Abstract
It has become increasingly obvious that the notion of a terminally differentiated cell is likely a simplified concept. Epithelial-mesenchymal transition (EMT), during which epithelial cells assume a mesenchymal phenotype, is a key event occurring during normal development and pathological processes. Multiple extracellular stimuli and transcriptional regulators can trigger EMT, but how such distinct signaling pathways orchestrate the complex cellular events that facilitate EMT is not well understood. In this issue of the JCI, Venkov et al. report on their examination of fibroblasts resulting from EMT and describe a novel protein-DNA complex that is essential for transcription of fibroblast-specific protein 1 (FSP1) and sufficient to induce early EMT events (see the related article beginning on page 482). Collectively, their results suggest that this complex is an important regulator of the EMT transcriptome.
During development and adult organ pathogenesis, cells are in a constant state of phenotypic transition. In pathological settings, differentiated adult cells from the kidney, lung, liver, or heart can undergo drastic phenotypic transitions. Such acts are likely undertaken to avoid cell death in a hostile environment. But if an insult, such as organ fibrosis, persists, then such transitions likely become semipermanent.
During epithelial-mesenchymal transition (EMT), epithelial cells gradually lose their epithelial signatures while acquiring the characteristics of mesenchymal cells. EMT is regarded as a critical regulator of metazoan embryogenesis and physiological processes such as wound healing. EMT also contributes significantly in pathologies such as tissue fibrosis and cancer metastasis. Hallmarks of EMT include: (a) the downregulation of cell adhesion molecules such as E-cadherin; (b) the increased expression of MMPs to assist in the degradation of the basement membrane; (c) the activation of the Rac/Rho/Cdc42 family small GTPase to bring about cytoskeleton rearrangement; and (d) the nuclear translocation of several transcription factors including β-catenin and the T cell factor/lymphocyte enhancer factor 1 (TCF/LEF1) complex, Snail1, Snail2, and Twist (1, 2). The adoption of a fibroblast-like transcription profile is crucial for the survival of the cells undergoing EMT. Several key transcription factors have been described (1); however, it is now clear that more such transcriptional regulators are required to govern the complex EMT transcriptome.
A novel protein-DNA complex directly activates fibroblast-specific protein 1 during EMT
The calcium-binding fibroblast-specific protein 1 (FSP1; also known as S100A4) is a crucial facilitator of EMT. The expression of FSP1 marks an early stage of EMT, and blockade of FSP1 expression suppresses the EMT induced by TGF-β and EGF signals (3, 4).
In this regard, a proximal cis-acting regulatory element in the FSP1 promoter, capable of interacting specifically with nuclear extracts from the fibroblasts but not the epithelium, was previously identified using EMSA. The element was termed fibroblast transcription site–1 (FTS-1), and the FTS-1 site has been shown to be crucial for the expression of FSP1 in fibroblasts (5).
Now, in this issue of the JCI, Venkov et al. report the use of FTS-1 as a probe to identify protein components of the FTS-1 complex in fibroblast nuclear extracts (6). Using 2 independent approaches, EMSA and DNA affinity chromatography, the authors identified 2 proteins, CArG box–binding factor–A (CBF-A) and KRAB-associated protein 1 (KAP-1), present in a complex with FTS-1. Furthermore, the authors report that in a kidney epithelial cell line engineered to conditionally express CBF-A, the expression of CBF-A coincided with formation of the CBF-A/KAP-1/FTS-1 complex and the de novo transcription of the FSP1 gene.
CBF-A has been described as both a transcriptional activator and a repressor in distinct genomic loci (7–9). Unlike traditional transcription factors, CBF-A recognizes a variety of DNA motifs and has an affinity for both single-stranded and double-stranded DNA (6–8). KAP-1 is generally considered a transcriptional repressor and also interacts with the nucleosome remodeling and deacetylase (NuRD) complex (10, 11). Such evidence suggests that the CBF-A/KAP-1/FTS-1 complex, whose function is likely to facilitate chromatin remodeling, is a direct and major activator of FSP1 transcription during EMT.
CBF-A as a regulator of the EMT transcriptome and a potential target for drug development
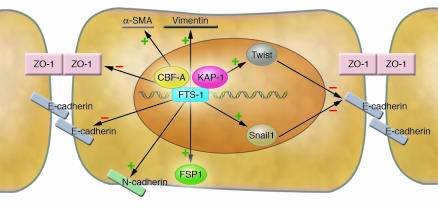
Venkov et al. (6) also show that the effects of CBF-A go beyond just the activation of the FSP1 gene. Cultured kidney epithelial cells initiate the EMT process when triggered to express CBF-A. These cells assume the spindle-shaped fibroblast morphology, exhibit increased migratory capacity, and display the transcriptional responses associated with EMT (Figure 1). CBF-A thus decreases the abundance of the epithelial cell markers zona occludens 1 (ZO-1) and E-cadherin and increases the expression of fibroblast markers N-cadherin, α-SMA, vimentin, Snail1, and Twist. FTS-1 sites are also present in the promoter regions of multiple genes involved in the EMT process, and these sites are more abundant than TCF/LEF1 sites and E-boxes (Snail family protein–binding sites) (6).
Figure 1. The CBF-A/KAP-1/FTS-1 complex is a master regulator of EMT.
In an epithelial cell undergoing EMT, the CBF-A and KAP-1 proteins recognize and bind to the FTS-1 sites in the genomic DNA. The CBF-A/KAP-1/FTS-1 complex controls the expression of a wide spectrum of EMT-responsive genes, possibly via the FTS-1 sites also present in their promoters. Arrows with + or – symbols indicate whether the abundance of a given protein is increased or decreased, respectively, via the action of the CBF-A/KAP-1/FTS-1 complex. ZO-1, zona occludens 1.
It has been well established that EMT plays a significant role in the pathology of tissue fibrosis and metastasis (1, 2). Bone morphogenic protein 7 has been shown to reverse TGF-β1–induced EMT and ameliorate TGF-β1–triggered fibrosis (12). Nevertheless, potential targets for the future development of drugs that can inhibit EMT need to be further explored.
In this regard, the CBF-A/KAP-1/FTS-1 complex qualifies as a promising therapeutic target upstream of the EMT transcriptome. This notion is supported by the broad presence of FTS-1 sites in EMT-responsive genes and also by the observation that CBF-A controls the expression of important transcription regulators of EMT such as Snail1 and Twist. Therefore, disruption of the CBF-A/KAP-1/FTS-1 complex may selectively interrupt chromatin remodeling, thus not allowing for the transcription of key EMT-inducible genes.
In summary, the work presented by Venkov et al. (6) has introduced us to the CBF-A/KAP-1/FTS-1 complex, a regulator of the early events during EMT. Future studies of this protein-DNA complex will enrich our knowledge regarding the complex transcriptional cascades that facilitate EMT and provide further insights into the treatment of EMT-dependent diseases.
Acknowledgments
The research work in the laboratory of the authors is supported by the NIH (grants DK 55001, DK 62987, DK 61688, and AA 13913) and program funds provided to the Division of Matrix Biology by the Beth Israel Deaconess Medical Center. Michael Zeisberg is funded by the NIH (IK08DK074558-01) and the Carl W. Gottschalk Award 2006.
Footnotes
Nonstandard abbreviations used: CBF-A, CArG box–binding factor–A; EMT, epithelial-mesenchymal transition; FSP1, fibroblast-specific protein 1; FTS-1, fibroblast transcription site–1; KAP-1, KRAB-associated protein 1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:304–306 (2007). doi:10.1172/JCI31200.
See the related article beginning on page 482.
References
- 1.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J.M., Dedhar S., Kalluri R., Thompson E.W. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strutz F. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada H., Danoff T.M., Kalluri R., Neilson E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 5.Okada H., et al. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. . Am. J. Physiol. 1998;275:F306–F314. doi: 10.1152/ajprenal.1998.275.2.F306. [DOI] [PubMed] [Google Scholar]
- 6.Venkov C.D., et al. 2007A proximal activator of transcription in epithelial-mesenchymal transitio n . J. Clin. Invest. 117482–491. 10.1172/JCI29544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamada S., Miwa T. A protein binding to CArG box motifs and to single-stranded DNA functions as a transcriptional repressor. Gene. 1992;119:229–236. doi: 10.1016/0378-1119(92)90276-u. [DOI] [PubMed] [Google Scholar]
- 8.Bemark M., Olsson H., Heinegard D., Leanderson T. Purification and characterization of a protein binding to the SP6 kappa promoter. A potential role for CArG-box binding factor-A in kappa transcription. J. Biol. Chem. 1998;273:18881–18890. doi: 10.1074/jbc.273.30.18881. [DOI] [PubMed] [Google Scholar]
- 9.Mikheev A.M., Mikheev S.A., Zhang Y., Aebersold R., Zarbl H. CArG binding factor A (CBF-A) is involved in transcriptional regulation of the rat Ha-ras promoter. Nucleic Acids Res. 2000;28:3762–3770. doi: 10.1093/nar/28.19.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman J.R., et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 11.Schultz D.C., Friedman J.R., Rauscher F.J., 3rd. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev. 2001;15:428–443. doi: 10.1101/gad.869501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg M., et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]