Abstract
OBJECTIVE
To determine the effectiveness of a short-term intervention to promote best practices for control of respiratory infections in primary care physicians’ offices.
DESIGN
Before-after observational study.
SETTING
Family physicians’ offices in Ottawa, Ont.
PARTICIPANTS
General practitioners and office staff.
INTERVENTIONS
Four infection-control practices (use of masks, alcohol-based hand gel, and signs, and asking patients to sit at least 1 m apart in the waiting room) were observed, and 2 reported infection-control practices (disinfecting surfaces and use of hand-gel dispensers in examining rooms) were audited before the intervention and 6 weeks after the intervention.
MAIN OUTCOME MEASURES
Percentage of patients asked to use masks and alcohol-based hand gel, number of relevant signs, and percentage of patients asked to sit at least 1 m away from other patients. Percentage of surfaces disinfected and percentage of physicians using hand-gel dispensers in examining rooms.
RESULTS
Of 242 practices invited, 53 agreed to participate (22% response rate), and within those practices, 143/151 (95%) physicians participated. Signs regarding respiratory infection control measures increased from 15.4% to 81.1% following the intervention (P < .001). At least 1 patient with cough and fever was given a mask in 17% of practices before the intervention; during the observation period after the intervention, at least 1 patient was given a mask in 66.7% of practices (P < .001). Patients were instructed to use alcohol-based hand gel in 24.5% of practices before the intervention and in 79.2% of practices after it (P < .001). Instruction to sit at least 1 m from others in the waiting area was given in 39.6% of practices before the intervention and in 52.8% of practices following the intervention (P < .001). Before the intervention, the percentage of practices using all 4 audited primary prevention measures was 3.8%; after the intervention, 52.8% of practices were using them (P < .001), demonstrating a 49% increase in adoption of best practices.
CONCLUSION
A multifaceted intervention by public health nurses successfully promoted best practices for control of respiratory infections in primary care offices. Collaboration between public health services and primary care can promote best practices and warrants further study and development in areas of common interest.
Abstract
OBJECTIF
Évaluer l’efficacité d’une intervention à court terme pour promouvoir des pratiques exemplaires visant le contrôle des infections respiratoires dans les cabinets de médecins de première ligne.
TYPE D’ÉTUDE
Étude par observation avant-après.
CONTEXTE
Cabinets de médecins de famille à Ottawa, en Ontario.
PARTICIPANTS
Omnipraticiens et personnel de bureau.
INTERVENTIONS
On a observé 4 pratiques de contrôle des infections (utilisation de masque, gel alcoolisé pour les mains, affiches et demandes aux patients de s’asseoir à au moins 1 mètre de distance dans la salle d’attente). On a vérifié 2 pratiques de contrôle des infections signalées (désinfection des surfaces et utilisation des dispensateurs de gel pour les mains dans les salles d’examen) avant l’intervention et 6 semaines après l’intervention.
PRINCIPAUX PARAMÈTRES ÉTUDIÉS
Pourcentage des patients à qui on a demandé d’utiliser des masques et du gel alcoolisé pour les mains, nombres d’affiches pertinentes et pourcentage des patients à qui on a demandé de s’asseoir à au moins 1 mètre de distance des autres patients. Pourcentage des surfaces désinfectées et pourcentage des médecins qui utilisent les dispensateurs de gel pour les mains dans les salles d’examen.
RÉSULTATS
Des 242 cabinets invités, 53 ont accepté de participer (taux de réponse de 22%). Dans les 53 cabinets participants, 143 médecins sur 151 (95%) ont pris part à l’étude. Le nombre d’affiches sur les mesures de contrôle des infections respiratoires a augmenté de 15,4% à 81,1% à la suite de l’intervention (P < ,001). Au moins 1 patient présentant de la toux et de la fièvre a reçu un masque dans 17% des cabinets avant l’intervention; durant la période d’observation après l’intervention, au moins 1 patient a reçu un masque dans 66,7% des cabinets (P < ,001). Avant l’intervention, on demandait aux patients d’utiliser du gel alcoolisé pour les mains dans 24,5% des cabinets et, après l’intervention, ce pourcentage est passé à 79,2% (P < .001). On conseillait aux patients de s’asseoir à au moins 1 mètre les uns des autres dans 39,6% des cabinets avant l’intervention et, après l’intervention, cette pratique était suivie dans 52,8% des cabinets (P < ,001). Avant l’intervention, le pourcentage des cabinets qui respectaient les 4 mesures de prévention à l’étude se situait à 3,8%; après l’intervention, 52,8% des cabinets (P < ,001) avaient adopté ces pratiques exemplaires, soit une augmentation de 49%.
CONCLUSION
Une intervention à multiples facettes entreprise par des infirmières en santé publique a permis de promouvoir avec succès des pratiques exemplaires pour le contrôle des infections respiratoires dans des cabinets de soins de première ligne. La collaboration entre les services de santé publique et les soins de première ligne favorise des pratiques exemplaires, et mérite d’être étudiée plus à fond et élargie dans les domaines d’intérêts communs.
EDITOR’S KEY POINTS.
This study assessed whether a short-term outreach intervention was effective in improving practices for controlling respiratory infections in family physicians’ offices.
Outcomes were the percentage of offices following the 4 observed infection-control practices (masks, alcohol gel, spaced seating, and signs) and the 2 reported infection-control practices (disinfection of potentially contaminated surfaces and use of hand-gel dispensers in examining rooms).
Before the intervention, all 4 infection-control practices were observed in fewer than 4% of offices; 6 weeks following the intervention, more than 50% of offices were using the infection-control practices.
This study is the first to use a facilitator-based intervention to promote guidelines for control of respiratory infections.
POINTS DE REPÈRE DU RÉDACTEUR.
Cette étude évaluait l’efficacité d’une intervention de sensibilisation à court terme pour améliorer les pratiques de contrôle des infections respiratoires dans des cabinets de médecins de famille.
Les résultats étaient mesurés en fonction du pourcentage de cabinets qui suivaient les 4 pratiques de contrôle des infections à l’étude (masque, gel alcoolisé, espace entre les patients, et affiches) et les 2 pratiques de contrôle des infections signalées (désinfection des surfaces possiblement infectées et utilisation des dispensateurs de gel alcoolisé dans les salles d’examen).
Avant l’intervention, les 4 pratiques de contrôle des infections étaient observées dans moins de 4% des cabinets; 6 semaines après l’intervention, plus de 50% des cabinets respectaient ces pratiques de contrôle des infections.
Cette étude est la première à avoir mis à l’essai une intervention utilisant un facilitateur pour promouvoir les guides de pratique en matière de contrôle des infections respiratoires.
Severe acute respiratory syndrome (SARS) disturbed the medical community’s complacency about control of respiratory infections. Severe acute respiratory syndrome disproportionately affected health care workers and innocent bystander patients1,2 and revealed the potential for spread of respiratory infection in primary care offices.3 More recently, occurrences of avian influenza in Asia, Europe, and North America with occasional spread to humans has increased concern about the risk of pandemic influenza.4 Added to these concerns is the recognition that common microbial pathogens are becoming increasingly resistant to antimicrobial therapy. In the face of a re-emerging threat of respiratory infections, prevention is increasingly important.
Several guidelines on control of respiratory infections were issued both before and after SARS.5-10 Guidelines, however, are not always implemented. There is a well documented gap between what ought to be done and what is being done. It is now clear that programs designed only to increase physicians’ knowledge, such as traditional continuing medical education courses, are ineffective in changing physicians’ behaviour.11-13
Growing evidence indicates that interventions involving multiple strategies are more likely to result in improved practice behaviour than single-strategy interventions are.14-18 Bero et al19 looked at 18 systematic reviews covering more than 400 research papers on improving professional performance and concluded that multifaceted facilitation interventions are effective in persuading physicians to incorporate good preventive practices into routine care. More recent reviews20-22 indicate that more research is needed to clarify whether multifaceted interventions are better than single interventions. Interventions tailored to overcome barriers appear to be the most effective.
One of the most effective multifaceted strategies is outreach facilitation. Outreach facilitation involves having trained professionals working directly with physicians in their offices and uses audit of current practice, evidence-based best practices, planning and consensus building, and feedback on performance change as means to improve practice.23 Several randomized controlled trials have shown outreach facilitation to be successful in improving delivery of preventive services and prescribing.24-28 One trial done in Ontario29 showed an absolute change of 11.5%, or a relative improvement of 36%, in preventive practices after an intervention, a result similar to those found in comparable trials.20,30-35
In keeping with the post-SARS recommendation that primary care and public health services work more collaboratively,36 this research was a joint initiative of the University of Ottawa’s Family Medicine Department and the City of Ottawa’s Public Health Branch. We trained public health nurses in outreach facilitation so they could conduct the intervention. We evaluated both process and outcomes. This paper focuses on outcomes. Our study was designed to assess whether a short-term outreach facilitated intervention could be effective in improving practices for control of respiratory infections in family physicians’ offices.
METHODS
Setting
Ottawa, Ont, is a bilingual city with a population of approximately 800 000 people living in both urban and rural areas. The study was conducted between February and May 2004.
Study population
We identified all 638 family physicians in 242 practices in Ottawa and faxed them an invitation to join the study. Nonrespondents received a second fax and a follow-up telephone call. Because of time constraints (the project had to be implemented in 12 weeks), participating practices were self-selected. Recruitment continued until the required number of practices had been enrolled. We estimated that a sample of 49 practices would have 95% power to detect a 15% improvement in the primary outcome measures. We included practices with 2 or more physicians participating in the study even if not all doctors in the office agreed to participate. The smallest clinically significant difference was determined to be a 15% improvement in practices for control of respiratory infections. All practices joining the study gave written consent. The study was approved by the Ottawa Hospital’s Research Ethics Board.
Identification of best practices
At the time of our intervention, there were Ontario guidelines on best practices for control of respiratory infections in hospitals37 and long-term care facilities38 but not in ambulatory care settings. We convened an Expert Advisory Committee to review literature from the Cochrane Library database; medical literature databases (MEDLINE); and major guideline, public health, and relevant professional association websites in Canada and abroad. We found fairly consistent advice on best practices for control of respiratory infections in primary care5-10:
give masks to patients with cough and fever;
direct patients with cough and fever to clean their hands with alcohol-based gel;
ensure patients with cough and fever sit at least 1 m from all others in the waiting area;
have signs to inform patients about these practices and prepare them to follow the directions;
disinfect surfaces that might have been contaminated with respiratory secretions following coughing or sneezing (arms of chairs, toys, etc); and
provide masks and alcohol-based hand gel to physicians and staff who have contact with patients.
Training nurses in outreach facilitation
Five public health nurses took 2 weeks’ training in outreach facilitation and best practices for control of respiratory infections. A mnemonic was developed for both the nurses and physicians to summarize best practices: MASKS (Masks for patients with cough and a fever, Alcohol gel for sanitizing hands, Seat potentially infectious patients apart from others, “Kleen” by disinfecting hard surfaces, and use Signs). A more detailed description and evaluation of the nurses’ training is given in the process evaluation.39
Before-after audit
Professional nurse auditors gathered data once before and then 6 weeks after the intervention. Auditors sat for an hour in physicians’ waiting rooms and noted whether signs informed patients of practices for control of respiratory infections and whether patients who presented with cough and fever received masks, were instructed to clean their hands with alcohol-based gel, and were instructed to sit at least 1 m away from others. Auditors also inquired how often potentially contaminated areas were cleaned with disinfectant and whether alcohol-based hand gel was used in examining rooms. Auditors were blinded to the outcome measures and aware only of data-gathering requirements.
Intervention
The intervention began with facilitators providing feedback to physicians and other practice staff on the baseline audit of practices for control of respiratory infections. Information on evidence-based best practices and a facilitative “tool kit” was presented directly to physicians or indirectly through them to other staff. The tool kit contained colourful signs outlining best practices for control of respiratory infections, a poster demonstrating proper hand-washing technique and use of alcohol-based gel, references listing the main sources of guidelines and websites, 4 articles on infection control,6-9 a box of procedure masks, wall-mounted alcohol gel dispensers with refills, alcohol gel pumps, and hospital-grade disinfectant wipes. During the 5-week intervention, the facilitators worked independently but corresponded with the project team daily and attended scheduled meetings each week to share information and strategies.
Outcome measures
Primary outcome measures were the number and percentage of offices that followed the 4 infection-control practices (masks, alcohol gel, spaced seating, and signs) both separately and together. A practice was considered implemented if it was executed at least once during the observation period. Secondary outcome measures included the 2 reported infection-control practices (disinfecting potentially contaminated surfaces and use of hand-gel dispensers in examining rooms).
Data analysis
Audit forms were coded and entered into SPSS, version 12.0. The quality of data entry was checked by initial frequency runs on all data elements to ensure that responses were correct and consistent. Frequency tables were generated and descriptive statistical procedures conducted. To compare practice performance over time, change in how often preventive measures were used was estimated. Paired t tests were applied to changes using SAS (version 8.1) to determine whether they were statistically significant.
RESULTS
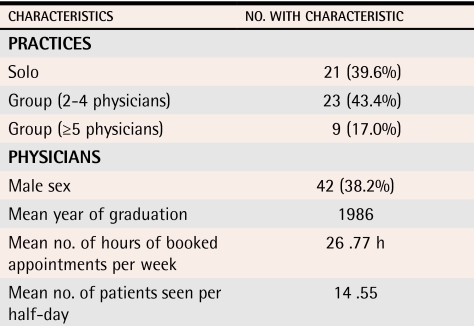
Response rate was 22%; 53 of the 242 practices invited agreed to participate in the study. All 53 practices completed the study, and 95% of physicians within the practices (143/151) agreed to see the nurse facilitators. There was an average of 4 physicians per practice (range 1 to 13). About 40% of physicians were male (Table 1).
Table 1. Characteristics of the 53 participating offices and 110 physicians.
110 participating physicians reported information on certain characteristics before the intervention; 7 physicians did not respond to either of the last 2 questions, so means were calculated on the basis of 103 respondents.
Each public health nurse had primary responsibility for 10 or 11 offices. Each office was visited at least twice during the 5-week intervention; 28 offices (55%) received a third visit, and 7 (13%) received a fourth visit. Most meetings were held during the lunch hour.
Statistically significant differences were observed in all 4 primary outcome measures, both together and separately (Table 2). Before the intervention, all 4 infection-control practices were observed in fewer than 4% of offices; 6 weeks after the intervention, they were observed in more than 50% of offices. The practice most frequently followed was posting signs about infection control guidelines in waiting areas for patients. Before the intervention, 15% of offices followed this practice; 6 weeks after the intervention, more than 81% of offices did so. The practice least frequently followed was offering masks to patients with fever and cough. Only 17% of offices did this before the intervention, but about 66% did so after the intervention.
Table 2. Results of before-after audit of best practices in control of respiratory infections in family physicians’ offices.
P = .0001 (N = 53).
CI—confidence interval.
*Percent change might not reflect the difference between percentages in the before and after columns exactly due to rounding or to missing data.
†Valid percentages are used (ie, missing data have been removed).
DISCUSSION
To our knowledge, this is the first study to use a facilitator-based intervention to promote guidelines for control of respiratory infections. Few family physicians’ offices followed these guidelines before the intervention. There was a marked improvement after the intervention with almost a 50% increase in adoption of the evidence-based best practices recommended in the guidelines.
Strengths of the study include sufficient statistical power from an adequate sample size and separation of the intervention from the data collection, so that physicians, office staff, and nurse facilitators were blinded to outcomes.
Limitations
This was an uncontrolled study. Factors that might lead to overestimation of the intervention’s effectiveness are the relatively low response rate of 22%, which suggests that only highly motivated physicians were involved; the release of provincial guidelines, Preventing Respiratory Illnesses in Community Settings,36 during the study; and practice staff’s awareness that they were being observed (Hawthorne effect). Despite efforts to minimize bias, auditors might have inferred the desired infection-control practices owing to their nursing background. They derived no benefit from the success or failure of the study, however.
With a low recruitment rate and without a control group, it is impossible to determine the extent to which the changes were brought about by release of the guidelines or by the intervention or to determine whether the sample of physicians was representative, thus limiting the generalizability of the findings. Previous research, however, suggests that publishing and distributing guidelines alone is rarely effective in changing physicians’ behaviour.11-13 Factors that might have led to underestimation of the intervention’s effectiveness include the short intervention period of 5 weeks and the timing of the intervention near the end of the respiratory infection outbreak season.
Conclusion
This before-after study demonstrated that facilitation of a multifaceted intervention by public health nurses helped promote best practices for control of respiratory infections in primary care offices. These findings add weight to the growing evidence that outreach facilitation is an effective strategy for knowledge transfer. A logical next step would be to offer this facilitated approach in a broader context. This could be done as part of a multicentre randomized controlled trial or through a broader program developed jointly by primary care and public health services. Finally, this study suggests that collaboration between public health services and primary care is possible and can lead to positive outcomes for all concerned.
Acknowledgments
We thank Ms Jessie McGowan for designing and implementing the search strategy to access guidelines for preventive care, Dr Virginia Roth for participating in the Expert Advisory Committee and the course for facilitators, and Mr James Jaffey for statistical analysis.
Biographies
Dr Hogg is Director of Research in the Department of Family Medicine at the University of Ottawa in Ontario.
Dr Huston was Associate Medical Officer of Health for the City of Ottawa and an Adjunct Professor of Epidemiology and Community Medicine at the University of Ottawa at the time of the study.
Dr Martin is Associate Professor of Family Medicine at the Northern Ontario School of Medicine and an Adjunct Professor at the Indigenous Peoples’ Health Research Centre at the First Nations University of Canada.
Dr Saginur practises in the Department of Medicine at the Ottawa Hospital.
Ms Newbury was Program Planning and Evaluation Officer in the City of Ottawa’s Mandatory Evaluation and Development Unit at the time of the study.
Ms Vilis was Facilitator Coordinator at the time of the study, and Dr Soto is Research Manager, in the Department of Family Medicine at the Institute of Population Health at the University of Ottawa.
Footnotes
Competing interests: None declared
References
- 1.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 2.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 3.Svoboda T, Henry B, Shulman L, Kennedy E, Rea E, Ng W, et al. Public health measures to control the spread of the severe acute respiratory syndrome during the outbreak in Toronto. N Engl J Med. 2004;350(23):2352–2361. doi: 10.1056/NEJMoa032111. [DOI] [PubMed] [Google Scholar]
- 4.Zambon M. The inexact science of influenza prediction. Lancet. 2004;363:582–583. doi: 10.1016/S0140-6736(04)15624-9. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health and Long-Term Care. Public health: hand washing. Toronto, Ont: Ministry of Health and Long-Term Care; 2003. [cited 2006 September 8]. Available from: http://www.health.gov.on.ca/english/public/program/pubhealth/handwashing/handwashing_mn.html. [Google Scholar]
- 6.Health Canada. Alcohol for hand hygiene: new comparative studies add to the evidence base. Ottawa, Ont: Health Canada; 2003. [cited 2006 June 26]. Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/03vol29/dr2901eb.html. [PubMed] [Google Scholar]
- 7.Institute for Clinical Evaluative Sciences (ICES) [cited 2006 June 26];“Out, damned spot! ”—hand hygiene. 2003 9(3):1. Available from: http://www.ices.on.ca/informed/periodical/issue/971-vol9issue3Art5.pdf.
- 8.Centers for Disease Control and Prevention. Respiratory hygiene/cough etiquette in healthcare settings. Bethesda Md: Centers for Disease Control and Prevention; 2003. [cited 2006 June 26]. Available from: http://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm. [Google Scholar]
- 9.Boyce JM, Pittet D. [cited 2006 June 26];Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. 2002 51(RR16):1–44. doi: 10.1067/mic.2002.130391. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5116a1.htm. [DOI] [PubMed]
- 10.American Academy of Pediatrics. Infection control in physicians’ offices. American Occupational Safety and Health Administration (OSHA). Pediatrics. 2000;105(6):1361–1369. [PubMed] [Google Scholar]
- 11.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–1322. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 12.Davis DA, Thompson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995;274(9):700–705. doi: 10.1001/jama.274.9.700. [DOI] [PubMed] [Google Scholar]
- 13.Tamblyn R, Battista R. Changing clinical practice: which interventions work? J Contin Educ Health Prof. 1993;13(4):273–288. [Google Scholar]
- 14.Leininger LS, Finn L, Dickey L, Dietrich AJ, Foxhall L, Garr D, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med. 1996;5(2):108–115. doi: 10.1001/archfami.5.2.108. [DOI] [PubMed] [Google Scholar]
- 15.Lomas J, Haynes RB. A taxonomy and critical review of tested strategies for the application of clinical practice recommendations: from “official” to “individual” clinical policy. Am J Prev Med. 1988;4(4 Suppl):77–94. [PubMed] [Google Scholar]
- 16.Wensing M, Grol R. Single and combined strategies for implementing changes in primary care: a literature review. Int J Qual Health Care. 1994;6(2):115–132. doi: 10.1093/intqhc/6.2.115. [DOI] [PubMed] [Google Scholar]
- 17.Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract. 1998;48(427):991–997. [PMC free article] [PubMed] [Google Scholar]
- 18.Solberg LI, Brekke ML, Kottke TE. Are physicians less likely to recommend preventive services to low-SES patients? Prev Med. 1997;26(3):350–357. doi: 10.1006/pmed.1997.0150. [DOI] [PubMed] [Google Scholar]
- 19.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ. 1998;317:465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39(8 Suppl 2):112–145. [PubMed] [Google Scholar]
- 21.Jamtvedt G, Young JM, Kristoffersen DT, Thomson MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003. CD000259. [DOI] [PubMed]
- 22.Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004. iii-iv,1-72. [DOI] [PubMed]
- 23.Harvey G, Loftus-Hills A, Rycroft-Malone J, Titchen A, Kitson A, McCormack B, et al. Getting evidence into practice: the role and function of facilitation. J Adv Nurs. 2002;37(6):577–588. doi: 10.1046/j.1365-2648.2002.02126.x. [DOI] [PubMed] [Google Scholar]
- 24.Manfredi C, Czaja R, Freels S, Trubitt M, Warnecke R, Lacey L. Prescribe for health. Improving cancer screening in physician practices serving low-income and minority populations. Arch Fam Med. 1998;7(4):329–337. doi: 10.1001/archfami.7.4.329. [DOI] [PubMed] [Google Scholar]
- 25.Kottke TE, Solberg LI, Brekke ML, Conn SA, Maxwell P, Brekke MJ. A controlled trial to integrate smoking cessation advice into primary care practice: Doctors Helping Smokers, Round III. J Fam Pract. 1992;34(6):701–708. [PubMed] [Google Scholar]
- 26.Cockburn J, Ruth D, Silagy C, Dobbin M, Reid Y, Scollo M, et al. Randomised trial of three approaches for marketing smoking cessation programmes to Australian general practitioners. BMJ. 1992;304:691–694. doi: 10.1136/bmj.304.6828.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsinger LS, Harris R, Qaqish B, Strecher V, Kaluzny A. Using an office system intervention to increase breast cancer screening. J Gen Intern Med. 1998;13(8):507–514. doi: 10.1046/j.1525-1497.1998.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubin M, Vezina L, Fortin JP, Bernard PM. Effectiveness of a program to improve hypertension screening in primary care. CMAJ. 1994;150:509–515. [PMC free article] [PubMed] [Google Scholar]
- 29.Lemelin J, Hogg W, Baskerville N. Evidence to action: a tailored multifaceted approach to changing family physician practice patterns and improving preventive care. CMAJ. 2001;164(6):757–763. [PMC free article] [PubMed] [Google Scholar]
- 30.Humair JP, Ward J. Smoking-cessation strategies observed in videotaped general practice consultations. Am J Prev Med. 1998;14:1–8. doi: 10.1016/s0749-3797(97)00010-x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman RM, Papenfuss MR, Buller DB, Moon TE. Attitudes and practices of primary care physicians for prostate cancer screening. Am J Prev Med. 1996;12:277–281. [PubMed] [Google Scholar]
- 32.Buntinx F, Winkens R, Grol R, Knottnerus JA. Influencing diagnostic and preventive performance in ambulatory care by feedback and reminders. A review. Fam Pract. 1993;10:219–228. doi: 10.1093/fampra/10.2.219. [DOI] [PubMed] [Google Scholar]
- 33.Frame PS, Zimmer JG, Werth PL, Hall WJ, Eberly SW. Computer-based vs manual health maintenance tracking. A controlled trial. Arch Fam Med. 1994;3:581–588. doi: 10.1001/archfami.3.7.581. [DOI] [PubMed] [Google Scholar]
- 34.Morrissey JP, Harris RP, Kincade-Norburn J, McLaughlin C, Garrett JM, Jackman AM, et al. Medicare reimbursement for preventive care. Changes in performance of services, quality of life, and health care costs. Med Care. 1995;33:315–331. [PubMed] [Google Scholar]
- 35.Hulscher M, Van Drenth B, van de Wouden J, Mokkink H, van Weel C, Grol R. Changing preventive practice: a controlled trial on the effects of outreach visits to organise prevention of cardiovascular disease. Qual Health Care. 1997;6(1):19–24. doi: 10.1136/qshc.6.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ontario Ministry of Health and Long Term Care. Preventing respiratory illnesses in community settings: guidelines for infection control and surveillance for febrile respiratory illness (FRI) in community settings in non-outbreak conditions. Toronto, Ont: Ontario Ministry of Health and Long Term Care; 2004. [cited 2006 June 28]. Available from: http://www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs3/guide_fri_comm_031104.pdf. [Google Scholar]
- 37.Ontario Ministry of Health and Long Term Care. Preventing respiratory illness, protecting patients and staff. Infection control and surveillance standards for febrile respiratory illness (FRI) in non-outbreak conditions in acute care hospitals. Toronto, Ont: Ontario Ministry of Health and Long Term Care; 2003. [cited 2006 June 28]. Available from: http://www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs3/dir_infec_control_010604.pdf. [Google Scholar]
- 38.Ontario Ministry of Health and Long Term Care. Final report of the Infection Control Standards Task Force: non-acute institutional settings. Preventing respiratory illnesses, protecting residents and staff in non-acute care institutions. Recommended infection control and surveillance standards for febrile respiratory illness (FRI) in non-outbreak conditions. Toronto, Ont: Ontario Ministry of Health and Long Term Care; 2004. [cited 2006 June 28]. Available from: http://www.health.gov.on.ca/english/providers/program/pubhealth/sars/docs/docs3/report_taskforce_non_acute_031104.pdf. [Google Scholar]
- 39.Huston P, Hogg W, Martin C, Soto E, Newbury A. A process evaluation of an intervention to improve respiratory infection control practices in family physicians offices. Can J Public Health In press. 2006. [DOI] [PMC free article] [PubMed]