Abstract
OBJECTIVE
To review the evidence on identifying and managing misuse of and dependence on opioids among primary care patients with chronic pain.
QUALITY OF EVIDENCE
MEDLINE was searched using such terms as “opioid misuse” and “addiction.” The few studies on the prevalence of opioid dependence in primary care populations were based on retrospective chart reviews (level II evidence). Most recommendations regarding identification and management of opioid misuse in primary care are based on expert opinion (level III evidence).
MAIN MESSAGE
Physicians should ask all patients receiving opioid therapy about current, past, and family history of addiction. Physicians should take “universal precautions” that include careful prescribing and ongoing vigilance for signs of misuse. Patients suspected of opioid misuse can be treated with a time-limited trial of structured opioid therapy if they are not acquiring opioids from other sources. The trial should consist of daily to weekly dispensing, regular urine testing, and tapering of doses of opioids. If the trial fails or is not indicated, patients should be referred for methadone or buprenorphine treatment.
CONCLUSION
Misuse of and dependence on opioids can be identified and managed successfully in primary care.
Abstract
OBJECTIF
Examiner les données scientifiques concernant l’identification et la prise en charge de l’usage abusif d’opiacés et de la dépendance à leur endroit chez des patients en soins de première ligne souffrant de douleur chronique.
QUALITÉ DES PREUVES
Une recension a été effectuée dans MEDLINE à l’aide des expressions en anglais «usage abusif d’opiacés» et «toxicomanie». Les quelques études sur la prévalence de la dépendance aux opiacés dans les populations de première ligne se fondaient sur une étude rétrospective de dossiers médicaux (preuves de niveau II). La plupart des recommandations portant sur l’identification et la prise en charge de l’usage abusif d’opiacés reposaient sur l’opinion d’experts (preuves de niveau III).
PRINCIPAL MESSAGE
Les médecins devraient demander à tous les patients traités aux opiacés s’ils ont actuellement ou s’ils ont eu un problème de dépendance, ou s’il y a des antécédents familiaux de ce problème. Les médecins devraient prendre des «précautions universelles», notamment prescrire avec prudence et surveiller constamment les signes d’usage abusif. S’ils soupçonnent un usage abusif d’opiacés chez leur patient, ils peuvent faire l’essai d’une thérapie structurée aux opiacés pour une période de temps limitée, si le patient n’obtient pas d’opiacés d’autres sources. L’essai devrait comporter une administration passant d’une fois par jour à une fois par semaine, des analyses d’urine régulières et la diminution des doses d’opiacés. Si l’essai échoue ou n’est pas indiqué, un traitement à la méthadone ou au buprénorphine devrait être recommandé.
CONCLUSION
Il est possible, dans les soins de première ligne, d’identifier et de prendre en charge avec succès l’usage abusif d’opiacés et la dépendance à leur endroit.
EDITOR’S KEY POINTS.
Family doctors are in the difficult position of trying to address legitimate needs for pain control and concerns about patients misusing opioids.
The exact prevalence of opioid misuse in primary care is unknown, but misuse appears to be increasing as reflected in the increasing number of emergency department visits related to drug abuse.
Risk factors for opioid misuse include younger age; current, past, or family history of substance abuse; concurrent psychiatric disorders; and childhood sexual abuse.
Managing patients at higher risk for misuse includes insisting on treatment contracts, structured prescribing, and urine testing, and when these fail, referring patients for methadone or buprenorphine treatment.
POINTS DE REPÈRE DU RÉDACTEUR.
Les médecins de famille vivent le dilemme de répondre à des besoins légitimes de contrôle de la douleur et à des préoccupations entourant l’usage abusif d’opiacés par leurs patients.
La prévalence exacte de l’usage abusif d’opiacés en soins de première ligne n’est pas connue, mais elle semble à la hausse si on en juge par le nombre accru de visites à l’urgence reliées à la toxicomanie.
Au nombre des facteurs de risque d’usage abusif d’opiacés figurent un plus jeune âge, des antécédents familiaux ou des problèmes passés ou présents de toxicomanie, des troubles psychiatriques simultanés et des abus sexuels durant l’enfance.
La prise en charge des patients à risque plus élevé d’usage abusif comporte l’insistance sur un contrat de traitement, des ordonnances structurées et des analyses d’urine. En cas d’échec, il faut recommander un traitement à la méthadone ou à la buprénorphine.
In a recent national survey, 35% of Canadian family physicians reported that they would never prescribe opioids for moderate-to-severe chronic pain, and 37% identified addiction as a major barrier to prescribing opioids.1 This attitude leads to undertreatment and unnecessary suffering.2
Physicians’ concerns about patients’ becoming dependent on opioids, however, are legitimate. The prevalence of opioid misuse is increasing, and untreated dependence can result in loss of productivity, family disruption, depression, overdose, and suicide.3-5 Family physicians must be able to prescribe opioids safely and effectively, and at the same time must identify and manage opioid misuse and dependence in their practices.
Quality of evidence
MEDLINE was searched using such terms as “opioid misuse” and “addiction.” The few studies on the prevalence of opioid dependence in primary care populations were based on retrospective chart reviews (level II evidence). Observational studies have documented a high prevalence of opioid misuse in certain primary care patient populations, although the population prevalence is unknown. Most recommendations regarding identification and management of opioid misuse in primary care are based on expert opinion (level III evidence). Screening instruments for detecting opioid dependence have not yet been fully validated in primary care. Level I evidence indicates that primary care physicians can manage opioid dependence safely and effectively with methadone or buprenorphine therapy.
Key concepts
Substance dependence (addiction).
Dependence occurs when patients find the psychoactive effects of a drug so reinforcing that they have difficulty controlling their use of the drug. Addiction is characterized by the four Cs: loss of Control over use, continued use despite knowledge of harmful Consequences, Compulsion to use, and Craving. The reinforcing effects of opioids range from a mild “mood leveling” to profound euphoria.
Physical dependence.
Dependence involves 2 related phenomena, tolerance and withdrawal. Tolerance occurs when patients must take more of the drug over time to achieve the same effect. Tolerance is due to compensatory changes in the number and sensitivity of central nervous system receptors. Tolerance to the analgesic effects of opioids develops slowly; tolerance to their mood-altering effects begins within days.
Physical symptoms of withdrawal include myalgia, and cramps and diarrhea. Psychological symptoms include anxiety, craving, and insomnia. Objective signs include lacrimation, acute rhinitis, yawning, sweating, chills, and piloerection. These signs are most evident several days after high doses of opioids are discontinued. Withdrawal peaks 2 to 3 days after last use, and physical symptoms largely resolve by 5 to 10 days after last use, although insomnia and dysphoria can last for months afterward. Opioid withdrawal does not have medical complications except during pregnancy when it can induce spontaneous abortion, premature labour, and neonatal withdrawal.
Opioid misuse.
Opioid misuse (or aberrant drug behaviour) refers to opioid use that is not medically sanctioned, such as dose escalation, running out of the drug early, bingeing on opioids, or crushing controlled-release tablets. While opioid misuse can result from opioid dependence, it can also reflect inadequately treated pain, patients’ attitudes toward medication, and other factors.
Potential for abuse.
Level II evidence suggests that oxycodone has a greater risk of abuse than morphine.6-8 Controlled-release opioids have a slower onset of action and in theory have lower abuse potential than short-acting opioids (although they can be easily converted into immediate-release by crushing the tablets). The abuse potential of a drug is dose-related9,10; controlled-release preparations contain larger doses of opioids than acetaminophen-opioid preparations.
Pseudoaddiction.
This is said to occur when patients with inadequately treated pain exhibit drug-seeking behaviour similar to that of true addicts. Consensus opinion (level III evidence) suggests that this behaviour resolves with reasonable dose increases. True addictive behaviour remains the same or worsens.11 One study found that patients with inadequate pain relief were no more likely to misuse opioids than those with adequate pain relief,12 suggesting that pseudoaddiction is an uncommon reason for opioid misuse.
Prevalence
The reported prevalence of opioid dependence among chronic pain patients varies among clinical settings. A review of studies conducted in tertiary care pain clinics found that prevalence ranged from 3% to 19% or more.13,14 Other studies, generally of older patients attending specialty clinics, found rates of 1% to 3%.15 In 3 retrospective chart reviews in primary care clinics, 7% to 31% of charts documented opioid misuse,16,17 and drug abuse was diagnosed in 6% of these patients.18 The true prevalence of prescribed opioid misuse is unknown. In the Health Care for Communities Study in the United States, which involved 14 000 patients,19 those taking prescription opioids had 4 times the risk of problems with use of prescribed and illicit drugs and of mood and anxiety disorders the other participants had. These studies must be interpreted with caution because opioid misuse is not synonymous with opioid dependence. A detailed diagnostic assessment of opioid users found that non-addicted patients frequently misuse opioids.20
The prevalence of prescription opioid misuse appears to be increasing. The Drug Abuse Warning Network in the United States reported a 7-fold increase in oxycodone-related emergency department visits from 1996 to 2002.10,11 A national surveillance system involving addiction experts confirmed that opioid abuse increased in the United States from 2002 to 2004, with oxycodone showing the greatest increase.21
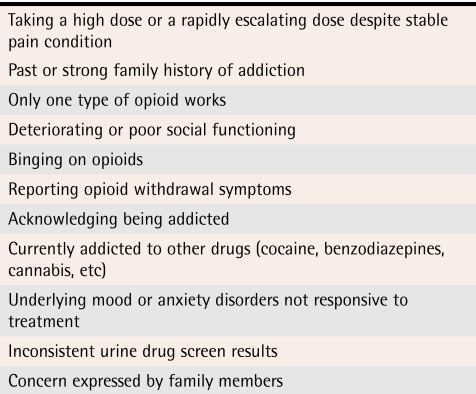
Risk factors for opioid dependence include youth; current, past, or family history of substance abuse; concurrent psychiatric disorders; and a childhood history of sexual abuse. Studies of the positive or negative predictive value of these risk factors have had inconsistent results.12,22,23
Identifying opioid misuse and dependence
Universal precautions.24
Physicians should take a careful baseline history of substance use on all patients, inquiring about current and past use of opioids, alcohol, benzodiazepines, cocaine, cannabis, and other drugs, as well as about history of previous treatment for substance abuse and family history. Physicians should routinely use treatment agreements, titrate opioid doses cautiously, and watch for signs of misuse.
Screening.
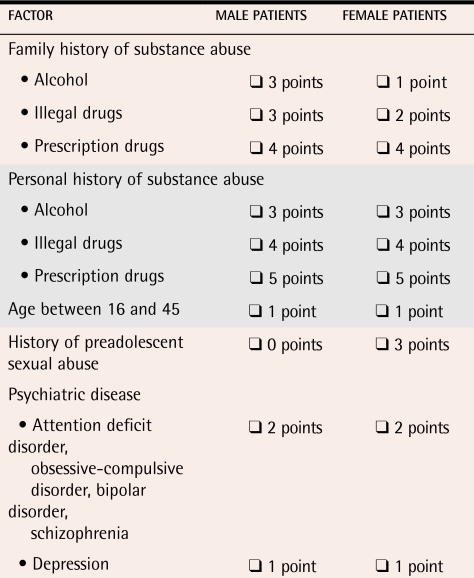
Screening instruments have not yet been shown in prospective studies to predict accurately which primary care patients suffering pain will become addicted to opioids.25 Several instruments are currently under development.26,27 Two brief screening instruments, the Opioid Risk Tool28 and the CAGE test,29 can be administered in primary care settings, although further validation research is needed (Tables 1 and 2).
Table 1. Opioid Risk Tool.
Check box if factor applies (0-3 points—low risk, 4-7 points—moderate risk, ≥8 points—high risk).
Table 2. CAGE test.
Two or more positive responses indicate misuse or dependence and suggest patients need further assessment.
Clinical features of opioid dependence.
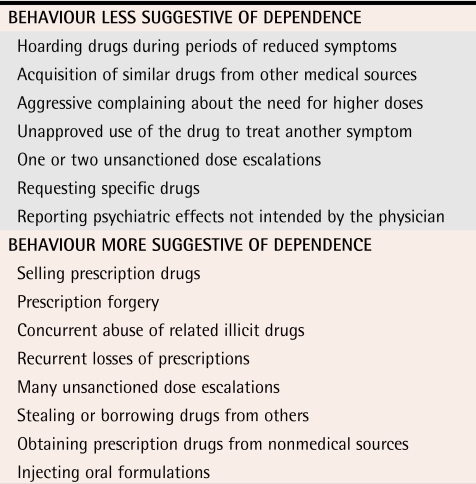
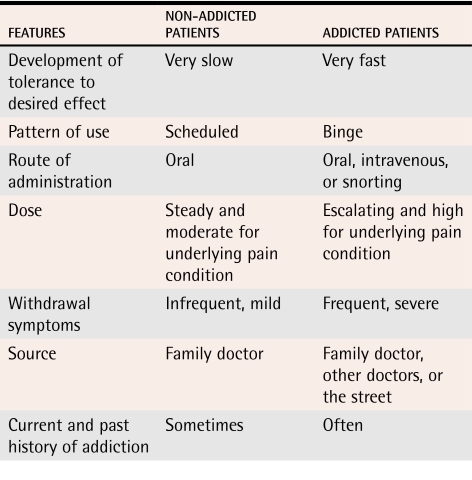
We do not know which opioid misuse behaviour most reliably predicts opioid dependence (Tables 3,30 4, and 5).30 Some behaviour, such as injecting or crushing tablets and buying opioids on the street, is probably more predictive than other behaviour.20 Such behaviour tends to be hidden from physicians.
Table 3.
Behaviour suggesting opioid dependence
Data from Passik et al.30
Table 4.
Additional clinical features of opioid dependence
Table 5.
Clinical features of pain patients with and without addictions
Opioid misuse can be grouped into several categories: unsanctioned use (running out early, bingeing); altering the route of delivery (injecting, crushing tablets); accessing opioids from other sources (friends, the street, other doctors); drug-seeking behaviour (anger, harassing office staff for fit-in appointments); and reluctance to use other methods of pain management. This behaviour stems from opioid-dependent patients’ need to overcome opioid tolerance, achieve desired psychoactive effects, and relieve withdrawal symptoms.
If asked, opioid-dependent patients might say that they experience withdrawal symptoms at the end of a dosing interval. They might even acknowledge that, although they use the drug for pain, they are also addicted to it. They typically experience depression, anxiety, and social isolation and often have a current, past, or strong family history of addiction.
It is sometimes difficult to distinguish patients with opioid dependence from patients with pain disorder, also known as chronic pain syndrome. Patients with pain disorder often describe their pain in dramatic terms, are prescribed high doses of opioids, focus on medications, and are depressed and socially isolated. They differ from opioid-dependent patients in that they are not usually seeking a psychoactive effect from their opioids, do not have a strong personal or family history of addiction, and generally comply with their medication schedules.
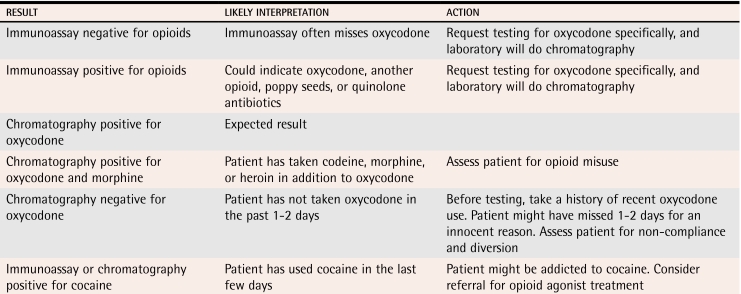
Urine testing.
Physicians who regularly prescribe opioids for chronic pain should be skilled in ordering and interpreting the results of urine tests. Such drug tests can help to identify noncompliance, opioid diversion, and concurrent drug abuse (Table 6 and 7). One study showed that 21% of chronic pain patients without any evidence of drug-seeking behaviour had unauthorized drugs in their urine.31,32
Table 6.
Urine testing for drugs
Table 7.
Interpretation of urine drug-screening results among patients receiving oxycodone
Assessment of suspected opioid misuse or dependence.
Physicians should take a complete history of substance use and carefully inquire about mood and occupational and family functioning. Urine should be tested, and records from previous care providers should be requested. Physicians should enquire about binge use, psychoactive effects, use from other sources, withdrawal symptoms, and other features of dependence. Spouses should be interviewed, if feasible, as they will notice features of dependence long before physicians do.
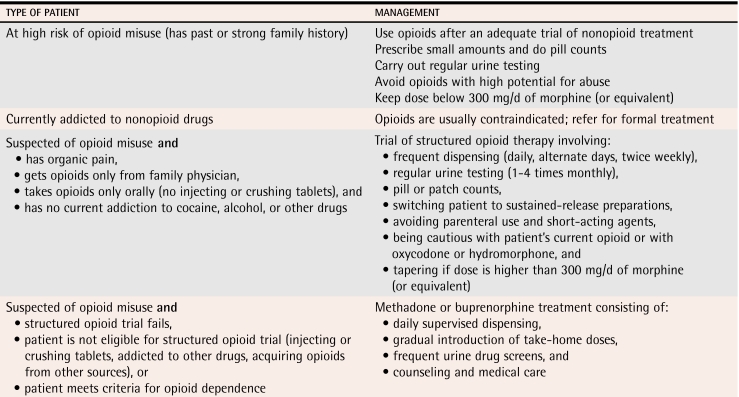
Management
Physicians should manage opioid dependence as they would any other medical condition, without defensiveness, avoidance, or anger (Table 8). Physicians can anticipate varying degrees of resistance from patients and must be comfortable with setting boundaries and saying “no.”
Table 8.
Management of suspected opioid misuse or dependence
Management strategies described below allow patients with chronic pain to receive opioids under controlled conditions based on their level of risk of addiction. The category “suspected opioid misuse or dependence” recognizes the difficulty in diagnosing opioid dependence in patients who will not disclose their true symptoms or behaviour.
Patients at high risk of opioid dependence.
Patients with a history or strong family history of addiction should be asked to sign specific treatment agreements and should be monitored regularly with urine tests. Physicians should avoid prescribing opioids (such as oxycodone and hydromorphone) with a higher potential for abuse. Doses well below 300 mg/d of morphine (or equivalent) should be adequate in almost all cases. Particularly at the beginning of therapy, physicians should prescribe small doses and schedule frequent follow-up visits, and patients should bring their medications in for pill or patch counting. The approach taken with these patients is similar to that described below for opioid misusers (structured opioid therapy), but might not be as stringent depending on patients’ degree of risk.
Currently dependent on nonopioid drugs.
Opioids are, in most cases, contraindicated in patients currently addicted to other drugs. Alcohol, benzodiazepines, and opioids are a dangerous combination. Cocaine users sometimes sell their opioids to pay for cocaine. Actively addicted patients should be referred for formal addiction treatment.
Suspected opioid misuse or dependence.
Several experts in the field (level III evidence) have suggested a time-limited trial of structured opioid therapy for patients suspected of opioid misuse or dependence who have pain that warrants opioid treatment.11,27,33 If the trial fails, options include an integrated pain and addiction treatment program,34 if available, or treatment with methadone or buprenorphine.
Structured opioid therapy.
A trial of structured opioid therapy is indicated for chronic pain patients suspected of opioid misuse who are not currently addicted to other substances, do not acquire opioids from other sources, and do not inject or crush opioid tablets. Patients should be asked to sign revised treatment agreements that specify the type and dose of opioid, the frequency of dispensing and of urine tests, and other components of care. Opioids should be dispensed daily, weekly, or biweekly for patients who frequently run out early. Urine testing should be obtained as often as weekly, depending on patients’ patterns of misuse and the reliability of their self-reports. Pill and patch counting should be done routinely.
Patients suspected of abusing a specific opioid should, in general, be switched to a different opioid. Oxycodone and hydromorphone should be used with caution. Tapering is indicated if the opioid dose is well above 300 mg/d of morphine. Tapering can improve patients’ mood and pain35 because the cycle of intoxication and withdrawal is less extreme once they have been stabilized at a lower dose. Tapering should be done with scheduled doses of controlled-release opioids, if possible. A suggested tapering schedule involves reductions of 10% every 2 to 4 weeks, slowing to reductions of 5% once a dose of one third of the initial dose is reached.36 The end point of successful tapering is either abstinence or a moderate scheduled dose that provides effective analgesia with minimal withdrawal symptoms. Patients who remain noncompliant with the trial after 1 to 3 months should be referred for opioid agonist treatment.
Opioid agonist treatment.
Treatment with methadone or buprenorphine is indicated for patients who have failed a trial of structured opioid therapy or were ineligible for such a trial because they injected drugs, acquired opioids from other sources, or had active addiction. Patients receiving opioid agonist treatment must meet the criteria for opioid dependence.
Opioid agonist treatment consists of daily supervised dosing, gradual introduction of take-home doses, frequent urine tests, and medical follow up and counseling. Methadone is an oral opioid with a slow onset and long duration of action. In appropriate doses it relieves symptoms of withdrawal and cravings for 24 hours without inducing sedation or euphoria. Methadone maintenance is highly effective in reducing drug use and its consequences (level I evidence).37-41 Buprenorphine, soon to be available in Canada as Subutex® and Suboxone®, is a sublingual partial opioid agonist. Buprenorphine has level I evidence of effectiveness42,43 and can be prescribed safely and effectively by primary care physicians.44-47 While buprenorphine might be less effective than high doses of methadone, it can be titrated more quickly and has a lower risk of overdose.48,49
Conclusion
Opioid misuse and dependence can be detected through careful assessment of patients, vigilance for opioid misuse, and urine testing. If opioid misuse or dependence is suspected, physicians could consider a trial of structured opioid therapy provided patients are not injecting opioids, crushing opioid tablets, or acquiring opioids from other sources. If the trial fails or is not indicated, patients should be referred for opioid agonist treatment with methadone or buprenorphine.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Biographies
Dr Kahan is Medical Director of the Addiction Medical Service at St Joseph’s Health Centre in Toronto, Ont, and Head of the Alcohol Clinic at the Centre for Addiction and Mental Health.
Dr Srivastava is a staff physician at St Joseph’s Health Centre and a clinical researcher at the Centre for Addiction and Mental Health.
Dr Wilson is Chief of the Department of Family Medicine at St Joseph’s Health Centre.
Dr Gourlay is an Addiction Consultant in the Wasser Pain Clinic at Mount Sinai Hospital in Toronto.
Ms Midmer is a researcher in the Department of Family and Community Medicine at the University of Toronto.
Footnotes
Competing interests: Dr Gourlay has received honoraria from Ligand Pharmaceuticals, Janssen-Ortho, Purdue Pharma, and Cephalon.
References
- 1.Morley-Forster PK, Clark AJ, Speechley M, Moulin DE. Attitudes toward opioid use for chronic pain: a Canadian physician survey. Pain Res Manag. 2003;8(4):189–194. doi: 10.1155/2003/184247. [DOI] [PubMed] [Google Scholar]
- 2.Auret K, Schug SA. Underutilisation of opioids in elderly patients with chronic pain: approaches to correcting the problem. Drugs Aging. 2005;22(8):641–654. doi: 10.2165/00002512-200522080-00002. [DOI] [PubMed] [Google Scholar]
- 3.Oyefeso A, Ghodse H, Clancy C, Corkery J, Goldfinch R. Drug abuse-related mortality: a study of teenage addicts over a 20-year period. Soc Psychiatry Psychiatr Epidemiol. 1999;34(8):437–441. doi: 10.1007/s001270050166. [DOI] [PubMed] [Google Scholar]
- 4.Wall R, Rehm J, Fischer B, Brands B, Gliksman L, Stewart J, et al. Social costs of untreated opioid dependence. J Urban Health. 2000;77(4):688–722. doi: 10.1007/BF02344032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krausz M, Degkwitz P, Haasen C, Verthein U. Opioid addiction and suicidality. Crisis. 1996;17(4):175–181. doi: 10.1027/0227-5910.17.4.175. [DOI] [PubMed] [Google Scholar]
- 6.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710–1714. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 7.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002. J Pain Symptom Manage. 2004;28:176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non–drug-abusing volunteers. Psychopharmacology (Berl) 2003;170:242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- 9.Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, et al. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- 10.Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299(3):1056–1065. [PubMed] [Google Scholar]
- 11.Weaver M, Schnoll S. Abuse liability in opioid therapy for pain treatment in patients with an addiction history. Clin J Pain. 2002;18(4 Suppl):61–69. doi: 10.1097/00002508-200207001-00007. [DOI] [PubMed] [Google Scholar]
- 12.Passik SD, Kirsch KL, McDonald MV. A pilot survey of aberrant drug-taking attitudes and behavior in samples of cancer and AIDS patients. J Pain Symptom Manage. 2000;19:274–286. doi: 10.1016/s0885-3924(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 13.Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain. 1992;8:77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Chelminski PR, Ives TJ, Felix KM, Prakken SD, Miller TM, Perhak JS, et al. A primary care, multi-disciplinary disease management program for opioid-treated patients with chronic non-cancer pain and a high burden of psychiatric comorbidity [abstract]. BMC Health Serv Res. 2005;5(1):3. doi: 10.1186/1472-6963-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis Rheum. 2005;52(1):312–321. doi: 10.1002/art.20784. [DOI] [PubMed] [Google Scholar]
- 16.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roeloffs CA, Wells KB, Ziedonis D, Tang L, Unutzer J. Problem substance use among depressed patients in managed primary care. Psychosomatics. 2002;43(5):405–412. doi: 10.1176/appi.psy.43.5.405. [DOI] [PubMed] [Google Scholar]
- 18.Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage. 2001;22:791–796. doi: 10.1016/s0885-3924(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan M, Edlund M, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119(1-3):95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355–363. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 21.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. J Pain. 2005;6(10):662–672. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23:131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 23.Michna E, Ross EL, Hynes WL, Nedeljkovik SS, Soumekh S, Janfaza D, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Gourlay D, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107–112. doi: 10.1111/j.1526-4637.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 25.Nedeljkovic SS, Wasan A, Jamison RN. Assessment of efficacy of long-term opioid therapy in pain patients with substance abuse potential. Clin J Pain. 2002;18(4 Suppl):39–51. doi: 10.1097/00002508-200207001-00005. [DOI] [PubMed] [Google Scholar]
- 26.Adams LL, Gatchel RJ, Robinson RC, Polatin P, Gajraj N, Deschner M, et al. Development of a self-report screening instrument for assessing potential opioid medication misuse in chronic pain patients. J Pain Symptom Manage. 2004;27:440–459. doi: 10.1016/j.jpainsymman.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Passik SD, Kirsh KL, Whitcomb L, Portenoy RK, Katz NP, Kleinman L, et al. A new tool to assess and document pain outcomes in chronic pain patients receiving opioid therapy. Clin Ther. 2004;26(4):552–561. doi: 10.1016/s0149-2918(04)90057-4. [DOI] [PubMed] [Google Scholar]
- 28.Webster LR, Webster RM. Predicting aberrant behaviours in opioid-treated patients: validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94(3):135–140. [PubMed] [Google Scholar]
- 30.Passik SD, Kirsh KL, Whitcomb L, Dickerson PK, Theobald DE. Pain clinicians’ rankings of aberrant drug-taking behaviors. J Pain Palliat Care Pharmacother. 2002;16(4):39–49. doi: 10.1080/j354v16n04_04. [DOI] [PubMed] [Google Scholar]
- 31.Katz NP, Sherburne S, Beach M, Rose RJ, Vielguth J, Bradley J, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097. doi: 10.1213/01.ANE.0000080159.83342.B5. 1097-102, table of contents. [DOI] [PubMed] [Google Scholar]
- 32.Vaglienti RM, Huber SJ, Noel KR, Johnstone RE. Misuse of prescribed controlled substances defined by urinalysis. W V Med J. 2003;99:67–70. [PubMed] [Google Scholar]
- 33.Passik SD, Kirsh KL. Opioid therapy in patients with a history of substance abuse. CNS Drugs. 2004;18(1):13–25. doi: 10.2165/00023210-200418010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Currie SR, Hodgins DC, Crabtree A, Jacobi J, Armstrong S. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91–100. doi: 10.1054/jpai.2003.17. [DOI] [PubMed] [Google Scholar]
- 35.Rome JD, Townsend CO, Bruce BK, Sletten CD, Luedtke CA, Hodgson JE. Chronic noncancer pain rehabilitation with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Mayo Clin Proc. 2004;79:759–768. doi: 10.4065/79.6.759. [DOI] [PubMed] [Google Scholar]
- 36.Gourlay D. Methadone for pain guidelines. Toronto, Ont: College of Physicians and Surgeons of Ontario; 2005. [Google Scholar]
- 37.Fiellin DA, O’Connor PG, Chawarski M, Pakes JP, Pantalon MV, Schottenfeld RS. Methadone maintenance in primary care: a randomized controlled trial. JAMA. 2001;286:1724–1731. doi: 10.1001/jama.286.14.1724. [DOI] [PubMed] [Google Scholar]
- 38.Farre M, Mas A, Torrens M, Moreno V, Cami J. Retention rate and illicit opioid use during methadone maintenance interventions: a meta-analysis. Drug Alcohol Depend. 2002;65:283–290. doi: 10.1016/s0376-8716(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 39.Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4-5 year follow-up results. Addiction. 2003;98:291–303. doi: 10.1046/j.1360-0443.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 40.Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 41.Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence: a randomized trial. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- 43.West SL, O’Neal KK, Graham CW. A meta-analysis comparing the effectiveness of buprenorphine and methadone. J Subst Abuse. 2000;12:405–414. doi: 10.1016/s0899-3289(01)00054-2. [DOI] [PubMed] [Google Scholar]
- 44.Caplehorn JR. A comparison of buprenorphine treatment in clinic and primary care settings: a randomised trial. Med J Aust. 2003;179:557–558. doi: 10.5694/j.1326-5377.2003.tb05417.x. Author reply Med J Aust 2003;179:558. [DOI] [PubMed] [Google Scholar]
- 45.Bouchez J, Vignau J. The French experience—the pharmacist, general practitioner and patient perspective. Eur Addict Res. 1998;4(Suppl 1):19–23. doi: 10.1159/000052037. [DOI] [PubMed] [Google Scholar]
- 46.Vignau J, Brunelle E. Differences between general practitioner- and addiction centre–prescribed buprenorphine substitution therapy in France. Preliminary results. Eur Addict Res. 1998;4(Suppl 1):24–28. doi: 10.1159/000052038. [DOI] [PubMed] [Google Scholar]
- 47.Bridge TP, Fudala PJ, Herbert S, Leiderman DB. Safety and health policy considerations related to the use of buprenorphine/naloxone as an office-based treatment for opiate dependence. Drug Alcohol Depend. 2003;70(2 Suppl):79–85. doi: 10.1016/s0376-8716(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 48.Lepere B, Gourarier L, Sanchez M, Adda C, Peyret E, Nordmann F, et al. [Reduction in the number of lethal heroin overdoses in France since 1994. Focus on substitution treatments]. Ann Med Interne (Paris) 2001. IS5-12. [PubMed]
- 49.Borron SW, Monier C, Risede P, Baud FJ. Flunitrazepam variably alters morphine, buprenorphine, and methadone lethality in the rat. Hum Exp Toxicol. 2002;21(11):599–605. doi: 10.1191/0960327102ht303oa. [DOI] [PubMed] [Google Scholar]