Abstract
L-type Ca2+ current (ICa) is reduced in myocytes from cardiac-specific Na+-Ca2+ exchanger (NCX) knockout (KO) mice. This is an important adaptation to prevent Ca2+ overload in the absence of NCX. However, Ca2+ channel expression is unchanged, suggesting that regulatory processes reduce ICa. We tested the hypothesis that an elevation in local Ca2+ reduces ICa in KO myocytes. In patch-clamped myocytes from NCX KO mice, peak ICa was reduced by 50%, and inactivation kinetics were accelerated as compared to wild-type (WT) myocytes. To assess the effects of cytosolic Ca2+ concentration on ICa, we used Ba2+ instead of Ca2+ as the charge carrier and simultaneously depleted sarcoplasmic reticular Ca2+ with thapsigargin and ryanodine. Under these conditions, we observed no significant difference in Ba2+ current between WT and KO myocytes. Also, dialysis with the fast Ca2+ chelator BAPTA eliminated differences in both ICa amplitude and decay kinetics between KO and WT myocytes. We conclude that, in NCX KO myocytes, Ca2+-dependent inactivation of ICa reduces ICa amplitude and accelerates current decay kinetics. We hypothesize that the elevated subsarcolemmal Ca2+ that results from the absence of NCX activity inactivates some L-type Ca2+ channels. Modulation of subsarcolemmal Ca2+ by the Na+-Ca2+ exchanger may be an important regulator of excitation-contraction coupling.
INTRODUCTION
The Na+-Ca2+ exchanger (NCX) is the main Ca2+ extrusion mechanism of the cardiac myocyte, maintaining Ca2+ homeostasis by removing Ca2+ that enters the myocyte during each cardiac cycle (1–3). Although functional excitation-contraction coupling would seem impossible without NCX, we have produced a murine cardiac-specific knockout (KO) of NCX. Strikingly, these animals survive into adulthood and exhibit only minor cardiac dysfunction (4,5). One mechanism that enables KO myocytes to maintain Ca2+ homeostasis in the absence of NCX is a reduction of Ca2+ entry via the L-type Ca2+ current (ICa) (5), which is accentuated by a shortening of the action potential (6).
The mechanism underlying the reduction in ICa is unknown. Because dihydropyridine receptor (DHPR) expression is unaltered in KO myocytes (7), regulatory processes must be responsible. We have demonstrated previously that the voltage dependence of ICa is similar in WT and KO, and ICa is reduced by a similar amount at all voltages in KO compared to WT (5,7). Thus, it seems unlikely that the mechanisms involved in the reduction of ICa in KO are primarily voltage dependent.
Intracellular Ca2+ itself potently inactivates ICa by a process known as Ca2+-dependent inactivation (8,9). Changes in local subsarcolemmal Ca2+ caused by the absence of normal Ca2+ extrusion via NCX could explain the reduction of ICa in NCX KO mice. Such a functional relationship between NCX and ICa has indeed been demonstrated in a noncardiac mammalian cellular expression system (10). However, the experimental investigation of this relationship in intact cardiac myocytes remains challenging: Pharmacological manipulation of NCX activity is difficult because of a lack of sufficiently specific inhibitors (11). Furthermore, when NCX is acutely blocked by removal of external Na+, there is an almost immediate increase in the sarcoplasmic reticular (SR) Ca2+ load and the Ca2+ transient (12). Thus, it is difficult to directly link changes in ICa to altered NCX activity and to establish a functional relationship between the two proteins.
Cardiac-specific NCX KO mice exhibit unaltered SR Ca2+ load, and their global systolic and diastolic Ca2+ concentrations measured with the Ca2+-sensitive indicator fura-2 are indistinguishable from those in wild-type (WT) littermates (5,7). Thus, these animals present a unique tool for investigating the effects of NCX on the behavior of the L-type Ca2+ current. An increase in subsarcolemmal Ca2+ might increase the degree of ICa inactivation and reduce current amplitude yet be undetectable by conventional fluorescent indicator techniques. We report here that Ca2+-dependent inactivation is indeed responsible for the reduction of ICa in NCX KO mice. We attribute this to elevated subsarcolemmal Ca2+ in the absence of NCX activity.
METHODS
Generation of transgenic mice
NCX cardiac-specific KO mice were generated using cre/lox technology as previously described (7). Animals used in this study were between 8 and 12 weeks of age, did not display any gross pathology, had good cardiac function, and exhibited no evidence of heart failure.
Isolation of ventricular myocytes from adult mouse hearts
Before (20 min) explantation of the hearts, mice were injected with 200 μl heparin (10,000 units/ml) i.p. Animals were then anesthetized with 200 μl nembutal (50 mg/ml) i.p., and hearts were quickly removed via thoracotomy. Isolation of ventricular myocytes by collagenase/protease digestion was performed as reported previously (5,7). All procedures were in accordance with the guidelines of the UCLA Office for Protection of Research Subjects. Isolated cardiomyocytes were stored for up to 6 h at room temperature in modified Tyrode solution containing (in mM) 136 NaCl, 5.4 KCl, 10 Hepes, 1.0 MgCl2, 0.33 NaH2PO4, 1.0 CaCl2, 10 glucose, pH 7.4 with NaOH. To prevent Ca2+ activated Cl− current, 0.1 mM DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate) was present when SR Ca2+ release was not depleted by thapsigargin and ryanodine. DIDS was prepared as a 100 mM stock solution by heating in 0.1 M KHCO3. The above solution was also used, with modifications described below, as the standard bath for electrophysiological recordings.
Electrophysiology
Whole-cell membrane currents were recorded as described previously (5,7). The pipette solution contained (in mM) 120 CsCl, 10 TEA-Cl, 10 NaCl, 20 Hepes, 5 MgATP, 0.05 cAMP, pH 7.2 with CsOH, with modifications described below. We recorded whole-cell membrane current using an Axopatch 200 or 200B patch-clamp amplifier (Axon Instruments, Union City, CA) in voltage-clamp mode and a Digidata 1322A (Axon Instruments) data acquisition system controlled by pCLAMP 9 software (Axon Instruments). We applied series resistance compensation to all recordings. To measure differences in decay kinetics of ICa, single exponential curves were fitted, and the time constant (τ) was calculated.
Rapid solution exchange
Miniature solenoid valves (The Lee Co., Westbrook, CT) controlled by PCLAMP digital outputs controlled the bath solution flow through a micromanifold (ALA Scientific Instruments, Westbury, NY). This enabled precise timing of solution exchanges in relation to the voltage-clamp protocol. The solution surrounding a patched cell exchanged with a half-time of <150 ms. The procedure has been described previously (5,7).
Statistical analysis
Data are expressed as means ± SE. Student's unpaired t-test was used for direct comparisons of WT versus KO.
RESULTS
Determination of cellular phenotype
It has been a common finding that cardiac-specific gene knockout using cre/lox technology does not occur with 100% efficiency (13,14). In NCX KO mice, those cells in which gene excision occurs have complete absence of NCX, and the remaining cells have normal (WT) expression levels. Immunofluorescence and functional data indicate that 80–90% of myocytes from NCX KO mice have no detectable NCX (5).
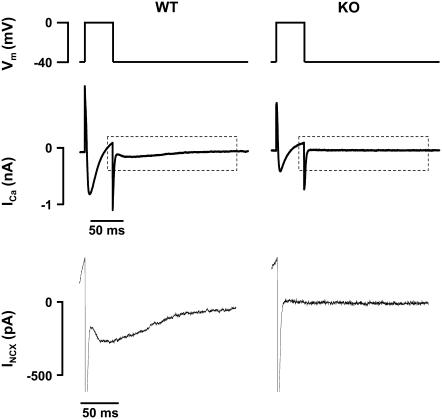
To identify the 10–20% of myocytes isolated from KO animals that express NCX, each myocyte was tested for NCX inward current (INCX) before exposure to thapsigargin and ryanodine. Cells were held at a voltage of −40 mV. After six 100-ms conditioning pulses from −40 to 0 mV (at 1 Hz), cells were depolarized from −40 to 0 mV for 50 ms to induce ICa and SR Ca2+ release. In WT myocytes, a slowly inactivating inward current is detected on repolarization to −40 mV, which is generated by the forward mode of NCX to remove the elevated cytosolic Ca2+ (15). This “tail” NCX current is absent in myocytes with a KO phenotype (Fig. 1). Because NCX KO myocytes have the same SR Ca2+ content and Ca2+ release on depolarization as WT, the absence of inward current is indicative of the absence of NCX. As expected, only 10–20% of myocytes isolated from NCX KO mice expressed INCX. This result is consistent with findings from our previous studies (5,7) and the published efficiency of the cre recombinase system (13,14). Cells with detectable INCX were excluded from the KO group.
FIGURE 1.
Identification of cellular phenotype. (Top panel) Cells were held at −40 mV and then depolarized to 0 mV for 50 ms. (Middle panel) Depolarization induces ICa. In cells with a WT phenotype, this led to a second inward current on repolarization generated by NCX. This NCX “tail current” is absent in myocytes with a KO phenotype. Insets from the middle panel are magnified in the bottom panel to visualize INCX on repolarization.
ICa amplitude and kinetics in NCX KO myocytes
We recorded ICa in WT and KO cells. After six prepulses to 0 mV (1 Hz; 100 ms) to ensure steady-state SR Ca2+ load, cells were depolarized from −75 to −40 mV for 100 ms to inactivate Na+ current and then further depolarized to 0 mV (200 ms) to elicit ICa. Consistent with our previous studies (5,7), we observed a reduced amplitude of ICa in KO myocytes as compared to WT myocytes (KO, −6.2 ± 0.8 pA/pF, n = 9; WT, −12.3 ± 0.8 pA/pF, n = 15; p < 0.01) (Fig. 2 A, Fig. 3). We also found that the decay rate of ICa was accelerated in KO compared to WT (KO, τ = 17.8 ± 2.5 ms; WT, τ = 27.6 ± 1.0 ms; p < 0.01) (Fig. 2 B, Fig. 3). These changes in amplitude and decay rate are consistent with increased Ca2+-dependent inactivation of the Ca2+ current, which we hypothesize is caused by increased subsarcolemmal Ca2+ resulting from the elimination of NCX.
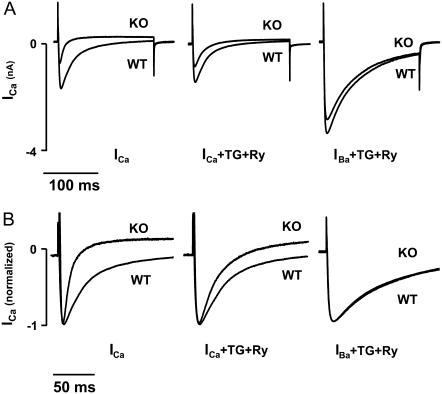
FIGURE 2.
Using Ba2+ as a charge carrier while depleting SR Ca2+ eliminates differences in ICa. (A) Representative traces of ICa (left), ICa after depletion of SR Ca2+ with Tg and Ry (middle), and IBa in the presence of Tg and Ry (right) for KO and WT myocytes. (B) Recordings shown in A were normalized to peak to emphasize the equivalent rates of inactivation after eliminating SR Ca2+ release (ICa in the presence of Tg and Ry, middle) and Ca2+ entry into the diadic cleft (IBa in the presence of Tg and Ry, right).
FIGURE 3.
Summary data comparing amplitude of ICa in KO and WT (KO, n = 9; WT, n = 15) under three conditions: 1), using Ca2+ as the charge carrier with intact SR, 2), using Ca2+ as the charge carrier with SR Ca2+ eliminated by Tg/Ry (KO, n = 5; WT, n = 4), and 3), using Ba2+ as the charge carrier (IBa) in the presence of Tg/Ry (KO, n = 6; WT, n = 4). Amplitude is shown on the left, and decay kinetics on the right. *p < 0.01 for WT versus KO.
Effect of SR Ca2+ on ICa in NCX KO myocytes
To assess the effect of SR Ca2+ on ICa in NCX KO cells, we recorded ICa as described above after exposing the cells to thapsigargin (Tg; 0.2 μM) and ryanodine (Ry; 10 μM) for 10–15 min to deplete SR Ca2+ stores. Tg/Ry eliminated the difference in decay kinetics between KO and WT (KO, τ = 26.5 ± 2.0 ms; WT, τ = 29.1 ± 0.9 ms; p > 0.05; Fig. 2 B, Fig. 3) but did not affect the amplitude of ICa in either KO or WT (KO, −6.0 ± 0.9 pA/pF, n = 5; WT, −12.0 ± 1.5 pA/pF, n = 4; p < 0.01) (Fig. 2 B, Fig. 3). Thus, although SR Ca2+ has an important influence on ICa kinetics in KO cells, this source of Ca2+ does not account for all differences in ICa between WT and KO. Interestingly, elimination of SR Ca2+ had a greater effect on ICa decay kinetics in KO myocytes than in WT. We discuss the significance of this finding in more detail below.
Effect of Ca2+ entry on ICa in NCX KO myocytes
We next tested the contribution of extracellular Ca2+ entry through L-type Ca2+ channels on ICa kinetics in KO myocytes. To do this, we replaced bath Ca2+ (1 mM) with Ba2+ (3 mM). These experiments were conducted in the presence of Tg and Ry to deplete SR stores so that no Ca2+ would be available to influence ICa. Ba2+ is readily conducted by L-type Ca2+ channels. However, unlike Ca2+, Ba2+ has no direct effect on ICa inactivation. Thus, under these conditions, inactivation of ICa is predominantly voltage dependent (9). When Ba2+ was used as the charge carrier, the differences in ICa amplitude between KO and WT were completely eliminated (KO, −15.9 ± 2.9 pA/pF, n = 6; WT, −17.8 ± 3.7 pA/pF, n = 4; p > 0.05) (Fig. 2 A, Fig. 3), and decay kinetics were further slowed (KO, τ = 57.8 ± 4.3 ms; WT, τ = 52.0 ± 6.9 ms; p > 0.05) (Fig. 2 B, Fig. 3). These data indicate that both intact SR Ca2+ stores and also Ca2+ entry through L-type Ca2+ channels are required to produce the differences in ICa amplitude and kinetics observed between WT and KO cells. Because global cytosolic Ca2+ concentration measured with fura-2 is the same in both cell types (5,7), this finding suggests that differences in subsarcolemmal Ca2+ are responsible for the smaller and more rapidly inactivating Ca2+ current we observe in NCX KO mice.
Effect of intracellular Ca2+ buffering on ICa in NCX KO myocytes
To further investigate the influence of intracellular Ca2+ on ICa in NCX KO myocytes, we dialyzed myocytes with the fast Ca2+ chelator BAPTA (10 mM) via the patch pipette to buffer cytosolic Ca2+ (Fig. 4). With Ca2+ as the charge carrier, BAPTA eliminated differences in ICa amplitude (KO, −11.8 ± 1.1 pA/pF, n = 9; WT, −13.9 ± 1.3 pA/pF, n = 11; p > 0.05) (Fig. 4, A and B) and decay kinetics (KO, τ = 44.7 ± 4.1 ms; WT, τ = 44.7 ± 3.0 ms; p > 0.05) (Fig. 4, A and B) between KO and WT myocytes. Thus, buffering cytosolic Ca2+ with BAPTA negates the effect of knocking out NCX on ICa amplitude and inactivation.
FIGURE 4.
Magnitude and decay kinetics of ICa are equal when cytosolic Ca2+ is buffered with BAPTA. (A) Representative tracings of a KO and a WT myocyte under control conditions (left) and from a separate set of KO and WT myocytes that had been dialyzed with BAPTA (right). (B) Summary data comparing ICa in the presence and absence of BAPTA (KO, n = 9; WT, n = 11). Amplitude is shown on the left-hand graph, and decay kinetics on the right. *p < 0.01 for WT versus KO.
Abrupt block of NCX
In cardiac myocytes, blockade of NCX leads to an almost immediate increase in SR Ca2+ load and the Ca2+ transient (12). To evaluate the effects of NCX blockade on ICa before loading of the SR can occur, we used the following protocol: ICa was measured during constant pulsing from a holding potential of −40 mV to 0 mV at 1 Hz. After seven pulses, external Na+ was rapidly replaced with Li+ using the rapid solution exchanger described in the Methods. To prevent reverse Na+-Ca2+ exchange, pipette Na+ was replaced with Cs+ for these experiments. ICa during the final pulse in control solution was then compared with ICa elicited during the first pulse in Li+ solution, i.e., before an increase in SR Ca2+ load and the Ca2+ transient could occur. An example is shown in Fig. 5. In WT cells, acute blockade of NCX reduced ICa amplitude to 86.8 ± 1.3% of control (n = 14; p < 0.05), whereas in KO cells, rapid removal of external Na+ had no effect on ICa (98.0 ± 6.5%; n = 10; p > 0.05). These results suggest that abrupt blockade of NCX in WT cells can raise subsarcolemmal Ca2+ and thereby inactivate Ca2+ channels sufficiently to reduce ICa. This is consistent with the report by Goldhaber et al. (16), who found that acute blockade of NCX (without reversal) rapidly raises subsarcolemmal Ca2+ and activates Ca2+ sparks in rat ventricular myocytes. In KO cells, removing Na+ has no effect on subsarcolemmal Ca2+ or ICa because NCX is already blocked.
FIGURE 5.
Effect of abrupt blockade of NCX on ICa. (A) Representative traces showing effect on ICa of rapidly replacing external Na+ with Li+ before loading of SR Ca2+ can occur. The left-hand traces show the seventh and final Ca2+ current elicited in Na+-containing solution, whereas the right hand traces show the first Ca2+ current elicited in Li+-containing solution. Note that Li+ replacement causes a small but significant reduction in ICa amplitude in the WT cell (upper panel) but no change in the KO cell (lower panel) because of the lack of NCX. (B) Summary results (*p < 0.05, n.s. = difference not significant).
Experimental limitations
As mentioned earlier, some myocytes (10–20%) isolated from KO hearts have a WT phenotype. To identify and exclude these cells from the study, we tested for the presence of Na+-Ca2+ exchange current as described above. However, testing for INCX depends on the presence of cytosolic Ca2+. It was not feasible, then, to test for INCX when cells were dialyzed with BAPTA. Thus, for this subset of experiments (Fig. 4), we cannot exclude the possibility that some cells in the KO group actually have a WT phenotype. However, no more than 20% of KO cells are likely to be of WT phenotype (5,7), which would not alter our conclusions.
DISCUSSION
The Na+-Ca2+ exchanger is the dominant Ca2+ efflux mechanism in cardiac myocytes. Surprisingly, knocking out the exchanger in a cardiac-specific manner is not lethal. NCX KO mice live into adulthood with only modest hemodynamic abnormalities (5,7). NCX KO mice do not up-regulate alternative Ca2+ extrusion mechanisms and therefore have severely impaired Ca2+ removal capacity (7). To compensate for the absence of NCX, KO mice limit Ca2+ entry via ICa, and the net effect is a decrease in transsarcolemmal Ca2+ flux without compromising contractility (5,7). Although ICa is reduced in NCX KO mice, DHPR expression is unaltered (5). Thus, regulatory mechanisms must underlie the reduction of ICa in NCX KO mice. How does this occur?
Knockout of NCX promotes Ca2+-dependent inactivation of ICa
Ca2+-dependent inactivation is one of the strongest modulators of the cardiac L-type Ca2+ channel and can regulate Ca2+ influx on a beat-to-beat basis. Ca2+-dependent inactivation of ICa occurs when Ca2+ binds to DHPR-bound calmodulin (17). Although the amount of Ca2+ released from the SR is severalfold higher than that entering the cell via the L-type Ca2+ channel, both sources of Ca2+ are major contributors to Ca2+-dependent inhibition of ICa (18–21), and both sources may thereby influence ICa amplitude (10).
In this study, we show that either buffering intracellular Ca2+ (Fig. 4), or eliminating both SR Ca2+ release and Ca2+ entry (Fig. 3) eliminates the differences in ICa between NCX KO and WT mice. Thus, we conclude that the mechanism underlying the reduced ICa in NCX KO mice is most likely Ca2+-dependent inactivation. Because global cytosolic Ca2+ levels are unchanged in NCX KO cells, we conclude that ICa is inactivated by elevated subsarcolemmal Ca2+.
Regulation of ICa by subsarcolemmal Ca2+
Suppression of SR Ca2+ release is not sufficient to normalize ICa amplitude in NCX KO myocytes (Figs. 2 and 3). Only the additional inhibition of Ca2+ entry (Figs. 2 and 3) or heavily buffering cytosolic Ca2+ (Fig. 4) leads to normalization of ICa amplitude and decay kinetics. These observations suggest that in NCX KO myocytes, the absence of a robust Ca2+ efflux mechanism keeps subsarcolemmal Ca2+ elevated, even when SR Ca2+ is eliminated. We do not completely understand the source of the elevated subsarcolemmal Ca2+ in the absence of SR Ca2+. One possibility is that stochastic openings of L-type Ca2+ channels are sufficient to raise subsarcolemmal Ca2+ (even before depolarization) in the absence of NCX. Also, we cannot exclude the possibility of other Ca2+ sources or adaptations of which we are not yet aware. For example, ablation of NCX may lead to an increase in the sensitivity of L-type Ca2+ channels to inactivating Ca2+, although we have no direct evidence for this.
ICa amplitude in WT myocytes is also regulated by subsarcolemmal Ca2+. However, in WT cells, with an active efflux of Ca2+ by NCX, subsarcolemmal Ca2+ is lower at rest than in KO myocytes. Thus, ICa amplitude is greater and inactivation slower in WT compared with KO even at baseline. Furthermore, because subsarcolemmal Ca2+ is relatively low in WT myocytes to begin with, elimination of SR Ca2+ using thapsigargin and ryanodine has a relatively small effect on subsarcolemmal Ca2+ (and therefore inactivation of ICa; see Fig. 3) compared to the effect in KO.
Conventional fluorescent indicator methods are incapable of measuring the local Ca2+ concentration in the diadic cleft or the subsarcolemmal space. Indeed, resting and systolic cytoplasmic Ca2+ levels measured by fura-2 and fluo-3 are similar in WT and KO myocytes (5,7). Nevertheless, as shown in this study, a Ca2+-dependent mechanism underlies the reduced ICa in NCX KO mice. The Ca2+ current thereby serves as a reporter of subsarcolemmal and diadic cleft Ca2+. Elevated Ca2+ in these restricted spaces caused by a lack of normal Ca2+ extrusion by the exchanger results in reduced ICa amplitude and accelerated inactivation kinetics.
Effect of acute NCX blockade on ICa
Unlike KO of NCX, rapid blockade of NCX by Li+ substitution in WT myocytes had only a small effect on the ICa recorded during the first pulse after blockade (Fig. 5). An explanation for the mild effect of acute NCX blockade on ICa could be that several cardiac cycles in the absence of NCX activity are necessary to sufficiently raise subsarcolemmal Ca2+ in WT cells to levels required to inactivate ICa. This is difficult to investigate in cardiac myocytes because the blockade of NCX leads to a rapid increase of SR Ca2+ load and Ca2+ transients during continued pulses (12,22). Isaev et al. (10) addressed this problem by investigating the effects of NCX on ICa in a noncardiac mammalian expression system in which DHPR and NCX were coexpressed. Cytosolic Ca2+ was raised to levels expected in the subsarcolemmal space of cardiomyocytes during systole. Under these conditions, they found that suppression of NCX leads to a reduction of ICa similar to what we have described here in KO myocytes (Fig. 2).
We cannot exclude the possibility that there are longer-term adaptive responses participating in the survival of the NCX KO mice. Adaptations could, for example, sensitize KO myocytes to Ca2+-induced inactivation of ICa; we do not presently have evidence for such adaptations.
Ca2+-dependent versus voltage-dependent inhibition of ICa
We have reported previously that there is no difference in the relative voltage dependence of ICa in WT versus KO myocytes (5,7). In this study, we demonstrate a complete reversal of the reduction in ICa in KO myocytes when regulatory Ca2+ is eliminated. We therefore conclude that the underlying mechanism is primarily Ca2+-dependent rather than voltage-dependent.
However, evidence has been presented recently that subsarcolemmal Ca2+ can alter the voltage sensor of the DHPR (10), and thus, voltage-dependent and Ca2+-dependent regulatory mechanisms may be interconnected. Therefore, we cannot exclude the possibility that differences in subsarcolemmal Ca2+ may differentially influence the voltage-dependent availability of ICa in KO versus WT. Future experimental work will be necessary to investigate this issue.
Functional implications
The reduction of ICa in NCX KO mice via a Ca2+-dependent mechanism suggests a functional coupling between NCX and the L-type Ca2+ channel (Fig. 6): Reduced Ca2+ extrusion capacity leads to an accumulation of subsarcolemmal Ca2+. This promotes Ca2+-dependent inactivation of ICa, leading to a reduction of ICa. The reduction of Ca2+ influx balances Ca2+ fluxes. There is then an overall reduction in transsarcolemmal Ca2+ traffic.
FIGURE 6.
Schematic diagram of functional coupling between NCX and the L-type Ca2+ channel (LTCC). (A) Under physiological conditions, Ca2+ influx via ICa is in balance with Ca2+ efflux via NCX. (B) In situations of reduced Ca2+ extrusion capacity, Ca2+ accumulates in the subsarcolemmal space. (C) The increased subsarcolemmal Ca2+ concentration promotes Ca2+-dependent inactivation of ICa. This reduces Ca2+ influx so that a new balance between influx and efflux is established. ec, extracellular; ic, intracellular.
This feedback mechanism may enable the cardiomyocyte to adapt to impaired Ca2+ extrusion and may stabilize the balance of Ca2+ fluxes. The interplay between the Na+-Ca2+ exchanger and the L-type Ca2+ channel may be essential for maintenance of Ca2+ homeostasis in both healthy and diseased heart.
Acknowledgments
The authors appreciate helpful discussions with Dr. R. Olcese.
This research was supported by Köln Fortune and the German Research Foundation (DFG PO 1004\1-1 and 1-2) (C.P.), National Institutes of Health grants HL70828 (J.I.G.) and HL48509 (K.D.P.), and the Laubisch Foundation.
References
- 1.Bers, D. M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205. [DOI] [PubMed] [Google Scholar]
- 2.Bridge, J. H., J. R. Smolley, and K. W. Spitzer. 1990. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science. 248:376–378. [DOI] [PubMed] [Google Scholar]
- 3.Philipson, K. D., and D. A. Nicoll. 2000. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62:111–133. [DOI] [PubMed] [Google Scholar]
- 4.Pott, C., J. I. Goldhaber, and K. D. Philipson. 2004. Genetic manipulation of cardiac Na+/Ca2+ exchange expression. Biochem. Biophys. Res. Commun. 322:1336–1340. [DOI] [PubMed] [Google Scholar]
- 5.Henderson, S. A., J. I. Goldhaber, J. M. So, T. Han, C. Motter, A. Ngo, C. Chantawansri, M. R. Ritter, M. Friedlander, D. A. Nicoll, J. S. Frank, M. C. Jordan, K. P. Roos, R. S. Ross, and K. D. Philipson. 2004. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac-specific knockout of NCX1. Circ. Res. 95:604–611. [DOI] [PubMed] [Google Scholar]
- 6.Pott, X. R., X. D. Tran, M.-J. Yang, S. Henderson, M. C. Jordan, K. P. Roos, A. Garfinkel, K. D. Philipson, and J. I. Goldhaber. 2006. Mechanism of shortened action potential duration in Na+-Ca2+ exchanger knockout mice. Am. J. Physiol. (Epub ahead of print). [DOI] [PubMed]
- 7.Pott, C., K. D. Philipson, and J. I. Goldhaber. 2005. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ. Res. 97:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuhlke, R. D., and H. Reuter. 1998. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc. Natl. Acad. Sci. USA. 95:3287–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass, R. S., and M. C. Sanguinetti. 1984. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J. Gen. Physiol. 84:705–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaev, D., K. Solt, O. Gurtovaya, J. P. Reeves, and R. Shirokov. 2004. Modulation of the voltage sensor of L-type Ca2+ channels by intracellular Ca2+. J. Gen. Physiol. 123:555–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reuter, H., S. A. Henderson, T. Han, T. Matsuda, A. Baba, R. S. Ross, J. I. Goldhaber, and K. D. Philipson. 2002. Knockout mice for pharmacological screening: testing the specificity of Na+-Ca2+ exchange inhibitors. Circ. Res. 91:90–92. [DOI] [PubMed] [Google Scholar]
- 12.Meme, W., S. O'Neill, and D. Eisner. 2001. Low sodium inotropy is accompanied by diastolic Ca2+ gain and systolic loss in isolated guinea-pig ventricular myocytes. J. Physiol. 530:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutstein, D. E., G. E. Morley, and G. I. Fishman. 2001. Conditional gene targeting of connexin43: exploring the consequences of gap junction remodeling in the heart. Cell Commun. Adhes. 8:345–348. [DOI] [PubMed] [Google Scholar]
- 14.Shai, S. Y., H. A. Babbitt, C. J. Jordan, M. C. Fishbein, M. C. Chen, J. Omura, M. Leil, T. A. Becker, K. D. Jiang, M. Smith, D. J. Cherry, Sr., J. C. Loftus, and R. S. Ross. 2002. Cardiac myocyte-specific excision of the β1 integrin gene results in myocardial fibrosis and cardiac failure. Circ. Res. 90:458–464. [DOI] [PubMed] [Google Scholar]
- 15.Choi, H. S., A. W. Trafford, C. H. Orchard, and D. A. Eisner. 2000. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J. Physiol. 529:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhaber, J. I., S. T. Lamp, D. O. Walter, A. Garfinkel, G. H. Fukumoto, and J. N. Weiss. 1999. Local regulation of the threshold for calcium sparks in rat ventricular myocytes: role of sodium-calcium exchange. J. Physiol. 520:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuhlke, R. D., G. S. Pitt, K. Deisseroth, R. W. Tsien, and H. Reuter. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162. [DOI] [PubMed] [Google Scholar]
- 18.Brette, F., L. Salle, and C. H. Orchard. 2004. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ. Res. 95:e1–e7. [DOI] [PubMed] [Google Scholar]
- 19.Puglisi, J. L., W. Yuan, J. W. Bassani, and D. M. Bers. 1999. Ca(2+) influx through Ca(2+) channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ. Res. 85:e7–e16. [DOI] [PubMed] [Google Scholar]
- 20.Sipido, K. R., G. Callewaert, and E. Carmeliet. 1995. Inhibition and rapid recovery of Ca2+ current during Ca2+ release from sarcoplasmic reticulum in guinea pig ventricular myocytes. Circ. Res. 76:102–109. [DOI] [PubMed] [Google Scholar]
- 21.Sham, J. S., L. Cleemann, and M. Morad. 1995. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 92:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuter, H., S. A. Henderson, T. Han, R. S. Ross, J. I. Goldhaber, and K. D. Philipson. 2002. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ. Res. 90:305–308. [DOI] [PubMed] [Google Scholar]