Abstract
In the phagocytic cell, NADPH oxidase (Nox2) system, cytoplasmic regulators (p47phox, p67phox, p40phox, and Rac) translocate and associate with the membrane-spanning flavocytochrome b558, leading to activation of superoxide production. We examined membrane targeting of phox proteins and explored conformational changes in p40phox that regulate its translocation to membranes upon stimulation. GFP-p40phox translocates to early endosomes, whereas GFP-p47phox translocates to the plasma membrane in response to arachidonic acid. In contrast, GFP-p67phox does not translocate to membranes when expressed alone, but it is dependent on p40phox and p47phox for its translocation to early endosomes or the plasma membrane, respectively. Translocation of GFP-p40phox or GFP-p47phox to their respective membrane-targeting sites is abolished by mutations in their phox (PX) domains that disrupt their interactions with their cognate phospholipid ligands. Furthermore, GFP-p67phox translocation to either membrane is abolished by mutations that disrupt its interaction with p40phox or p47phox. Finally, we detected a head-to-tail (PX–Phox and Bem1 [PB1] domain) intramolecular interaction within p40phox in its resting state by deletion mutagenesis, cell localization, and binding experiments, suggesting that its PX domain is inaccessible to interact with phosphatidylinositol 3-phosphate without cell stimulation. Thus, both p40phox and p47phox function as diverse p67phox “carrier proteins” regulated by the unmasking of membrane-targeting domains in distinct mechanisms.
INTRODUCTION
In phagocytic cells, reactive oxygen species (ROS) are produced by NADPH oxidase (Nox2 system). The enzyme is a multiprotein complex assembled from a membrane-spanning flavocytochrome b558 (composed of gp91phox [Nox2] and p22phox) and four cytoplasmic components (p47phox, p67phox, p40phox, and Rac) (Leto, 1999; Nauseef, 2004; Quinn and Gauss, 2004). In unstimulated phagocytes, the oxidase is dissociated and inactive: the flavocytochrome b558 is stored on the membranes of intracellular granules (Jesaitis et al., 1990), Rac is maintained in a GDP-bound cytoplasmic complex dimerized with Rho-guanine nucleotide dissociation inhibitor (GDI) (Bokoch et al., 1994), and the other phox proteins associate in a separate ternary cytoplasmic complex (p47phox-p67phox-p40phox) in a dephosphorylated state (Bolscher et al., 1989; Rotrosen and Leto, 1990; Kuribayashi et al., 2002; Lapouge et al., 2002). During phagocyte activation, intracellular granules containing flavocytochrome b558 fuse with phagosomes; p47phox is phosphorylated, thereby inducing conformational changes in p47phox that promote the interaction of the cytoplasmic complex with the flavocytochrome b558; and Rac dissociates from Rho-GDI and translocates independently to the membrane after exchange of GDP for GTP (Heyworth et al., 1994; Zhao et al., 2003), resulting in generation of superoxide anion by the transfer of electrons from cytoplasmic NADPH to molecular oxygen.
Chronic granulomatous disease (CGD), characterized by defective microbial killing by phagocytic cells, is caused by defects or deficiencies in any one of four oxidase components: Nox2, p22phox, p47phox, or p67phox (Leto, 1999). An essential role for Rac1 or Rac2 in Nox2 activation was also identified in cell-free reconstitution studies (Abo et al., 1991; Knaus et al., 1991). This role was later confirmed in an oxidase-deficient patient who expressed mutant Rac2 (Ambruso et al., 2000; Williams et al., 2000) and in mice rendered genetically deficient in Rac2 (Roberts et al., 1999; Gu et al., 2003). Rac and p67phox together have a direct roles in regulating electron flow through the flavocytochrome b558 through GTP-dependent interactions; hence, p67phox is called an “activator” component (Bokoch and Diebold, 2002). Alternatively, p47phox is called an “adaptor” or “organizer” component because it binds to membrane lipids [phosphatidylinositol-(3,4)-bisphosphate [PI(3,4)P2] and phosphatidic acid (PA)] through its phox (PX) domain (Kanai et al., 2001; Karathanassis et al., 2002), is tethered to the flavocytochrome b558 through direct interactions between p22phox and its Src homology (SH) 3 domain, and is linked to other cytoplasmic phox proteins to this complex (Leto et al., 1994; Sumimoto et al., 1994). CGD patients who lack p47phox show impaired translocation of p67phox to the particulate or membrane fraction, whereas CGD patients who lack p67phox show normal translocation of p47phox to the particulate fraction, indicating the adaptor function of p47phox in recruitment of p67phox to the membrane (Heyworth et al., 1991; Dusi et al., 1996; Allen et al., 1999). However, Nox2 activity can be reconstituted in vitro in the absence of p47phox, when p67phox and Rac1 are provided in excess (Freeman and Lambeth, 1996; Koshkin et al., 1996) or when p67phox is adapted with the membrane-binding sequences from Rac1, although GTP-bound Rac is still required for oxidase activation (Gorzalczany et al., 2000; Alloul et al., 2001), indicating p67phox and Rac1 are minimum essential cytoplasmic components in the Nox2 system. p40phox also has a PX domain that specifically binds to phosphatidylinositol 3-phosphate [PI(3)P] (Bravo et al., 2001; Kanai et al., 2001), a phospholipid enriched in the early endosome (Gillooly et al., 2000) and produced in the phagosomal membrane during phagocytosis (Ellson et al., 2001a; Gillooly et al., 2001). Thus, p40phox is also thought to serve as an adaptor component that recruits p67phox to phagosomal membranes (Kuribayashi et al., 2002). There are, however, no reports of p40phox defects or deficiencies resulting in CGD. There is some controversy on the precise function of p40phox, because both Nox2-inhibitory (Sathyamoorthy et al., 1997; Vergnaud et al., 2000; Lopes et al., 2004) and Nox2-supporting effects of p40phox (Tsunawaki et al., 1996; Cross, 2000; Ellson et al., 2001b; Kuribayashi et al., 2002; He et al., 2004) have been reported. However, recent studies in p40phox-deficient mice or in FcγIIA receptor-reconstituted cells indicate p40phox is an essential component of the Nox2 system (Ellson et al., 2006; Suh et al., 2006).
Arachidonic acid (AA) has been used frequently as a activator of Nox2 in cell-free assay systems (Bromberg and Pick, 1984; Curnutte, 1985). Although relatively high concentrations of AA (50–200 μM) are required for activation of Nox2 both in vitro and in vivo, functional roles for cytosolic phospholipase A2 (cPLA2), which produces AA, in Nox2 activation have been demonstrated at the cellular level (Dana et al., 1998; Zhao et al., 2002; Shmelzer et al., 2003). Furthermore, recent studies have shown that the orchestration of low concentrations of AA produced by PLA2 together with protein kinase C (PKC)-dependent phosphorylation promotes translocation of p47phox and enhances ROS production by Nox2 (Shiose and Sumimoto, 2000; Peng et al., 2003).
In recent work, we described mechanisms for translocation of p47phox (Ueyama et al., 2004) and isoform-specific translocation of Rac to the phagosome (Ueyama et al., 2005). To understand and clarify targeting of p67phox, an essential cytoplasmic Nox2 activator that acts through Rac, we used AA as a stimulant and explored the adaptor functions of p40phox and p47phox in recruitment of p67phox to membranes. In the present study, we show that p67phox is not targeted to the membrane by itself but that it relies on diverse targeting and adaptor functions of p40phox and p47phox for its translocation to membranes. Furthermore, we examine the mechanism by which p40phox acquires its function as a p67phox “adaptor protein.” We propose here that an intramolecular interaction between the PX and the PB1 domains of p40phox in the resting state renders p40phox inaccessible to bind PI(3)P and that upon stimulation the intramolecular interaction can be disrupted, enabling p40phox to bind to PI(3)P-enriched membranes.
MATERIALS AND METHODS
Materials
Goat polyclonal antibody (Ab) against human p47phox or p67phox and rabbit polyclonal Ab against human p40phox were described previously (Leto et al., 1991; Sathyamoorthy et al., 1997). Mouse monoclonal Ab against human p47phox or p67phox was from BD Biosciences (San Jose, CA). Rabbit polyclonal Ab against green fluorescent protein (GFP) or early endosome antigen-1 (EEA1) was from Clontech (Mountain View, CA) or from ABR-Affinity BioReagents (Golden, CO), respectively. Rabbit polyclonal Ab against glutathione S-transferase (GST) and mouse monoclonal Ab against (His)6 were from Santa Cruz Biotechnology (Santa Cruz, CA) and GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom), respectively. AA was purchased from Doosan Serdary Research Laboratories (Kyungki-Do, Korea).
Cell Culture
RAW 264.7 macrophages (Ueyama et al., 2005) and COS-7 cells (American Type Culture Collection, Manassas, VA) were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C in 5% CO2.
Construction of Plasmids
The pEGFP-C1 plasmids (Clontech) containing human p47phox, p47phox (W193R), p40phox, p40phox(PX; aa 1-167), p40phox(PX:R105K), and p47phox(R90K) were described previously (Ago et al., 2001, 2003; Ueyama et al., 2004). cDNA encoding p67phox and p67phox(ΔSH3, aa 1-457) were described previously (de Mendez et al., 1994; Ueyama et al., 2004), transferred into pEGFP-C1, and designated GFP-p67phox and GFP-p67phox(ΔSH3), respectively. The fragment encoding p67phox(ΔAD, deletion of aa 199-212) was made by polymerase chain reaction (PCR) by using appropriate primers, transferred into pEGFP-C1, and designated GFP-p67phox(ΔAD). The fragments encoding p47phox, p47phox(ΔPR; aa 1-359), and p40phox were amplified by PCR, cloned into pIRES2-DsRed2, and designated p47phox-IRES2-DsRed2, p47phox(ΔPR)-IRES2-DsRed2, and p40phox-IRES2-DsRed2, respectively. The fragments encoding p47phox and GFP-p67phox(ΔAD) were amplified by PCR, cloned into BglII and EcoRI sites of multiple cloning site of pIRES2-DsRed2 and BstXI and XbaI sites of pIRES2-DsRed2 in place of DsRed2, respectively, and designated p47phox-IRES2-GFP-p67phox(ΔAD). GFP-p40phox(R105K), GFP-p67phox(ΔAD:K355A), and p40phox (D289A)-IRES2-DsRed2 were made using the QuikChange II XL site-directed mutagenesis kit protocol (Stratagene, La Jolla, CA). We confirmed that GFP-p47phox and GFP-p67phox support Nox2 activity at levels comparable with unfused, wild-type p47phox, and p67phox (data not shown).
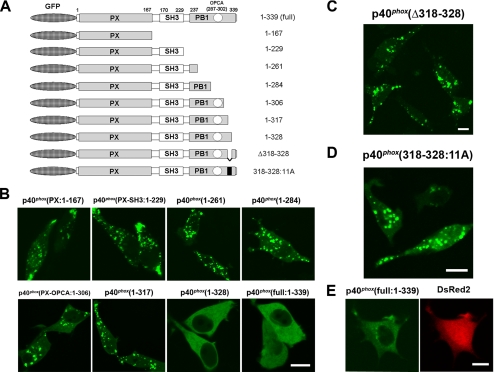
To study the intramolecular binding of p40phox, the DNA fragments encoding p40phox(aa 1-229), p40phox(aa 1-261), p40phox(aa 1-284), p40phox(aa 1-306), p40phox(aa 1-317), and p40phox(aa 1-328) were amplified by PCR, cloned into pEGFP-C1, and designated GFP-p40phox(1-229), GFP-p40phox(1-261), GFP-p40phox(1-284), GFP-p40phox(1-306), GFP-p40phox(1-317), and GFP-p40phox(1-328), respectively (Figure 9A). The DNA fragment encoding p40phox(Δ318-328; deletion of aa 318-328) was amplified by PCR by using GFP-p40phox(1-328) as a template with appropriate primers, cloned into pEGFP-C1, and designated GFP-p40phox(Δ318-328). p40phox(318-328:11A) in pEGFP-C1, in which residues 318-328 are replaced by 11 alanine residues, was made using GFP-p40phox as a template by the QuikChange II XL site-directed mutagenesis kit. All modified expression vectors were sequenced to confirm their identities.
Figure 9.
Inhibitory effects of the p40phox PB1 domain (residues 318-328) in PX domain-mediated targeting of p40phox to vesicular structures in RAW 264.7 cells. (A) Structure of GFP-tagged p40phox constructs used in the present study. (B) GFP-p40phox(PX), GFP-p40phox(1-229), GFP-p40phox(1-261), GFP-p40phox(1-284), GFP-p40phox(1-306), and GFP-p40phox(1-317) are localized on vesicular structures. In contrast, full-length GFP-p40phox(1–339) and GFP-p40phox(1-328) remain localized in the cytoplasm. Bar, 10 μm. Representative of n ≥ 6. (C) GFP-p40phox(Δ318-328) is localized on vesicular structures. Bar, 10 μm. Representative of n ≥ 6. (D) GFP-p40phox(318-328:11A) is localized on vesicular structures. Bar, 10 μm. Representative of n ≥ 6. (E) Exogenously expressed wild-type p40phox is primarily detected by immunofluorescence in the cytoplasm in COS-7 cells (left). p40phox expressing cell is detected by DsRed2 fluorescence by using p40phox-IRES2-DsRed2 plasmid (right). Bar, 10 μm. Representative of n ≥ 6.
Immunoprecipitation and Immunoblotting
COS-7 cells were transfected using electroporation methods (Shirai et al., 1998). Forty-eight hours after the transfection, cells were lysed in homogenizing buffer in the presence of protease inhibitors (Ueyama et al., 2001) by sonication. The total cell lysates were centrifuged at 20,000 × g for 30 min at 4°C, the supernatants were incubated with the Ab (α-p47phox, α-p67phox or α-p40phox, α-GFP) or control IgG (goat or rabbit; Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C and then with protein G-Sepharose 4B (GE Healthcare) for an additional 12 h at 4°C. The precipitates were washed three times, and the aliquots of precipitates were subjected to SDS-PAGE and followed by immunoblotting using primary Ab for α-p47phox, α-p67phox, α-p40phox, or α-GFP (1/2000, room temperature [RT] for 2 h). Bound antibodies were detected with secondary antibody-horseradish peroxidase (HRP) conjugates (Jackson ImmunoResearch Laboratories, West Grove, PA) by using the ECL detection system (GE Healthcare).
Cell Imaging Studies
Fluorescently labeled (Alexa-568) and IgG-opsonized phagocytosis targets (BIgG) were prepared using 2-μm glass beads (Duke Scientific, Fremont, CA), as described previously (Larsen et al., 2002). In total, 1.0 × 105 RAW 264.7 cells were seeded on 35-mm glass bottom dishes (MatTek chambers; MatTek, Ashland, MA) and transfected using Superfect (QIAGEN, Valencia, CA). Twenty-four to 32 h after the transfection, the culture medium was replaced with Hanks' balanced salt solution (HBSS)++ (Larsen et al., 2002). As a stimulant, HBSS++ containing BIgG (five targets per cell) or HBSS++ containing AA (indicated final concentrations) was added to each plate. Images were collected using an LSM 510 invert (Carl Zeiss, Thornwood, NY) confocal laser scanning fluorescence microscope with a heated stage and objective (40× oil or 63× oil) as described previously (Ueyama et al., 2004). The images were collected at 5 s intervals for 5 min.
For immunocytochemical studies, cells (transfected or untransfected) were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (Ueyama et al., 2001). After permeabilization, cells were stained using primary Ab (p40phox, diluted 1/500; EEA-1, diluted 1/250) for 2 h at RT. Primary Abs were visualized by confocal microscopy using Alexa-488 or -594–conjugated anti-rabbit IgG (1/2000, 0.5 h at RT; Invitrogen).
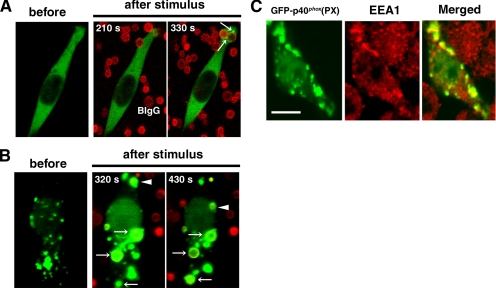
All time-lapsed imaging experiments were performed in triplicate and were repeated in at least three independent transfection experiments (n ≥ 9). All imaging experiments were very reproducible (≥80%), except for the experiment of GFP-p40phox stimulated with BIgG (≤10%; Figure 2A and Supplemental Video 1).
Figure 2.
Accumulation of GFP-p40phox(PX) and full-length GFP-p40phox on membranes during FcγR-mediated phagocytosis in RAW 264.7 cells. (A) During FcγR-mediated phagocytosis, transient vesicular accumulation of GFP-p40phox (arrows) is observed occasionally, which fuses with newly forming phagosomes. Time-lapsed photography of this process is available in Supplemental Video 1. BIgG, Alexa-568 labeled– and IgG-opsonized 2-μm glass beads. Addition of BIgG occurs at time 0. Representative of n ≥ 30. (B) GFP-p40phox(PX) is localized prominently on vesicular structures in the cytoplasm, which accumulates on phagosomes (arrows and arrowheads) during FcγR-mediated phagocytosis after phagosome sealing. Time-lapsed photography is available in Supplemental Video 2. Representative of n ≥ 9. (C) Vesicular structures containing GFP-p40phox(PX) in the cytoplasm are reactive with antibodies against EEA-1, a marker of early endosomes in RAW 264.7 cells. Bar, 10 μm. Representative of n ≥ 6.
In Vitro Binding Assay
The cDNA for the PCR-amplified PX domain of p40phox(aa 1-167) was cloned into BamHI and EcoRI sites of pProEx-Htb (Invitrogen) and designated (His)6-p40phox(PX). Forward and reverse oligonucleotides for p40phox(aa 307-317), p40phox(aa 318-328), and p40phox(aa 329-339) were annealed and cloned into the BamHI and EcoRI sites of pGEX-6P-1 and designated GST-p40phox(307-317), GST-p40phox(318-328), and GST-p40phox(329-339), respectively. PCR-amplified fragments of p40phox(SH3 domain; aa 170-229) and p40phox(PB1 domain; aa 237-339) were cloned into the BamHI and EcoRI sites of pGEX-6P-1 and designated GST-p40phox(SH3) and GST-p40phox(237-339), respectively. All constructs were sequenced to confirm their identities. Tagged proteins were expressed in Escherichia coli strain BL21-CodonPlus (DE3)-RIL (Stratagene). When the bacteria reached an OD600 of ∼0.2, protein expression was induced by 0.1 mM isopropyl β-d-thiogalactoside at 21°C for 16 h and purified using nickel-Agarose [Invitrogen; in the case of (His)6-p40phox(PX), the tagged protein was solubilized in 50 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate] or glutathione-Sepharose 4B (GE Healthcare). The purified (His)6-p40phox(PX) (500 nM) was mixed with each purified GST-tagged fragment of p40phox (500 nM) in 500 μl of buffer (25 mM HEPES, 150 mM NaCl, and 2% fatty acid-free bovine serum albumin, pH 7.5). Glutathione-Sepharose 4B beads were added to the solution, rotated for 30 min at 4°C, and then washed three times with the same buffer. The material absorbed to beads was eluted with 10 mM glutathione, and the elutants were separated on a 15% SDS-polyacrylamide gel. Western blotting was performed using primary (His)6 Ab and secondary Ab-HRP conjugates, and detected by the ECL detection system.
Online Supplemental Material
The supplemental time-lapsed photography shows the “kiss and run” fusion-like accumulation of GFP-p40phox on phagosomes (Video 1; total time 330 s) and the transient accumulation of GFP-p40phox(PX) on phagosomes by fusion of early endosomes and from a cytoplasmic pool of GFP-p40phox(PX) (Video 2; total 310 s) during Fcγ receptor (FcγR)-mediated phagocytosis in RAW 264.7 cells. The supplemental time-lapsed photography shows translocation of GFP-p67phox(ΔAD) both to early endosomes and to the plasma membrane (Video 3; total time 150 s) after AA stimulation in RAW 264.7 cells coexpressing p40phox and p47phox.
RESULTS
Accumulation of p40phox(PX) at the Phagosome during FcγR-mediated Phagocytosis
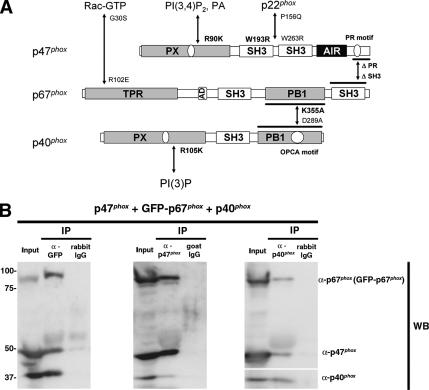
Although a recent study reported that p40phox and p67phox exist as a heterodimer in the resting state and that the p47phox-containing ternary complex forms only after cell activation (Brown et al., 2003), most other studies to date indicate that the phox proteins exist as a stable 1:1:1 ternary cytoplasmic complex (p47phox-p67phox-p40phox) in their dephosphorylated state even without cell stimulation (Figure 1A; Bolscher et al., 1989; Rotrosen and Leto, 1990; Lapouge et al., 2002; Kuribayashi et al., 2002). We examined ternary complex formation by using GFP-p67phox coexpressed with p47phox and p40phox in COS-7 cells and confirmed that all three proteins exist at least partially in a complex that could be detected by immunoprecipitation with antibodies against any of these components (Figure 1B). Furthermore, we examined ROS production using GFP-p67phox or p67phox plus p47phox and Nox2 in human embryonic kidney 293 and COS-7 cell models, and confirmed that GFP-p67phox supports Nox2 activity at levels comparable with the unfused, wild-type p67phox (data not shown). These results indicate that GFP-p67phox behaves like wild-type p67phox, at least with regard to its interactions and assembly with other phox proteins and its ability to support ROS production in the Nox2 system. Therefore, we used GFP-p67phox to monitor its interactions with other oxidase components in intact cells.
Figure 1.
Phox proteins exist in a complex in resting COS-7 cells. (A) Schematic representation of structural domains and their interactions among p47phox, p67phox, and p40phox. Additional interactions between Rac and p67phox, p22phox and p47phox, PI(3,4)P2, PA and the PX domain of p47phox, and PI(3)P and the PX domain of p40phox are also shown. AIR, autoinhibitory region; TPR, tetratricopeptide repeat; AD, activation domain; PB1, Phox and Bem1; OPCA, OPR-PC-AID. Mutations that disrupt these interactions are shown beside arrows; those used in the current study are in bold. (B) Supernatants from COS-7 cells expressing p47phox, GFP-p67phox, and p40phox were immunoprecipitated by rabbit polyclonal antibodies against GFP or rabbit control IgG, goat polyclonal antibodies against p47phox or goat control IgG, or rabbit polyclonal antibodies against p40phox or rabbit control IgG and then immunoblotted with antibodies against p47phox (monoclonal), p67phox (monoclonal), and p40phox (polyclonal). p47phox and p40phox are coimmunoprecipitated with GFP-p67phox (left), GFP-p67phox and p40phox are coimmunoprecipitated with p47phox (middle), and p47phox and GFP-p67phox are coimmunoprecipitated with p40phox (right). Similar results were obtained in two independent experiments
Because we described the mechanism of GFP-p47phox accumulation on phagosomal cups and phagosomes during FcγR-mediated phagocytosis (et al., 2004), the present study focused on p40phox. GFP-p40phox is detected diffusely throughout the cytoplasm of resting RAW 264.7 cells (Figure 2A). During FcγR-mediated phagocytosis, the accumulation of GFP-p40phox on newly forming phagosomes occurs as a kiss and run phenomenon, in which a transient fusion of GFP-p40phox is observed between phagosomes and endosomes without showing much evidence of complete intermixing of their membranes (Duclos et al., 2000). This phenomenon is not observed constantly (≤10%), but it occurred only occasionally when followed by time-lapsed photography (Figure 2A and Supplemental Video 1). We then used GFP-p40phox(PX) for further studies, because it was reported to bind specifically to PI(3)P. Consistent with a previous report (Ellson et al., 2001a), GFP-p40phox(PX) is localized faintly in the cytoplasm and predominantly on vesicular structures (Figure 2B), which could be colabeled with antibodies against early endosomal antigen (EEA)-1 (Figure 2C), a marker of the early endosome that is enriched in PI(3)P (Gillooly et al., 2000) (Figure 2B). The vesicular structures and free-form of GFP-p40phox(PX) seems to fuse readily with phagosomes in later stages of FcγR-mediated phagocytosis, after the phagosome is sealed (Figure 2B and Supplemental Video 2). The fusion of early endosomes, as detected by GFP fluorescence, resembles the same kiss and run pattern (transient interaction); however, the GFP-p40phox(PX) fluorescence seems to reflect significant amounts membrane material fusing with the phagosomes (Supplemental Video 2). This accumulation of GFP-p40phox(PX) on the phagosome persists longer on phagosomes (>100 s) than observed with GFP-p47phox, which starts accumulating before phagosome sealing (on phagosomal cups) and is observed on phagosomal cups and retained on phagosomes for 67 ± 7 s (Ueyama et al., 2004). Accumulation of GFP-p67phox on phagosomes was not evident in RAW 264.7 during FcγR-mediated phagocytosis, perhaps reflecting low levels of expression of the endogenous phox proteins (data not shown). However, GFP-p67phox accumulated at phagosomal cups and phagosomes after coexpression with p47phox (data not shown). The differences in localization patterns between GFP fusion proteins of full-length p40phox and its isolated PX domain suggest that the PX domain of p40phox is masked or is otherwise maintained in a state that is relatively inaccessible to early endosomes or PI(3)P-enriched phagosomes. The differences in localization between p40phox and p47phox fusions proteins suggest that the two proteins are targeted to phagosomes by distinctly different signals.
Translocation of p40phox and p47phox, but Not p67phox, to Membranes after AA Stimulation
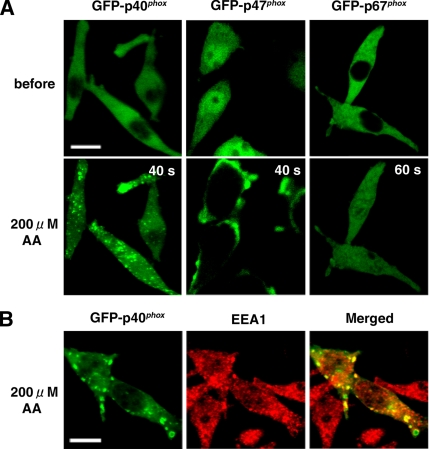
To examine the mechanism by which p40phox acquires the capacity to bind PI(3)P, we explored the effects of cell stimulation by phorbol 12-myristate 13-acetate (PMA), N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP), or AA. Among these stimulants, only AA can induce any detectable translocation of GFP-p40phox in our real-time confocal microscopy system, which shows that GFP-p40phox translocates to vesicular structures in the cytoplasm (Figure 3A, left) that are colabeled with antibodies against EEA-1 (Figure 3B). The same translocation pattern of AA-induced GFP-p40phox translocation is observed using a C-terminally tagged GFP fusion with p40phox, p40phox-GFP (data not shown). In contrast, GFP-p47phox translocates to the plasma membrane after cell stimulation by AA (Figure 3A, middle), whereas GFP-p67phox shows no translocation to any membranes after treatments by any of these three stimulants (Figure 3A, right). These data suggest that AA induces conformational changes in p40phox that render the PX domain accessible to bind to early endosomes, as occurs in AA-treated p47phox to expose its SH3 domains to bind to p22phox (Shiose and Sumimoto, 2000; Zhao et al., 2002). It is intriguing that GFP-p40phox and GFP-p47phox, but not GFP-p67phox, have the ability to bind to the membrane only after AA stimulation, which likely induces conformational change in both proteins.
Figure 3.
AA-stimulated translocation of GFP-p40phox and GFP-p47phox, but not GFP-p67phox, to the membrane in RAW 264.7 cells. (A) Left, AA triggers GFP-p40phox translocation to vesicular structures in the cytoplasm. (A) Middle, AA stimulates GFP-p47phox translocation to the plasma membrane. (A) Right, GFP-p67phox shows no significant translocation to membranes. AA was added at time 0. Bar, 10 μm. Representative of n ≥ 9. (B) Vesicular structures in the cytoplasm containing GFP-p40phox are labeled with antibodies against EEA-1, a marker of early endosomes. Bar, 10 μm. Representative of n ≥ 6.
There are reports that phosphorylation occurs within p40phox at Thr154 and Ser315 (Bouin et al., 1998) and that phosphorylation of Thr154, but not Ser315, inhibits Nox2 activation (Lopes et al., 2004). Therefore, we made mutants of GFP-p40phox that could not be phosphorylated [GFP-p40phox(T154A) and GFP-p40phox(S315A)] or mimic the phosphorylated states [GFP-p40phox(T154D) and GFP-p40phox (S315D)]. However, these mutants are localized throughout the cytoplasm and seem to translocate normally to early endosomes after AA stimulation, like GFP-p40phox (data not shown).
Function of PX Domains of p40phox and p47phox for Their Translocation
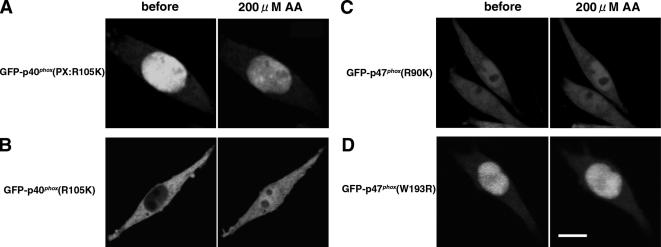
We then explored the translocation mechanisms of p40phox and p47phox by examining the effects of mutating their PX domains. GFP-p40phox(PX:R105K), which loses its capacity to bind PI(3)P (Ago et al., 2001), shows no vesicular localization pattern, nor does it translocate to early endosomes after AA stimulation (Figure 4A). GFP-p40phox(R105K) also shows no translocation to the early endosome after AA stimulation (Figure 4B). These observations indicate that translocation of p40phox to early endosomes requires binding of the PX domain to PI(3)P.
Figure 4.
Requirement of phosphatidylinositol phosphate-specific binding of PX domains for translocation of p40phox and p47phox in AA-stimulated RAW 264.7 cells. (A) GFP-p40phox(PX:R105K), which loses the capacity for binding PI(3)P, shows no vesicular localization pattern nor translocation to early endosomes. Representative of n ≥ 9. (B) GFP-p40phox(R105K) shows no translocation to early endosomes. Representative of n ≥ 9. (C) GFP-p47phox(R90K), which loses the capacity for binding PI(3,4)P2, shows no translocation to the plasma membrane. Representative of n ≥ 9. (D) GFP-p47phox(W193R), which cannot bind to p22phox, shows no translocation to the plasma membrane. Bar, 10 μm. Representative of n ≥ 9.
In the case of p47phox, GFP-p47phox(R90K), which loses its capacity to bind PI(3,4)P2 (Ago et al., 2003), shows no translocation to the plasma membrane by AA (Figure 4C). GFP-p47phox(W193R), which cannot translocate to the phagosome to bind to proline-rich (PR) motif of p22phox (Ueyama et al., 2004) nor produce ROS (Sumimoto et al., 1996; de Mendez et al., 1997), also shows no translocation to the plasma membrane after AA stimulation (Figure 4D). Together, these data indicate that translocation of p47phox to the plasma membrane after AA stimulation requires both the binding of the PX domain to PI(3,4)P2 and the SH3 domain interaction with p22phox.
Targeting of p67phox to Early Endosomes or to the Plasma Membrane by Coexpressed Carrier Proteins, p40phox or p47phox
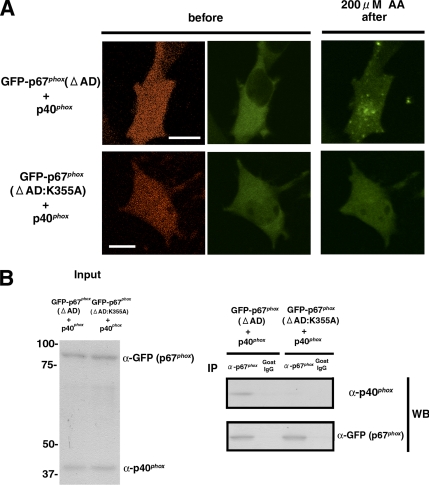
To clarify the function of p40phox as a potential membrane “adaptor protein” for p67phox, we expressed GFP-p67phox and p40phox-IRES2-DsRed2, which identifies transfected cells expressing wild-type p40phox by DsRed2 fluorescence. When GFP-p67phox and p40phox-IRES2-DsRed2 are cotransfected into RAW 264.7 cells, we cannot easily detect cells coexpressing these products. We speculate that overexpression of p67phox and p40phox may be too toxic to cells due to the enhanced production of ROS. Therefore, further studies were performed using p67phox(ΔAD), which cannot support ROS production (Han et al., 1998). In RAW 264.7 cells coexpressing GFP-p67phox(ΔAD) and p40phox-IRES2-DsRed2, GFP-p67phox(ΔAD) translocates to vesicular structures (Figure 5A, top). However, in cells coexpressing p40phox-IRES2-DsRed2 and GFP-p67phox(ΔAD:K355A), which does not bind p40phox (Kuribayashi et al., 2002), GFP-p67phox(ΔAD:K355A) does not translocate to the vesicular structures (Figure 5A, bottom). This result is further confirmed by complementary experiments using GFP-p67phox(ΔAD) and p40phox(D289A)-IRES2-DsRed2, which does not interact with p67phox (Kuribayashi et al., 2002). In cells coexpressing GFP-p67phox (ΔAD) and p40phox(D289A)-IRES2-DsRed2, GFP-p67phox(ΔAD) shows no translocation to the vesicular structure (data not shown). The interaction between p40phox and GFP-p67phox(ΔAD), but not GFP-p67phox(ΔAD:K355A), is confirmed by immunoprecipitation experiments (Figure 5B).
Figure 5.
Targeting of GFP-p67phox(ΔAD) to early endosomes by coexpression of p40phox in RAW 264.7 cells. (A) Top, coexpression of GFP-p67phox(ΔAD) and p40phox-IRES2-DsRed2 enables GFP-p67phox(ΔAD) translocation to vesicular structures. DsRed2 fluorescence (left) identifies cells expressing p40phox. Bar, 10 μm. Representative of n ≥ 9. (A) Bottom, GFP-p67phox(ΔAD:K355A) shows no translocation to vesicular structures when coexpressed with p40phox-IRES2-DsRed2. DsRed2 fluorescence (left) identifies cells expressing p40phox. Bar, 10 μm. Representative of n ≥ 9. (B) Supernatants from COS-7 cells expressing p40phox and GFP-p67phox(ΔAD) or GFP-p67phox(ΔAD:K355A) were immunoprecipitated by goat polyclonal antibodies against p67phox or goat control IgG and immunoblotted with rabbit polyclonal antibodies against p40phox and GFP. p40phox is coimmunoprecipitated with GFP-p67phox(ΔAD) but not GFP-p67phox(ΔAD:K355A). Similar results were obtained in two independent experiments.
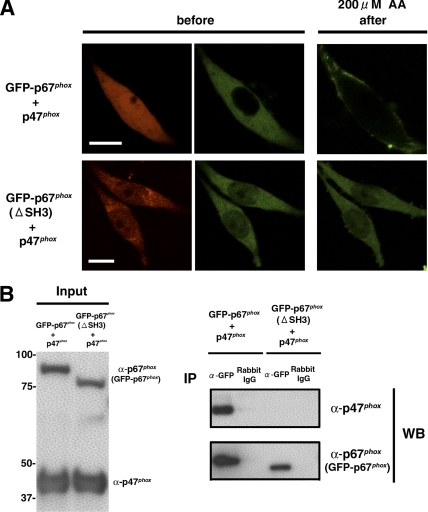
In the case where p47phox is used as a membrane adaptor protein for p67phox, GFP-p67phox translocates to the plasma membrane in RAW 264.7 cells (Figure 6A, top). However, in cells coexpressing p47phox-IRES2-DsRed2 and GFP-p67phox(ΔSH3), which disrupts the interaction with p47phox (de Mendez et al., 1994), no GFP-p67phox(ΔSH3) translocation to the plasma membrane is seen (Figure 6A, bottom). This result was further confirmed by complementary experiment using GFP-p67phox and p47phox(ΔPR)-IRES2-DsRed2, which also disrupts the interaction with p67phox (Kami et al., 2002; Massenet et al., 2005). In the cells coexpressing GFP-p67phox and p47phox(ΔPR)-IRES2-DsRed2, GFP-p67phox shows no the translocation to the plasma membrane (data not shown). The interaction between p47phox and GFP-p67phox, but not GFP-p47phox(ΔSH3), is confirmed in immunoprecipitation experiments (Figure 6B). These data indicate that both p40phox and p47phox can function as adaptors of p67phox to translocate p67phox to the early endosome and to the plasma membrane, respectively.
Figure 6.
Targeting of GFP-p67phox to the plasma membrane by coexpression of p47phox in RAW 264.7 cells. (A) Top, coexpression of GFP-p67phox and p47phox-IRES2-DsRed2 enables GFP-p67phox translocation to the plasma membrane. DsRed2 fluorescence (left) shows cells expressing p47phox. Bar, 10 μm. Representative of n ≥ 9. (A) Bottom, GFP-p67phox(ΔSH3) shows no translocation to the plasma membrane when coexpressed with p47phox-IRES2-DsRed2. DsRed2 fluorescence (left) shows cells expressing p47phox. Bar, 10 μm. Representative of n ≥ 9. (B) Supernatants from COS-7 cells expressing p47phox and GFP-p67phox or GFP-p67phox(ΔSH3) were immunoprecipitated by rabbit polyclonal antibodies against GFP or control rabbit IgG and then immunoblotted with goat polyclonal antibodies against p47phox and p67phox. p47phox is coimmunoprecipitaed with GFP-p67phox, but not GFP-p67phox(ΔSH3). Similar results were obtained in two independent experiments.
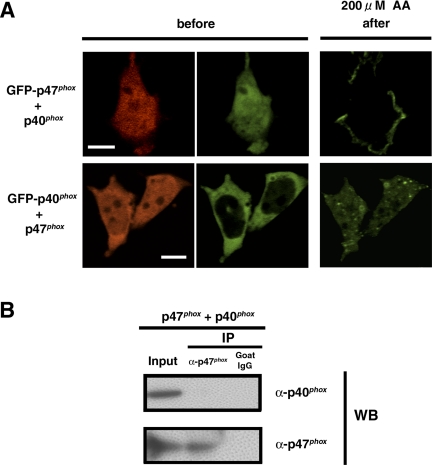
Because there are a few studies reporting weak interactions between p47phox and p40phox detected in vitro and in the yeast two-hybrid system (Sathyamoorthy et al., 1997; Grizot et al., 2001; Lapouge et al., 2002; Massenet et al., 2005), we examined the effect of this interaction on membrane translocation of p47phox and p40phox. Coexpression of p40phox-IRES2-DsRed2 does not influence the translocation of GFP-p47phox to the plasma membrane in RAW 264.7 cells (Figure 7A, top). Furthermore, coexpression of p47phox-IRES2-DsRed2 does not influence the translocation of GFP-p40phox to the vesicular structures (Figure 7A, bottom). Finally, no apparent interaction is detected between p40phox and p47phox (Figure 7B) by immunoprecipitation experiments. These data indicate that the interaction between p40phox and p47phox is below detectable levels and does not seem to be a factor in membrane targeting of these proteins in this system.
Figure 7.
Independent membrane targeting of p40phox and p47phox in RAW 264.7 cells. (A) Top, p40phox does not influence GFP-p47phox targeting to the plasma membrane after AA stimulation. DsRed2 fluorescence (left) shows cells expressing p40phox. Bar, 10 μm. Representative of n ≥ 9. (A) Bottom, p47phox does not influence the GFP-p40phox targeting to vesicular structures after AA stimulation. DsRed2 fluorescence (left) shows cells expressing p47phox. Bar, 10 μm. Representative of n ≥ 9. (C) Supernatants from COS-7 cells expressing p40phox and p47phox were immunoprecipitated by goat polyclonal antibodies against p47phox or goat control IgG and were immunoblotted with rabbit polyclonal antibodies against p40phox and goat polyclonal antibodies against p47phox. p40phox is not coimmunoprecipitated with p47phox. Similar results were obtained in two independent experiments.
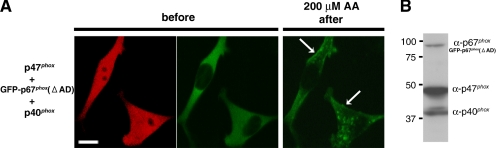
Finally, we examined the case where all of three phox proteins are overexpressed in RAW 264.7 cells using p47phox-IRES2-GFP-p67phox(ΔAD) and p40phox-IRES2-DsRed2, which identifies transfected cells expressing wild-type p47phox and GFP-p67phox(ΔAD) by GFP fluorescence and wild-type p40phox by DsRed2 fluorescence. In the RAW 264.7 cells coexpressing GFP-p67phox(ΔAD), p47phox, and p40phox, GFP-p67phox(ΔAD) translocates both to the plasma membrane and to vesicular structures after AA stimulation (Figure 8A and Supplemental Video 3). Expression of all three phox proteins by the internal ribosome entry site (IRES) plasmids is confirmed by Western blotting by using COS-7 cells (Figure 8B).
Figure 8.
Targeting of GFP-p67phox(ΔAD) both to early endosomes and to the plasma membrane by coexpression of p40phox and p47phox in RAW 264.7 cells. (A) Cotransfection of p47phox-IRES2-GFP-p67phox(ΔAD) and p40phox-IRES2-DsRed2 enables GFP-p67phox(ΔAD) translocation both to vesicular structures and to the plasma membrane (arrows). DsRed2 fluorescence (left) shows cells expressing p40phox, and GFP fluorescence shows cells expressing p47 phox and GFP-p67phox(ΔAD) (middle). Time-lapsed photography is available in supplemental Video 3. Bar, 10 μm. Representative of n ≥ 9. (B) Supernatants from COS-7 cells expressing p47phox and GFP-p67phox(ΔAD) by p47phox-IRES2-GFP-p67phox(ΔAD) and p40phox by p40phox-IRES2-DeRed2 were immunoblotted with antibodies against p47phox, p67phox, and p40phox. Expression of all three phox proteins by IRES plasmids is confirmed. Similar results were obtained in two independent experiments.
PB1 Domain of p40phox Masks the PX Domain of p40phox
It has been suggested that p47phox exists in the cytoplasm in the resting state because of two intramolecular interactions masking both the N- and C-terminal SH3 domains and the PX domain (Hiroaki et al., 2001; Karathanassis et al., 2002; Groemping et al., 2003; Durand et al., 2006). When phagocytes are activated after cell stimulation, the autoinhibitory region (AIR) (aa 286-340) is phosphorylated, thereby disrupting the intramolecular interactions and rendering both SH3 domains and the PX domain accessible to bind p22phox and membrane lipids, respectively (Karathanassis et al., 2002; Ago et al., 2003; Groemping et al., 2003). It was reported that the interaction between the PR motif in the PX domain and the C-terminal SH3 domain of p47phox (Hiroaki et al., 2001). p40phox also has a PR motif in the PX domain and one SH3 domain, which have the possibility of interacting each other. Therefore, we examined this possibility using purified (His)6-p40phox(PX) and GST-p40phox(SH3) in in vitro pull-down binding assays. However, we did not detect binding between these two proteins (data not shown). This result is consistent with crystallographic data on p40phox(PX) showing that the PR motif in the PX domain of p40phox is buried within the PX domain, although this study was performed using the lipid-bound form of p40phox(PX) (Bravo et al., 2001).
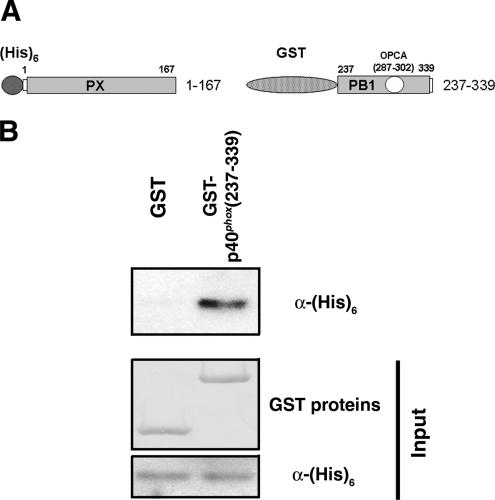
Then, we examined other regions within p40phox that might interact with its own PX domain, by using serially deleted mutants of GFP-p40phox expressed in RAW 264.7 cells. Among the various C-terminal deletions of GFP-p40phox, GFP-p40phox(1-328) loses the punctate localization on early endosomes, as seen with full-length GFP-p40phox (Figure 9B). GFP-p40phox(Δ318-328) and GFP-p40phox(318-328:11A) are localized at vesicular structures in the cytoplasm (Figure 9, C and D). The cytoplasmic localization of wild-type p40phox is confirmed by indirect immunofluorescence of COS-7 cells transfected with wild-type p40phox (Figure 9E). To examine further, we used in vitro pull-down assays between purified (His)6-p40phox(PX) and various forms of purified GST-tagged proteins. We could not detect interactions between (His)6-p40phox(PX) and GST-p40phox (307-317), GST-p40phox(318-328), GST-p40phox(329-339), or GST alone; however, (His)6-p40phox(PX) interacts strongly with GST-p40phox(PB1:237-339) (Figure 10).These results suggested that the PB1 domain of p40phox masks the PX domain of p40phox, making this domain inaccessible to bind to early endosomes in the absence of AA stimulation (Figure 11).
Figure 10.
Interaction of PX domain and PB1 domain of p40phox. (A) Structure of (His)6-tagged PX domain (1–167) of p40phox and GST-tagged PBI domain (237–339) of p40phox constructs used in pull-down assays. (B) Top blot, purified GST-p40phox(PBI: 237–339), but not GST, interacts with (His)6-p40phox(PX) in GST-based pull-down assays. Middle and bottom blots, comparable amounts of input proteins are confirmed by Ponsceau S staining (GST proteins) and by (His)6 blotting [(His)6-p40phox(PX)], respectively. Representative of three independent experiments.
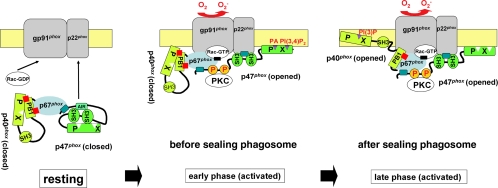
Figure 11.
Model of activation and assembly of Nox2 system during phagocytosis. In the resting state, the ternary complex of p47phox, p67phox, and p40phox is in the cytoplasm. p47phox is folded and inactive, due to inhibitory intramolecular interactions (Hiroaki et al., 2001; Groemping et al., 2003; Durand et al., 2006). p40phox is also folded and inactive due to inhibitory intramolecular head-to-tail (PX–PB1) interactions. In the early stages of activation of Nox2, the ternary complex translocates to the phagosome after conformational changes of p47phox that render the PX domain and SH3 domains of p47phox accessible to phospholipids [PI(3,4)P2, and PA; produced during this stage] and the PR motif of p22phox, respectively. In later stages of activation of Nox2, the ternary complex is retained on the phagosome or may exchange with a cytoplasmic pool after conformational changes in p40phox that render the PX domain of p40phox accessible to bind PI(3)P. In both stages, PKC may stay on the phagosome and phosphorylate p47phox, whereas AA may be a key modulator of conformational changes in p40phox and p47phox. Rac independently translocates to the phagosome.
DISCUSSION
In this study, we propose that both p40phox and p47phox function as regulated carrier or adaptor proteins of p67phox through distinct membrane-targeting mechanisms during NADPH oxidase assembly. We reported that p47phox accumulates transiently on phagocytic cups and mature phagosomes during FcγR-mediated phagocytosis (Ueyama et al., 2004), consistent with earlier studies showing p47phox and p67phox associate with both nascent and mature phagosomes in neutrophils and neutrophil-like PLB-985 cells (Allen et al., 1999; van Bruggen et al., 2004). The present study shows that GFP-p40phox also transiently associates on phagosomes, although this targeting is initiated at later stages after phagosome sealing. This accumulation is even more apparent with GFP-p40phox(PX), suggesting the PX domain of p40phox is masked or otherwise inhibited from interacting with membranes in the context of the full-length protein, because the isolated PX domain binds readily to PI(3)P-enriched endosome-like vesicles even before phagocytic stimulation. Finally, we show that AA has dramatic affects in unmasking the PX domain, thereby enabling p40phox to bind these vesicles along with p67phox. We suggest two mechanisms for the targeting p40phox to phagosomes during FcγR-mediated phagocytosis: 1) p40phox can bind initially to PI(3)P-enriched early endosomes, followed by their fusion to phagosomes, and/or 2) p40phox can target directly to phagosomes, where PI(3)P is transiently produced during phagosytosis (Ellson et al., 2001a; Gillooly et al., 2001). Because the cytosolic phox proteins exist as a ternary complex (Kuribayashi et al., 2002; Lapouge et al., 2002), we propose a model for Nox2 complex assembly involving these spatiotemporal factors during the FcγR-mediated respiratory burst (Figure 11): 1) in early stages, before phagosome sealing, p47phox functions as the predominant carrier, mediating translocation of the ternary complex to newly forming phagosomes, 2) in later stages after phagosome sealing, the carrier or adaptor function of p47phox is replaced by p40phox, whereby the ternary complex interacts with sealed phagosome through PI(3)P specific-binding of the PX domain of p40phox. In this series of interactions, both PKC and AA derived from PLA2 likely orchestrate conformational changes in p40phox and p47phox that promote their assembly with Nox2 complexes. This model is consistent with recent studies showing that p40phox contributes significantly to phagosomal superoxide production in mouse neutrophils or in FcγIIA receptor and phox-reconstituted COS-7 cells (Ellson et al., 2006; Suh et al., 2006) and that this requires interactions with the PX, SH3, and PB1 domains of p40phox (Suh et al., 2006).
Several lines of evidence suggest AA has important roles in activation of the Nox2 system, although the mechanism of its action is still debated. Many reports emphasize the effects of AA on p47phox. Early studies showed AA or other anionic amphiphiles activate ROS production in cell-free assays of Nox2 activity (Bromberg and Pick, 1984; Curnutte, 1985). It is thought that AA induces conformational changes in p47phox that may mimic the effects of phosphorylation, resulting in exposure of its binding PX and SH3 domains and translocation of p47phox and p67phox (Shiose and Sumimoto, 2000). It was reported that PLA2 is required for translocation of phox proteins without their phosphorylation (Uhlinger et al., 1993; Zhao et al., 2002). Others suggested both AA produced by PLA2- and PKC-dependent phosphorylation together promote translocation of p47phox and enhance ROS production by Nox2 (Sellmayer et al., 1996; Shiose and Sumimoto, 2000; Peng et al., 2003). We confirmed AA can trigger membrane translocation of p47phox, through interactions involving its PX domain and SH3 domain and that this enables cotranslocation of p67phox (Figures 4 and 6). Other reports suggest exogenous AA has indirect effects on Nox2 activation through downstream AA-derived mediators (Liu et al., 2003; Kerkhoff et al., 2005; Kim and Dinauer, 2006). Direct involvement of cPLA2 in human Nox2 activation (neutrophils, PLB-985 cells, or monocytes) has been demonstrated using inhibitors or antisense molecules targeted to cPLA2 (Dana et al., 1994; Li and Cathcart, 1997; Dana et al., 1998; Zhao et al., 2002). However, macrophages or neutrophils from cPLA2-deficient mice exhibited normal stimulated ROS release (Gijon et al., 2000; Rubin et al., 2005), suggesting the murine systems are less dependent on AA. Surprisingly, human cPLA2-deficient cells show normal translocation of phox proteins (Dana et al., 1994; Dana et al., 1998; Shmelzer et al., 2003), suggesting cPLA2 serves other roles in oxidase activation during Nox2 assembly. Interestingly, both cPLA2 and secretory PLA2 accumulate on newly formed phagosomes (Shmelzer et al., 2003; Girotti et al., 2004; Balestrieri et al., 2006). Furthermore, cells lacking Nox2 show no translocation of cPLA2 to membranes, and it seems that human cPLA2 associates directly with the cytoplasmic phox proteins and the membrane-bound Nox2 complex after stimulation by several agonists that activate the oxidase (Shmelzer et al., 2003).
We have now shown that AA triggers the movement of p40phox to PI(3)P-enriched membranes independently of other cytoplasmic phox proteins and that this can enable cotranslocation of p67phox. Although high concentrations of AA (200 μM) were used to observe this phenomenon, 50–100 μM AA also induced translocation (data not shown). Based on our experience, high concentrations of agonists (including AA) are usually required to visualize translocation of GFP-tagged molecules (Shirai et al., 1998). It is difficult to estimate the concentrations of AA achieved in whole cells, although high local concentrations of AA may be reached on phagosomes when PLA2 is recruited during phagocytosis. AA-dependent translocation of p40phox to early endosomes occurs with either N- or C-terminally GFP-tagged p40phox, suggesting the fusions do not influence this process. We propose that this translocation involves AA-induced conformational changes in p40phox that disrupt intramolecular interactions and expose its PX domain to bind membrane lipids, as was suggested in p47phox (Shiose and Sumimoto, 2000). However, in this case we mapped the intramolecular contacts of the p40phox PX domain to its C-terminal PB1 domain.
It is known that p40phox and p67phox associate through homotypic interactions between their PB1 domains (Ito et al., 2001; Kuribayashi et al., 2002; Wilson et al., 2003). The region of p40phox involved was reported as the PC motif (residues 287-302) (Nakamura et al., 1998; Ito et al., 2001), now referred as the OPR-PC-AID (OPCA) motif (Ponting et al., 2002). Later, Wilson et al. (2003) showed that Thr337 and Pro339 in the region C-terminal region also participate in the heterodimerization. The importance of this interaction with p67phox was demonstrated with the p40phox(D289A) mutant, which, unlike wild-type p40phox, is unable to stimulate Nox2 activity in PMA or muscarinic receptor-stimulated cells (Kuribayashi et al., 2002). This mutation not only interferes with p40phox membrane translocation but also leads to decreased p67phox and p47phox translocation stimulated by PMA. In contrast, in our AA-stimulated model, wild-type or mutant (D289A) GFP-p40phox translocates to early endosomes, even in the absence of p67phox (Figure 3; data not shown). However, we show that a mutation of the PB1 domain in p67phox(K355A) inhibits its cotranslocation with p40phox in AA-stimulated cells (Figure 5). This is the first report proposing an autoinhibitory intramolecular interaction within p40phox between its PX domain and its PB1 domain, because deleting residues 318-328 or replacing this sequence with alanine residues resulted in localization of GFP-p40phox on early endosomes without AA stimulation (Figure 9). Furthermore, we observed the interaction between (His)6-p40phox(PX) and the GST-p40phox(PB1:237-339) (Figure 10B). Crystallographic studies indicate that residues 318-328, encompassing the β-5 strand, do not participate in heterodimerization of p40phox and p67phox (Wilson et al., 2003). However, we could not determine which part of the PB1 domain participates in the PX–PB1 interaction of p40phox, because β-5 is part of a β-sheet core structure and deletion or changes of residues 318-328 to alanines could affect the global folding of the PB1 domain of p40phox. It is interesting that sites of phosphorylation in p40phox (Thr154 and Ser315) lie close to the PX domain and residues 318-328 (Bouin et al., 1998). Phosphorylation of Thr154, but not Ser315, was proposed as a negative regulator of ROS production by Nox2 (Lopes et al., 2004). However, we could not observed significant differences in the subcellular localization between GFP-p40phox and its phosphorylation site mutants [GFP-p40phox(T154A), GFP-p40phox(T154D), GFP-p40phox(S315A), or GFP-p40phox(S315D)], suggesting phosphorylation does not disrupt intramolecular interactions within GFP-p40phox, as seen with AA stimulation. Further studies are needed to clarify whether phosphorylation of p40phox can affect interactions with its PX or PB1 domain or the dynamics of its assembly with the oxidase on phagosomes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a grant-in-aid for scientific research from the 21st Century Center of Excellence Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a grant-in-aid for scientific research from the Young Scientist of the Ministry of Education, Culture, Sports, Science and Technology in Japan; and by the Uehara Memorial Foundation of Life Science.
Abbreviations used:
- AA
arachidonic acid
- FcγR
Fcγ receptor
- PA
phosphatidic acid
- ROS
reactive oxygen species.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0731) on November 22, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. USA. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago T., Takeya R., Hiroaki H., Kuribayashi F., Ito T., Kohda D., Sumimoto H. The PX domain as a novel phosphoinositide-binding module. Biochem. Biophys. Res. Commun. 2001;287:733–738. doi: 10.1006/bbrc.2001.5629. [DOI] [PubMed] [Google Scholar]
- Allen L. A., DeLeo F. R., Gallois A., Toyoshima S., Suzuki K., Nauseef W. M. Transient association of the nicotinamide adenine dinucleotide phosphate oxidase subunits p47phox and p67phox with phagosomes in neutrophils from patients with X-linked chronic granulomatous disease. Blood. 1999;93:3521–3530. [PubMed] [Google Scholar]
- Alloul N., Gorzalczany Y., Itan M., Sigal N., Pick E. Activation of the superoxide-generating NADPH oxidase by chimeric proteins consisting of segments of the cytosolic component p67phox and the small GTPase Rac1. Biochemistry. 2001;40:14557–14566. doi: 10.1021/bi0117347. [DOI] [PubMed] [Google Scholar]
- Ambruso D. R. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA. 2000;97:4654–4659. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri B., Hsu V. W., Gilbert H., Leslie C. C., Han W. K., Bonventre J. V., Arm J. P. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J. Biol. Chem. 2006;281:6691–6698. doi: 10.1074/jbc.M508314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Bohl B. P., Chuang T. H. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Bokoch G. M., Diebold B. A. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2695. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- Bolscher B. G., van Zwieten R., Kramer I. M., Weening R. S., Verhoeven A. J., Roos D. A phosphoprotein of Mr 47,000, defective in autosomal chronic granulomatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. J. Clin. Investig. 1989;83:757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouin A. P., Grandvaux N., Vignais P. V., Fuchs A. p40phox is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- Bravo J., et al. The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol. Cell. 2001;8:829–839. doi: 10.1016/s1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol. 1984;88:213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Brown G. E., Stewart M. Q., Liu H., Ha V. L., Yaffe M. B. A novel assay system implicates PtdIns(3,4)P2, PtdIns(3)P, and PKCδ in intracellular production of reactive oxygen species by the NADPH oxidase. Mol. Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Cross A. R. p40phox participates in the activation of NADPH oxidase by increasing the affinity of p47phox for flavocytochrome b558. Biochem. J. 2000;349:113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J. Clin. Investig. 1985;75:1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R., Leto T. L., Malech H. L., Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1998;273:441–445. doi: 10.1074/jbc.273.1.441. [DOI] [PubMed] [Google Scholar]
- Dana R., Malech H. L., Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem. J. 1994;297:217–223. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendez I., Garrett M. C., Adams A. G., Leto T. L. Role of p67-phox SH3 domains in assembly of the NADPH oxidase system. J. Biol. Chem. 1994;269:16326–16332. [PubMed] [Google Scholar]
- de Mendez I., Homayounpour N., Leto T. L. Specificity of p47phox SH3 domain interactions in NADPH oxidase assembly and activation. Mol. Cell. Biol. 1997;17:2177–2185. doi: 10.1128/mcb.17.4.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos S., Diez R., Garin J., Papadopoulou B., Descoteaux A., Stenmark H., Desjardins M. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J. Cell Sci. 2000;113:3531–3541. doi: 10.1242/jcs.113.19.3531. [DOI] [PubMed] [Google Scholar]
- Durand D., Cannella D., Dubosclard V., Pebay-Peyroula E., Vachette P., Fieschi F. Small-angle X-ray scattering reveals an extended organization for the autoinhibitory resting state of the p47phox modular protein. Biochemistry. 2006;45:7185–7193. doi: 10.1021/bi060274k. [DOI] [PubMed] [Google Scholar]
- Dusi S., Donini M., Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J. 1996;314:409–412. doi: 10.1042/bj3140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C. D., Anderson K. E., Morgan G., Chilvers E. R., Lipp P., Stephens L. R., Hawkins P. T. Phosphatidylinositol 3-phosphate is generated in phagosomal membranes. Curr. Biol. 2001a;11:1631–1635. doi: 10.1016/s0960-9822(01)00447-x. [DOI] [PubMed] [Google Scholar]
- Ellson C. D., Davidson K., Ferguson G. J., O'Connor R., Stephens L. R., Hawkins P. T. Neutrophils from p40phox−/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J. Exp. Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C. D., et al. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat. Cell Biol. 2001b;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- Freeman J. L., Lambeth J. D. NADPH Oxidase Activity Is Independent of p47phox in Vitro. J. Biol. Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- Gijon M. A., Spencer D. M., Siddiqi A. R., Bonventre J. V., Leslie C. C. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. J. Biol. Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- Gillooly D. J., Morrow I. C., Lindsay M., Gould R., Bryant N. J., Gaullier J. M., Parton R. G., Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D. J., Simonsen A., Stenmark H. Phosphoinositides and phagocytosis. J. Cell Biol. 2001;155:15–17. doi: 10.1083/jcb.200109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M., Evans J. H., Burke D., Leslie C. C. Cytosolic Phospholipase A2 Translocates to Forming Phagosomes during Phagocytosis of Zymosan in Macrophages. J. Biol. Chem. 2004;279:19113–19121. doi: 10.1074/jbc.M313867200. [DOI] [PubMed] [Google Scholar]
- Gorzalczany Y., Sigal N., Itan M., Lotan O., Pick E. Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly. J. Biol. Chem. 2000;275:40073–40081. doi: 10.1074/jbc.M006013200. [DOI] [PubMed] [Google Scholar]
- Grizot S., et al. Small angle neutron scattering and gel filtration analyses of neutrophil NADPH oxidase cytosolic factors highlight the role of the C-terminal end of p47phox in the association with p40phox. Biochemistry. 2001;40:3127–3133. doi: 10.1021/bi0028439. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Gu Y., et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- Han C. H., Freeman J. L., Lee T., Motalebi S. A., Lambeth J. D. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67phox. J. Biol. Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- He R., Nanamori M., Sang H., Yin H., Dinauer M. C., Ye R. D. Reconstitution of chemotactic peptide-induced nicotinamide adenine dinucleotide phosphate (reduced) oxidase activation in transgenic COS-phox cells. J. Immunol. 2004;173:7462–7470. doi: 10.4049/jimmunol.173.12.7462. [DOI] [PubMed] [Google Scholar]
- Heyworth P., Bohl B., Bokoch G., Curnutte J. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. Evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. J. Clin. Investig. 1991;87:352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroaki H., Ago T., Ito T., Sumimoto H., Kohda D. Solution structure of the PX domain, a target of the SH3 domain. Nat. Struct. Biol. 2001;8:526–530. doi: 10.1038/88591. [DOI] [PubMed] [Google Scholar]
- Ito T., Matsui Y., Ago T., Ota K., Sumimoto H. Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions. EMBO J. 2001;20:3938–3946. doi: 10.1093/emboj/20.15.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Buescher E. S., Harrison D., Quinn M. T., Parkos C. A., Livesey S., Linner J. Ultrastructural localization of cytochrome b in the membranes of resting and phagocytosing human granulocytes. J. Clin. Investig. 1990;85:821–835. doi: 10.1172/JCI114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami K., Takeya R., Sumimoto H., Kohda D. Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67phox, Grb2 and Pex13p. EMBO. J. 2002;21:4268–4276. doi: 10.1093/emboj/cdf428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F., Liu H., Field S. J., Akbary H., Matsuo T., Brown G. E., Cantley L. C., Yaffe M. B. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- Karathanassis D., Stahelin R. V., Bravo J., Perisic O., Pacold C. M., Cho W., Williams R. L. Binding of the PX domain of p47phox to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhoff C., Nacken W., Benedyk M., Dagher M. C., Sopalla C., Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- Kim C., Dinauer M. C. Impaired NADPH oxidase activity in Rac2-deficient murine neutrophils does not result from defective translocation of p47phox and p67phox and can be rescued by exogenous arachidonic acid. J. Leukoc. Biol. 2006;79:223–234. doi: 10.1189/jlb.0705371. [DOI] [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Evans T., Curnutte J. T., Bokoch G. M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Koshkin V., Lotan O., Pick E. The cytosolic component p47phox is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal superoxide production. J. Biol. Chem. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- Kuribayashi F., Nunoi H., Wakamatsu K., Tsunawaki S., Sato K., Ito T., Sumimoto H. The adaptor protein p40phox as a positive regulator of the superoxide-producing phagocyte oxidase. EMBO J. 2002;21:6312–6320. doi: 10.1093/emboj/cdf642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge K., Smith S. J., Groemping Y., Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. J. Biol. Chem. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- Larsen E. C., Ueyama T., Brannock P. M., Shirai Y., Saito N., Larsson C., Loegering D., Weber P. B., Lennartz M. R. A role for PKC-ε in FcγR-mediated phagocytosis by RAW 264.7 cells. J. Cell Biol. 2002;159:939–944. doi: 10.1083/jcb.200205140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T., Garrett M., Fujii H., Nunoi H. Characterization of neutrophil NADPH oxidase factors p47phox and p67phox from recombinant baculoviruses. J. Biol. Chem. 1991;266:19812–19818. [PubMed] [Google Scholar]
- Leto T. L. The respiratory burst oxidase. In: Gallin J. I., Snyderman R., editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott Williams ' Wilkins; 1999. pp. 769–787. [Google Scholar]
- Leto T. L., Adams A. G., de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. USA. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Cathcart M. K. Selective inhibition of cytosolic phospholipase A2 in activated human monocytes. Regulation of superoxide anion production and low density lipoprotein oxidation. J. Biol. Chem. 1997;272:2404–2411. doi: 10.1074/jbc.272.4.2404. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Z., Chuai S., Shen X. Phospholipase C and phosphatidylinositol 3-kinase signaling are involved in the exogenous arachidonic acid-stimulated respiratory burst in human neutrophils. J. Leukoc. Biol. 2003;74:428–437. doi: 10.1189/jlb.1102537. [DOI] [PubMed] [Google Scholar]
- Lopes L. R., Dagher M. C., Gutierrez A., Young B., Bouin A. P., Fuchs A., Babior B. M. Phosphorylated p40phox as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- Massenet C., Chenavas S., Cohen-Addad C., Dagher M.-C., Brandolin G., Pebay-Peyroula E., Fieschi F. Effects of p47phox C Terminus Phosphorylations on Binding Interactions with p40phox and p67phox. J. Biol. Chem. 2005;280:13752–13761. doi: 10.1074/jbc.M412897200. [DOI] [PubMed] [Google Scholar]
- Nakamura R., Sumimoto H., Mizuki K., Hata K., Ago T., Kitajima S., Takeshige K., Sakaki Y., Ito T. The PC motif: a novel and evolutionarily conserved sequence involved in interaction between p40phox and p67phox, SH3 domain-containing cytosolic factors of the phagocyte NADPH oxidase. Eur. J. Biochem. 1998;251:583–589. doi: 10.1046/j.1432-1327.1998.2510583.x. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- Peng G., Huang J., Boyd M., Kleinberg M. E. Properties of phagocyte NADPH oxidase p47-phox mutants with unmasked SH3 (Src homology 3) domains: full reconstitution of oxidase activity in a semi-recombinant cell-free system lacking arachidonic acid. Biochem. J. 2003;373:221–229. doi: 10.1042/BJ20021629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C. P., Ito T., Moscat J., Diaz-Meco M. T., Inagaki F., Sumimoto H. OPR, PC and AID: all in the PB1 family. Trends Biochem. Sci. 2002;27:10. doi: 10.1016/s0968-0004(01)02006-0. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Gauss K. A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Roberts A. W. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Leto T. L. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. J. Biol. Chem. 1990;265:19910–19915. [PubMed] [Google Scholar]
- Rubin B. B., et al. Cytosolic phospholipase A2-α is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection. J. Biol. Chem. 2005;280:7519–7529. doi: 10.1074/jbc.M407438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamoorthy M., de Mendez I., Adams A. G., Leto T. L. p40(phox) down-regulates NADPH oxidase activity through interactions with its SH3 domain. J. Biol. Chem. 1997;272:9141–9146. doi: 10.1074/jbc.272.14.9141. [DOI] [PubMed] [Google Scholar]
- Sellmayer A., Obermeier H., Danesch U., Aepfelbacher M., Weber P. C. Arachidonic acid increases activation of NADPH oxidase in monocytic U937 cells by accelerated translocation of p47-phox and co-stimulation of protein kinase C. Cell Signal. 1996;8:397–402. doi: 10.1016/0898-6568(96)00077-0. [DOI] [PubMed] [Google Scholar]
- Shiose A., Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000;275:13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- Shirai Y., Kashiwagi K., Yagi K., Sakai N., Saito N. Distinct effects of fatty acids on translocation of γ- and ε-subspecies of protein kinase C. J. Cell Biol. 1998;143:511–521. doi: 10.1083/jcb.143.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelzer Z., Haddad N., Admon E., Pessach I., Leto T. L., Eitan-Hazan Z., Hershfinkel M., Levy R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J. Cell Biol. 2003;162:683–692. doi: 10.1083/jcb.200211056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh C.-I., Stull N. D., Li X. J., Tian W., Price M. O., Grinstein S., Yaffe M. B., Atkinson S., Dinauer M. C. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcγIIA receptor-induced phagocytosis. J. Exp. Med. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H., Hata K., Mizuki K., Ito T., Kage Y., Sakaki Y., Fukumaki Y., Nakamura M., Takeshige K. Assembly and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1996;271:22152–22158. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Kagara S., Yoshikawa K., Yoshida L. S., Kuratsuji T., Namiki H. Involvement of p40phox in activation of phagocyte NADPH oxidase through association of its carboxyl-terminal, but not its amino-terminal, with p67phox. J. Exp. Med. 1996;184:893–902. doi: 10.1084/jem.184.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T., Eto M., Kami K., Tatsuno T., Kobayashi T., Shirai Y., Lennartz M. R., Takeya R., Sumimoto H., Saito N. Isoform-specific membrane targeting mechanism of Rac during FcγR-mediated phagocytosis: positive charge-dependent and independent targeting mechanism of Rac to the phagosome. J. Immunol. 2005;175:2381–2390. doi: 10.4049/jimmunol.175.4.2381. [DOI] [PubMed] [Google Scholar]
- Ueyama T., et al. Superoxide production at phagosomal cup/phagosome through βI protein kinase C during FcγR-mediated phagocytosis in microglia. J. Immunol. 2004;173:4582–4589. doi: 10.4049/jimmunol.173.7.4582. [DOI] [PubMed] [Google Scholar]
- Ueyama T., Ren Y., Sakai N., Takahashi M., Ono Y., Kondoh T., Tamaki N., Saito N. Generation of a constitutively active fragment of PKN in microglia/macrophages after middle cerebral artery occlusion in rats. J. Neurochem. 2001;79:903–913. doi: 10.1046/j.1471-4159.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- Uhlinger D. J., Tyagi S. R., Inge K. L., Lambeth J. D. The respiratory burst oxidase of human neutrophils. Guanine nucleotides and arachidonate regulate the assembly of a multicomponent complex in a semirecombinant cell-free system. J. Biol. Chem. 1993;268:8624–8631. [PubMed] [Google Scholar]
- van Bruggen R., Anthony E., Fernandez-Borja M., Roos D. Continuous translocation of Rac2 and the NADPH oxidase component p67phox during phagocytosis. J. Biol. Chem. 2004;279:9097–9102. doi: 10.1074/jbc.M309284200. [DOI] [PubMed] [Google Scholar]
- Vergnaud S., Paclet M. H., El Benna J., Pocidalo M. A., Morel F. Complementation of NADPH oxidase in p67phox-deficient CGD patients p67phox/p40phox interaction. Eur. J. Biochem. 2000;267:1059–1067. doi: 10.1046/j.1432-1327.2000.01097.x. [DOI] [PubMed] [Google Scholar]
- Williams D. A., et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96:1646–1654. [PubMed] [Google Scholar]
- Wilson M. I., Gill D. J., Perisic O., Quinn M. T., Williams R. L. PB1 domain-mediated heterodimerization in NADPH oxidase and signaling complexes of atypical protein kinase C with Par6 and p62. Mol. Cell. 2003;12:39–50. doi: 10.1016/s1097-2765(03)00246-6. [DOI] [PubMed] [Google Scholar]
- Zhao T., Benard V., Bohl B. P., Bokoch G. M. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Clin. Investig. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Bey E. A., Wientjes F. B., Cathcart M. K. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. J. Biol. Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.