Abstract
ARF6 and Rac1 are small GTPases known to regulate remodelling of the actin cytoskeleton. Here, we demonstrate that these monomeric G proteins are sequentially activated when HEK 293 cells expressing the angiotensin type 1 receptor (AT1R) are stimulated with angiotensin II (Ang II). After receptor activation, ARF6 and Rac1 transiently form a complex. Their association is, at least in part, direct and dependent on the nature of the nucleotide bound to both small G proteins. ARF6-GTP preferentially interacts with Rac1-GDP. AT1R expressing HEK293 cells ruffle, form membrane protrusions, and migrate in response to agonist treatment. ARF6, but not ARF1, depletion using small interfering RNAs recapitulates the ruffling and migratory phenotype observed after Ang II treatment. These results suggest that ARF6 depletion or Ang II treatment are functionally equivalent and point to a role for endogenous ARF6 as an inhibitor of Rac1 activity. Taken together, our findings reveal a novel function of endogenously expressed ARF6 and demonstrate that by interacting with Rac1, this small GTPase is a central regulator of the signaling pathways leading to actin remodeling.
INTRODUCTION
Reorganization of the actin cytoskeleton is an essential cellular response in various physiological and pathological conditions triggered by a broad variety of external stimuli such as hormones and growth factors. In mammalian cells, these stimuli promote the assembly of actin structures by activating signaling cascades regulated by small GTP-binding proteins of the Rho family. Like all GTPases, these cycle between an inactive (GDP-bound) and active (GTP-bound) state. Cycling between these two states is regulated by guanine-nucleotide exchange factors (GEFs), which facilitate the exchange of bound GDP for GTP and GTPase-activating proteins (GAPs) that catalyze GTP hydrolysis (Geyer and Wittinghofer, 1997; Schmidt and Hall, 2002; Moon and Zheng, 2003; Rossman et al., 2005). In addition, the activation of Rho-like GTPases is regulated by guanine-nucleotide dissociation inhibitors (GDIs), which retain the small G proteins in the cytosol (Olofsson, 1999). The best characterized members of the Rho family are RhoA, Rac1, and Cdc42. Although all promote actin reorganization, these small GTPases have distinct effects on cell shape and movement (Hall, 1998). For instance, Rho proteins have been classically associated with stress fiber formation (Ridley and Hall, 1992), Rac1 protein regulates ruffling and lamellipodia formation (Ridley et al., 1992), whereas Cdc42 is important for filopodia formation (Nobes and Hall, 1995).
Several studies have contributed to an understanding of the molecular mechanisms by which small GTP-binding proteins regulate actin remodeling, leading to membrane ruffling and cell migration after extracellular stimuli. The ADP-ribosylation factor 6 (ARF6), a small GTPase that regulates vesicular trafficking and the remodelling of membrane lipids, has also been shown to play an important role in actin rearrangement (reviewed in D'Souza-Schorey and Chavrier, 2006). It was recently demonstrated that aluminum fluoride and epidermal growth factor treatment can promote the relocalization of ARF6 to the ruffling membranes (Fang et al., 2006). Interestingly, Radhakrishna et al. (1999) have suggested that this small GTP-binding protein is an important upstream regulator of Rac1-mediated ruffle formation because expression of a dominant negative mutant (ARF6T27N) prevents the aluminum fluoride–activated effect in Rac1-expressing cells. Similarly, Zhang et al. (1999) demonstrated that ARF6 was required for Rac1-mediated membrane ruffling in macrophages after growth factor stimulation. Recently, Nishiya et al. (2005) suggested that the localized formation of a complex including α4 integrin, paxillin, and an ARF GAP is required for polarized Rac activity and directional cell migration, providing a mechanism for the spacial redistribution of activated Rac necessary for cell movement.
ARF6-dependent Rac1 activation has been suggested to require the involvement of adaptor proteins. ARF6-mediated peripheral actin rearrangement is proposed to involve POR1 (Arfaptin 2), a Rac1-interacting protein (D'Souza-Schorey et al., 1997). Furthermore, other proteins such as Arfaptin 1 and p95-APP1 promote the formation of a complex including both ARF6 and Rac1 (Di Cesare et al., 2000; Tarricone et al., 2001). Many other cellular events including neurite outgrowth and epithelial cell scattering are also regulated by the coordinated action of ARF6 and Rac1 (Santy and Casanova, 2001; Albertinazzi et al., 2003; Palacios and D'Souza-Schorey, 2003). Thus, it is well established that cross-talk between ARF6 and Rac plays an important role in cell shape remodelling. The molecular mechanisms by which ARF6 regulates Rac activity remain, however, somewhat obscure.
We have previously shown that ARF6 is important for the agonist-dependent internalization of the angiotensin II (Ang II) type 1 receptor (AT1R) in HEK 293 cells, suggesting that this G protein–coupled receptor (GPCR) can activate ARF6-dependent signaling pathways (Houndolo et al., 2005). Ang II is a potent hypertensive hormone that also affects cell proliferation, migration, and invasion (Lucius et al., 1999). Targeting of the Ang II system is therefore the therapy of choice for diseases such as hypertension, heart failure, and cardiac hypertrophy (de Gasparo et al., 2000; Kim and Iwao, 2000). In HEK 293 cells, Ang II promotes the activation of several signaling pathways such as stimulation of the heterotrimeric G protein Gαq/11 and the recruitment of the scaffolding protein β-arrestin 1 to coordinately activate RhoA and the formation of stress fibers (Barnes et al., 2005). In this study, we have examined a potential role for ARF6 in mediating Ang II-dependent actin remodelling. Specifically, we investigated the role of endogenous ARF6 as a regulator of Rac1 activity using AT1R-dependent membrane ruffling and migration of HEK 293 cells as a model system.
MATERIALS AND METHODS
Reagents and Antibodies
Minimal essential medium and fetal bovine serum were purchased from Sigma (Oakville, Ontario, Canada). All other tissue culture reagents were purchased from Invitrogen (Burlington, Ontario, Canada). Monoclonal anti-myc 4A6 and anti-Rac1 23A8 were purchased from Upstate (Lake Placid, NY). The anti-ARF6 3A1 (monoclonal), anti-Rac1 C-11 (polyclonal) antibodies and protein G PLUS agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-ARF6 polyclonal antibody was a generous gift from J. Donaldson (National Institutes of Health). The anti-ARF1 antibody was from AbCam (Cambridge, United Kingdom). The β-PIX antibody was from Chemicon (Temecula, CA). HA-beads Affinity Matrix and anti-HA antibodies were from Roche Applied Science (Laval, Quebec, Canada). Alexa Fluor 488 phalloidin, Alexa Fluor 568 goat anti-mouse, Alexa Fluor 633 goat anti-mouse, and Alexa Fluor 568 goat anti-rabbit were from Molecular Probes (Eugene, OR). Ang II, fluorescein isothiocyanate (FITC)-conjugated secondary antibodies, and other reagents were obtained from Sigma.
DNA Plasmids and Small Interfering RNA
Human angiotensin II type 1 receptor (AT1R) was obtained form S. A. Laporte (McGill University, Montreal, Quebec, Canada). GST-Rac1(Δ CAAX) was a gift from J. D. Lambeth (Emory University, Atlanta, GA). Rac1-myc, Rac1T17N-myc, Rac1Q61L-myc, and GST-PAK(CRIB) were obtained from N. Lamarche-Vane (McGill University). ARF6-HA and ARF6 T157A-HA were from L. C. Santy (University of Virginia, Charlottesville, VA). GST-RhoA and GST-Cdc42 were from R. Cerione (Cornell University, Ithaca, NY). GST-GGA3 was from J.-L. Parent (Université de Sherbrooke, Sherbrooke, Quebec, Canada), and β-PIX-Flag was from R.T. Premont (Duke University, Durham, NC). Double-stranded small interfering RNA (siRNA) targeting human ARF6 or ARF1 was synthesized as previously described (Houndolo et al., 2005) using the Silencer siRNA construction kit from Ambion (Austin, TX). The 21-nucleotide sequence from siRNA #1 (Houndolo et al., 2005) and #2 (Hashimoto et al., 2004) were previously characterized. To design ARF1-specific siRNA duplexes, we choose a 21-nucleotide sequence corresponding to region 7–28 from the human ARF1 mRNA (5′-AACATCTTCGCCAACCTCTTC-3′). The scrambled siRNA targets a nonrelevant region in the human genome (5′- AACAGGATAGTCGAGCAGAGT-3′).
Cell Culture and Transfection
HEK 293 cells stably expressing the AT1R-HA (Fessart et al., 2005) or AT1R-Flag were a gift from S. A. Laporte (McGill University). HEK 293 cells were maintained in minimal essential medium supplemented with 1 mM nonessential amino acids, 1 mM sodium pyruvate, and 10% fetal bovine serum at 37°C, 5% CO2. Transfection of DNA plasmids and siRNAs were performed as previously described (Houndolo et al., 2005) using Lipofectamine 2000 according to the manufacturer's instructions. However, in these experiments, cells were used 48 h after siRNA transfection. Hep2 cells were maintained Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal calf serum (Sigma), and penicillin and streptomycin (Sigma) at 37°C, 5% CO2. Cells were grown to 60–70% confluency before transfection by electroporation in HEBS buffer (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 6 mM d-glucose) using two 450-V, 125-μF pulses (Gene Electropulser II, Bio-Rad, Hercules, CA) and 1 μg of the relevant cDNA or 200 nM ARF6 siRNA. Cells were harvested and processed 48 h after transfection (Cant and Pitcher, 2005).
Western Blotting
All proteins were run on polyacrylamide gels (12%) and transferred onto nitrocellulose membranes. The membranes were blotted for relevant proteins using specific antibodies described in the following sections. Secondary antibodies were all FITC-conjugated, and fluorescence was detected using a Typhoon 9410 scanner (Amersham Biosciences, Baie D'Urfe, Quebec, Canada). Quantification of the digital images obtained was performed using ImageQuant 5.2 software (Amersham Biosciences).
Activation of ARF6 and Rac1
HEK 293 cells stably expressing the AT1R-HA receptor (Fessart et al., 2005) were serum-starved overnight. The cells were stimulated with Ang II (1 μM) at 37°C for the indicated times. Cells were lysed in 400 μl of ice-cold lysis buffer E (pH 7.4, 50 mM Tris HCl, 1% NP-40, 137 mM NaCl, 10% glycerol, 5 mM MgCl2, 20 mM NaF, 1 mM NaPPi, 1 mM Na3VO4, and protease inhibitors). Samples were incubated for 30 min (4°C) and spun for 10 min at 10,000 rpm. GST-PAK(CRIB) (Rac1 activation), or GST-GGA3 (ARF6 activation) fusion proteins coupled to glutathione-Sepharose 4B beads were added to each tube, and samples were rotated at 4°C for 1 h. Proteins were eluted into 25 μl of SDS sample buffer containing 5% β-mercaptoethanol by heating to 95°C for 5 min, resolved on 12% SDS-PAGE, and detected by immunoblot using a specific anti-Rac (Upstate) or anti-ARF6 (gift from J. Donaldson) antibody. The secondary antibodies were FITC-conjugated (Sigma), and the proteins were detected using a Typhoon 9410 scanner (Amersham). The same protocol was followed to study the activation of transiently expressed Rac1-myc and ARF6-HA. The proteins were detected using respectively anti-myc and anti-HA antibodies.
GST Pulldown Assays
Equal amounts of GST, GST-Rac1(Δ CAAX), GST-Cdc42, and GST-RhoA were incubated in buffer SM (pH 7.4, 25 mM HEPES, 1 mM EDTA, 1 mM DTT, 2.5 mM MgCl2, 1 mM ATP, 0.2% Triton X-100, and protease inhibitors) with 1 μg of purified recombinant nonmyristoylated ARF6. For nucleotide-loaded small GTPase assays, GST-Rac1(Δ CAAX) was mixed with either GDPβS (100 μM) or GTPγS (10 μM) in buffer E to a final volume of 250 μl. Nucleotide loading was stopped with MgCl2 (60 mM) after a 30-min incubation at 30°C. The beads were then washed and resuspended in buffer SM containing 1 μg of purified recombinant ARF6. For ARF6 and ARF1 loading experiments, purified G proteins were incubated with either GDPβS (100 μM) or GTPγS (10 μM) in buffer E in a final volume of 100 μl. These incubations were then mixed with GST-Rac1(Δ CAAX), in a total volume of 250 μl. In both types of experiments, samples were tumbled for 3 h at 4°C. Beads were recovered by centrifugation and washed five times, and proteins were eluted into 20 μl of SDS sample buffer by heating to 95°C for 5 min. Samples were run on polyacrylamide gels (12%), and the amount of interacting ARF6 or ARF1 was detected by Western blotting using the monoclonal anti-ARF6 antibody (Santa Cruz) or anti-ARF1 (AbCam).
Coimmunoprecipitation Experiments
For ARF6/Rac1 coimmunoprecipitations, HEK293 cells stably expressing AT1R-HA were plated in 10-cm dishes. Before the experiments, cells were serum-starved overnight and stimulated with Ang II (1 μM) for the indicated times before being solubilized in 300 μl of TGH buffer (pH 7.3, 1% Triton, 10% glycerol, 50 mM NaCl, 50 mM HEPES, 5 mM EDTA) containing protease inhibitors (4°C for 1 h). Lysates were centrifuged at 10,000 rpm for 5 min, and equal concentrations of soluble protein were incubated with the monoclonal anti-ARF6 or polyclonal anti-Rac1 antibodies and protein G-PLUS agarose beads. The beads were washed, and bound proteins were eluted into 20 μl of SDS sample buffer containing 5% β-mercaptoethanol and heated to 95°C for 5 min. Proteins were resolved on 12% gels and detected by immunoblot analysis using specific antibodies (polyclonal anti-ARF6, polyclonal anti-Rac1). In a third set of experiments, HEK 293 cells stably expressing AT1R-Flag were transfected with ARF6-HA or ARF6T157A-HA and either Rac1-myc, Rac1T17N-myc or empty vector. Immunoprecipitations were performed as described above with the following exceptions: samples were incubated with affinity matrix HA beads (15 μl; Roche) overnight at 4°C and interacting/immunoprecipitated proteins were detected using specific monoclonal anti-HA and monoclonal anti-myc antibodies. The interactions were quantified as described previously, using ImageQuant v5.2 (Molecular Dynamics).
Immunofluorescence
For Rac1 and ARF6 localization experiments, HEK 293 cells stably expressing the AT1R-Flag were transfected with Rac1-myc and ARF6-HA constructs. Cells were then stimulated with Ang II (1 μM) for the indicated times and fixed using paraformadehyde (4%), and overexpressed proteins were successively labeled using a polyclonal HA antibody, a secondary anti-rabbit antibody coupled to Alexa-568, a monoclonal anti-myc antibody, and a secondary anti-mouse antibody coupled to Alexa-633 in a permeabilizing media (MEM; 0.1% BSA, 10 mM HEPES, 0.05% saponin). Finally, cells were incubated with Alexa-488 phalloidin in the same media for 1 h. For the ruffling experiments, HEK 293 cells stably expressing the AT1R-HA receptor were transfected with Rac1Q61L-myc construct, siRNA targeting ARF6 (#1), siRNA targeting ARF1 or siRNA targeting ARF6 (#1), and Rac1T17N. Forty-eight hours after transfection, cells were serum-starved overnight and then stimulated for 10 min with Ang II (1 μM) or left untreated. For time-course observations (untreated and ARF1 siRNA treated), cells were stimulated for 1, 2, 10, 15, 30, or 60 min. Cells were fixed using 4% paraformaldehyde and incubated with Alexa-488 phalloidin (1 h). For ruffling experiments performed in Hep2 cells, cells were transfected with the muscarinic receptor (M1MR) and either control siRNA, siRNA targeting ARF6, Rac1T21N-myc, or Rac1Q61L-myc. Similarly, cells were stimulated with acetylcholine (ACh, 100 μM) for 10 min, fixed, permeabilized, and stained for actin. Expression of the different constructs was verified by immunofluorescence. For β-PIX experiments, HEK 293 cells stably expressing the AT1R-HA were transfected with either β-PIX-Flag and ARF6 siRNA(#1) or β-PIX-Flag and a scrambled siRNA. After a 30-min Ang II stimulation, cells were fixed and incubated with Alexa-488 phalloidin for 1 h, a polyclonal anti-Flag antibody, and then with a secondary anti-rabbit antibody coupled to Alexa-568. Confocal images were acquired using a Zeiss LSM-510 META laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Migration Assay
Transfected HEK293 cells were serum-starved overnight and seeded into Boyden chambers (24-well inserts with 8-μm pore collagen-coated membranes). One hour after plating, cells were stimulated with Ang II (100 nM) or left untreated. After 4 h, cells were fixed using paraformaldehyde (4%) for 20 min and incubated with crystal violet (0.1% in 20% MeOH: overnight). Membranes were washed five times in dH2O, and cells were removed from the upper chamber, leaving those that migrated through the membrane to the lower chamber. Pictures of five different fields were taken, and the average number of migrating cells was determined for each condition.
β-PIX Membrane Recruitment
HEK 293 cells stably expressing the AT1R-HA were transfected with ARF6 siRNA(#1) or a scrambled one and stimulated with Ang II (1 μM) for 30 min after overnight serum-starving or were left untreated. Cells were then harvested in 300 μl of PBS buffer (pH 7.4; 2.5 mM KCl; 150 mM NaCl; 1.5 mM KH2PO4; 8 mM Na2HPO4) containing protease inhibitors. Cell membranes were disrupted passing three times through the needle of a tuberculine syringe. Cell lysates were then centrifuged for 10 min at 500 × g to discard the nucleus and cellular debris, and the supernatants were further ultracentrifuged at 100,000 × g (30 min, 4°C), in order to separate cytosolic and membrane fractions. Membranes pellets were then lysed for 10 min in 100 μl of ice-cold TGH buffer. Lysates were boiled for 5 min in SDS sample buffer two times and migrated on a 12% polyacrylamide gel. Proteins were detected by immunoblot analysis using specific antibodies (polyclonal anti-ARF6, polyclonal anti-β-PIX; Chemicon). Quantification of the data were performed using Image Quant v5.2 (Molecular Dynamics).
Statistical Analysis
Statistical analysis was performed using a one-way analysis of variance followed by a Bonferroni's multiple comparison test or by a Student's t test using GraphPad Prism (ver. 4.0a; San Diego, CA).
RESULTS
Ang II Stimulation Promotes the Activation of Endogenous ARF6 and Rac1 in HEK 293 Cells
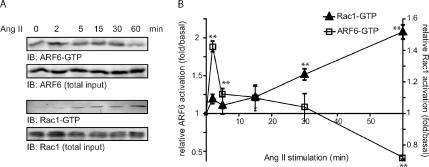
To delineate the molecular mechanisms by which the AT1R, a G protein–coupled receptor, promotes cytoskeleton reorganization, we examined the activation profile of ARF6 and Rac1 after agonist stimulation of HEK 293 cells stably expressing this receptor. Activation of endogenous ARF6 is rapid and transient with maximal levels of ARF6-GTP detected after 2 min of agonist-stimulation (Figure 1). Consistently, we observed that the amount of ARF6-GTP was significantly lower after 60 min of Ang II stimulation compared with that observed in unstimulated cells (0 min). Conversely, the activation of endogenous Rac1 was much slower than ARF6. GTP loading of Rac1 was found to increase gradually with time. Maximal activation was observed after 60 min of agonist treatment (Figure 1) and remained stable for at least 3 h (data not shown). Overexpression of Rac1-myc (1.6-fold/endogenous) markedly altered its kinetic of activation. In these conditions, GTP-binding on Rac1-myc was maximal 2 min after Ang II stimulation and remained sustained for at least 60 min. In addition, Rac1 overexpression led to an increased fold activation of Rac1 after Ang II stimulation (4-fold/basal compared with 1.5-fold/basal for endogenous proteins; Supplementary Figure 1). In contrast, overexpression of ARF6 had no effect on its activation profile (data not shown).
Figure 1.
Ang II promotes the activation of ARF6 and Rac1. (A) HEK293 cells stably expressing HA-AT1R were treated with Ang II for the indicated times. Cells were lysed and activated ARF6 and Rac1 captured using, respectively, GST-GGA3, and GST-PAK(CRIB) coupled to glutathione-Sepharose 4B beads in a GST pulldown assay. Endogenous levels of activated ARF6 and Rac1 were subsequently detected by Western blotting. The levels of ARF6 and Rac1 in total cell lysates (3% of the GST pulldown input) were similarly assessed. These results are representative of five independent experiments for each small GTP-binding protein. Quantification of these experimental results is shown in B. Data (fold/basal) are the mean ± SEM of four independent experiments. **p < 0.01.
Previous studies have suggested a role for ARF6 in regulating Rac1 activity (D'Souza-Schorey et al., 1997; Radhakrishna et al., 1999; Zhang et al., 1999). In an attempt to determine if, in our model system, ARF6 is required for Rac1 activation, we initially investigated whether ARF6 and Rac can associate in an agonist-dependent manner.
Ang II Stimulation Promotes the Association of ARF6 and Rac1 in HEK 293 Cells
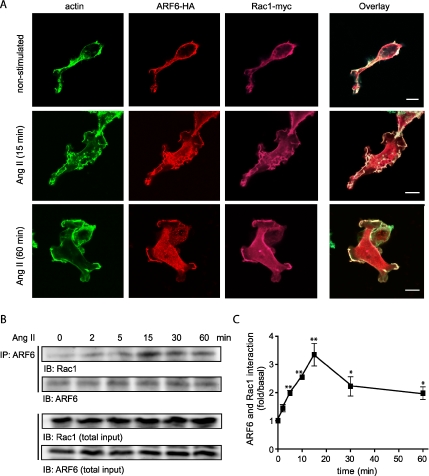
The molecular mechanisms by which ARF6 regulates Rac1 activity after stimulation of a G protein-coupled receptor remain unclear. A previous study has reported the colocalization of ARF6 and Rac1 in a perinuclear recycling compartment in HeLa cells and the subsequent translocation of both GTPases to the plasma membrane in response to aluminum fluoride treatment (Radhakrishna et al., 1999), suggesting a G protein–dependent relocalization of ARF6 and Rac1. We therefore sought to examine the localization of both small GTP-binding proteins before and after Ang II treatment in our AT1R expressing HEK 293 cells. To perform these experiments, we coexpressed ARF6-HA together with Rac1-myc and examined their distribution using confocal microscopy. Before agonist stimulation, both monomeric G proteins were present at the plasma membrane colocalized with actin. On Ang II treatment, remodeling of the actin cytoskeleton was observed; activation of the AT1R led to the formation of membrane protrusions and ruffles, which appeared between 10 and 15 min after Ang II treatment. Sixty minutes after treatment, both GTPases remained present at the site of ruffling. Colocalization of ARF6 and Rac1 in these protrusions is consistent with a potential role for these GTPases in this Ang II–dependent remodeling event (Figure 2A).
Figure 2.
AT1R-stimulation promotes the association of ARF6 and Rac1. (A) HEK 293 cells stably expressing the AT1R-Flag were transiently transfected with Rac1-myc and ARF6-HA constructs. Cells were treated or not with Ang II (1 μM), fixed and labeled for overexpressed proteins using anti-HA and myc specific antibodies. Primary labeling was visualized using secondary antibodies coupled, to Alexa-568 (anti-HA), and Alexa-633 (anti-myc). Finally, polymerized actin was visualized using Alexa-488 phalloidin. Scale bar, 10 μm. (B) HEK293 cells stably expressing AT1R-HA were stimulated with Ang II (1 μM) for the indicated times and solubilized. Endogenous ARF6 was immunoprecipitated using a monoclonal anti-ARF6 antibody. Endogenous interacting Rac1 proteins were then detected by Western blot analysis, using a specific anti-Rac1 antibody. The ARF6 and Rac1 content of the total cell lysate (4% of the initial input) were also assessed. Quantification of the coimmunoprecipitation experiments is presented in C. Data are the mean ± SEM of four independent experiments. *p < 0.05, **p < 0.01.
Using coimmunoprecipitation experiments, we subsequently investigated whether endogenous ARF6 and Rac1 could be found in complex upon agonist stimulation. Figure 2, B and C, illustrates that stimulation of the AT1R promotes the transient association of ARF6 and Rac1 in a time-dependent manner, with maximal association being observed after 15 min of agonist treatment. Quantification of the data reveals that agonist stimulation promotes a 3.3-fold enhancement of ARF6/Rac1 complex formation (Figure 2C). In addition, the association of the two GTPases could also be observed when endogenous Rac1 was immunoprecipitated. In these conditions, the maximal association between Rac1 and ARF6 also occurred after 15 min of Ang II treatment (Supplementary Figure 2).
The Association between ARF6 and Rac1 Is Direct and Dependent on the Activation State of Both Proteins
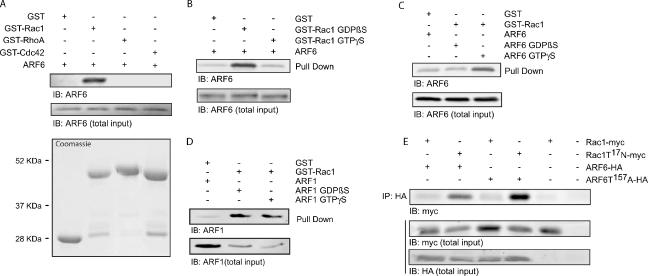
It is likely that, in cells, the formation of a complex between ARF6 and Rac1 is highly regulated by the recruitment of regulatory/scaffold proteins such as exchange factors and/or GTPase-activating proteins. However, the possibility exists that the two small G proteins directly interact after receptor activation. The ability of ARF6 to bind directly to Rac1 was assessed in vitro using purified proteins. Pulldown assays revealed a direct and specific interaction between GST-Rac1 and ARF6 (Figure 3A). In contrast, GST-RhoA and GST-Cdc42 did not bind purified ARF6. To our knowledge, these experiments represent the first demonstration of a direct interaction between two small GTP-binding proteins. Because ARF6 and Rac1 both cycle between an inactive (GDP) and an active (GTP) state, we next examined the nucleotide specificity of ARF6/Rac1 complex formation. Preloading GST-Rac1 with GTPγS markedly impaired its ability to interact with soluble ARF6, suggesting that ARF6 binds the inactive (GDP-bound) form of Rac1 (Figure 3B). Conversely, preloading recombinant ARF6 with GTPγS increased its ability to interact with Rac1, suggesting that the activated form of ARF6 binds preferentially to Rac1 (Figure 3C). To further characterize the specificity of this interaction, we examined whether GST-Rac1 could also directly bind ARF1, another ARF isoform. As depicted in Figure 3D, Rac1 can also directly bind ARF1 in a GST pulldown assay. However, the interaction of the two small GTPases does not appear to be dependent on the nature of the nucleotide bound to ARF1. Several groups have identified interacting partners common to both ARF6 and Rac1 (D'Souza-Schorey et al., 1997; Di Cesare et al., 2000; Tarricone et al., 2001) and have proposed models for cross-talk between these two small GTPases. Our findings raise the possibility of an alternative mechanism whereby ARF6 could influence the activity of Rac1, via direct association.
Figure 3.
Activated ARF6 binds directly to the GDP-bound form of Rac1. (A) Equal amounts of GST, GST-Rac1(ΔCAAX), GST-RhoA, or GST-Cdc42 fusion proteins (Coomassie staining) were incubated with purified ARF6. Using a GST pulldown assay, interacting ARF6 was precipitated and detected by Western blot analysis using a specific anti-ARF6 antibody. These results are representative of three independent experiments, and total input represents 6% of the total protein present in the sample. (B) The GST-Rac1(ΔCAAX) fusion protein coupled to glutathione-Sepharose 4B beads was preloaded with either GDPβS or GTPγS. The nucleotide-bound proteins were incubated with purified ARF6. Interacting proteins were precipitated by a GST pulldown assay, and amounts of associated ARF6 were detected by Western blot analysis using a specific anti-ARF6 antibody. These results are representative of three independent experiments, and the total input represents 6% of the total protein present in the sample. (C) GST-Rac1(ΔCAAX) coupled to glutathione-Sepharose 4B beads was incubated with GDPβS- or GTPγ S-bound purified ARF6. The GST-Rac1 was precipitated and interacting ARF6-GDPβS or ARF6-GTPγS detected by Western blot analysis. These results are representative of four independent experiments. Total input represents 6% of the total protein present in the sample. (D) GST-Rac1(ΔCAAX) coupled to glutathione-Sepharose 4B beads was incubated with GDPβS- or GTPγ S-bound purified ARF1. The GST-Rac1 was precipitated and interacting ARF1-GDPβS or ARF1-GTPγS was detected by Western blot analysis. These results are representative of six independent experiments. Total input represents 30% of the total protein present in the sample. (E) HEK 293 cells stably expressing AT1R-Flag were transfected with Rac1-myc or Rac1T17N-myc and either ARF6-HA, ARF6T157A-HA or empty vector. Using an anti-HA antibody coupled to agarose beads, HA-tagged proteins were immunoprecipitated from the cell lysates, and interacting Rac1 (wild type and mutants) was detected by Western blot analysis using an anti-myc antibody. These results are representative of five independent experiments, and the total input represents 4% of the total protein present in the sample.
To confirm that, in cells, activated ARF6 preferentially interacts with inactive Rac1, we also examined the ability of Rac1 and ARF6 mutants to coimmunoprecipitate. Consistent with the results obtained using purified proteins, the fast cycling mutant of ARF6, ARF6 T157A, and the dominant negative form of Rac1, Rac1T17N, represented the strongest interacting partners (Figure 3E). The observation that an association between wild type ARF6 and Rac1T17N could also be detected in these cells (Figure 3E) suggests the possibility that ARF6 is, at least in part, in an active conformation when expressed in HEK 293 cells.
Depletion of ARF6 in HEK 293 Cells Leads to Enhanced Basal Rac1 Activation
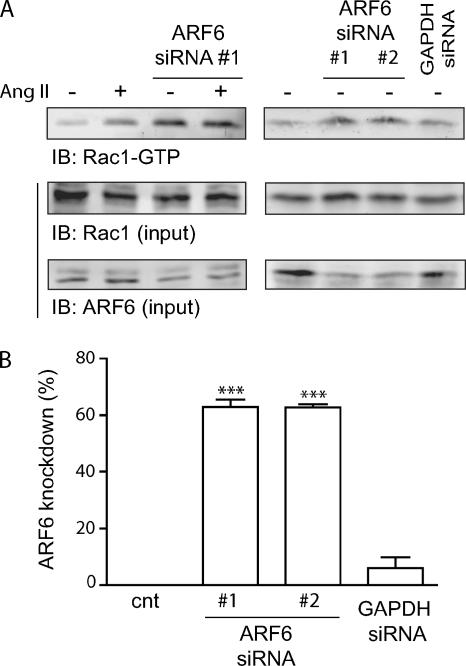
Several studies, using mutants mimicking the inactive or active states of ARF6, have been useful in demonstrating a role for this small G protein in Rac1 activation (Donaldson, 2003). However, a recent report has pointed out that ARF6 mutants, in particular ARF6T27N, which mimics the GDP-bound form of the GTPase, may not recapitulate the effects observed with endogenous inactive ARF6 proteins in a cellular setting (Macia et al., 2004). To study the role endogenous ARF6 plays in regulating Rac1 activity after Ang II treatment, we used RNA interference (siRNA) strategies to silence the expression of ARF6 in our AT1R expressing HEK293 cells. Surprisingly, depletion of this small GTPase dramatically altered the pattern of basal Rac1 activation (Figure 4A). Transfection of siRNA directed against ARF6 led to a marked activation of Rac1 that was not further increased by a 60-min Ang II treatment, which we have shown results in maximal Rac1 activation. To verify that the increased Rac1 activity observed was a specific effect resulting from the depletion of ARF6, and therefore independent of the nature of the siRNA, we compared the effect of our siRNA to another one designed against a different portion of the ARF6 sequence (Hashimoto et al., 2004) or an irrelevant protein (GAPDH). As illustrated in Figure 4A, depletion of ARF6 by a siRNA designed by Hashimoto et al. (2004) resulted in a similar increase of endogenous Rac1 activity. In contrast, transfection of an siRNA directed against GAPDH or a scrambled siRNA (data not shown) did not significantly affect ARF6 expression or basal Rac1 activation. These results support the hypothesis that, in unstimulated cells, ARF6 is responsible for maintaining Rac1 in an inactive state.
Figure 4.
Depletion of ARF6 increases basal Rac1 activation. (A) HEK293 cells stably expressing HA-AT1R were transiently transfected with siRNA targeting ARF6 (#1 or #2, 60 nM), or GAPDH (60 nM). Cells were treated with Ang II for 60 min, and activated Rac1 was captured using GST-PAK(CRIB) coupled to glutathione-Sepharose 4B beads in a GST pulldown assay. Endogenous levels of activated and total Rac1/ARF6 (4% of total input) were detected by Western blotting using specific antibodies. These results are representative of three independent experiments. (B) Quantification of the inhibition of ARF6 expression by transfection of different siRNA. Data are the mean ± SEM of three to eight independent experiments. ***p < 0.001.
Depletion of ARF6 Leads to Spontaneous Membrane Ruffling of HEK 293 Cells
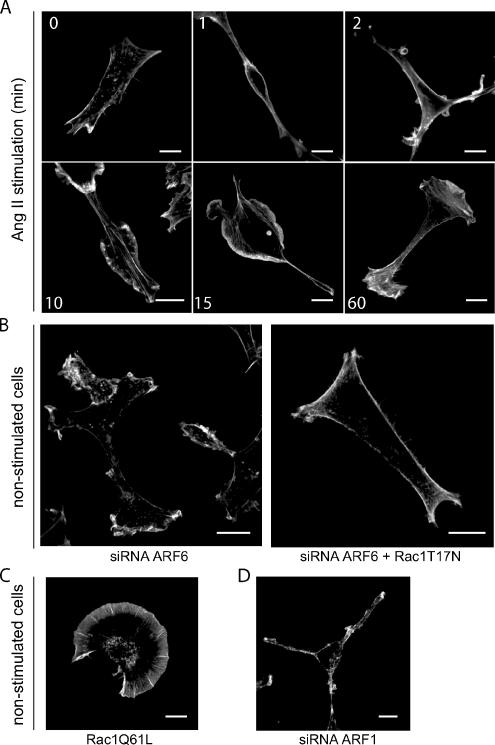
The principal cellular event associated with Rac1 activation, in HEK 293 cells, is remodelling of cortical actin leading to membrane ruffling as shown in Figure 2A. We thus compared the effects of Rac1 activation mediated by either depletion of ARF6 or activation of the AT1R on this cellular response. Stimulation of AT1R expressing HEK 293 cells with Ang II results in the formation of actin-rich membrane protrusions, which appear 10–15 min after agonist treatment and remain present for at least 60 min (Figure 5A). Transfection of ARF6 siRNA, which as shown in Figure 4 leads to Rac1 activation, promotes agonist-independent membrane ruffling (Figure 5B). This phenomenon can be observed as soon as 24 h after transfection. To confirm that this spontaneous ruffling is dependent upon Rac1 activation, we cotransfected the dominant negative form of Rac1 together with the ARF6 siRNA. Rac1T17N expression abolished the spontaneous membrane ruffling induced by ARF6 depletion (Figure 5B). In these cells, membrane ruffling can be spontaneously initiated by the expression of a constitutively active mutant form of Rac1, Rac1Q61L-myc (Figure 5C). The reorganization of cortical actin in ARF6-depleted cells was very similar to that observed in Ang II–stimulated control cells. Indeed, in these two conditions, formation of protrusions as well as ruffles were observed. This is in contrast to actin remodeling induced by overexpression of Rac1Q61L, which results exclusively in membrane ruffle formation. These findings may suggest an impaired ability of the Rac1Q61L mutant to interact with its full range of effectors compared with endogenous activated Rac1. The ability of both AT1R activation and ARF6 siRNA transfection to induce membrane ruffling suggests similar functional capabilities for Rac1 activated via these two different stimuli and demonstrates that modulating ARF6 expression levels has profound functional consequences in a cellular setting. In contrast, depletion of ARF1 did not initiate spontaneous ruffling (Figure 5D) or alter the Ang II–dependent ruffling response (Supplementary Figure 3A).
Figure 5.
Ang II stimulation or ARF6 depletion promotes membrane ruffling in HEK293 cells. (A) Cells stably expressing the HA-AT1R were stimulated with Ang II for the indicated times, fixed, and stained for the distribution of actin using phalloidin coupled to Alexa-488. (B) HA-AT1R stably expressing cells were transfected with siRNA directed against ARF6 (#1, 60 nM) or with both the siRNA for ARF6 and the inactive form of Rac1 (T17N), and staining of actin was performed as in A. (C and D) Cells were transfected with a constitutively active mutant form of Rac1 (Q61L; C) or an siRNA directed against ARF1 (25 nM; D), and actin staining was performed as in A and B. Scale bar, 10 μm. This figure is representative of more than 30 cells observed in three to six independent experiments.
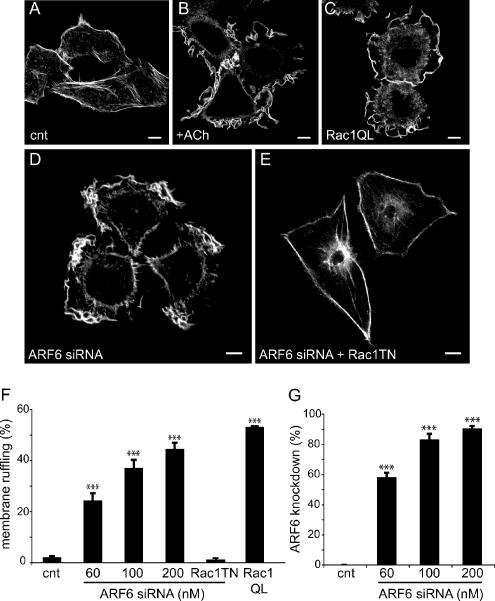
To determine whether this phenomenon can also be observed in other cell types, we depleted ARF6 from Hep2 cells, a cell line that we have previously used to study receptor-mediated cytoskeletal reorganization (Cant and Pitcher, 2005). As depicted in Figure 6, ruffling can be initiated by activation of transfected muscarinic M1 receptor (M1MR), but also by overexpression of Rac1Q61L (Figure 6, B and C). As in HEK 293 cells, inhibition of ARF6 expression resulted in spontaneous membrane ruffling, suggesting that the ability of endogenous ARF6 to inhibit endogenous Rac1 activity is not unique to HEK 293 cells (Figure 6D). Similarly, spontaneous ruffling induced by ARF6 depletion was abolished when a dominant negative form of Rac1 was overexpressed (Figure 6E). In these cells, the number of ruffling cells was proportional to the inhibition of ARF6 expression (Figure 6, F and G).
Figure 6.
Ang II stimulation or ARF6 depletion promotes membrane ruffling in Hep2 cells. (A and B) Hep2 cells expressing the M1MR were left untreated (A) or stimulated with ACh for 10 min (B), fixed, and stained for the distribution of actin using phalloidin coupled to Alexa-488. (C) Hep2 cells were transfected with Rac1 (Q61L)-myc, and staining of actin was assessed as in A and B. (D and E) Hep 2 cells were transfected with siRNA directed against ARF6 (#1, 200 nM; D), or with both the siRNA for ARF6 and the inactive form of Rac1, Rac1(T17N)-myc (E). Staining of actin was performed as described above. Scale, 10 μm. This figure is representative of more than 30 cells observed in three to six independent experiments. (F) Data (% of ruffling cells) are presented as mean ± SEM for three to six independent experiments. RacT17N-myc was overexpressed in cells transfected with 200 nM of ARF6 siRNA. These data are the mean ± SEM of three independent experiments. (G) Quantification of the inhibition of ARF6 expression by transfection of ARF6 siRNA. Data are the mean ± SEM of three independent experiments. ***p < 0.001.
Remodelling of the Actin Cytoskeleton Induced by ARF6 Depletion Promotes Cell Migration
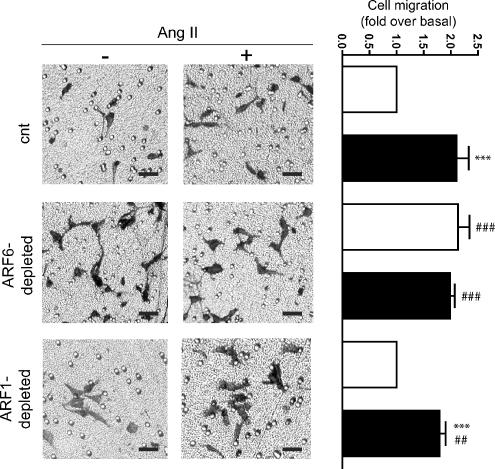
The formation of actin-rich protrusions is one of the first steps required for cell migration. We therefore investigated the role of ARF6-mediated Rac1 activation in this important cellular process. We seeded HEK 293 cells into collagen-coated Boyden chambers and analyzed migratory phenotypes under different conditions. As illustrated in Figure 7, Ang II stimulation resulted in a 2.1-fold increase of cell motility as previously reported (Barnes et al., 2005). Notably, siRNA-mediated depletion of ARF6 also stimulated cell migration to a similar extent (2.1-fold). Agonist treatment had no additional effect on the migratory phenotype of ARF6-depleted cells. In contrast, depletion of ARF1 did not alter basal or Ang II-stimulated migration of HEK 293 cells. These results support the hypothesis that Rac1 activated, via ARF6 depletion or AT1R activation, is functionally equivalent. In addition, our findings highlight the importance of ARF6-mediated Rac1 activation in the process of AngII-dependent cell migration. Expression of a constitutively active mutant form of Rac1 (RacQ61L-myc), effective in promoting membrane ruffling, but not surface protrusion, had no effect on cell migration (data not shown). These results further suggest that RacQ61L expression is not functionally equivalent to activated endogenous Rac1.
Figure 7.
Ang II stimulation or depletion of ARF6 promotes HEK 293 cell migration. Cells stably expressing the HA-AT1R were transiently transfected with either empty vector, siRNA directed against ARF6 (#1) or ARF1 siRNA. Cells were trypsinized and equal cell numbers were reseeded into Boyden chambers and left for 1 h. One set of cells was then stimulated with Ang II, and the second set was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. The left panels are representative of pictures taken from each set of data. Scale bar, 50 μm. The right panel shows the results of the cell migration assay ± SEM for three to five independent experiments performed in duplicate. Statistical analysis was performed using a Bonferroni's multiple comparison test, where ***p < 0.001 are values compared with its paired unstimulated conditions. ###p < 0.001, ##p < 0.01 are values compared with the control (nonstimulated) condition of cells expressing normal levels of endogenous ARF6 and ARF1.
The Rac1 Guanine-Nucleotide Exchange Factor β-PIX Is Relocalized to the Plasma Membrane in ARF6-depleted Cells
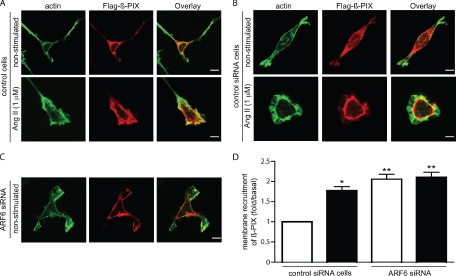
It is generally accepted that relocalization of small GTPases is necessary for their activation, although the mechanisms that control targeting of Rho GTPases in general are poorly understood. It was recently demonstrated that Rac1 binds directly to β-PIX (p21-activated kinases [PAK]-interacting exchange factor) and that this interaction is necessary and sufficient for Rac1 recruitment to membrane ruffles, providing a model for the intracellular targeting and localized activation of Rac1 (ten Klooster et al., 2006). To begin to address why Rac1 is found basally activated in ARF6-depleted cells, we examined the localization of the Rac/Cdc42 GEF β-PIX. As illustrated in Figure 8A, β-PIX is present mainly in the cytosol when overexpressed in HEK 293 cells. Ang II stimulation promotes the relocalization of β-PIX to the membrane ruffles, where it is found colocalized with actin. The agonist-dependent relocalization of β-PIX is consistent with a role for this protein in mediating receptor-dependent Rac1 activation. In ARF6-depleted cells, β-PIX is found at the plasma membrane (Figure 8C). Transfection of a control scrambled siRNA has no effect on the localization of β-PIX in basal and agonist-stimulated conditions (Figure 8B). Similar results can be obtained using a biochemical approach. Ang II treatment as well as depletion of ARF6 promotes the recruitment of endogenous β-PIX to the membrane fraction (Figure 8D). That ARF6 depletion promotes membrane recruitment of β-PIX in a similar manner to AT1R activation provides a potential explanation of how ARF6 depletion may regulate Rac1 activity.
Figure 8.
β-PIX is localized to the plasma membrane in ARF6-depleted cells. (A–C) Cells stably expressing the HA-AT1R were transfected with Flag-β-PIX (A) and Flag-β-PIX and a control scrambled siRNA (60 nM; B) or with Flag-β-PIX and ARF6 siRNA (#1, 60 nM; C). Cells were stimulated with Ang II for 0 or 30 min, fixed, and stained for the distribution of actin, using phalloidin coupled to Alexa-488, and β-PIX, using a specific anti-Flag polyclonal antibody and a secondary anti-rabbit antibody coupled to Alexa-568. This figure is representative of more than 20 cells observed in four independent experiments. (D) HEK 293 cells stably expressing the HA-AT1R were transfected with a control scrambled siRNA (60 nM) or with our ARF6 siRNA (#1, 60 nM). Cells were left untreated (□) or stimulated with Ang II (30 min; ■). Membranes fractions were prepared, and amounts of associated β-PIX protein were assessed by Western blotting using an anti-β-PIX antibody. Results represent the mean ± SEM of four independent experiments. *p < 0.05, **p < 0.01.
DISCUSSION
In this study, we show that stimulation of a G protein–coupled receptor (AT1R) leads to the activation of endogenous ARF6 and Rac1 in HEK 293 cells, promoting the formation of actin-rich membrane protrusions and cell migration. We also demonstrate that upon Ang II treatment, ARF6 and Rac1 are relocalized to the edge of the membrane protrusions, where they transiently associate. In vitro assays suggest that this interaction can be direct and is regulated by the nature of the nucleotide bound to both ARF6 and Rac1. Our experiments demonstrate that ARF6, when bound to GTP, preferentially interacts with Rac1-GDP, suggesting that once Rac1 becomes loaded with GTP, the two small G proteins dissociate. In cells, it is likely that the signaling complex necessary to promote migration involves other signaling partners/regulators/effectors of both small GTPases. For example, we and others have shown that GIT proteins, characterized as ARF GAPs, and β-PIX proteins, a Rac GEF are tightly associated (Bagrodia et al., 1999; Premont et al., 2004). One would therefore expect these proteins to associate with ARF6 and Rac1 to promote signal transduction in a cellular context.
In our experiments, the maximal activation of endogenous ARF6 after Ang II stimulation occurs very rapidly (2 min) and can return to levels lower than basal in ruffling cells exposed to agonist for 60 min. These data suggest that under basal (unstimulated) conditions, a certain proportion of ARF6 is already GTP bound. Interestingly, activation of endogenous Rac1 is much slower (maximal at 60 min) and remains sustained for several hours. The activation profile of overexpressed Rac1, in contrast to ARF6, significantly differs from its endogenous counterpart, being much faster. This represents an important consideration when performing experiments with overexpressed proteins. For this reason, we have largely focused our effort on examining the role of endogenously expressed proteins. However, because of a lack of commercially available antibodies that recognize endogenous levels of these small GTPases by microscopy, we were unable to localize endogenous ARF6 and Rac1 in cells. To visualize their distribution, we had to expressed tagged versions of the wild-type proteins. In these conditions, both proteins were relocalized to the membrane ruffles upon Ang II treatment.
Stimulation of the AT1R not only promotes relocalization of the GTPases to the ruffling membrane but also their association. We have observed that 2 min after Ang II treatment, ARF6 and Rac1 coimmunoprecipitate. This interaction is transient and maximal at 15 min. At this specific time point, ARF6 has been maximally activated and Rac1 is in the process of being maximally activated. Using confocal microscopy, we were able to visualize morphological changes in the actin cytoskeleton after 2 min of agonist stimulation, indicating that early after receptor activation, when ARF6 is maximally activated, specific proteins and signaling cascades are activated to initiate actin reorganization. In our cells, formation of membrane protrusions and ruffling appeared 10–15 min after Ang II treatment (depending on the cell), coinciding with the time of maximal ARF6/Rac1 association and suggesting that Rac1 does not need to be fully activated to induce this morphological effect. Although the peak of interaction was observed at 15 min, association of the two proteins to a level comparable to what is observed at 10, 30, and 60 min is sufficient to promote ruffling. Because the activation/inactivation process of ARF6 after stimulation of a G protein–coupled receptor involves a yet to be characterized complex cascade of events, we suspect that relocalization of proteins and the assembly of signaling complexes is important for the interaction of both small GTPases. This argument is supported by the findings of Fang et al. (2006), who showed that GTP hydrolysis is required for the ARF6-dependent membrane remodeling.
Because ARF6 and Rac1 were found to associate in cells, we hypothesized that depletion of ARF6 would prevent the transmission of the signal that leads to activation of Rac1. Previous studies had demonstrated the coordinated action of ARF6 and Rac1 during the remodeling of the actin cytoskeleton through the use of wild-type or mutant forms of these two GTPases (Radhakrishna et al., 1999). Upon stimulation, ARF6 and Rac1 were previously shown to act in concert to promote remodelling of actin (Radhakrishna et al., 1999; Zhang et al., 1999; Boshans et al., 2000; Palacios and D'Souza-Schorey, 2003). Interestingly, our siRNA approach to study the role of endogenous ARF6 in regulating Rac1 activity has revealed a hitherto unsuspected role for ARF6: ARF6-dependent regulation of basal Rac1 activity. The use of RNA interference has allowed us to demonstrate that, in the two cell lines examined (HEK293 and Hep2), the presence of ARF6 is required to maintain Rac1 in an inactive state under basal conditions. Depletion of ARF6 led to activation of Rac1 that was as robust as that observed when stimulating the cells with Ang II.
The functional consequences of activating Rac1 via ARF6 depletion, or alternatively, AT1R stimulation, appear similar in that both resulted in membrane ruffling and migration of HEK 293 cells. We have demonstrated that increased Rac1 activity resulting from the depletion of ARF6 is responsible for this actin remodeling because expression of a dominant negative mutant form of Rac1 inhibits this process in ARF6-depleted cells. However, our data reveal the complex nature of ARF6-dependent Rac1 regulation. Overexpression of a mutant mimicking the activated form of Rac1 promotes membrane ruffling but does not induce the formation of actin-rich protrusions, observed when endogenous Rac1 is activated by the stimulus-independent (ARF6 siRNA) and stimulus-dependent (AT1R activation) strategies. Notably, expression of the constitutively active Rac1 mutant does not promote cell migration, whereas activation of Rac1 by agonist-stimulation or ARF6-depletion do. In addition, our data suggests that the interaction between ARF6 and Rac1 is specific and associated with distinct biological effects. ARF6 does not directly interact with other small GTPases of the Rho family (Cdc42 and RhoA). Its direct association with ARF1, although very interesting, does not appear to be important for the Ang II–dependent membrane ruffling and migration process of HEK 293 cells.
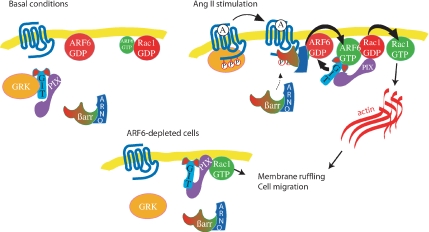
To gain a better understanding of why ARF6 depletion leads to Rac1 activation in HEK 293 cells, we examined the localization of the Rac1 GEF, β-PIX. It was recently reported that the interaction with β-PIX was necessary and sufficient for Rac1 recruitment to membrane ruffles and to focal adhesions (ten Klooster et al., 2006). We therefore hypothesized that the depletion of ARF6 might allow the relocalization of β-PIX to the plasma membrane and the activation of Rac1. This is indeed what we observed. In ARF6-depleted cells, β-PIX is found mainly in the membrane ruffles. The molecular mechanism by which the endogenous expression of ARF6 prevents the translocation of β-PIX to the plasma membrane in normal conditions remains however to be defined. Figure 9represents a model of the sequence of events that may occur in basal, Ang II–stimulated and ARF6-depleted cells. Before agonist-stimulation, ARF6 and Rac1 are largely found in a GDP-bound state because their exchange factors (ARNO and β-PIX) are mainly cytosolic. Stimulation of the AT1R results in the activation of ARF6, which we have previously suggested occurs via the agonist-dependent recruitment of a β-arrestin/ARNO complex (Claing et al., 2001). This study indicates that ARF6-GTP can directly interact with Rac1-GDP and that Ang II treatment ultimately leads to Rac1 activation and actin remodelling. We suspect that ARF6 could result in the activation of Rac1, via recruitment of its GEF β-PIX, which directly interacts with GIT1, an ARF GAP. In ARF6-depleted cells, we have observed that β-PIX is mainly localized to the plasma membrane and that Rac1 is mostly bound to GTP, resulting in cell ruffling and migration.
Figure 9.
Schematic diagram depicting a potential role for ARF6 and Rac1 in Ang II–stimulated membrane ruffling and cell migration. Under basal conditions, ARF6 and Rac1 are mainly found in their inactive state, associated with the plasma membrane. β-PIX, the Rac/Cdc42 guanine-nucleotide exchange factor is mostly present in the cytosol. On AT1R stimulation, ARF6 and Rac1 are subsequently activated and transiently associate to promote remodeling of the actin cytoskeleton, leading to membrane ruffling and cell migration. To allow the loading of GTP on Rac1, β-PIX is relocalized to the plasma membrane. In ARF6-depleted cells, β-PIX is found principally at the plasma membrane, and Rac1 is largely GTP bound. This results in spontaneous ruffling and migration of these cells.
Taken together, our results suggest that changes in ARF6 expression may have important cellular consequences. ARF6 may exhibit differential effects in cells with variable basal levels of Rac1 activity. It is possible that noninvasive cells could acquire spontaneously a migratory phenotype when ARF6 expression is reduced, suggesting that ARF6-dependent regulation of Rac1 activity may be of pathological importance. However, in other cell types and experimental conditions, the function of endogenous ARF6 might be different. It was reported previously that ARF6 depletion blocks the invasive activity of breast cancer cells (Hashimoto et al., 2004). In these cells, the signaling events regulating ARF6 activity might involve different proteins and be regulated through distinct molecular mechanisms.
How exactly ARF6 functions in cells remains an important biological question. This GTPase is well known for its role in vesicle trafficking and remodeling of the membrane lipids (reviewed in D'Souza-Schorey and Chavrier, 2006). We have previously shown that depletion of ARF6 in AT1R-expressing HEK 293 cells leads to the inhibition of the endocytosis of a variety of G protein–coupled receptors, namely the AT1R (Houndolo et al., 2005). However, the spontaneous activation of Rac1 leading to membrane ruffling that we observed in ARF6-depleted cells is not linked to the agonist-promoted block of receptor internalization. Indeed, unstimulated, untransfected Hep2 cells still spontaneously ruffle when transfected with siRNA targeted against ARF6. In normal conditions, however, it is likely that the processes of endocytosis, lipid remodeling and actin rearrangement are intimately related to regulate agonist-promoted cellular responses.
Our data demonstrate that an imbalance between ARF6 and Rac1 activity/expression levels can have profound cellular consequences. A change in the expression or in the activation mechanisms of ARF6 may thus contribute to the development of new cell phenotype, which may lead to important pathological conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. S. A. Laporte (McGill University) for the use of his confocal microscope. This work was supported by the Canadian Institutes of Health Research Grant MOP-53199 to A.C. A.C. is a scholar of the Fonds de Recherche sur la Nature et les Technologies and a member of the Groupe d'étude des proteines membranaires (GÉPROM). J.A.P. is a Wellcome Senior Fellow.
Abbreviations used:
- ARF
ADP-ribosylation factor
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- GPCR
angiotensin II type 1 receptor
- GEF
guanine-nucleotide exchange factor
- GAP
GTPase-activating protein
- PIX
p21-activated kinases [PAK]-interacting exchange factor
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-06-0567) on November 22, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Albertinazzi C., Za L., Paris S., de Curtis I. ADP-ribosylation factor 6 and a functional PIX/p95-APP1 complex are required for Rac1B-mediated neurite outgrowth. Mol. Biol. Cell. 2003;14:1295–1307. doi: 10.1091/mbc.E02-07-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S., Bailey D., Lenard Z., Hart M., Guan J. L., Premont R. T., Taylor S. J., Cerione R. A. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- Barnes W. G., Reiter E., Violin J. D., Ren X. R., Milligan G., Lefkowitz R. J. β-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- Boshans R. L., Szanto S., van Aelst L., D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol. Cell Biol. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant S. H., Pitcher J. A. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol. Biol. Cell. 2005;16:3088–3099. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A., Chen W., Miller W. E., Vitale N., Moss J., Premont R. T., Lefkowitz R. J. β-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J. Biol. Chem. 2001;276:42509–42513. doi: 10.1074/jbc.M108399200. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Boshans R. L., McDonough M., Stahl P. D., Van Aelst L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Di Cesare A., Paris S., Albertinazzi C., Dariozzi S., Andersen J., Mann M., Longhi R., de Curtis I. p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat. Cell Biol. 2000;2:521–530. doi: 10.1038/35019561. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G. Multiple roles for Arf6, sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Fang Z., et al. Proteomic identification and functional characterization of a novel ARF6 GTPase-activating protein, ACAP4. Mol. Cell Proteomics. 2006;5:1437–1449. doi: 10.1074/mcp.M600050-MCP200. [DOI] [PubMed] [Google Scholar]
- Fessart D., Simaan M., Laporte S. A. c-Src regulates clathrin adap6 ter protein 2 interaction with β-arrestin and the angiotensin II type 1 receptor during clathrin-mediated internalization. Mol. Endocrinol. 2005;19:491–503. doi: 10.1210/me.2004-0246. [DOI] [PubMed] [Google Scholar]
- Geyer M., Wittinghofer A. GEFs, GAPs, GDIs and effectors: taking a closer (3D) look at the regulation of Ras-related GTP-binding proteins. Curr. Opin. Struct. Biol. 1997;7:786–792. doi: 10.1016/s0959-440x(97)80147-9. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Onodera Y., Hashimoto A., Tanaka M., Hamaguchi M., Yamada A., Sabe H. Requirement for Arf6 in breast cancer invasive activities. Proc. Natl. Acad. Sci. USA. 2004;101:6647–6652. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houndolo T., Boulay P. L., Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J. Biol. Chem. 2005;280:5598–5604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Kim S., Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol. Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- Lucius R., Gallinat S., Busche S., Rosenstiel P., Unger T. Beyond blood pressure: new roles for angiotensin II. Cell Mol. Life Sci. 1999;56:1008–1019. doi: 10.1007/s000180050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E., Luton F., Partisani M., Cherfils J., Chardin P., Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J. Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- Moon S. Y., Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Nishiya N., Kiosses W. B., Han J., Ginsberg M. H. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11:545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Palacios F., D'Souza-Schorey C. Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J. Biol. Chem. 2003;278:17395–17400. doi: 10.1074/jbc.M300998200. [DOI] [PubMed] [Google Scholar]
- Premont R. T., Perry S. J., Schmalzigaug R., Roseman J. T., Xing Y., Claing A. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell Signal. 2004;16:1001–1011. doi: 10.1016/j.cellsig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Radhakrishna H., Al-Awar O., Khachikian Z., Donaldson J. G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 1999;112(Pt 6):855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Santy L. C., Casanova J. E. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Tarricone C., Xiao B., Justin N., Walker P. A., Rittinger K., Gamblin S. J., Smerdon S. J. The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature. 2001;411:215–219. doi: 10.1038/35075620. [DOI] [PubMed] [Google Scholar]
- ten Klooster J. P., Jaffer Z. M., Chernoff J., Hordijk P. L. Targeting and activation of Rac1 are mediated by the exchange factor beta-Pix. J. Cell Biol. 2006;172:759–769. doi: 10.1083/jcb.200509096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Calafat J., Janssen H., Greenberg S. ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting. Mol. Cell Biol. 1999;19:8158–8168. doi: 10.1128/mcb.19.12.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.