Abstract
INTRODUCTION
Transient hypoxia and subsequent reoxygenation are common phenomena in solid tumors that greatly influence the outcome of radiation therapy. This study was designed to determine how varying cycles of hypoxia/reoxygenation affect the response of cervical carcinoma cells irradiated under oxic and hypoxic conditions and whether this could be modulated by proteasome inhibition.
MATERIALS AND METHODS
Plateau-phase SiHa cervical carcinoma cells in culture were exposed to varying numbers of 30-minute cycles of hypoxia/reoxygenation directly before irradiation under oxic or hypoxic conditions. 26S Proteasome activity was blocked by addition of MG-132. Clonogenic survival wasmeasured by a colonyforming assay.
RESULTS
Under oxic conditions, repeated cycles of hypoxia/reoxygenation decreased the clonogenic survival of SiHa cells. This effect was even more pronounced after the inhibition of 26S proteasome complex. In contrast, under hypoxic conditions, SiHa cells were radioresistant, as expected, but this was increased by proteasome inhibition.
CONCLUSIONS
Proteasome inhibition radiosensitizes oxygenated tumor cells but may also protect tumor cells from ionizing radiation under certain hypoxic conditions.
Keywords: Reoxygenation, hypoxia, radiation response, MG-132, 26S proteasome
Introduction
The basis of the curative effect of radiotherapy is DNA damage in tumor cells. This cytotoxic effect of ionizing radiation is modified by many factors, one of which is oxygen. Hypoxic cells are two to three times more resistant to ionizing irradiation [oxygen enhancement ratio (OER)] than well-oxygenated cells [1]. The hypoxic cell fraction of a tumor is, therefore, one of the most important factors determining the probability of local tumor control after radiation treatment [2]. Hypoxia is frequently found in human tumors and can be divided into chronic and acute hypoxia. Although chronic hypoxia results from limited diffusion of oxygen from capillaries to tumor cells, acute hypoxia occurs because of perfusion defects. Irregular tumor growth and the abnormal tumor microenvironment cause existing blood vessels to open and close because of microthrombosis or high intramural pressure. This makes transient hypoxia and reoxygenation common phenomena in tumors that have grown beyond a critical diameter [3,4]. As a corollary, the oxygenation status of cells within tumors undergoes frequent changes [5]. Changing oxygen tensions cause the production of high levels of reactive oxygen intermediate, which acts to select cells that can adapt to changing environmental conditions [6,7].
In the last few years, knowledge on the mechanisms of adaptation to oxidative stress in mammalian cells has grown [8,9]. The suggestion is that this results from the activation of important signal transduction pathways that involve 26S proteasome function [9,10], but little is known about the relevance of these pathways to radiosensitivity (for a review, see Adams et al. [11]).
26S Proteasome is a multicatalytic ATP-dependent protease complex that is responsible for the posttranslational control of all short-lived and many long-lived proteins [12]. The activity of this protease can be blocked by specific inhibitors such as MG-132, lactacystein, or bortezomib [13,14]. Proteasome inhibition effectively induces apoptosis and sensitizes cancer cells to chemotherapy [15] and ionizing radiation [16,17]. Bortezomib (Velcade; formally known as PS-341), the first specific proteasome inhibitor, has recently been approved by the Food and Drug Administration to treat patients with multiple myeloma [18] and is presently on clinical trial for the treatment of solid cancers and hematologic malignancies. The mechanisms leading to induction of apoptosis and radiosensitization are currently not fully understood. Nevertheless, knowledge on the effects of proteasome inhibitors on tumor cells in different tumor microenvironments will possibly aid decision making in situations where a combination of this new class of anticancer drugs and classic treatment modalities might be thought beneficial.
In this study, we tested if the proteasome inhibitor MG-132 can be used to overcome the radioprotective effects of acute hypoxia in a cervical cancer cell line. Although MG-132 sensitized oxygenated cells to ionizing radiation, we found that proteasome inhibition had no effect on acutely hypoxic cells. In fact, proteasome inhibition protected hypoxic cells that had undergone three cycles of hypoxia/reoxygenation from ionizing radiation, indicating that pathophysiological conditions might exist where proteasome inhibition may not necessarily be cytotoxic to tumor cells.
Materials and Methods
Cell Culture
SiHa (ATCC, Manassas, VA) cervical carcinoma cells were cultured in 75-cm2 flasks (BD Bioscience, San Jose, CA) in Leibowitzs' 15 medium (Gibco BRL, Gaithersburg, MD), 10%bovine serum(Seromed BiochromKG, Berlin, Germany), MEM vitamins (Gibco BRL), 1% sodium pyruvate (Gibco BRL; 100 mM), 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO), and 0.5 mg/ml fungizone (amphotericin B; Gibco BRL), and incubated in a humidified atmosphere at 37°C. Seventy-two hours before irradiation, cells were plated at a density of 2.5 x 105 cells/cm2 in glass Petri dishes. Thirty minutes before starting the first hypoxic cycle, MG-132 (Calbiochem, San Diego, CA) was added at a concentration of 50 µM. The inhibitor remained in the medium during periods of preconditioning and radiation. Immediately after irradiation, the medium was changed to wash out the inhibitor, and the cells were incubated for an additional 4 hours to allow repair before plating.
Hypoxic Conditions
A polyacrylic chamber was used to provide hypoxic conditions. The chamber was placed into a 37°C shaking water bath at low frequency. Prewarmed and moisturized gas (nitrogen or air) was flushed into the chamber at a constant flow rate of 1.5 l/min. To monitor the actual oxygen partial pressure, an oxygen electrode (CellOx 325, Weilheim, Germany) was placed into a medium-filled culture dish during all experiments. Movement of the chamber in the shaking water bath guaranteed a constant flow of medium around the electrode, which is a precondition for exact measurements of oxygen tension. After 20 minutes of flushing the chamber with nitrogen, oxygen content in the medium remained < 0.1%. pH values were also monitored and remained constant. In reoxygenation experiments, each cycle of hypoxia (30 minutes in total) was followed by a 30-minute incubation interval, during which the chamber was flushed with air at a flow rate of 1.5 l/min.
Irradiation
The cells were irradiated at the plateau phase of growth. Irradiation was performed at room temperature using a linear accelerator (Clinac; Varian, Palo Alto, CA). During transport and irradiation, the chamber was flushed with a constant nitrogen flow of 1.5 l/min to maintain hypoxic conditions, or with air to maintain oxic conditions.
Colony-Forming Assay
Cells were trypsinized, counted, and diluted to a final concentration of 106 cells/ml. Colony-forming assays were performed by plating an appropriate number of cells into culture dishes, in triplicate. After 14 days, cells were fixed and stained with 1% crystal violet, and colonies containing > 50 cells were counted. The surviving fraction was normalized to the surviving fraction of the corresponding control, and survival curves were fitted by a linear-quadratic model [19]. Dq and D0 were calculated from the survival curves using the single-hit multitarget model of radiation dose survival. Dq is a measure of the shoulder region of the curve and is where the exponential region extrapolates to the x (dose) axis, whereas D0 represents the slope of the exponential region.
Results
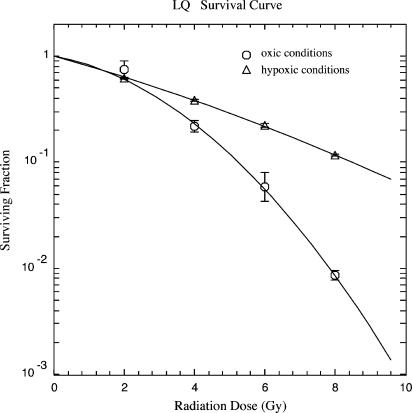
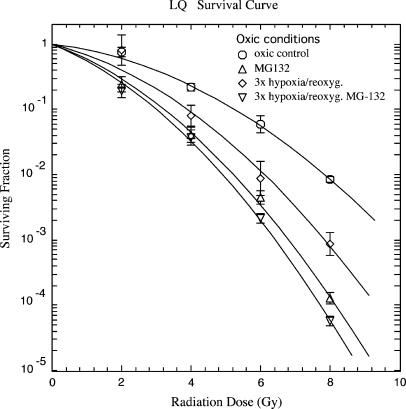
The clonogenic survival of SiHa cells treated with radiation doses of 0, 2, 4, 6, and 8 Gy under oxic and hypoxic conditions is shown in Figure 1. As expected, under hypoxic conditions, clonogenic survival increased with an OER of 2.9 (OER measured at D0 values). Under oxic conditions, inhibition of proteasome function by MG-132 (50 µM) for 3 hours before irradiation sensitized SiHa cells. The increase in radiosensitivity was 1.6-fold (± 0.2 for LD10; P < .05, Student's t test). Three cycles of hypoxia/reoxygenation, followed by irradiation under oxic conditions, also resulted in radio-sensitization (1.35 ± 0.24-fold for LD10) that was not further enhanced by proteasome inhibition (1.75 ± 0.19-fold for LD10, not significant) with hypoxia/reoxygenation alone (Figure 2). Under oxic conditions, none of the treatments significantly changed plating efficacy (PE; Table 1).
Figure 1.
Clonogenic survival of SiHa cervical carcinoma cells in the plateau phase, irradiated under oxic (○) and hypoxic (△) conditions. Data from three independent experiments, each performed in triplicate (mean ± SEM).
Figure 2.
Clonogenic survival of SiHa cervical carcinoma cells in the plateau phase, irradiated under oxic conditions. Data from three independent experiments, each performed in triplicate (mean ± SEM). MG-132 was added 30 minutes before the start of the first cycle of hypoxia and remained in the medium until cells were irradiated. Control cells (○), three cycles of hypoxia/reoxygenation (◊), MG-132 at 50 µM (△), three cycles of hypoxia/reoxygenation, and preincubation with MG-132 at 50 µM (▽).
Table 1.
Radiobiologic Parameters of SiHa Cervical Cancer Cells (mean ± SEM).
| Treatment | PE (%) | D0 (Gy) | Dq (Gy) | n | LD50 (Gy) | Dose Enhancement Ratio | LD10 (Gy) | Dose Enhancement Ratio | |
| Oxic | Control | 50.6 ± 3.8 | 1.91 [0.16] | 2.46 [1.0] | 7.92 | 2.48 ± 0.48 | - | 5.26 ± 0.38 | - |
| MG-132 | 38.1 ± 5.4 | 0.75 [0.39] | 1.51 [3.7] | 7.57 | 1.26 ± 0.25* | 1.96 ± 0.54 | 3.23 ± 0.32* | 1.63 ± 0.2 | |
| 3x Reoxygenation | 47.6 ± 4.2 | 0.9 [0.16] | 2.05 [1.1] | 11.18 | 1.63 ± 0.6 | 1.52 ± 0.64 | 3.9 ± 0.63 | 1.35 ± 0.24 | |
| 3x Reoxygenation MG-132 | 42.4 ± 6.4 | 0.7 [0.37] | 1.44 [3.5] | 7.95 | 1.16 ± 0.18* | 2.14 ± 0.53 | 3.01 ± 0.24* | 1.75 ± 0.19 | |
| Hypoxia | Control | 44.8 ± 2.1 | 3.41 [0.98] | 0.81 [1.9] | 1.27 | 2.97 ± 0.11 | - | 8.5 ± 0.13 | - |
| MG-132 | 41.7 ± 3.5 | 3.05 [1.14] | 1.84 [4.9] | 1.82 | 3.62 ± 0.44 | 0.82 ± 0.10 | 8.46 ± 0.36 | 1.00 ± 0.05 | |
| 3x Reoxygenation | 44.3 ± 10 | 3.63 [1.6] | 0.0 [3.7] | 1 | 2.52 ± 0.33 | 1.17 ± 0.15 | 8.4 ± 0.46 | 1.02 ± 0.06 | |
| 3x Reoxygenation MG-132 | 33.1 ± 1.8* | 2.46 [0.85] | 4.29 [1.1] | 5.74 | 5.2 ± 0.16* | 0.57 ± 0.03 | 9.47 ± 0.19* | 0.9 ± 0.02 | |
| 1x Reoxygenation | 47.2 ± 1.9 | 3.27 [1.3] | 1.56 [1.9] | 1.6 | 3.64 ± 0.13* | 0.86 ± 0.1 | 8.6 ± 0.20 | 0.99 ± 0.03 | |
| 15x Reoxygenation | 28.1 ± 1.1* | 1.96 [0.42] | 1.63 [1.0] | 2.3 | 2.45 ± 0.31 | 1.12 ± 0.09 | 6.1 ± 0.23* | 1.38 ± 0.06 |
Ninety-five percent confidence intervals are presented in square brackets.
P < .05.
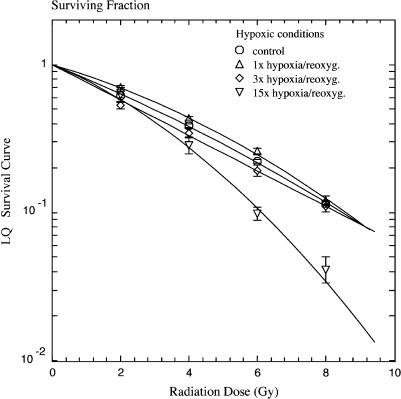
Interestingly, one or three cycles of hypoxia/reoxygenation did not affect intrinsic radiosensitivity under hypoxic conditions (Figure 3). Only after 15 cycles were cells sensitized to irradiation (Figure 3) (1.38 ± 0.06-fold for LD10; P < .05, Student's t test).
Figure 3.
Clonogenic survival of SiHa cervical carcinoma cells in the plateau phase, irradiated under hypoxic conditions. Cells were preconditioned with 0 (○), 1 (△), 3 (◊), or 15 (▽) cycles of hypoxia/reoxygenation. Data from three independent experiments, each performed in triplicate (mean ± SEM).
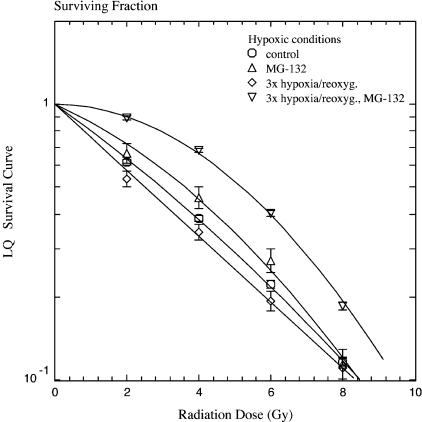
Cells that had been preconditioned with three cycles of hypoxia/reoxygenation and had been treated with MG-132 (50 µM) for 3 hours were remarkably radioresistant under hypoxic conditions (1.75 ± 0.06-fold; P < .05, Student's t test), as measured by LD50 values, although addition of MG-132 alone did not significantly change clonogenic survival under such conditions (Figure 4). The observed effect was less pronounced for LD10 values, increased the survival curve's shoulder, and caused a 5.3-fold increase in Dq (Table 1).
Figure 4.
Clonogenic survival of SiHa cervical carcinoma cells in the plateau phase, irradiated under hypoxic conditions. Control cells (○) and cells preconditioned with three cycles of hypoxia/reoxygenation (◊), pretreatment with MG-132 at 50 µM (△), or combined treatment of hypoxia/reoxygenation and MG-132 at 50 µM (▽). Data from three independent experiments, each performed in triplicate (mean ± SEM).
Although treatment of SiHa cells with either MG-132 alone, one cycle of hypoxia/reoxygenation, or three cycles of hypoxia/reoxygenation did not alter PE under hypoxic conditions, the combination of MG-132 treatment and cyclic reoxygenation, as well as 15 cycles of reoxygenation, significantly decreased PE to 33.1 ± 1.8% (P < .05, Student's t test) and 28.1 ± 1.1% (P < .05, Student's t test), respectively.
Discussion
The hypoxic fraction of cells in a tumor, in particular acute hypoxia resulting from fluctuations in blood supply, is one of the most important factors determining the curability of cancer by radiation therapy [2]. In contrary to in vitro conditions, the oxygenation status of tumor cells in vivo is characterized by frequent fluctuations between oxic and hypoxic conditions [5]. Recent reports demonstrated not only that hypoxia selects for cells defective in apoptotic pathways [6] but also that survival-related signal transduction pathways involving NF-κB, AP-1, and HIF-1 that are switched on during the hypoxic response might make cells more resistant to radiation therapy [20–22]. Because many of these pathways are tightly controlled by the 26S proteasome, we became interested on whether specific inhibitors of this multicatalytic protease, which is being targeted in clinical cancer trials [18], would alter the response of cervical cancer cells exposed to rapidly changing oxygen tensions when irradiated under oxic and hypoxic conditions. To investigate the radiation response of cells under changing oxygen tensions, we designed an experimental setting that allowed controlled and reproducible changes between hypoxia and reoxygenation. Responses were studied in plateau-phase cells because it has been previously suggested that plateau-phase cells could be a better in vitro model for tumors than exponentially growing cultures [23] because they are likely to repair DNA damage better, which would contribute to the radioresistance of tumors in vivo [24].
Using SiHa cervical cancer cells, we found an OER of 2.9 for acute hypoxic cells when compared to control cells under oxic conditions. This value compared well to published OERs [1] and verified that low oxygen tensions were reached in our experimental setting, as measured by the oxygen electrode placed on a control Petri dish.
Exposure of the cells to three cycles of hypoxia/reoxygenation sensitized the cells to ionizing radiation under oxic conditions as did pretreatment with the proteasome inhibitor MG-132.We have previously shown that this treatment inhibits proteasome function almost completely and radiosensitizes HD-MyZ cancer cells; thus, the latter was expected [16].
However, the combined treatment of proteasome inhibition and cyclic exposure to hypoxia and reoxygenation was not additive, indicating that both effects may have overlapping mechanisms. Nevertheless, our findings indicate that treatment with proteasome inhibitors will not negatively affect the radiation response of oxygenated and reoxygenated cells under oxic conditions and, if anything, will radiosensitize them.
The outcome was very different under hypoxic conditions. Treatment with either one or three cycles of hypoxia/reoxygenation did not cause any significant change in radiation response. Only after 15 cycles of hypoxia/reoxygenation were cells sensitized (1.38-fold) to radiation, with a 28.1% reduction in overall PE. This decrease in PE is in accordance with previous studies showing that cycles of hypoxia/reoxygenation induced apoptosis in a portion of cells, thereby selecting for cells with diminished apoptotic potential [6,25]. It was surprising to see that the proteasome inhibitor MG-132 was unable to sensitize hypoxic cells to ionizing radiation. Furthermore, in cells pretreated with three cycles of hypoxia/reoxygenation, there was a substantial increase of the shoulder portion of the survival curve. Therefore, cells were protected from radiation especially at lower doses, such as those given clinically (around 2 Gy). The lack of a sensitizing effect of proteasome inhibition under hypoxic conditions might be related to the fact that at least part of the toxicity of proteasome inhibition in tumor cells relates to the generation of endoplasmic reticulum stress-reactive oxygen species (ROS) [26], which are, in general, known to be reduced under hypoxic conditions, and that the 26S proteasome is redox-sensitive itself [27–29]. So far, the process of DNA repair by nonhomologous end joining, the major repair mechanism for radiation-induced DNA double-strand breaks, has not been directly linked to proteasome function. However, there is increasing evidence that it may be [30], as are nucleotide excision repair [31], base excision repair, and homologous recombination [30]. Although these reports may explain the radiosensitizing effects of MG-132 under oxic conditions, they failed to explain the radioprotective properties of proteasome inhibition after cyclic reoxygenation when cells were irradiated under hypoxic conditions.
Taken together, one may speculate that proteasome inhibition acts in two different ways: on one hand, it activates the cellular death program and sensitizes to cytotoxic treatment by a ROS-dependent pathway; on the other hand, proteasome inhibition causes a cellular stress response that counter-balances its own cytotoxic effects. In the absence of oxygen and in the presence of additional stressors such as hypoxia/reoxygenation, cytotoxic effects might be outbalanced and proteasome inhibition could even lead to cytoprotection.
Footnotes
Frank Pajonk and Thorsten Grumann contributed equally to this study.
References
- 1.Zolzer F, Streffer C. Increased radiosensitivity with chronic hypoxia in four human tumor cell lines. Int J Radiat Oncol Biol Phys. 2002;54:910–920. doi: 10.1016/s0360-3016(02)02963-2. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P. Tumor hypoxia and anemia: impact on the efficacy of radiation therapy. Semin Hematol. 2000;37:4–8. doi: 10.1016/s0037-1963(00)90061-1. [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 4.Denekamp J, Fowler JF, Dische S. The proportion of hypoxic cells in a human tumor. Int J Radiat Oncol Biol Phys. 1977;2:1227–1228. doi: 10.1016/0360-3016(77)90140-7. [DOI] [PubMed] [Google Scholar]
- 5.Rak JW, St Croix BD, Kerbel RS. Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anticancer Drugs. 1995;6:3–18. doi: 10.1097/00001813-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [see comments] [DOI] [PubMed] [Google Scholar]
- 7.Das DK, Engelman RM, Kimura Y. Molecular adaptation of cellular defences following preconditioning of the heart by repeated ischaemia. Cardiovasc Res. 1993;27:578–584. doi: 10.1093/cvr/27.4.578. [DOI] [PubMed] [Google Scholar]
- 8.Pahl HL, Baeuerle PA. Oxygen and the control of gene expression. Bioessays. 1994;16:497–502. doi: 10.1002/bies.950160709. [DOI] [PubMed] [Google Scholar]
- 9.Rupec RA, Baeuerle PA. The genomic response of tumor cells to hypoxia and reoxygenation. Differential activation of transcription factors AP-1 and NF-kappa B. Eur J Biochem. 1995;234:632–640. doi: 10.1111/j.1432-1033.1995.632_b.x. [DOI] [PubMed] [Google Scholar]
- 10.Muller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11:301–312. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- 11.Adams GE, Hasan NM, Joiner MC. The Klaas Breur Lecture, Radiation, hypoxia and genetic stimulation: implications for future therapies. Radiother Oncol. 1997;44:101–109. doi: 10.1016/s0167-8140(97)00090-x. [DOI] [PubMed] [Google Scholar]
- 12.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Goldberg AL. Selective inhibitors of the proteasomedependent and vacuolar pathways of protein degradation in Saccharomyces cerevisiae. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 14.Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J Biochem (Tokyo) 1996;119:572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- 15.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, Baldwin AS., Jr Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factorkappaB inhibition. Cancer Res. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 16.Pajonk F, Pajonk K, McBride WH. Apoptosis and radiosensitization of Hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys. 2000;47:1025–1032. doi: 10.1016/s0360-3016(00)00516-2. [DOI] [PubMed] [Google Scholar]
- 17.Russo SM, Tepper JE, Baldwin AS, Jr, Liu R, Adams J, Elliott P, Cusack JC., Jr Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 18.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: US FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 19.Albright N. Computer programs for the analysis of cellular survival data. Radiat Res. 1987;112:331–340. [PubMed] [Google Scholar]
- 20.Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Li CY. Enhancement of hypoxiainduced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139–8142. doi: 10.1158/0008-5472.CAN-03-2301. [DOI] [PubMed] [Google Scholar]
- 21.Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene. 2003;22:3213–3220. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- 22.Potapova O, Haghighi A, Bost F, Liu C, Birrer MJ, Gjerset R, Mercola D. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. J Biol Chem. 1997;272:14041–14044. doi: 10.1074/jbc.272.22.14041. [DOI] [PubMed] [Google Scholar]
- 23.Hahn GM, Little JB. Plateau-phase cultures of mammalian cells: an in vitro model for human cancer. Curr Top Radiat Res Q. 1972;8:39–43. [PubMed] [Google Scholar]
- 24.Deschavanne PJ, Fertil B, Chavaudra N, Malaise EP. The relationship between radiosensitivity and repair of potentially lethal damage in human tumor cell lines with implications for radioresponsiveness. Radiat Res. 1990;122:29–37. [PubMed] [Google Scholar]
- 25.Weinmann M, Jendrossek V, Guner D, Goecke B, Belka C. Cyclic exposure to hypoxia and reoxygenation selects for tumor cells with defects in mitochondrial apoptotic pathways. FASEB J. 2004;18:1906–1908. doi: 10.1096/fj.04-1918fje. [DOI] [PubMed] [Google Scholar]
- 26.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377:65–68. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- 29.Pajonk F, McBride WH. Ionizing radiation affects 26S proteasome function and associated molecular responses, even at low doses. Radiother Oncol. 2001;59:203–212. doi: 10.1016/s0167-8140(01)00311-5. [DOI] [PubMed] [Google Scholar]
- 30.Krogan NJ, Lam MH, Fillingham J, Keogh MC, Gebbia M, Li J, Datta N, Cagney G, Buratowski S, Emili A, et al. Proteasome involvement in the repair of DNA double-strand breaks. Mol Cell. 2004;16:1027–1034. doi: 10.1016/j.molcel.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 31.Wang QE, Wani MA, Chen J, Zhu Q, Wani G, El-Mahdy MA, Wani AA. Cellular ubiquitination and proteasomal functions positively modulate mammalian nucleotide excision repair. Mol Carcinog. 2005;42:53–64. doi: 10.1002/mc.20065. [DOI] [PubMed] [Google Scholar]