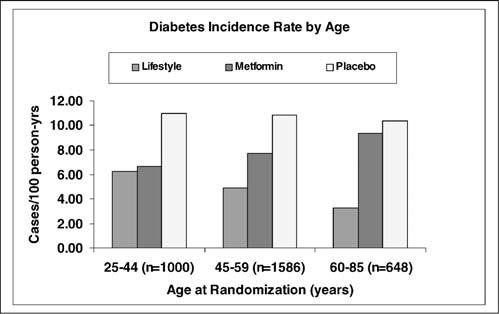
Diabetes incidence rate by age and treatment group.
Variables related to ILS participation by age group are shown in Table 2. Weight loss, reduction in waist circumference, and percentage of participants who achieved the 7% weight loss goal all increased with increasing age. Older participants also reported greater MET-h/wk of recreational activity and were more likely to achieve the exercise goal of 150 min/wk. There were no differences in reduction of reported caloric intake or in the percent reverting to normal glucose tolerance among the age groups.
Table 2.
Lifestyle Changes During DPP, by Age at Randomization
| Metabolic and Activity Variable | 25–44 Years (A) | 45–59 Years (B) | 60–85 Years (C) | Overall p Value |
|---|---|---|---|---|
| Weight change, kg | −4.1 (0.4) | −5.0 (0.3) | −6.4 (0.3) | <.001#,*,† |
| Waist circumference change, cm | −4.3 (0.4) | −4.7 (0.3) | −6.7 (0.4) | <.001*,† |
| Percent at weight loss goal (7% of body weight) | 33 | 39 | 55 | <.001#,*,† |
| Recreational activity change (MET-h/wk) | 4.4 (1.6) | 5.8 (1.3) | 18.7 (2.1) | <.001*,† |
| Leisure activity change (MET-h/wk) | 5.1 (0.8) | 5.2 (0.7) | 8.5 (1.0) | .02*,† |
| Percent at exercise goal (150 min/wk) | 34 | 38 | 48 | <.001*,#,† |
| Percent change in calorie intake (at 1 y) | −18 (2) | −14 (2) | −10 (2) | .05 |
Notes: Continuous data are presented as mean (standard error) and change is from baseline, averaged throughout the study from repeated-measures models. Means are estimated with baseline values adjusted. Percent at goal variables are estimated from generalized estimating equation (GEE) models except for caloric intake. Pair-wise comparisons (p < .05) are marked as:
(A vs B)
(A vs C)
(B vs C).
DPP = Diabetes Prevention Program; MET = metabolic equivalent.
Table 3 displays similar variables for the metformin and placebo groups. As has been previously observed with this drug (21), the metformin participants experienced modest weight loss, which was greatest in the oldest age group. Likewise, waist circumference was reduced, with the greatest change in the 60–85 year age group. In contrast, there were no significant changes in weight or waist circumference at any age in the placebo group. In both the metformin and placebo groups, there was a trend toward increased medication adherence and increased recreational activity with age.
Table 3.
Metformin and Placebo Changes During DPP, by Age at Randomization
| Treatment Group | Metabolic and Activity Variables | 25–44 Years (A) | 45–59 Years (B) | 60–85 Years (C) | Overall p Value |
|---|---|---|---|---|---|
| Metformin | Weight change, kg | −1.5 (0.3) | −1.7 (0.2) | −2.7 (0.3) | .001*,† |
| Waist circumference change, cm | −1.2 (0.4) | −1.7 (0.3) | −2.8 (0.3) | .001*,† | |
| Percent adherent to medication | 66 | 73 | 71 | .01# | |
| Recreational activity change (MET-h/wk) | −3.0 (1.7) | −1.1 (1.2) | 15.6 (2.1) | <.001*,† | |
| Leisure activity change (MET-h/wk) | −0.9 (1.0) | 1.8 (0.8) | 3.1 (1.2) | .04 | |
| Percent change in calorie intake (at 1 y) | −11 (2) | −3 (1) | −4 (1) | .08 | |
| Placebo | Weight change, kg | 0.5 (0.3) | 0.1 (0.2) | −0.2 (0.3) | .19 |
| Waist circumference change, cm | −0.0 (0.3) | −0.5 (0.2) | −0.4 (0.3) | .46 | |
| Percent adherent to medication | 70 | 77 | 81 | .001#,* | |
| Recreational activity change (MET-h/wk) | −0.9 (1.5) | 3.7 (1.4) | 12.8 (2.5) | <.001#,*,† | |
| Leisure activity change (MET-h/wk) | −1.6 (0.8) | 0.2 (0.7) | 4.2 (1.4) | <.01† | |
| Percent change in calorie intake (at 1 y) | −5 (2) | −6 (2) | 1 (1) | .06 |
Notes: Continuous data are presented as mean (standard error) and change is from baseline, averaged throughout the study from repeated-measures models. Means are estimated with baseline values adjusted. Percent at goal variables are estimated from generalized estimating equation (GEE) models except for caloric intake. Pair-wise comparisons (p < .05) are marked as:
(A vs B)
(A vs C)
(B vs C).
DPP = Diabetes Prevention Program; MET = metabolic equivalent.
Diabetes hazard ratios were compared for the age groups (Table 4) by using models that successively controlled for sex and ethnicity (Model 1), baseline metabolic variables (Model 2), and time-dependent change in weight, activity, calorie intake, and metformin adherence (Model 3). For the ILS group, diabetes risk in the 60–85 year age group was approximately one half that in the 25–44 year age group (hazard ratio, 0.47; 95% confidence interval [CI], 0.28–0.78); and there was little change following adjustment for baseline glucose, insulin sensitivity, and insulin secretion (hazard ratio, 0.50; 95% CI, 0.29–0.89). After adjusting for time-dependent change in weight, calorie intake, and activity, the age difference disappeared (hazard ratio, 0.83; 95% CI, 0.46–1.50). Using Model 1 for the metformin group, diabetes risk was greater for the 60–85 year age group (hazard ratio, 1.45; 95% CI, 0.98–2.16), although this difference was of marginal significance. This trend was attenuated following adjustment for baseline metabolic parameters (hazard ratio, 1.26; 95% CI, 0.80–1.93) and remained nonsignificant with adjustment for weight and activity change and medication adherence (hazard ratio, 1.37; 95% CI, 0.87–2.16), which were all greater in the 60–85 year age group.
Table 4.
Age and Diabetes Risk in the Metformin and Lifestyle Group
| Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|
| Treatment Group | Age Group (y) | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
| Metformin | 60–85 vs 25–44 | 1.45 (0.98–2.16) | .06 | 1.26 (0.80–1.93) | .33 | 1.37 (0.87–2.16) | .17 |
| Lifestyle | 60–85 vs 25–44 | 0.47 (0.28–0.78) | <.01 | 0.50 (0.29–0.89) | .02 | 0.83 (0.46–1.50) | .54 |
Notes: Model 1 = Effect of age is adjusted for gender and race/ethnicity. Model 2 = same as Model 1, with further adjustment for baseline weight, activity, fasting glucose, 2-hour glucose, homeostasis model assessment (HOMA), and corrected insulin response at 30 minutes (CIR). Model 3 = same as Model 2, with further adjustment for time-dependent change in weight, activities, and caloric intake at 1 year. For the metformin group, time-dependent adherence is also adjusted.
CI confidence interval.
We also looked for age differences in glycemic patterns at conversion to diabetes. The diagnosis of diabetes in the DPP could be documented by an elevated fasting or 2-hour glucose level, confirmed on retesting. Although these glucose parameters were similar among the age groups at baseline, among those participants who developed diabetes during the DPP, the youngest group was more likely to convert by fasting criteria (62% vs 53% vs 45%), and the oldest group by 2-hour criteria (38% vs 47% vs 55%; p < .01), an observation consistent with previous reports (22). This pattern of diabetes conversion was most apparent in the metformin group, although differences by treatment group were not statistically significant.
Serious adverse events (AEs) were infrequent among DPP participants (1). When reported AEs were analyzed by age and treatment group (Table 5), hospital admissions were more common in the oldest age group, but did not differ by treatment assignment, likely because these AEs were not study-related. A similar pattern was seen for musculoskeletal AEs. Gastrointestinal complaints were more common in the metformin group (as expected), with rates slightly lower in the middle-age and older groups, although this difference was not statistically significant at an overall .05 level.
Table 5.
Adverse Events During DPP by Age and Treatment Groups
| Placebo |
Metformin |
Lifestyle |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | 25–44 | 45–59 | 60–85 | 25–44 | 45–59 | 60–85 | 25–44 | 45–59 | 60–85 |
| Gastrointestinal (No. of events/100 person-years)* | 32.4 | 30.8 | 27.8 | 82.2 | 77.5 | 72.2 | 13.1 | 14.2 | 9.7 |
| Musculoskeletal (No. of events/100 person-years)† | 16.1 | 21.9 | 26.7 | 13.3 | 21.4 | 26.1 | 19.9 | 25.4 | 28 |
| Hospitalization | |||||||||
| One or more admissions (% of participants) | 11.1 | 16.9 | 21.9 | 10.1 | 16.8 | 22.4 | 15.4 | 13.3 | 20.6 |
| Rate (No. of admissions/100 person-years) | 6.3 | 7.9 | 10.6 | 4.4 | 8.8 | 13.3 | 7.5 | 6.4 | 12.3 |
| Median stay, days | 3 | 3 | 4 | 3 | 3 | 4 | 3 | 3 | 3 |
| Deaths (No./100 person-years) | 0 | 0 | 0.86 | 0.11 | 0.13 | 0.48 | 0.1 | 0 | 0.31 |
Notes: DPP = Diabetes Prevention Program.
Gastrointestinal symptoms included diarrhea, flatulence, nausea, and vomiting.
Most participants with musculoskeletal symptoms had myalgia, arthritis, or arthralgia.
Discussion
The prevention of diabetes assumes increasing importance for the growing elderly population in the United States and elsewhere. Other studies have also demonstrated that diabetes can be prevented (or delayed) by lifestyle change (23,24) and medication (25,26), but the DPP provides unique information about prevention in older adults. DPP data indicate that there are significant age differences in response to either lifestyle modification or metformin, which appear to reflect variation in both behavior and biology.
Age-related deterioration in glucose tolerance reflects both impaired insulin secretion and declining insulin sensitivity (27-29). Insulin resistance is closely linked to obesity, and changes in body composition that are typical of aging (increased visceral fat and decreased skeletal muscle mass) are thought to be the major determinants of increasing insulin resistance with age. Diet-induced weight loss improves insulin sensitivity and insulin secretion in older patients (30), but data are conflicting about whether exercise can enhance insulin action and improve glucose tolerance, independent of weight loss (31). Several studies in older patients with type 2 diabetes suggested that resistance training can improve glycemic control and insulin sensitivity, independent of weight loss (32-34). Improvements in insulin action and reduction in hyperinsulinemia have also been reported in older, nondiabetic men following short-term strength training (35). In contrast, the effect of aerobic (endurance) training on insulin sensitivity in the elderly population is uncertain, with some reporting a clear benefit (36,37) and others no effect, despite increased muscle GLUT4 and mitochondrial oxidative capacity (38). Most DPP ILS participants achieved their exercise goal through low-level aerobic activity, such as brisk walking or cycling, with a small contribution from resistance training. The DPP ILS program was not designed to determine the individual effectiveness of diet change versus exercise or to determine the most effective form of exercise. However, our data suggest that the effectiveness of a comprehensive ILS program in preventing deterioration of glucose tolerance is enhanced in older adults. These results appear to be due to more active participation in ILS activities by the older group, although other (biological) factors are suggested by their better baseline body size and glycemic profile and the stronger effect on postchallenge glycemia.
The reasons for differences in ILS participation among the age groups were not formally assessed in DPP. As expected, younger participants were more likely to be employed (85% vs 82% vs 39%) and to live in households with four or more members (50% vs 29% vs 12%). These data would support anecdotal evidence that younger participants had family and work responsibilities that could interfere with lifestyle changes, whereas older participants had fewer competing obligations and more time to devote to the ILS program. Older individuals may also be more aware of disease vulnerability, now being the same age when parents or good friends died or experienced serious illness.
We observed a trend (in comparison to ILS) toward decreased effectiveness of metformin with increasing age, despite greater weight loss and comparable (if not enhanced) medication adherence among older metformin-treated participants. Although baseline glucose variables were almost identical in the three age groups, the strength of the association between age and declining metformin effect was attenuated when the small differences in baseline fasting glucose values (106 vs 107 mg/dL, in the 25–44 and 60–85 year age groups, respectively) were included in the model. This finding suggests that the older metformin group may have had a somewhat higher risk of diabetes at baseline, which could account for some of the observed difference in treatment effect. Nonetheless, the pattern of decreased metformin effect with age suggests that there may be true differences in physiology and pharmacologic response.
The three age groups were generally well matched at baseline for glucose parameters, but the older group was significantly more insulin-sensitive and had greater impairment of insulin secretion than the young or middle-aged groups. This observation is in contrast to data from some cross-sectional studies, which have reported increased obesity and insulin resistance with aging (39,40). One explanation for these results is a “survivor effect”—that is, those persons who survived to a later age without developing diabetes tended to be somewhat leaner and less insulin resistant than were age-mates who did become diabetic. As reported previously (9), baseline insulin sensitivity and secretion were strong and independent predictors of diabetes risk in the DPP. Within the metformin group, those participants with the highest insulin sensitivity and lowest insulin secretion (the pattern typical of older adults) had a greater hazard ratio for diabetes than did those participants with the lowest insulin sensitivity and greatest insulin secretion (typical of younger adults) (9). However, this pattern was not observed in the placebo or lifestyle groups, suggesting that metformin effectiveness is tied to metabolic profile, being most effective in insulin-resistant individuals and less so in those individuals whose predominant metabolic defect is impaired insulin secretion.
The primary mechanism for the antidiabetic effect of metformin is through suppression of hepatic glucose production, which is the major determinant of fasting plasma glucose levels but plays a more minor role in postchallenge (or postprandial) hyperglycemia (21). Although elevated hepatic glucose production is a classic feature of type 2 diabetes, some have reported that this may be less of a factor in older diabetic persons (39), thus providing another potential explanation for reduced metformin effect. Among those persons who progressed to diabetes within DPP, we observed that younger participants were more likely to convert by fasting glucose criteria, and older participants by 2-hour criteria. This pattern was most apparent in the metformin group, and is consistent with the relative ineffectiveness of this agent in reducing postchallenge hyperglycemia. Age differences in patterns of diabetes conversion have been reported by others (6,22) and may be related to the varying contributions of defects in insulin secretion and sensitivity. In the Rancho Bernardo Study, isolated postchallenge hyperglycemia was much more common than fasting hyperglycemia as a diagnostic criterion for diabetes (4). Data from the Baltimore Longitudinal Study of Aging suggest that there may be two distinct phenotypes for the development of diabetes—one through increased fasting glucose and the other through elevated 2-hour glucose levels (22). In that study, older adults (>65 years) had substantially accelerated rates of progression to abnormal 2-hour glucose compared to younger persons. We propose that reduced metformin effectiveness in preventing diabetes in the elderly population may be due to the interaction of a specific metabolic profile (insulin secretory defect, leading to postchallenge hyperglycemia) with the drug's pharmacologic action. However, metformin may be useful in treating elderly patients with established diabetes, when both fasting and postprandial hyperglycemia are typically present.
Some limitations to these analyses should be acknowledged. Ethnic and sex distributions were not equal in all age groups, with men and Caucasians over-represented among older participants. Male ILS participants lost more weight and reported more exercise than did female ILS participants, although diabetes risk reduction did not differ significantly (62% for men vs 52% for women; p = .25). Similarly, although Caucasians and Hispanics lost more weight than African Americans did, diabetes risk reduction did not differ by ethnic group. Among metformin participants, diabetes risk reduction was similar in men and women (37% and 27%; p = .41) and among ethnic groups, suggesting that the observed age differences in treatment effects are unlikely to be explained solely by variations in sex and ethnic composition of the age groups. Although age differences in response to intervention was a predetermined secondary outcome in DPP, the study had limited power to detect these differences, and this may have been a factor in the borderline statistical significance of some of our findings. The numbers also preclude meaningful stratified analyses to examine the effect of unequal ethnic and sex distributions in some age groups.
As noted, the DPP cohort was a relatively healthy and motivated group, so these results may not apply to frail or disabled elderly persons. Individuals with significant physical limitations (i.e., those unable to walk at a moderate pace) were not enrolled in DPP. However, the DPP ILS program was successfully modified for participants who developed such limitations (for example, due to injury or arthritis) during the course of the study. On the whole, the DPP ILS program of low-intensity aerobic activity and low-fat, reduced-calorie diet was well-tolerated by older participants and has broad potential applicability for community-dwelling seniors.
In conclusion, results of the DPP demonstrate that an intensive lifestyle modification program is exceptionally effective in preventing diabetes in older individuals with IGT. This robust response is in large part due to greater weight loss and more active participation in ILS activities among the older participants. The limited effectiveness of metformin, in contrast, may reflect true age-related differences in glucose metabolism and glycemic patterns at diabetes conversion. These results indicate that a program of moderate-intensity exercise and modest weight loss should be recommended for older individuals at high risk for type 2 diabetes. Implementation of these recommendations has the potential to reduce or delay the burden of diabetes in the growing elderly population.
Acknowledgments
This work was supported by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the Office of Women's Health, and the National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, and two pharmaceutical companies (Bristol-Myers Squibb and Parke-Davis) contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the clinical centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a Cooperative Agreement, except for the Southwestern American Indian Centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service.
References
- 1.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris M, Flegal K, Cowie C, et al. Prevalence of diabetes, impaired fasting glucose and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Resnick H, Harris M, Brock D, Harris T. American Diabetes Association diabetes diagnostic criteria, advancing age and cardiovascular disease risk profiles. Diabetes Care. 2000;23:176–180. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. Diabetes Care. 1998;21:1236–1239. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 5.Balkau B. New diagnostic criteria for diabetes and mortality in older adults. Lancet. 1999;353:68–69. doi: 10.1016/s0140-6736(05)74840-6. [DOI] [PubMed] [Google Scholar]
- 6.Wahl P, Savage P, Psaty B, et al. Diabetes in older adults: comparison of 1997 American Diabetes Association classification of diabetes mellitus with 1985 WHO classification. Lancet. 1998;352:1012–1015. doi: 10.1016/S0140-6736(98)04055-0. [DOI] [PubMed] [Google Scholar]
- 7.The DECODE Study Group Consequences of the new diagnostic criteria for diabetes in older men and women. Diabetes Care. 1999;22:1667–1671. doi: 10.2337/diacare.22.10.1667. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JE, Hodge AM, deCourten M, Chitson P, Zimmet P. Isolated post-challenge hyperglycaemia confirmed as a risk factor for mortality. Diabetologia. 1999;42:1050–1054. doi: 10.1007/s001250051269. [DOI] [PubMed] [Google Scholar]
- 9.The DPP Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effect of lifestyle intervention and metformin. Diabetes. 2005;54:2402–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The DPP Research Group The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The DPP Research Group The Diabetes Prevention Program: description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report on the Expert Committee on Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization: Diabetes Mellitus: report of a WHO study group. World Health Organization; Geneva, Switzerland: 1985. (Technical Report Series No. 727). [PubMed] [Google Scholar]
- 14.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29(suppl):S5–S9. [PubMed] [Google Scholar]
- 15.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann EL. Nonparametric Statistical Methods Based on Ranks. McGraw-Hill; New York: 1975. [Google Scholar]
- 17.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. Sas System for Mixed Models. SAS Institute Inc.; Cary, NC: 1996. [Google Scholar]
- 18.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; New York: 1994. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc. Series B. 1972;34:187–220. [Google Scholar]
- 20.Holm S. A simple sequentially rejective Bonferroni test procedure. Scandinavian J Statistics. 1979;6:65–70. [Google Scholar]
- 21.Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 22.Meigs J, Muller D, Nathan D, Blake D, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 23.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 24.Pan XR, Li GW, Hu YU, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 25.Chaisson J, Josse R, Gomis R, et al. STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan TA, Xiang AH, Peters RJK, et al. Preservation of pancreatic beta cell function and prevention of type 2 diabetes by pharmacologic treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 27.Basu R, Breda E, Oberg A, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 28.Chen M, Bergman R, Pacini G, Port D. Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta cell function. J Clin Endocrinol Metab. 1985;60:13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 29.Utzschneider K, Carr D, Hull R, et al. Impact of intra-abdominal fat and age on insulin sensitivity and b-cell function. Diabetes. 2004;53:2867–2872. doi: 10.2337/diabetes.53.11.2867. [DOI] [PubMed] [Google Scholar]
- 30.Utzschneider KM, Carr DB, Barness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab. 2004;89:2704–2710. doi: 10.1210/jc.2003-031827. [DOI] [PubMed] [Google Scholar]
- 31.Williams KV, Kelley DE. Metabolic consequences of weight loss on glucose metabolism and insulin action in type 2 diabetes. Diabetes Obes Metab. 2000;2:121–129. doi: 10.1046/j.1463-1326.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- 32.Holten M, Zacho M, Gaster M, et al. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes. 2004;53:294–305. doi: 10.2337/diabetes.53.2.294. [DOI] [PubMed] [Google Scholar]
- 33.Dunstan D, Daly R, Owen N, et al. High intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 34.Castaneda C, Layne J, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 35.Miller J, Pratley R, Goldberg A, et al. Strength training increases insulin action in healthy 50-65 year old men. J Appl Physiol. 1994;77:1122–1127. doi: 10.1152/jappl.1994.77.3.1122. [DOI] [PubMed] [Google Scholar]
- 36.Kahn S, Larson V, Beard J, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol. 1990;258:E937–E943. doi: 10.1152/ajpendo.1990.258.6.E937. [DOI] [PubMed] [Google Scholar]
- 37.Cox J, Cortright R, Dohm GL, Houmard J. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol. 1999;86:2019–2025. doi: 10.1152/jappl.1999.86.6.2019. [DOI] [PubMed] [Google Scholar]
- 38.Short K, Vittone J, Bigelow M, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 39.Meneilly G, Elliott T. Metabolic alterations in middle-aged and elderly obese patients with type 2 diabetes. Diabetes Care. 1999;22:112–118. doi: 10.2337/diacare.22.1.112. [DOI] [PubMed] [Google Scholar]
- 40.Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U. Insulin action and age. European Group for the Study of Insulin Resistance (EGIR) Diabetes. 1996;45:947–953. doi: 10.2337/diab.45.7.947. [DOI] [PubMed] [Google Scholar]


