Abstract
Introduction. Given the considerable time and research cost of analyzing biomedical images to quantify adipose tissue volumes, automated image analysis methods are highly desirable. Hippo Fat™ is a new software program designed to automatically quantify adipose tissue areas from magnetic resonance (MR) images without user inputs. Hippo Fat™ has yet to be independently validated against commonly-used image analysis software programs. Objective. Our aim was to compare estimates of VAT (visceral adipose tissue) and SAT (subcutaneous adipose tissue) using the new Hippo Fat™ software against those from a widely-used, validated, computer-assisted manual method (slice-O-matic version 4.2, Tomovision, Montreal, CA) to assess its potential utility for large-scale studies. Methods. A Siemens Magnetom Vision 1.5-Tesla whole body scanner and a T1-weighted fast spin echo pulse sequence were used to collect multiple, contiguous axial images of the abdomen from a sample of 40 healthy adults (20 men) aged 18-77 years of age, with mean BMI of 29 kg/m2 (range=19−43 kg/m2). Results. Hippo Fat™ provided estimates of VAT and SAT that were highly correlated with estimates using slice-O-matic™ (R2>0.9). Average VAT was 9.4% lower and average SAT was 3.7% higher using Hippo Fat™ compared to slice-O-matic™; the overestimation of SAT tended to be greater among individuals with greater adiposity. Individual-level differences for VAT were also substantial; Hippo Fat™ gave estimates of VAT ranging from 1184 cm3 less to 566 cm3 more than estimates for the same person using slice-O-matic™. Conclusion. Hippo Fat™ provides a rapid method of quantifying total VAT, although the method does not provide estimates that are interchangeable with slice-O-matic™ at either the group (mean) or individual level.
Keywords: visceral adipose tissue, subcutaneous adipose tissue, adiposity, magnetic resonance imaging, methods, measurement
Introduction
It has become apparent in recent years that the metabolic and hormonal function of adipose tissue is not uniform, but instead varies from site to site in the body. Visceral adipose tissue (adipose tissue deposited around the internal organs of the abdomen, trunk, and pelvis) appears to hold particular risk for the Metabolic Syndrome and cardiovascular disease (CVD) (Rebuffe-Scrive et al. 1987; Reaven 1988; Pouliot et al. 1992; Kissebah 1996; Despres 1998; Reaven 1999; Bjorntorp 2000; Montague and O'Rahilly 2000; Wajchenberg 2000; Wajchenberg et al. 2002). This increased risk may operate though the association between visceral adipose tissue (VAT) and vascular inflammation, hormonal variations, and insulin resistance.
Safe and cost-effective methods of quantifying internal adipose tissue mass such as VAT are needed, as an increasing number of pharmaceuticals are being targeted toward adipose tissue reduction (e.g., Depres et al. 2005), while others, such as anti-depressive and anti-psychotic medications, have known side effects on adipose tissue mass and distribution (e.g., Schwartz et al. 2004). It is therefore increasingly desirable to monitor changes in specific adipose tissue compartments in clinical studies. Both computed tomography (CT) and magnetic resonance imaging (MRI) are well-established methods for the estimation of VAT mass (Ross 2003), while proton magnetic resonance spectroscopy (1HMRS) has emerged as a useful noninvasive method of quantifying lipid depositions within hepatic and skeletal muscle cells in vivo (Krssak et al. 1999; Seppala-Lindroos et al. 2002). CT provides high resolution images that facilitate image segmentation, but it necessitates exposure of subjects to ionizing radiation. Without ionizing radiation, MRI provides a safer alternative for adipose tissue quantification that has excellent agreement with adipose tissue values produced by dissection and chemical analysis (Fowler et al. 1991; Abate et al. 1994). A number of commercial and freeware biomedical image analysis software packages are available for segmenting MR images, each of which differs in the algorithms used to identify and measure anatomical structures, as well as in their flexibility, time efficiency, and ease of use. Popular biomedical image analysis software packages include Analyze™ (Mayo Clinic, Rochester, MN), slice-O-matic™ (Tomovision, Montreal, Canada), and the public domain programs NIH Image and ImageJ (National Institutes of Health, Bethesda, MD).
Positano and colleagues (Positano et al. 2004) recently introduced Hippo Fat™, an IDL Virtual Machine 6.1-based freeware designed to quantify adipose tissue areas from MR images automatically, without user supervision or manual editing (Positano 2004). Given the considerable time and research cost associated with manual tagging of VAT, an automated image analysis system is highly desirable, especially for large-scale studies. Compared to a free-hand method of contour fitting (Lancaster et al. 1991), Hippo Fat™ produced comparable results in less time (Positano et al. 2004), but Hippo Fat™ has yet to be independently compared to image analysis software currently in use in clinical and epidemiological studies.
slice-O-matic™ (slice-O-matic version 4.2, Tomovision, Montreal, Canada) has been used to estimate visceral adipose tissue volumes in over 200 peer-reviewed scientific publications since 1991 (e.g., Ross et al. 1991; Ross et al. 1992; Heymsfield et al. 2000; Park et al. 2001; Janssen et al. 2002; Slentz et al. 2005). It has been validated against chemical analysis and other image analysis methods (Ross et al. 1991; Mitsiopoulos et al. 1998) and has been used as a reference standard method for comparison in several methodological reports (Kim et al. 2002; Potretzke et al. 2004; Janssen et al. 2000). The goal of this study was to compare the reliability and the validity of the new Hippo Fat™ software against slice-O-matic™ software to assess its utility in measuring visceral and subcutaneous adipose tissues in epidemiological studies.
Methods
Subjects
Forty healthy subjects (20 men; 20 women) aged 18 to 77 years were randomly selected from those enrolled in the Southwest Ohio Family Study for participation in this validation study. The Southwest Ohio Family Study aims to identify genomic regions influencing cardiovascular disease risk factors, including body composition and inflammation, in five large extended families ascertained on a male proband with diastolic blood pressure over 95 mmHg. Participants for this sub-study were pre-screened to insure they were free of any contraindications for MRI (including pacemakers, and metal implants). The study protocols and informed consent documents were approved by the Wright State University Institutional Review Board prior to subject participation.
Acquisition of MR images
MR images were collected at the Good Samaritan Hospital Greater Dayton MRI Consortium in Dayton, Ohio. Subjects were instructed to lie in the magnet in a supine position with arms extended above the head. Images were obtained with a Siemens Magnetom Vision 1.5 Tesla whole body scanner using a T1-weighted fast-spin echo pulse sequence (TR 322 ms, TE 12 ms). A breath-hold sequence (approximately 22 seconds per acquisition) was used to minimize the effects of respiratory motion on the images. All images were acquired on a 256 × 256 mm matrix, with a rectangular 480 × 360 mm field of view. Slice thickness was 1 cm and contiguous slices were obtained every 1 cm from the 9th thoracic vertebra (T9) to the first sacral vertebra (S1). Depending on the height of the person, this resulted in a range of 21 to 35 MRI axial images per person. The images were retrieved from the scanner using the DICOM® (Digital Imaging and Communications in Medicine) protocol (National Electrical Manufacturer's Association, Rosslyn, VA). We begin tagging adipose tissue from the first image that contains the upper margin of the liver down to the L5-S1 image, which increases the likelihood that all intra-abdominal adipose tissue will be included. It should be borne in mind that the anatomic region covered by these images also includes lower mediastinal and pericardial adipose tissue, so that these thoracic depots are also, for the purposes of this study, classified as abdominal visceral adipose tissue.
Slice-O-matic analysis
Image analyses for this study were performed by a single trained observer. All raw MR images between T9 and S1 were opened in slice-O-matic™ version 4.2 and sorted into anatomical order; tagging was initiated at the image containing the upper margin of the liver and continued down to the image containing the L5-S1 intervertebral space. To maximize contrast and maintain consistency, the slice-O-matic™ “Color Scheme” or brightness settings were set at zero for the lowest signal intensity level and at 1278 for the highest signal intensity level for the grayscale images. Both SAT and VAT were tagged by using the program's “Region Growing / Painting” mode. Within the “Region growing / Painting” control panel, the upper threshold was set to the maximum possible setting and the lower threshold was set to a value within the range of 350-650, with the exact value dependent on the pixel intensity of the SAT and VAT in the particular series of images. Each consecutive slice was tagged using the same region growing procedure. SAT and VAT tags resulting from the “Region Growing / Painting” mode were then manually inspected and edited as needed in the “Edit” mode to identify image artifacts and to manually tag adipose tissue not previously tagged (adipose tissue areas below the chosen threshold). Intra-observer reliability for estimation of total VAT volume using slice-O-matic™ is good, and similar to previously reported values (CV=4.5%; ICC=0.997) (Ross et al. 1992)(Table 1), and there was no significant variation in intra-observer reliability depending on which particular image was compared.
Table 1.
Intra-observer reliabilities of SAT and VAT volume estimated from slice-O-matic and HippoFat
| N (pairs) | Reading 1 (mean, cm3) | Reading 2 (mean, cm3) | Difference (cm3) | S.D. | T.E. (cm3) | C.V. (%) | I.C.C. | ||
|---|---|---|---|---|---|---|---|---|---|
| Slice-O-matic | |||||||||
| SAT | 10 | 5040.00 | 5146.41 | 106.59 | 84.08 | 94.14 | 1.85 | 0.9996 | |
| VAT | 10 | 3112.13 | 3195.36 | 151.28 | 141.40 | 142.97 | 4.53 | 0.9967 | |
| HippoFat | |||||||||
| SAT | 10 | 5261.46 | 5201.94 | 81.88 | 108.13 | 92.81 | 1.77 | 0.9996 | |
| VAT | 10 | 2550.81 | 2677.73 | 190.05 | 199.49 | 189.65 | 7.25 | 0.9914 |
Abbreviations: SAT: Abdominal subcutaneous adipose tissue; VAT: Abdominal visceral adipose tissue; Difference: Mean difference between repeated readings by the same observer; S.D.: standard deviation; T.E.: technical error of measurement; C.V.: coefficient of variation, I.C.C.: intraclass correlation coefficient
Hippo Fat analysis
Hippo Fat™ version 6.0 uses a fuzzy c-mean (FCM) algorithm to compute a measure of membership (the fuzzy membership) at each pixel for each of three tissue classes (air, adipose tissue, and non-adipose tissue) (Udapa and Samarasekera 1996; Positano et al. 2004). The FCM algorithm results in a roughly bimodal distribution of pixel intensities in the visceral compartment for each axial image. A Gaussian distribution (with a mean value equal to the representative adipose tissue value and a height equal to the number of pixels with that range of values) is then fit to the peak that corresponds to adipose tissue to estimate SAT and VAT areas. Three contour lines are then automatically generated for each image: 1) along the outer margin of the SAT, 2) along the inner margin of the SAT, and 3) around the smallest possible region in the visceral region that includes all VAT. A histogram showing the distribution of pixel intensity values within the SAT and VAT contours is then automatically generated for each image, and the upper (adipose tissue-specific) peak for each is identified and a Gaussian curve automatically fit to the peak, allowing the adipose tissue area to be calculated. In our case, the analyst then manually adjusted both the contour lines and the shape of Gaussian curve by eye, as necessary, using the edit function. The analyst used Hippo Fat™ to conduct repeated SAT and VAT analyses for a set of N=10 subjects to calculate intra-observer reliability. The two observation days were separated by at least two weeks, and the observer was blinded to the results from day 1 before re-running the program on day 2. Intra- observer reliability for estimation of VAT using Hippo Fat™ was good (CV=7.25%; ICC=0.991), but lower than for slice-O-matic™ (Table 1). Intra-observer reliability tended to be somewhat better for images that were higher in the abdomen (CV=3-8%) than for images lower in the abdomen (CV=11%) (data not shown).
Statistical methods
Means and standard deviations were used to describe the study sample. The intraclass correlation (ICC) was used to determine the strength of association between VAT and SAT estimates from reading 1 and reading 2 to estimate the intra-observer reliability of each method. To quantify the measurement error of each method, the coefficient of variation (CV) and the technical error of measurement (TEM) were used. A paired t-test was used to detect differences in mean VAT and mean SAT using slice-O-matic™ and Hippo Fat™. The agreement between the methods was ascertained by linear regression analysis, with VAT (or SAT) from HippoFat™ as the dependent variable, and with VAT (or SAT) from slice-O-matic™ as the independent variable. Simultaneous tests were conducted to determine if the slopes of the fitted regression lines were significantly different from 1, and if the intercepts were significantly different from zero. Bland-Altman plots were used to examine the relationship, if any, between the inter-method differences in VAT and SAT and the average level of VAT and SAT, respectively, and to examine the 95% confidence intervals of the individual-level agreement between the two methods. Volumetric units were used throughout (cm3), except where mass was calculated (using the conversion equation of 1 liter adipose tissue=0.923 kg) in order to illustrate the inter-method differences in units that are more familiar in the context of obesity and weight loss.
All analyses were conducted using SAS, version 9.1 (SAS Institute, Cary, NC, USA) and results were considered statistically significant at p<0.05.
Results
Descriptive statistics of the study population are presented in Table 2. The participants in the study were overweight on average (BMI=28 kg/m2 in men and 29 kg/m2 in women), and BMI ranged from 19 to 43 kg/m2. Using the average of the SAT and VAT values from the two analysis methods, women tended to have higher SAT (p=0.09) and lower VAT (p=0.004) than men.
Table 2.
Description of study sample (Mean ± S.D., range)
| Males (N = 20) | Females (N = 20) | |
|---|---|---|
| Age (years) | 46.4±13.9 (22.4-69.2) | 49.6±13.4 (18.2-77.4) |
| Height (cm) | 179.6±5.3 (171.2-191.7) | 161.8±8.0 (147.9-174.0) * |
| Weight (kg) | 91.3±13.6 (69.5-118.2) | 77.1±18.2 (54.9-127.2) * |
| BMI (kg/m2) | 28.3±4.0 (22.8-36.9) | 29.4±6.0 (19.4-43.0) |
| Average SAT (cm3) | 4841±2550 (1572-10,133) | 6120± 3032 (1285-13,392) |
| Average VAT (cm3) | 3617±1613 (513-6,824) | 2671±1358 (204-4582) * |
Abbreviations: BMI=body mass index; Average SAT=average subcutaneous adipose tissue for two compared methods; Average VAT=average visceral adipose tissue for two compared methods
Sex difference significant at p<0.05
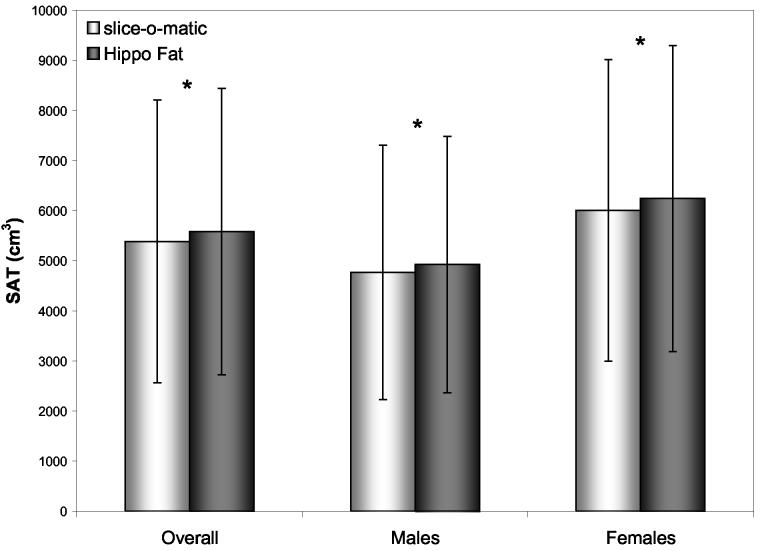
Agreement between the two analysis methods was very good; R2 values were 0.9275 for VAT and 0.9989 for SAT (sexes combined) (Table 3). Adjusting for age and BMI did not substantially change the R2 values (data not shown). R2 was substantially lower in males (0.93 for VAT and 0.91 for SAT) than females (0.99 for VAT and 0.99 for SAT). Despite the relatively strong correlation between the two methods, Hippo Fat™ resulted in significantly lower mean estimates of VAT (2989.6 ± 1492.0 cm3 vs. 3298.7 ± 1632.2 cm3, p<0.0001, sexes combined) and significantly higher mean estimates of SAT (5579.5 ± 2861.2 cm3 vs. 5382.7 ± 2819.4 cm3, p<0.0001, sexes combined) compared to slice-O-matic™ (see Figures 1 and 2). At the group level, therefore, the average inter-method difference in VAT was 9.4% and the average inter-method difference in SAT was 3.7%. The mean inter-method differences did not vary by sex. We examined inter-method agreement for mean SAT and VAT volume at the L4-L5 image to test if inter-method differences might depend on which abdominal location was chosen for comparison. Agreement was somewhat better for the L4-L5 image alone (VAT underestimated by 7.8%; SAT overestimated by 1.5%) as compared to the agreement for all images combined.
Table 3.
Agreement between Hippo Fat and slice-O-matic in the estimation of Abdominal subcutaneous (SAT) and visceral adipose tissue volume (VAT)
| Intercept (s.e.) | Slope (s.e.) | R2 | ||
|---|---|---|---|---|
| VAT | ||||
| Males | 114.0 (241.4) | 0.87 (0.06)* | 0.9260 | |
| Females | 53.2 (202.5)* | 0.89 (0.06) | 0.9984 | |
| Sexes Combined | 85.5 (146.5) | 0.88 (0.04)** | 0.9275 | |
| SAT | ||||
| Males | 122.4 (50.5) | 1.01 (0.01)* | 0.9130 | |
| Females | 145.3 (40.6)** | 1.01 (0.01)* | 0.9994 | |
| Sexes Combined | 120.0 (32.9)** | 1.01 (0.01) ** | 0.9989 |
Abbreviations: s.e.=standard error; p values refer to significance of the difference between the estimate and 0.0 (in the case of the intercept) and between the estimate and 1.0 (in the case of the slope)
p<0.05
p<0.01
Figure 1.
Mean (SD) Abdominal Subcutaneous Adipose Tissue (SAT): Hippo Fat vs. slice-O-matic (*p<0.05)
Figure 2.
Mean (SD) Abdominal Visceral Adipose Tissue (VAT): Hippo Fat vs. slice-O-matic (*p<0.05)
Bland-Altman plots show that as the mean SAT level increased, the differences between the methods increased (r=0.41, p=0.009) (Figure 3); inter-method differences in VAT did not vary significantly with VAT level (Figure 4). At the individual level, the 95% confidence intervals for the prediction of slice-O-matic™ adipose tissue volumes from Hippo Fat™ volumes ranged from −4 to +398 cm3 for SAT, and from −1184 cm3 to +566 cm3 for VAT.
Figure 3.
Bland Altman plot: Abdominal Subcutaneous Adipose Tissue (SAT) Differences, HippoFat vs. slice-O-matic
Figure 4.
Bland Altman plot: Abdominal Visceral Adipose Tissue (VAT) Differences, HippoFat vs. slice-O-matic
Discussion
In this sample of healthy adults aged 18-77 years of age, estimates of visceral adipose tissue volume using the recently-introduced Hippo Fat™ program were highly correlated with estimates using slice-O-matic™, a semi-automated image segmentation program widely used to measure visceral adiposity (e.g., Janssen et al. 2002; Janssen et al. 2002; Lee et al. 2004; Potretzke et al. 2004; Shen et al. 2004; Shen et al. 2004) that has been validated against directly measured chemical composition (Mitsiopoulos et al. 1998). Although there was good overall agreement between the methods (R2>0.9 and slope of nearly 1.0), they produced significantly different estimates of SAT and VAT at both the group and the individual levels. Average VAT was underestimated by 9.4% and average SAT was overestimated by 3.7% using Hippo Fat™ compared to slice-Omatic™. The overestimation of SAT tended to be greater among individuals with higher SAT levels. Converting the volumetric units to mass, the inter-method difference in SAT was 0.18 kg and the difference in VAT was 0.38 kg. Individual-level error for VAT was also substantial; Hippo Fat™ gave estimates of VAT ranging from 1.07 kg less to 0.51 kg more than estimates from the same person using slice-O-matic™. Therefore, slice-O-matic™ and HippoFat™ do not provide interchangeable results for total abdominal VAT or SAT volume. When designing a clinical or epidemiological study, the same image analysis method should be used for all observations and at all study centers. This would be particularly important for longitudinal and intervention studies where a change in image analysis software from baseline to follow-up could substantially bias the results for or against an effect on visceral adiposity.
While we did not examine the agreement between the methods on a slice-by-slice basis, it is possible that agreement may be greater or lesser at specific individual slices or when examining particular adipose tissue depots (e.g., mediastinal, pericardial, retroperitoneal). We found, for example, that for the image taken at the L4-L5 intervertebral space, the single image location most commonly used for MRI studies of visceral adiposity, the degree of overestimation of SAT area and the degree of underestimation of VAT area were lower than we found for total SAT and VAT volumes.
Hippo Fat™ software can be run without user supervision or manual editing, and can process 32 images from a single subject in approximately six minutes. This compares very favorably to the 60 minutes required to process the same number of images using slice-O-matic™. However, when we first attempted to use Hippo Fat™ without performing manual editing, the inter-method difference was ∼19% for VAT and ∼10% for SAT (data not presented). We therefore took advantage of the manual editing features of Hippo Fat™ to adjust both the VAT contour and the Gaussian distribution settings, which increased the processing time to 30 minutes per individual having a set of ∼28 MR images. In the case of individuals with low levels of adipose tissue, the non-adipose tissue and the adipose tissue peaks in the histogram somewhat overlapped one another, creating the need to edit the Gaussian curves manually. This issue has been previously noted (Positano et al. 2004).
Even in the case of heavier subjects with large amounts of visceral adipose tissue, the automated contours for the visceral compartment appeared to exclude some of the adipose tissue from the subsequent analysis, perhaps because of varying intensity of the VAT signal across a single image, or poor resolution of the boundary between the abdominal wall and the visceral compartment. If the signal intensity for VAT is low in a region, the automated VAT contour may not detect it, and the VAT level may be underestimated. Image quality may therefore determine if Hippo Fat™ can be used successfully in its completely automated mode, or if manual adjustments will be required. Subjects in the present analysis were imaged on a 1.5 T scanner using a standard body coil with a T-1 weighted fast spin-echo pulse sequence — a fairly standard protocol for the acquisition of abdominal VAT data in existing studies and similar to the protocol used to initially test the Hippo Fat™ method (Positano et al. 2004). Better resolution images may improve the ability of Hippo Fat™ to generate accurate estimates of VAT without manual editing.
A disadvantage of the Hippo Fat™ program is that it is designed to estimate the total amount of SAT and VAT in the abdominal region only. Recent work has established that there are sub-compartments of adipose tissue within both the subcutaneous compartment, including deep and superficial adipose tissue (Kelley et al. 2000; Smith et al. 2001) as well as the visceral adipose tissue compartment, including pericardial adipose tissue, mediastinal adipose tissue, and others (Iacobellis et al. 2003) that may play particularly important roles in the development of disease (Shen et al. 2003). Computer-assisted manual editing programs such as slice-O-matic™ allow particular adipose tissue sub-compartments to be visually identified and tagged and therefore hold a key advantage for flexible body composition assessment.
In conclusion, there was good overall agreement between Hippo Fat™, a newly introduced automated method for measuring visceral adipose tissue in humans, and a widely used, validated semi-automated software. However, the two methods produced significantly different estimates of SAT and VAT at both the group and the individual levels and are therefore not interchangeable. When designing a clinical or epidemiological study, the same image analysis method should be used for all observations and at all study centers. Advantages of Hippo Fat™ are low cost and a significant reduction in the time required for image analyses, which becomes particularly important in studies where large numbers of participants and/or large numbers of images per participant must be analyzed. Disadvantages of Hippo Fat™ are that the program does not allow for measurement of sub-compartments within the subcutaneous and visceral adipose tissue depots and that manual editing may be required, unless the MR images being used have excellent resolution and quality.
Footnotes
Supported by Grants HD12252 and DK064870 from the National Institutes of Health, Bethesda, Maryland
References
- Abate N, Burns D, et al. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. Journal of Lipid Research. 1994;35(8):1490–6. [PubMed] [Google Scholar]
- Bjorntorp P. [Metabolic difference between visceral fat and subcutaneous abdominal fat] Diabetes & Metabolism. 2000;26(Suppl 3):10–2. [PubMed] [Google Scholar]
- Depres J-P, Golay A, et al. Effects of Rimonabant on metabolic risk factors in overweight patients with dyslipidemia. NEJM. 2005;353:2121–34. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Despres J. The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients' risk. Obes Res. 1998;6:8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Fuller MF, et al. Total and subcutaneous adipose tissue in women: the measurement of distribution and accurate prediction of quantity by using magnetic resonance imaging. Am J Clin Nutr. 1991;54(1):18–25. doi: 10.1093/ajcn/54.1.18. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Nunez C, et al. Anthropometry and methods of body composition measurement for research and field application in the elderly. European Journal of Clinical Nutrition. 2000;54(Suppl 3):S26–32. doi: 10.1038/sj.ejcn.1601022. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Assael F, et al. Epicardial Fat from Echocardiography: a New Method for Visceral Adipose Tissue Prediction. Obes Res. 2003;11(2):304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, et al. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. The American Journal of Clinical Nutrition. 2002;75(4):683–8. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. Journal of Applied Physiology. 2000;89:465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, et al. Application of simple anthropometry in the assessment of health risk: implications for the Canadian Physical Activity, Fitness and Lifestyle Appraisal. Canadian Journal of Applied Physiology. 2002;27(4):396–414. doi: 10.1139/h02-021. [DOI] [PubMed] [Google Scholar]
- Kelley D, Thaete F, et al. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- Kim J, Wang Z, et al. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. The American Journal of Clinical Nutrition. 2002;76(2):378–83. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- Kissebah A. Intra-abdominal fat: is it a major factor in developing diabetes and coronary artery disease? Diabetes Res Clin Pract. 1996;30:25–30. doi: 10.1016/s0168-8227(96)80035-0. [DOI] [PubMed] [Google Scholar]
- Krssak M, Petersen K, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H-NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Ghiatas AA, et al. Measurement of abdominal fat with T1-weighted MR images. Journal of Magnetic Resonance Imaging : JMRI. 1991;1(3):363–9. doi: 10.1002/jmri.1880010315. [DOI] [PubMed] [Google Scholar]
- Lee S, Janssen I, et al. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. Journal of Applied Physiology. 2004;97(3):948–54. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- Mitsiopoulos N, Baumgartner RN, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. Journal of Applied Physiology. 1998;85(1):115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- Montague C, O'Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000 Jun;49:883–8. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- Park YW, Allison DB, et al. Larger amounts of visceral adipose tissue in Asian Americans. Obesity Research. 2001;9(7):381–7. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- Positano V. CNR Institute of Clinical Physiology; Pisa, Italy: 2004. Hippo Fat. [Google Scholar]
- Positano V, Gastaldelli A, et al. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. Journal of Magnetic Resonance Imaging : JMRI. 2004;20(4):684–9. doi: 10.1002/jmri.20167. [DOI] [PubMed] [Google Scholar]
- Potretzke AM, Schmitz KH, et al. Preventing overestimation of pixels in computed tomography assessment of visceral fat. Obesity Research. 2004;12(10):1698–701. doi: 10.1038/oby.2004.210. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- Reaven G. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Reaven G. Syndrome X: 10 years after. Drugs. 1999;58(Suppl 1):19–20. doi: 10.2165/00003495-199958001-00006. discussion 75-82. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Lonnrothe P, et al. Regional adipose tissue metabolism in men and postmenopausal women. Int J Obes Relat Metab Disord. 1987;11:347–355. [PubMed] [Google Scholar]
- Ross R. Advances in the application of imaging methods in applied and clinical physiology. Acta Diabetologica. 2003;40(Suppl 1):S45–50. doi: 10.1007/s00592-003-0025-y. [DOI] [PubMed] [Google Scholar]
- Ross R, Leger L, et al. Adipose tissue volume measured by magnetic resonance imaging and computerized tomography in rats. Journal of Applied Physiology. 1991;70(5):2164–72. doi: 10.1152/jappl.1991.70.5.2164. [DOI] [PubMed] [Google Scholar]
- Ross R, Leger L, et al. Quantification of adipose tissue by MRI: relationship with anthropometric variables. Journal of Applied Physiology. 1992;72(2):787–95. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- Schwartz T, Nihalani N, et al. Psychiatric medication-induced obesity: treatment options. Obesity Reviews. 2004;5:233–8. doi: 10.1111/j.1467-789X.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- Seppala-Lindroos A, Vehkavaara S, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independing of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–28. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of Applied Physiology. 2004;97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, et al. Visceral adipose tissue: relations between single-slice areas and total volume. The American Journal of Clinical Nutrition. 2004;80(2):271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Wang Z, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obesity Research. 2003;11(1):5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slentz CA, Aiken LB, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. Journal of Applied Physiology. 2005;99(4):1613–8. doi: 10.1152/japplphysiol.00124.2005. [DOI] [PubMed] [Google Scholar]
- Smith S, Lovejoy J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism: Clin Experimental. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- Udapa J, Samarasekera S. Fuzzy connectedness and object definition: theory, algorithm and application in image segmentation. Graph Models Image Process. 1996;58:246–61. [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL, Giannella-Neto D, et al. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Hormone and Metabolic Research. 2002;34(1112):616–21. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]