Abstract
Cdk5, a cyclin-dependent kinase, is critical for neuronal development, neuronal migration, cortical lamination, and survival. Its survival role is based, in part, on “cross-talk” interactions with apoptotic and survival signaling pathways. Previously, we showed that Cdk5 phosphorylation of mitogen-activated protein kinase kinase (MEK)1 inhibits transient activation induced by nerve growth factor (NGF) in PC12 cells. To further explore the nature of this inhibition, we studied the kinetics of NGF activation of extracellular signal-regulated kinase (Erk)1/2 in cortical neurons with or without roscovitine, an inhibitor of Cdk5. NGF alone induced an Erk1/2-transient activation that peaked in 15 min and declined rapidly to baseline. Roscovitine, alone or with NGF, reached peak Erk1/2 activation in 30 min that was sustained for 48 h. Moreover, the sustained Erk1/2 activation induced apoptosis in cortical neurons. Significantly, pharmacological application of the MEK1 inhibitor PD98095 to roscovitine-treated cortical neurons prevented apoptosis. These results were also confirmed by knocking down Cdk5 activity in cortical neurons with Cdk5 small interference RNA. Apoptosis was correlated with a significant shift of phosphorylated tau and neurofilaments from axons to neuronal cell bodies. These results suggest that survival of cortical neurons is also dependent on tight Cdk5 modulation of the mitogen-activated protein kinase signaling pathway.
INTRODUCTION
Signal transduction cascades translate extracellular signals into cytoplasmic and nuclear compartments that control cell proliferation, differentiation, and survival in neurons as well as other cell types. The mitogen-activated protein kinase (MAPK) signaling network comprises a cascade of sequential kinase phosphorylations to elicit specific cellular behaviors. The duration of the activation determines the cellular response in neurons or neuron progenitors such as PC12 cells (Marshall, 1995; Stork, 2002). A transient extracellular signal-regulated kinase (Erk) activation (10–20 min) as in epidermal growth factor activation of PC12 cells (Heasley and Johnson, 1992; Traverse et al., 1992) induces cell proliferation, a sustained activation (several hours) initiates neurite outgrowth and differentiation, whereas a more chronic activation of the Erk1/2 pathway (24 h), particularly in neurons under stress, is responsible for neuronal apoptosis (Subramaniam et al., 2003, 2004; Cheung and Slack, 2004). Specific pharmacological inhibition of mitogen-activated protein kinase kinase (MEK)1, the upstream kinase from Erk1/2, prevents apoptosis (Alessandrini et al., 1997), which suggests that neuronal survival requires a precisely timed down-regulation of the activated Erk1/2 kinase. Timing depends, in part, on down-regulation of receptors, or feedback inhibition by phosphatases (Keyse, 2000), and/or cross-talk interactions with other signaling cascades. One of these cross-talk regulatory interactions may be exclusive to neurons, which, in contrast to most cells, possess an active Cdk5 kinase.
Cdk5, a cyclin-dependent kinase, regulated by its neuron-specific activators, p35 and p39 (Dhavan and Tsai, 2001), is essential for neuronal morphogenesis (Nikolic et al., 1996) and survival (Tanaka et al., 2001; Li et al., 2002). It sustains neuronal migration, axon guidance, cytoskeletal protein phosphorylation, and synaptic transmission (Smith and Tsai, 2002; Gupta and Tsai, 2003; Kesavapany et al., 2003). Cdk5 is also involved in “cross-talk” interactions with various signal transduction pathways, including modulation of the MAPK pathway (Sharma et al., 2002). Cdk5 promotes neuronal survival by phosphorylation and inactivation of the c-Jun NH2-terminal kinase (JNK) 3 kinase, a key player in an apoptotic pathway (Li et al., 2002) and activation of the neuregulin/phosphatidylinositol 3-kinase (PI3K)/Akt survival pathway (Li et al., 2003). Cdk5 also has a darker side; when deregulated, it has been implicated in apoptosis in several cell systems, including neurons, and it may play a key role in the pathology of several neurodegenerative disorders, such as Alzheimers disease (Cheung and Slack, 2004; Cruz and Tsai, 2004). In a recent study, for example, cortical neurons exposed to staurosporine, a generic inhibitor of kinases, undergo apoptosis preceded by increased expression of Cdk5, p35, and p25, a more active fragment of p35 (Zhang et al., 2004).
Cdk5/p35 seemed to act as a “molecular switch” to modulate the duration of Erk1/2 activation in nerve growth factor (NGF)-stimulated PC12 cells (Harada et al., 2001; Sharma et al., 2002). Transient activation of Erk1/2 phosphorylation (1–2 h) is essential for neurite outgrowth and differentiation (Harada et al., 2001). The transient decline of Erk1/2 activity in NGF-treated PC12 cells after 1 h coincided with the observed increases in p35 and Cdk5 activity, which “turned off” Erk1/2 kinase activity by phosphorylating and inactivating MEK1 as neurons differentiated (Sharma et al., 2002). If, as we have suggested, Cdk5 activity acts in neurons to temporally modulate the activated MAPK pathway, then inhibition of Cdk5 should deregulate Erk1/2 activity and affect neuronal survival. To test this hypothesis, we specifically inhibited Cdk5 activity in postmitotic cortical neurons (which exhibit high levels of Cdk5/p35 expression) and showed that Erk1/2 activation was abnormally sustained up to 12 h, resulting in deregulated phosphorylation of cytoskeletal proteins and induction of cell death as measured by terminal deoxynucleotidyl transferase dUTP nick-end labeling assay (TUNEL) and caspase-3 expression. Significantly, these cells were rescued from apoptosis by pharmacological inhibition of MEK1. We propose that neurons may uniquely possess a tightly regulated mechanism in which Cdk5/p35 prevents apoptosis induced by sustained activation of the MAPK pathway.
MATERIALS AND METHODS
Materials
Antibodies to Cdk5 (J-3, C-8) and p35 (C-19), used at a dilution of 1:500, were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho (P)-JNK (G-7 and total (T)-JNK (c-17 antibodies were also obtained from Santa Cruz Biotechnology). Monoclonal antibody (mAb) to phospho-glycogen synthase kinase (GSK)3β was purchased from Upstate Biotechnology (Lake Placid, NY). Phospho-tau-S199/202 and Tau-5 monoclonal antibodies were obtained from BioSource International (Camarillo, CA) and used at 1:1000 and 1:500 dilutions, respectively. AT8 antibody was purchased from Innunogenetics (Ghent, Belgium) and used at 1:500. Antibodies to phospho-Erk1/2, total-Erk1/2, MEK1/2, and cleaved caspase-3 were obtained from Cell Signaling Technology (Beverly, MA) and used at 1:1000 dilution. α-Tubulin antibody from Sigma-Aldrich (St. Louis, MO) was used at 1:2000. Secondary horseradish peroxidase-conjugated antibodies were obtained from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom) and used at 1:2000. Secondary fluorescence-conjugated Oregon Green and Texas Red antibodies (Invitrogen, Carlsbad, CA) were used at 1:400. Anti-NF200 antibody and NGF were obtained from Sigma-Aldrich. RT97, a phospho-NF-H antibody was a gift from Drs. R. A. Nixon and Veeranna (Nathan Kline Institute for Psychiatric Research, Orangeburg, NY). Roscovitine was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA).
Cell Cultures and Treatment
Primary cultures of rat cortical neurons were prepared from E-18 rat fetuses as described previously (Zheng et al., 2003). After 7 d, neurons were treated with 50 ng/ml NGF and/or 20 μM roscovitine for different times. The cells were either fixed for immunohistochemical analyses or lysed with lysis buffer for immunoprecipitation and Western blot analyses. Rat hippocampal neuronal cultures were prepared from embryonic E-18 rat embryos at a density of 100,000 cells ml−1 on polyornithine- and fibronectin-coated coverslips as described previously (Goslin et al., 1998). Cultures were plated on a 7- to 10-d-old glial bed and grown for 2–3 d in a 40:60 mix of conditioned glial feed (10% fetal bovine serum in neurobasal medium; Invitrogen) and neuronal feed (1× B27 supplemented with 100× l-glutamine; Invitrogen). Starting at 2–3 d in vitro (DIV), cultures were maintained in conditioned medium (1:50 B27/neurobasal medium) with half-feed changes of neuronal feed. After 3 wk, cells were treated as described for the cortical neurons above and fixed for immunohistochemical analyses.
Immunoblotting
Western blot analysis was performed as described previously (Zheng et al., 2002). In brief, cortical neurons were harvested by scraping from dishes and lysed in ice-cold lysis buffer and incubated for 30 min on ice. After centrifugation for 20 min at 13,000 × g at 4°C, the protein concentrations of the supernatants were determined using bicinchoninic acid protein reagent. An equal amount of total protein (25 μg of protein/lane) was resolved on a 4–20% SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane. This membrane was incubated in blocking buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.1% (vol/vol) Tween 20 (TTBS) plus 5% dry milk (wt/vol) for 1 h at room temperature. This was followed by incubation overnight at 4°C in primary antibodies: anti-Cdk5 (1:500), anti-p35 (1:500), anti-MEK1/2 (1:1000), cleaved caspase-3 (1:1000), anti-tubulin (1:2000), phospho-tau (AT8; 1:500) and total tau (1:1000), phospho-NF-H (RT97; 1:5000) and anti-NF-H (1:2000), phospho- or phospho-independent Erk1/2 antibodies (1:2000 and 1:1000), phospho- and total-JNK (1:500), and phospho- and total GSK3 (1:1000). The membranes were then washed four times in TTBS (5 min/each). This was followed by incubation in secondary antibody (goat anti-mouse or goat anti-rabbit IgG [H+L]-horseradish peroxidase conjugate at a dilution of 1:3000) for 2 h at room temperature. Western blots were analyzed using the GE Healthcare enhanced chemiluminescence kit following the manufacturer's instructions. Quantitative assay of antigen expression was based on density measurements of protein bands using ImageJ software (http://rsb.info.nih.gov/ij/).
Immunocytochemical Analyses
Immunofluorescence was performed as described previously (Zheng et al., 2003). In brief, cortical neurons were grown on glass coverslips coated with poly-l-lysine. Cells were washed twice in phosphate-buffered saline (PBS) and fixed for 30 min at room temperature in 4% (wt/vol) paraformaldehyde in PBS, permeabilized in 0.1% (vol/vol) Triton X-100 in PBS for 20 min, blocked with 5% (vol/vol) fetal bovine serum-PBS for 30 min, and then probed with primary antibodies: phospho-Erk (1:100), cleaved caspase-3 (1:100), AT8 (1:500), anti-Cdk5 (1:50), RT97 (1:500), and anti-NF-H (1:50). Antibody was diluted in blocking solution at room temperature for 1 h. After a wash in PBS (three times for 15 min each), the cells or coverslips were incubated with Oregon Green- and Texas Red-conjugated secondary antibodies at 1:400 for 1 h at room temperature, followed by three PBS washes, and mounted in aqueous medium. Fluorescent images were observed using 63× oil immersion objective on a Zeiss LSM510 laser-scanning confocal microscope. Images were combined using Zeiss LSM510 image software and managed in Adobe Photoshop (Adobe Systems, Mountain View, CA).
In Situ Cell Death Detection (TUNEL Assays)
In situ cell death detection was performed as described previously (Zheng et al., 2004). In brief, after primary cortical neurons were cultured and treated, cells were fixed and prepared for TUNEL staining according to the manufacturer's instructions (In Situ Cell Death Detection kit, Roche Diagnostics, Indianapolis, IN). TUNEL staining fluorescent images were captured with a Zeiss LSM510 laser-scanning confocal microscope, and images were managed with Adobe Photoshop. Cell counts were performed as described in figure legends.
Small Interference RNA (siRNA) Transfection
Control nonsilencing and Cdk5 siRNAs (silencing) were designed as follows. Control siRNA (nonsilencing) sense and antisense sequences were 5′-r(UUUUCCGAACGUGUCACGU)d(TT)3′and5′r(ACGUGACACGUUCGGAGAA)d(TT)-3′, respectively. Cdk5 siRNA (silencing) sense and antisense sequences were 5′-r(AAGCCGUACCCGAUGUAUC)d(TT)-3′ and 5′r(GAUACAUCGGGUACGGCUU)d(TT)-3′, respectively. The sense and antisense strands were annealed to create the double-stranded siRNA at a 20 μM concentration. Cdk5 siRNA is designed against the cDNA nucleotide sequence spanning from 859 to 877. Control siRNA and Cdk5 siRNA were dissolved in Danieu's buffer (Nasevicius and Ekker, 2000) before transfection. Final concentrations (40 nM) of siRNAs were transfected into E18 primary cortical neurons (5DIC) by using Lipofectamine 2000 reagent according to the manufacturer's instructions. After 24-h transfection, the cells were either fixed for immunohistochemical analyses or lysed with lysis buffer for immunoprecipitation and Western blot analyses.
Immunoprecipitation and Cdk5 Kinase Assay
Immunoprecipitations and kinase assays were performed as described previously (Veeranna et al., 1998; Zheng et al., 2002; Kesavapany et al., 2004).
RESULTS
Erk1/2 Activation Was Increased and Sustained by Roscovitine Inhibition of Cdk5 Activity in Primary Cortical Neurons: A Time-Course Study
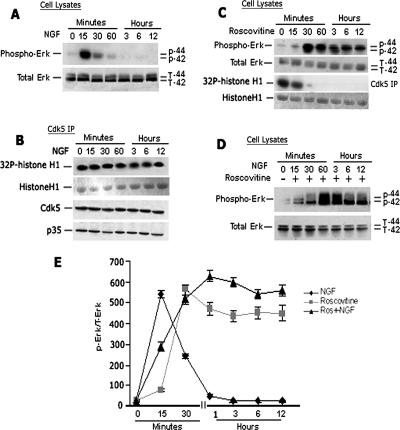
In a previous study, we demonstrated that Cdk5 inhibited the MAPK pathway in NGF-stimulated PC12 cells by phosphorylating MEK1 (Sharma et al., 2002). Although proliferating PC12 cells express Cdk5, its activity is low and only after NGF stimulation is p35 synthesized, activating Cdk5, which remains active during neurite outgrowth (Harada et al., 2001). Transient Erk1/2 activity began to decline at 2 h as Cdk5 activity increased. We suggested that the decline in Erk activity reflected cross-talk by activated Cdk5 (Sharma et al., 2002). To explore the role of Cdk5/p35 in neurons as a modulator of the MAPK cascade, we chose to use E18 rat cortical neurons cultured for 7 d. These postmitotic, differentiated neurons express various neurotrophin receptors, including the NGF TrkA, brain-derived neurotrophic factor TrkB as well as p75NTR involved in regulating neuronal survival (Miller and Kaplan, 2001). In contrast to PC12 cells, they also express high endogenous levels of active Cdk5/p35. To study the kinetics of activation of the MAPK pathway, we exposed cortical neurons to NGF and sampled cells at intervals up to 12 h. In Figure 1A, Western blots of lysates taken at different times show rapid activation of Erk1/2 phosphorylation, reaching a peak at 15 min, followed by a gradual decline to baseline levels at 1–12 h (Figure 1, A and E). The kinetics of the NGF-induced Erk1/2 response differs from that in PC12 cells, which peaks acutely at 5 min and declines more slowly over a period of 2 h and remains sustained at low levels (Harada et al., 2001).
Figure 1.
Inhibition of Cdk5 activity induced sustained Erk1/2 activation in primary cortical neurons. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 50 ng/ml NGF or 20 μM roscovitine, or both and lysates prepared for analysis over a 12-h period. (A) Kinetics of Erk1/2 activation after NGF treatment. Peak Erk1/2 phosphorylation is seen at 15 min followed by a decline to baseline at 3–12 h. (B) Cdk5 immunoprecipitates of lysates (J-3 antibody) during the above-mentioned NGF activation show that the expressions of Cdk5, p35, and Cdk5 activity are constant. (C) Time course of Erk1/2 activation after treatment with roscovitine. An initial peak of activity is reached at 30 min and sustained at that level for 12 h. (D) Combined treatment, pretreated with roscovitine for 30 min and then NGF is added. Erk1/2 activation was sustained up to 12 h at a higher level than roscovitine alone. (E) Line graph shows the corresponding quantification of Erk1/2 phosphorylation in the three different treatment groups. Data represent mean ± SEM of three experiments.
Does this kinetic pattern in cortical neurons reflect changes in Cdk5 activity similar to those seen in PC12 cells? To explore this possibility, these same lysates were used for Cdk5 immunoprecipitation and kinase assay (Figure 1B). In contrast to PC12 cells, there was no significant change in the expression of Cdk5, nor p35, whereas Cdk5 kinase activity was constant and robust throughout the time period. Does this mean that the initial rise and subsequent decline in Erk1/2 phosphorylation after 15 min was unrelated to Cdk5 activity? Can we assume that in these postmitotic neurons there is no cross-talk regulation of the MAPK pathway by Cdk5?
To answer this question, we first treated cortical neurons over the same time period with roscovitine, an inhibitor of Cdk5, to study its effect on Erk1/2 phosphorylation (Figure 1C). At the dose of roscovitine used (20 μM), Cdk5 activity was completely inhibited after 30 min (Figure 1C, third panel). Note that in nontreated cortical neurons, baseline levels of Erk1/2 activity were low, but within 15 min of treatment, Erk1/2 phosphorylation increased more slowly than after NGF, rising rapidly to a peak at 30 min and sustaining this level to 12 h. In the absence of Cdk5 activity, the activation of Erk1/2 is delayed, as if released from an inhibition, and then sustained at high levels (Figure 1E). It seems that in this system inhibition of Cdk5 activity not only delays the Erk1/2 activation but also sustains it. We suggest that in these cortical neurons, there is a balanced cross-talk interaction between Cdk5 and the endogenous MAPK activity. Abolishing Cdk5 activity induced sustained activation of Erk1/2 and suggests a Cdk5 feedback regulation of MAPK signaling cascade in neurons.
Does the application of NGF in the presence of roscovitine change the kinetics of this phosphorylation pattern? Cortical neurons exposed to roscovitine for 30 min were then treated with NGF for different times (Figure 1D). The kinetics of Erk1/2 phosphorylation were similar to that observed after roscovitine alone, sustained at a slightly higher level, however. It seems that the time course of Erk1/2 phosphorylation, when Cdk5 is inactive, is unaffected by NGF stimulation. A gradual decline does not occur and instead, Erk1/2 phosphorylation is sustained at high levels. The data from all three experimental situations are quantified in Figure 1E. These results suggest that endogenous Cdk5 in cortical neurons is acting to modulate the activity of the MAPK pathway.
Inhibition of Cdk5 Activity Induced Neuronal Apoptosis by Increased and Sustained Erk1/2 Activation
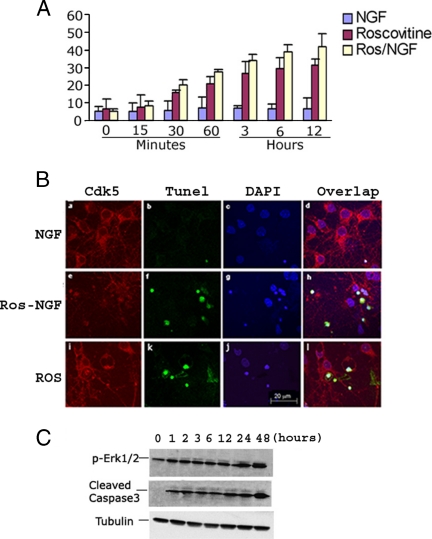
The above-mentioned studies show that the inhibition of Cdk5 activity resulted in increased and sustained Erk1/2 activation in cortical neurons. If Erk1/2 activation is sustained over a much longer period (6–24 h), as in stressed or injured neurons, this chronic activation is responsible for cell death (Cheung and Slack, 2004). Therefore, we asked whether sustained Erk1/2 activation by inhibition of Cdk5 causes cortical neuron apoptosis. To answer this question, we chose to study the kinetics of apoptosis after NGF and/or roscovitine induction of Erk activation by using the same experimental paradigm as previously. TUNEL assays were performed on samples of cells fixed at different times after treatment. Five hundred cells in total were counted at each time point, and the percentages of TUNEL-positive cells were determined (Figure 2A). Baseline levels of apoptosis (5%) were recorded in cells treated only with NGF. Roscovitine-treated cells, with or without NGF, however, exhibited a detectable increment of apoptosis beginning at 30 min after the peak of Erk1/2 kinase activity had been reached and sustained. The level of apoptosis gradually increased to 30–40% after 12 h of sustained activity and continued through 24 h, reaching almost 50% apoptosis (data not shown). Immunocytochemical assays of these cells under the different conditions for 6 h are shown in Figure 2B. At this time, most roscovitine-treated cells were apoptotic (or necrotic), showing cytological as well as nuclear signs of degeneration and fragmentation. To further confirm the long-term apoptotic effect of sustained Erk1/2 activity, we repeated the experiment with roscovitine and followed Erk1/2 activity and cleaved caspase-3 expression (an apoptotic marker) for 48 h (Figure 2C). Cortical neurons deficient in Cdk5 activity began to show signs of apoptosis early on, after 1 h, as soon as peak levels of sustained Erk activity were attained. We propose that in the absence of Cdk5 activity, apoptosis was induced by sustained Erk1/2.
Figure 2.
Roscovitine inhibition of Cdk5 activity induced cortical neuron apoptosis during sustained activation of Erk1/2. (A) Bar graph showing the time course of apoptosis as assayed by the TUNEL procedure. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 50 ng/ml NGF, 20 μM roscovitine, or both for 12 h. Cells were fixed at different times and stained for TUNEL assay. The percentage of TUNEL-positive cells gradually increased from 1 to 12 h after roscovitine or roscovitine plus NGF treatment. Data represent mean ± SEM of three experiments. (B) Immunocytochemistry (ICC) assay of cells after 6 h of each treatment, when a high proportion of cells were apoptotic. Fixed cells were stained for Cdk5, and TUNEL fragmented, condensed apoptotic nuclei are green (b, f, and k). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop. Bar, 20 μm. (C) Treatment with roscovitine for different times induces a sustained activation of Erk1/2 at the high level for 1–48 h and causes an increasing apoptosis in cortical neurons. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with roscovitine. Cell lysates at different times were subjected to Western blot analysis to measure the levels of phospho-Erk1/2, cleaved caspase3, and tubulin. Sustained Erk1/2 activation correlates with elevated cleaved caspase-3 expression.
Roscovitine-induced Neuronal Apoptosis Was Rescued by Inhibition of MEK1 Activation
To test the above-mentioned possibility, in a separate series of experiments we treated cortical neurons with roscovitine with or without the MEK1 inhibitor PD98095 for 2 h, and we repeated the TUNEL assay (Figure 3, A and B). As seen in Figure 3A, there are many more TUNEL-positive cells after roscovitine treatment alone than after roscovitine and PD98095 (Figure 3A, compare j with f and n). Quantification of the results shows that almost 35% of cells treated with roscovitine were apoptotic, whereas ∼6–8% of cells treated with PD98095 only, or PD98095 plus roscovitine, were apoptotic (Figure 3B). To further confirm apoptosis under these conditions, cortical neuron lysates, treated as in Figure 3A, were assayed in Western blots for Erk1/2 activation and cleaved caspases-3 expression, a downstream marker of apoptosis (Figure 3C). In lane 4, after roscovitine treatment, we see a dramatic increase in the expression of phosphorylated Erk1/2 together with caspase-3, and this elevation is dramatically reduced in the presence of the MEK1 inhibitor PD98095 (Figure 3C, lane 3). The histogram below shows the quantitative assay of the caspase-3 expression pattern.
Figure 3.
Roscovitine induced apoptosis of cortical neurons is rescued by inhibiting MEK1. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 20 μM roscovitine, 50 μM PD98095, or both for 2 h and fixed for ICC assay, or lysed for Western assay. (A) Fixed cells were stained for Cdk5 and TUNEL. Most fragmented apoptotic nuclei seen after roscovitine treatment alone (j and l), whereas apoptosis was decreased when Erk1/2 activation was inhibited by PD98095 (n compared with j). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm. (B) Bar graph shows the percentage of cell apoptosis. TUNEL-positive cell counts were obtained from 10 independent fields with a total of 500 cells. Results were expressed as mean ± SEM from three separate treatment groups. (C) Cleaved caspase-3 assay in neurons. Neuronal cell lysates were subjected to Western blot analysis to measure the levels of the phospho-Erk1/2, total Erk1/2, cleaved caspase-3, one of the apoptosis markers, and tubulin. High levels of phosphorylated Erk1/2 (first panel, lane 4) and apoptotic caspase-3 were expressed (third panel, lane 4), reduced in each case after treatment with the MEK1 inhibitor PD98095 (first and third panels, lane 3). Bar graph shows mean density measurement of the cleaved caspase-3. Results are expressed as mean ± SEM of three separate experiments.
Induced Neuronal Apoptosis by Ckd5 Knockdown with Cdk5 siRNA Was Rescued by Inhibition of MEK1 Activation
To further confirm that lack of Cdk5 activity induces cell apoptosis by increasing Erk1/2 activation, we designed siRNAs to specifically silence Cdk5 expression. We transfected control (nonsilencing) and Cdk5 siRNAs (silencing) into 5DIV primary cortical neurons with or without adding the MEK1 inhibitor PD98095 after 5-h transfection. We see an increase in the expression of phosphorylated Erk1/2 together with caspase-3 in the Cdk5 knockdown cells (Figure 4A, lane 2), and this elevation is reduced in the presence of the MEK1 inhibitor PD98095 (Figure 4A, lane 3). The histogram (Figure 4B) shows the quantitative assay of the caspase-3 expression pattern. These results are consistent with the results that were produced by roscovitine treatment (Figure 3). At the cellular level, the effect of the Cdk5 siRNA inhibition is seen in Figure 4B. Cells expressing both pErk1/2 and caspase-3 are seen in Figure 4C, a, b, and d, and rescue by the MEK1 inhibitor PD98095 is seen in Figure 4C, e, f, and h. In cortical neurons, we can induce elevated Erk1/2 activity and apoptosis by a knockdown of Cdk5 with either roscovitine or with a specific siRNA, and in each case rescue with the MEK1 inhibitor.
Figure 4.
Induced apoptosis of cortical neurons by Cdk5 siRNA is rescued by inhibiting MEK1 activity. E18 rat embryonic cortical neurons were cultured for 5 d in B27/neurobasal medium and then transfected with 1) control siRNA, 2) Cdk5 siRNA, and 3) Cdk5 siRNA plus 50 μM PD98095 treatment. After 24 h of transfection, cells were fixed and/or lysed. (A) Western blots of cell lysates to measure the levels of the phospho-Erk1/2, cleaved caspase-3, and tubulin. Only in the presence of the Cdk5 siRNA were high levels of phosphorylated Erk1/2 and apoptotic caspase-3 expressed (lane 2), these were reduced in each case after treatment with the MEK1 inhibitor PD98095 (lane 3). Lanes 2 and 3 show reduced expression of cdk5 in the presence of Cdk5 siRNA. Lane 1 shows Cdk5 expression in presence of con siRNA. (B) Bar graph shows the percentage of apoptosis in each situation. Caspase-3–positive cell counts were obtained from 10 independent fields with a total of 500 cells. Results are expressed as mean ± SEM from three separate treatment groups. (C) ICC assay of cells after 24 h of transfection shows p-Erk1/2 and caspase-3–positive cells. Increased p-Erk1/2 and apoptotic cells seen only in b and d when Cdk5 activity is reduced by Cdk5 siRNA. Expressions of both were decreased when Erk1/2 activation was inhibited by PD98095 (f compared with b). Images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm.
Inhibition of Cdk5 Deregulates the Topographic Phosphorylation of Cytoskeletal Proteins Tau and NF-H in Primary Neurons
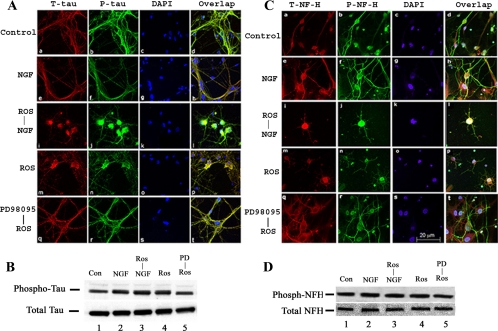
In addition to being targeted to nuclei to regulate transcription, active Erk1/2 is also cytosolic and interacts with the cytoskeleton (Reszka et al., 1995; Veeranna et al., 2000). Erk1 copurifies with tubulin, whereas Erk2 binds to mitogen-activated proteins (Morishima-Kawashima and Kosik, 1996). The Erks are among a number of kinases that phosphorylate cytoskeletal proteins such as tau, which in neurodegenerative disorders, become hyperphosphorylated to form abnormal aggregates within neuronal cell bodies. These pathological changes are usually correlated with neuronal apoptosis (Zhang and Johnson, 2000). Because sustained Erk1/2 activation leads to apoptosis in cortical neurons, we investigated tau and NF phosphorylation immunocytochemically, by using antibodies specific for phosphorylated tau and NFs. Cortical neurons treated with roscovitine (with or without NGF) for 3 h were fixed and immunoreacted for tau expression (Figure 5A). Tau and p-tau expression in control and NGF-treated cells was primarily localized in the extensive axonal outgrowth (Figure 5A, a, b, e, f). Three hours after roscovitine treatment, however, when >30% of the cells were apoptotic, and a significant shift of tau expression to the soma was observed in most cells (Figure 5A, i, j, m, and n), accompanied by a decrease in axon localization. Prior treatment of cells with PD98095 for 30 min before roscovitine treatment resulted in an expression pattern similar to the controls (Figure 5A, compare q–t with a–d). Western blots of these cell lysates with the same AT8 tau antibody showed that the levels of tau phosphorylation increased over control levels (Figure 5B, lanes 2–4), but they were restored to control levels after treatment with PD98095, the MEK1 inhibitor (Figure 5B, lane 5).
Figure 5.
Topographic phosphorylation of cytoskeletal proteins tau and NF-H is deregulated in cortical neurons by roscovitine treatment. (A) Immunocytochemical staining of total tau and p-tau in cortical neurons treated with roscovitine with or without NGF. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated with 1) 1× PBS as a control, 2). 50 ng/ml NGF only, 3) 20 μM roscovitine only, 4) roscovitine plus NGF, and 5) 50 μM PD98095 plus roscovitine. All groups were treated for 3 h before fixation. Control and NGF-treated neurons show normal morphology with tau (nonphospho- and phospho-tau) expression primarily in axons (a–h). Cells treated with roscovitine with or without NGF exhibit intense condensed staining in the soma with minimal staining in the axons (i–p). Cells first treated with 50 μM PD98095, followed by roscovitine show the same appearance as the control and NGF-treated cells (q–t). (B) Immunoblots of total tau and p-tau of cell lysates treated as described above. The cells were harvested and subjected Western blot by using the same phospho-tau antibody AT8 to detect phosphorylated tau and tau5 antibody for analysis total tau. (C) E18 rat hippocampal neurons, cultured for 3 d in B27/Neurobasal medium, were treated as described above, fixed, and stained for total NF-H with anti-NF-H antibody and for p-NF-H with R97 antibody. The control and NGF treatment cells display strong p-NF-H expression in axons with low expression in cell bodies (a–h). In the presence of roscovitine, however, total NF-H and p-NF-H accumulate in cell bodies, whereas axons are deleted of any expression (i–p). Cells pretreated with 50 μM PD98095 followed by roscovitine show the same appearance as the control and NGF-treated cells (q–t). (D) Immunoblots of total NF-H and p-NF-H of cell lysates treated as described above. The cells were harvested and subjected to Western blot to detect phosphorylation of NF-H with RT97 antibody and total NF-H with anti-NF-H antibody. For immunocytochemical analysis, all images were captured using a Zeiss LSM510 laser-scanning confocal microscope and managed using Adobe Photoshop software. Bar, 20 μm.
A similar shift of neurofilament expression was seen in hippocampal cells treated with antibodies to nonphosphorylated and phosphorylated NF-H (Figure 5C). Control and NGF-treated cells showed localization of NF-H, primarily to axons as seen in (Figure 5C, a, b, e, and f). The overlap displays the expression of axonal phospho-NF-H most clearly (Figure 5C, d and h). Exposure to roscovitine changes the expression pattern so that the most intense expression of NF-H is confined to cell bodies, with a significant reduction in the axons (Figure 5C, i–l and m–p). Here, too, prior treatment with PD98059 for 30 min protected cells from the roscovitine effect on NF localization (Figure 5C, q–t). Western blots of these lysates using the same mAb to phosphorylated NF-H showed no significant changes in the levels of NF-H phosphorylation in all cases (Figure 5D). These results are consistent with the hypothesis that inhibition of Cdk5 in neurons evokes sustained activation of Erk1/2, apoptosis, and abnormal localization of phosphorylated cytoskeletal proteins such as tau and NF.
Roscovitine Inhibition of Cdk5 Affects Cross-Talk Interactions with Other Signaling Pathways
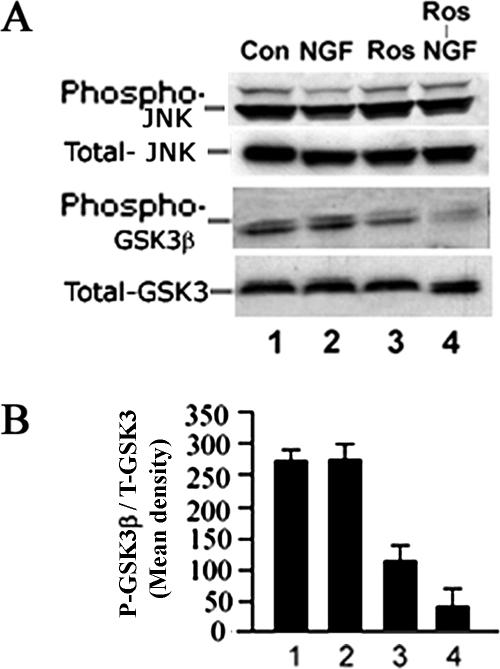
JNK is a stress-activated protein kinase that, when activated, can induce neuronal apoptosis. In a previous study, we showed that cdk5 may play a role in neuronal survival by negatively regulating JNK-3 activation (Li et al., 2002). It has also been demonstrated that cdk5 modulates the expression of GSK3, i.e., inhibition of cdk5 has been reported to activate GSK3 in neurons (Hallows et al., 2003; Morfini et al., 2004). More recently, hyperactive cdk5 activity in p25 transgenic mice inhibits GSK3 activity in hippocampus of young mice, but in older mice this inhibition is eliminated, leading to hyperphosphorylation of tau (Plattner et al., 2006). Hence, the question arises whether roscovitine inhibition of cdk5 activity in cortical neurons, in addition to sustaining Erk1/2 activity, also activated other pathways that might contribute to apoptosis. To answer this question, the same roscovitine experimental paradigm was used over a 6-h period to assay for the expression of phospho-JNK and phospho-GSK3 (Figure 6). Significantly, the levels of phospho-JNK exhibited no change in roscovitine-treated cells compared with controls (Figure 6A, first panel, compare lanes 3 and 4 with 1 and 2), although these cells did exhibit apoptosis as seen in Figure 2. Thus, roscovitine induced apoptosis in neurons without affecting the JNK pathway. In contrast, phospho-GSK3 activity decreased in the presence of roscovitine (Figure 6A, second lanes, 3 and 4, and histogram), suggesting that GSK3 was activated. This activation may have contributed to the additional phosphorylation of tau seen in Figure 5B, but did it also contribute to the apoptosis seen in these cells? That cell morphology was normal, however, and no apoptosis occurred after treatment with PD98095, the MEK1 inhibitor (Figure 5A, q–t), suggests that at least the apoptotic effect was not due to activated GSK3, consistent with our hypothesis that sustained Erk1/2 activation was responsible.
Figure 6.
Cdk5 cross-talk with other kinase pathways, JNK and GSK3. E18 rat embryonic cortical neurons were cultured for 7 d in B27/neurobasal medium and then treated as follows: 1) nontreated, 2) 50 ng/ml NGF, 3) 20 μM roscovitine, and 4) roscovitine plus NGF. After 6-h treatment, lysates were prepared and subjected to Western blot analysis to detect the activation of JNK and GSK3 using phospho-specific antibodies. (A) JNK and GSK3 activation after NGF treatment. (B) Bar graph shows the mean densities of phosphorylated GSK3 in each situation. Results are expressed as mean ± SEM from three separate treatment groups.
DISCUSSION
Controlling neuronal survival during development is one of the principal functions of the neuron-specific kinase Cdk5/p35. A Cdk5 “knockout,” an embryonic lethal with major defects in the cortex and cerebellum (Ohshima et al., 1996), was rescued by expressing Cdk5 primarily in the nervous system (Tanaka et al., 2001). How does this kinase exercise this survival function? We suggest that in addition to other functions, Cdk5 modulates several interacting survival and apoptotic signaling pathways, among which is the MAPK pathway. Our data are consistent with this hypothesis. Cortical neurons treated with roscovitine begin apoptosis within 30–60 min after exposure. Moreover, the signaling pathway leading to cell death seems to be a sustained (12- to 24-h) activation of the MAPK cascade via an activated MEK1, released from Cdk5 modulation (Sharma et al., 2002). This conclusion is supported by 1) sustained Erk1/2 activation correlated in time with cortical neuron apoptosis (assayed by TUNEL and caspase-3 expression); 2) pharmacological inhibition of MEK1 with PD98095 rescued roscovitine-treated cortical neurons from apoptosis; and 3) silencing Cdk5 in cortical neurons by using Cdk5 siRNA up-regulated Erk1/2 and caspace-3 expression, an effect eliminated by the MEK1 inhibitor. Furthermore, these results are consistent with other reports. The increased level of MEK1 activity in the cortex of p35 null mice, exhibiting only low basal levels of Cdk5 activity (Sharma et al., 2002; Hallows et al., 2003) and roscovitine induction of elevated MEK1 activity in NGF-treated PC12 cells imply a role for Cdk5 in modulating the MAPK pathway via MEK1 phosphorylation. Finally, evidence of elevated levels of apoptosis in the cortex of E16 Cdk5 null mice, the greater susceptibility of Cdk5−/− cortical neurons to apoptotic stimuli (Li et al., 2002), and the appearance of many brain stem neurons in E18 Cdk5 null mice with hyperphosphorylated cytoskeletal protein accumulations (Sharma et al., 2002) are all in agreement with the view that Cdk5 activity in neurons may act as a rheostat modulating the MAPK signaling pathway among others, to promote neuronal survival.
We have shown that the PI3K/Akt survival pathway is also modulated by Cdk5 (Li et al., 2003). Although apoptosis observed in our experiments could be attributed to a dysfunctional PI3K/Akt signaling pathway, that apoptosis was prevented by the MEK1 inhibitor PD98095 suggests otherwise. Our results also preclude a role for activation of JNK apoptotic pathways in roscovitine-treated neurons. Finally, although GSK3 is activated in roscovitine-treated cortical neurons, that neurons are rescued from apoptosis by the MEK1 inhibitor PD98095 also argues against a role for GSK3 in apoptosis.
An altered cytoskeleton or scaffolding proteins may be involved because these are essential in localizing the activated Erk1/2 to cytosol, membrane, or nucleus (Pouyssegur and Lenormand, 2003). The dying neurons (cortical and hippocampal) during roscovitine exposure exhibited a profound change in the localization of phosphorylated cytoskeletal proteins tau and NF-H: an abnormal shift from axons to cell bodies. Normally, these cytoskeletal proteins are assembled and phosphorylated in axons by a variety of kinases, including Erk1/2, Cdk5, and GSK3 (Veeranna et al., 1998, 2000; Reynolds et al., 2000). In stress-induced neurons undergoing apoptosis (Zhang and Johnson, 2000) and in neurodegenerative disorders (Buee et al., 2000), abnormal accumulation of hyperphosphorylated tau and NF proteins occur in cell bodies. Cell death could have been caused by aberrant phosphorylation of the neuronal cytoskeleton, which altered cellular trafficking and transport, inducing neuronal dysfunction (Smith and Tsai, 2002). This is consistent with pathological accumulations of phosphorylated NF proteins in the soma of Cdk5 knockout brain stem neurons (Sharma et al., 2002), and with evidence of up-regulation of GSK3 and hyperactivation of Erk kinases in p35 null mouse brain coupled to a redistribution of phosphorylated cytoskeletal proteins (Hallows et al., 2003). Our own results are consistent with the above-mentioned findings, because the up-regulation of GSK3 does correlate with increased tau phosphorylation in roscovitine-treated cortical neurons. No doubt Erk1/2 activity was also involved because tau phosphorylation decreased in the presence of PD98095. Because Erk1/2 is the principal kinase phosphorylating rat and mouse NF proteins (Veeranna et al., 1998), the elevated Erk1/2 activity could account for the abnormal phosphorylation and cytoskeletal reorganization of hippocampal neurons.
Our data are consistent with the hypothesis that roscovitine-induced apoptosis results from a sustained activation of Erk1/2 activity evoked by an “escape” from Cdk5/p35 regulation. Nevertheless, there may be an alternative explanation. In some neurons, the effect of roscovitine can be complex depending on cell phenotype, functional state, and Cdk5 target sites. One critical site is at the synapse. Cdk5/p35 is implicated in the exocytotic and endocytotic events of transmitter release at the synapse (Cheung et al., 2006). For example, roscovitine induced a rapid transmitter release in hippocampal slices and synaptosomes that was coupled to enhanced Ca2+ influx from P/Q-type voltage-dependent calcium channels (VDCCs) (Tomizawa et al., 2002). Evidently, Cdk5/p35 modulates transmitter release through phosphorylation of a P/Q-type Ca2+ channel subunit and down-regulation of channel activity. Can the release of channel activity by roscovitine inhibition of Cdk5 elicit sustained Erk1/2 activity and cell death? Cerebellar granule neurons treated with roscovitine become either apoptotic or necrotic, the latter correlated with an excitotoxicity at the synapse involving functionally linked α-amino-3-hydroxy-5- methyl-4-isoxazolepropionic acid and N-methyl-d-aspartate (NMDA) receptors also coupled to Ca2+ influx via P/Q-type VDCCs (Monaco and Vallano, 2005). How does this relate to sustained activated Erk1/2 and neuronal cell death? It is well documented that in neuronal cultures subjected to NMDA-meditated toxicity, okadaic acid, and oxidative stress, Erk activity is elevated and cell death follows, an effect inhibited by MEK1 inhibitors (Stanciu et al., 2000). Stress induced ischemia evoke similar excitotoxic effects in neurons correlated with activated sustained Erk1/2 and cell death rescued by MEK1 inhibitors (Irving and Bamford, 2002). These effects resemble the cortical neuron response to roscovitine seen in our data, which suggests that down-regulation of Cdk5 activity at the synapse may induce excitotoxicity and a sustained Erk kinase, which, in turn, activates a still unknown cell death process.
Is it possible that Cdk5/p35 in the nervous system has evolved to become a “surveillance system” that, among its other functions, monitors and integrates fluctuations in the activities of signaling cascades involved in growth, differentiation, and survival? In our laboratory, we have demonstrated Cdk5 mediation of the MAPK, JNK3, PI3K/Akt, and GRF2/Rac pathways and others have implicated it in the GSK3 signaling pathway (Hallows et al., 2003). Cdk5 activity is tightly regulated in the nervous system, but it may be deregulated under neuronal stress (e.g., oxidative injury, excitotoxic stimulation, and β-amyloid exposure), resulting in apoptosis marked by aggregates of hyperphosphorylated tau and other cytoskeletal proteins. These neuronal pathologies may also arise if stress induces a calpain-mediated cleavage of p35 to a more stable p25 activator, which hyperactivates Cdk5, promoting tau hyperphosphorylation and cell death, a model proposed for some neurodegenerative disorders (Patrick et al., 1999; Lee et al., 2000). Cdk5 in the nervous system is a “two-faced” kinase, it promotes neuronal cell death or survival (Cheung and Ip, 2004; Cruz and Tsai, 2004). An up- or down-regulation of Cdk5 can evoke survival challenges to neurons. Such changes in Cdk5 activity, perhaps even subtle perturbations in its cellular localization (membrane, cytosol, cytoskeleton, or nucleus), may have profound, simultaneous effects on networks of interacting kinase cascades, leading to totally opposing outcomes.
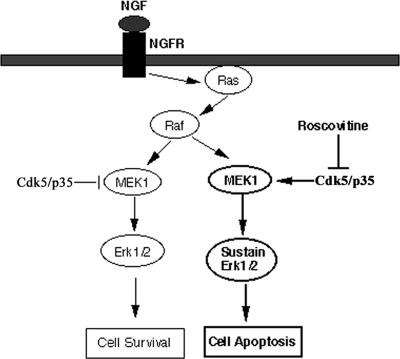
The diagram in Figure 7illustrates and describes our model of the role of cdk5 in modulating the expression of the MAPK pathway in cortical neurons.
Figure 7.
Model of Cdk5/p35 cross-talk modulation of the MAPK cascade to insure neuronal survival. In normal functioning neurons, the left side of the figure shows how Cdk5/p35 inhibits MEK1 activity to terminate the MAPK transient activation response after a short duration, thereby ensuring survival. This is based on previously reported data (Harada et al., 2001; Sharma et al., 2002). On the right side, the current data, showing the induction of sustained activation of Erk1/2 and apoptosis by roscovitine inhibition of Cdk5 activity, support the modulating role of Cdk5/p35 shown on the left side.
ACKNOWLEDGMENTS
This work was supported by funds from the Divisions of Intramural Research of the National Institute of Neurological Disorders and Stroke.
Abbreviations used:
- Cdk5
cyclin-dependent kinase-5
- Erk
extracellular signal-regulated protein kinase
- MAPK
mitogen-activated protein kinase
- MEK1
mitogen-activated protein kinase kinase 1
- NGF
nerve growth factor
- TUNEL
terminal deoxynucleotidyl-transferase enzyme-mediated dUTP nick end labeling
- PI3K
phosphatidylinositol 3-kinase
- siRNA
small interference RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0851) on November 15, 2006.
REFERENCES
- Alessandrini A., Brott B. K., Erikson R. L. Differential expression of MEK1 and MEK2 during mouse development. Cell Growth Differ. 1997;8:505–511. [PubMed] [Google Scholar]
- Buee L., Bussiere T., Buee-Scherrer V., Delacourte A., Hof P. R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Cheung E. C., Slack R. S. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE. 2004;2004:PE45. doi: 10.1126/stke.2512004pe45. [DOI] [PubMed] [Google Scholar]
- Cheung Z. H., Ip N. Y. Cdk 5, mediator of neuronal death and survival. Neurosci. Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
- Cheung Z. H., Fu A. K., Ip N. Y. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron. 2006;50:13–18. doi: 10.1016/j.neuron.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Cruz J. C., Tsai L. H. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr. Opin. Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Dhavan R., Tsai L. H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Goslin K., Asmussen H., Branker G. Culturing Nerve Cells. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Gupta A., Tsai L. H. Cyclin-dependent kinase 5 and neuronal migration in the neocortex. Neurosignals. 2003;12:173–179. doi: 10.1159/000074618. [DOI] [PubMed] [Google Scholar]
- Hallows J. L., Chen K., DePinho R. A., Vincent I. Decreased cyclin-dependent kinase 5 (cdk5) activity is accompanied by redistribution of cdk5 and cytoskeletal proteins and increased cytoskeletal protein phosphorylation in p35 null mice. J. Neurosci. 2003;23:10633–10644. doi: 10.1523/JNEUROSCI.23-33-10633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T., Morooka T., Ogawa S., Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat. Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. The β-PDGF receptor induces neuronal differentiation of PC12 cells. Mol. Biol. Cell. 1992;3:545–553. doi: 10.1091/mbc.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving E. A., Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J. Cereb. Blood Flow and Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Kesavapany S., et al. p35/Cyclin-dependent kinase 5 phosphorylation of ras guanine nucleotide releasing factor 2 (RasGRF2) mediates Rac-dependent extracellular signal-regulated kinase 1/2 activity, altering RasGRF2 and microtubule-associated protein 1b distribution in neurons. J. Neurosci. 2004;24:4421–4431. doi: 10.1523/JNEUROSCI.0690-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavapany S., Li B. S., Pant H. C. Cyclin-dependent kinase 5 in neurofilament function and regulation. Neurosignals. 2003;12:252–264. doi: 10.1159/000074627. [DOI] [PubMed] [Google Scholar]
- Keyse S. M. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Li B. S., Ma W., Jaffe H., Zheng Y., Takahashi S., Zhang L., Kulkarni A. B., Pant H. C. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J. Biol. Chem. 2003;278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- Li B. S., Zhang L., Takahashi S., Ma W., Jaffe H., Kulkarni A. B., Pant H. C. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 2002;21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Miller F. D., Kaplan D. R. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol. Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco E. A., Vallano M. L. Roscovitine triggers excitotoxicity in cultured granule neurons by enhancing glutamate release. Mol. Pharmacol. 2005;68:1331–1342. doi: 10.1124/mol.105.012732. [DOI] [PubMed] [Google Scholar]
- Morfini G., Szebenyi G., Brown H., Pant H. C., Pigino G., DeBoer S., Beffert U., Brady S. T. A novel CDK-5 dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Kosik K. S. The pool of map kinase associated with microtubules is small but constitutively active. Mol. Biol. Cell. 1996;7:893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A., Ekker S. C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nikolic M., Dudek H., Kwon Y. T., Ramos Y. F., Tsai L. H. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward J. M., Huh C. G., Longenecker G., Veeranna , Pant H. C., Brady R. O., Martin L. J., Kulkarni A. B. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Plattner F., Angelo M., Giese K. P. The roles of cyclin-dependent kinase 5 and glycogen synthase 3 in tau hyperphosphorylation. J. Biol. Chem. 2006;281:25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J., Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Eur. J. Biochem. 2003;270:3291–3299. doi: 10.1046/j.1432-1033.2003.03707.x. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Seger R., Diltz C. D., Krebs E. G., Fischer E. H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Natl. Acad. Sci. USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. H., Betts J. C., Blackstock W. P., Nebreda A. R., Anderton B. H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J. Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Sharma P., Veeranna , Sharma M., Amin N. D., Sihag R. K., Grant P., Ahn N., Kulkarni A. B., Pant H. C. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J. Biol. Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- Smith D. S., Tsai L. H. Cdk5 behind the wheel: a role in trafficking and transport? Trends Cell Biol. 2002;12:28–36. doi: 10.1016/s0962-8924(01)02181-x. [DOI] [PubMed] [Google Scholar]
- Stanciu M., et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Stork P. J. ERK signaling: duration, duration, duration. Cell Cycle. 2002;1:315–317. doi: 10.4161/cc.1.5.145. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Strelau J., Unsicker K. Growth differentiation factor-15 prevents low potassium-induced cell death of cerebellar granule neurons by differential regulation of Akt and ERK pathways. J. Biol. Chem. 2003;278:8904–8912. doi: 10.1074/jbc.M210037200. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Zirrgiebel U., von Bohlen Und Halbach O., Strelau J., Laliberte C., Kaplan D. R., Unsicker K. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J. Cell Biol. 2004;165:357–369. doi: 10.1083/jcb.200403028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Veeranna, Ohshima T., Rajan P., Amin N. D., Cho A., Sreenath T., Pant H. C., Brady R. O., Kulkarni A. B. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J. Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K., Ohta J., Matsushita M., Moriwaki A., Li S.-T., Takei K., Matsui H. Cdk5/p35 regulates neurotransmitter release through phosphorylation and downregulation of P/Q-type voltage-dependent calcium channel activity. J. Neurosci. 2002;22:2590–2597. doi: 10.1523/JNEUROSCI.22-07-02590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S., Gomez N., Paterson H., Marshall C., Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna , Amin N. D., Ahn N. G., Jaffe H., Winters C. A., Grant P., Pant H. C. Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J. Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna , Shetty K. T., Takahashi M., Grant P., Pant H. C. Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res. Mol. Brain Res. 2000;76:229–236. doi: 10.1016/s0169-328x(00)00003-6. [DOI] [PubMed] [Google Scholar]
- Zhang B. F., Peng F. F., Zhang W., Shen H., Wu S. B., Wu D. C. Involvement of cyclin dependent kinase 5 and its activator p35 in staurosporine-induced apoptosis of cortical neurons. Acta Pharmacol. Sin. 2004;25:1105–1111. [PubMed] [Google Scholar]
- Zhang J., Johnson G. V. Tau protein is hyperphosphorylated in a site-specific manner in apoptotic neuronal PC12 cells. J. Neurochem. 2000;75:2346–2357. doi: 10.1046/j.1471-4159.2000.0752346.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y. L., Kesavapany S., Gravell M., Hamilton R. S., Schubert M., Amin N., Albers W., Grant P., Pant H. C. A Cdk5 inhibitory peptide reduces tau hyperphosphorylation and apoptosis in neurons. EMBO J. 2004;24:209–220. doi: 10.1038/sj.emboj.7600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur. J. Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y. L., Li B. S., Veeranna , Pant H. C. Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J. Biol. Chem. 2003;278:24026–24032. doi: 10.1074/jbc.M303079200. [DOI] [PubMed] [Google Scholar]