Abstract
Marked heterogeneity exists in the patterns of parasitic infection between individuals, households and communities. Analysis of parasite distributions within populations is complicated by the fact that parasite distributions are highly aggregated, and few studies have explicitly incorporated this distribution when investigating small-scale spatial heterogeneities. This study aimed to quantify the small-scale (within and between household) heterogeneity of helminth infection in an area of Minas Gerais State, Brazil with rural and urban sectors. Parasitological data from a cross-sectional survey of 1,249 individuals aged 0-86 years from 242 households were analysed. Within household clustering of infection was assessed using random effect logistic regression models and between household spatial heterogeneity was assessed using a Bayesian negative binomial spatial model. The overall prevalence of hookworm (Necator americanus) was 66.9%, the prevalence of Schistosoma mansoni was 44.9%, and the prevalence of Ascaris lumbricoides was 48.8%. Statistical analysis indicated significant (within) household and (between household) spatial clustering of hookworm in both rural and urban areas and of S. mansoni in rural areas. There was no evidence of either household or spatial clustering of S. mansoni in urban areas. The spatial correlation of S. mansoni was estimated to reduce by half over a distance of 700m in the rural area. Rural hookworm had a much smaller half-distance and urban hookworm showed an even smaller half-distance (12m). We suggest that such species-specific differences in patterns of infection by environment are primarily due to variation in exposure and parasite life cycle, although host genetic factors cannot be ruled out.
Keywords: Hookworm (Necator americanus and Ancylostoma duodenale), schistosomiasis, Schistosoma mansoni, household clustering, spatial clustering, spatial analysis, negative binomial distribution, Brazil
1. Introduction
Marked heterogeneity exists in the patterns of parasitic infection between individuals, households and communities (Anderson and May, 1991). For example, studies of human helminth infections have shown that the distributions of parasites within populations are highly aggregated, such that a majority of the parasite population is concentrated in a minority of the host population (Bundy et al., 1987, 1988; Bundy & Medley, 1992; Shaw and Dobson, 1995; Woolhouse et al., 1997). Within-household clustering of helminth infections has also been observed (Werneck et al., 2002; Terhell et al., 2000; Forrester et al., 1988; Behnke et al., 2000; Shapiro et al., 2005).
Such heterogeneities may be a consequence of small-scale spatial heterogeneity in infection and/or in risk factors, although this aspect remains less well explored (Hess et al., 2001; Cornell et al., 2003). One reason for the lack of work on spatial patterns of infection was the practical difficulties presented by traditional cartography. This has changed dramatically with the easy geo-positioning of locations, using handheld global positioning systems, and the use of geographical information systems for data capture, storage and analysis (Hay et al., 2000). Initial applications of these technologies for helminth infections focused on the determination of large-scale (between community) spatial patterns and their environmental covariates. This work has yielded new insights into the ecology of infection at a geographical scale that has proven impossible to address using more traditional approaches, and has led to the development of low cost ways to identify target populations for treatment (Brooker and Michael, 2000; Brooker et al., 2002, 2004, 2006; Raso et al., 2005, 2006). More recent improvements in the accuracy of geo-location have enabled finer-scale investigation of spatial patterns, with studies investigating the small-scale (between household) spatial variation of infection among school-aged children (Handzel et al., 2003; Utzinger et al., 2003; Clennon et al., 2004; Booth and Dunne, 2004; Saathoff et al., 2005).
However, because of their aggregated distributions, spatial heterogeneity in helminth and other parasitic infections is difficult to analyze. One reason for this is the need to take account of spatial correlation. If this is not done, then spatial patterns may not always be evident in maps of the mean intensity of infection because of sampling variation (Bernardinelli and Montomoli, 1992). This is particularly important if standard errors are high, for example, because of small units, such as households, or high aggregation of parasites. The latter can often be fitted well by negative binomial distribution (Bliss and Fisher, 1953). Infection densities also vary strongly by demographic variables, such as age and sex, which should be taken into account.
In this paper we address these issues by using a negative binomial spatial model, which adjusts for individual-level covariates such as age and sex (Alexander et al., 2000), to investigate the spatial variation of human schistosome and intestinal nematode infection patterns in an entire community. The community is separated into a rural and an urban area, and we investigate how spatial heterogeneity, along with patterns of (within) household clustering, might vary between these environments.
2. Material and methods
The study was reviewed and approved by the ethical review boards of George Washington University (USA) (100310) and the ethical committee of Centro de Pesquisas René Rachou-FIOCRUZ and the Federal Brazilian Ethical Review Board (CONEP).
2.1. Study area and population
The study was conducted in Americaninhas in Minas Gerais State of Brazil. Americaninhas is located in the northwest of the state, lying between 17°02'12.310” - 17°13'13.857”S and 41°20'18.334” - 41°07'39.737” W and is divided into a rural area and a central municipality, which we refer to as the urban area. The area is hilly and characterized by a tropical climate, with an average temperature of 24°C, and experiences a long rainy season between November and March; annual rainfall is 1300-2000mm. The majority of inhabitants are involved in rural subsistence farming, growing mainly coffee, manioc (cassava) and beans. Cattle-ranching is another important source of income. Houses are predominately made from concrete, or from a combination of wood and mud, and have either tiles or iron sheets for roofing. Only approximately 50% of these homes have a latrine and people commonly collect their water from local springs. There is only one health post in the area with two auxiliary health workers who are paid by the municipality.
2.2. Recruitment
A series of meetings were held with community members to explain the purpose and methodology of the study, that participation was voluntary, and that they were able to withdraw from the study at any time. Written or oral consent was obtained from all adult subjects and from parents or guardians of minors. Individuals were included in the study if they were (1) resident in the study area over the last 24 months and (2) willing and able to give informed consent to study protocol. Individuals were excluded if they: (1) attended school outside of study area; (2) worked full-time outside of study area; or (3) received anthelmintic treatment within the last 24 months as determined by interview. All participants excluded from the study were offered a faecal exam and treated for all helminth infections, but were not considered part of the data set for analysis. Women who were evidently pregnant, or who tested positive on a urine pregnancy test received treatment for all helminth infections after the end of the pregnancy or the termination of breast feeding.
2.3. Parasitological data
Participants were instructed to deposit one faecal sample per day into each container and return the container immediately to one of several collection points, where the sample was stored at 4°C. Faecal samples returned later than 24 hours after date of distribution were not accepted, and new containers were issued. Presence of infection with hookworm (Necator americanus and/or Ancylostoma duodenale), Ascaris lumbricoides and Schistosoma mansoni as well as with other minor species was determined by using the formalin-ether sedimentation technique. Individuals positive for any helminth in the formalin-ether sedimentation were then analyzed by Kato-Katz fecal thick technique for assessment of eggs per gram of faeces (epg). These same patients were asked for another fecal sample. Two slides were taken from each day's faecal sample for a total of four slides from each individual and the average of the four slides presented as the mean egg count. Slides were examined within 45 minutes of slide preparation to avoid clearing of hookworm eggs. To test for quality control, a random sample of 10% of the slides were re-examined by a supervisor.
From 27 albendazole-treated patients, faeces were searched on three consecutive days for expelled worms. Worms were washed in phosphate-buffered saline and stored in 70% ethanol. For clarification and determination of the species, the worms were clarified in a phenol solution (70%) and the mouthparts were examined under the microscope (400x magnification). A total number of 120 male and female worms were determined to be exclusively of the species Necator americanus.
2.4. Household mapping
A hand-held Trimble GeoExplorer global positioning system (GPS) receiver (Trimble Navigation, Sunnyvale, CA, USA) with ArcPad 6.0.3 (NT) (Environmental Systems Research Institute Inc., Redlands, CA, USA) was used to calculate the latitude and longitude of each household participating in the study. Readings, with a resolution of 2 metres, were taken at the front door, or as near as possible in order to receive a sufficient satellite reception and an average of 10 readings of the co-ordinates were taken. Maps were created using ArcView 3.3 (Environmental Systems Research Institute Inc., Redlands, CA, USA)
2.5. Data analysis
Because helminth transmission dynamics are primarily determined by the number of worms present in the host (intensity of infection) rather than the number of hosts infected, and intensity of infection is a key determinant of morbidity, analysis focused on the intensity of infection and the prevalence of heavy infection. Intensity of infection was expressed as arithmetic means. This is justified biologically since intensity of infection is assumed to be proportional to clinical outcomes and no saturation occurs at high intensities. For example, intensity of hookworm infection is linearly related to faecal blood loss (Stoltzfus et al., 1996) and intensity of S. mansoni is associated with risk of hepatosplenic morbidity (Gryseels, 1992; Kardorff et al., 1996, 1997). Negative binomial regression models were applied to investigate whether mean egg counts of hookworm and S. mansoni significantly differed between those with living in rural areas and those in urban areas. Comparisons were not undertaken for A. lumbricoides because its egg counts were truncated at 500 per slide (12, 000 eggs per gram of faeces).
Heavy infection was defined according to thresholds proposed by WHO (2002): hookworm 4,000 epg; A. lumbricoides 10,000 epg; and S. mansoni 400 epg. We investigated household clustering of heavy infection using separate non-Bayesian random effects logistic regression models for the urban and rural areas, defining the household as the group variable. This approach accounts for the non-independence of individuals within a single household and estimates the proportion of total variance accounted for by the clustering effect within households. A likelihood ratio test was used to assess whether this proportion differed significantly from zero. These analyses were performed in Stata 8.2 (College Station, TX, USA).
We also looked for spatial structure in egg counts (intensity of infection) using a Bayesian statistical spatial model. This is not synonymous with household clustering because, in principle at least, high risk houses could be distributed in a spatially uncorrelated manner. The spatial model of Alexander et al. (2000) was used to investigate the spatial heterogeneity (and spatial clustering, if evident) of hookworm and S. mansoni egg counts. For each parasite, the model was used to fit a negative binomial distribution to the total egg count of each person's four slides. Maximum likelihood estimates of k, an inverse measure of aggregation, were used to assess aggregation of egg counts. Again, this analysis was not done for A. lumbricoides because its egg counts were truncated at 500 per slide. The spatial random-effect was modelled as a stationary Gaussian process with mean 0, variance σ2 and correlation function exp(−dij /α), where dij is the distance between houses i and j and the parameter α measures the rate at which the spatial correlation decays to zero with increasing distance. The extent of between-house variation is measured by the parameter 1/ϕ, which is the variance in log-mean egg count between houses separated by a negligible distance. The scale of spatial correlation is measured by the exponential decay constant α, where loge2(α) is the ‘half-distance’, that is, the distance over which the spatial correlation reduces by half. These parameters are fitted while adjusting for the age (as a quadratic function) and sex composition of each house. This yields a ratio by which the house's mean egg count is higher or lower than expected. By analogy with the Standardized Mortality Ratio, this may be called the Standardized Parasite Ratio (SPR). The SPRs incorporate a degree of smoothing which is dependent on the fitted spatial correlation. For this Bayesian analysis we used prior distributions as previously described (Alexander et al. 2000). For sensitivity analysis of ϕ we used, in addition to the Jeffreys (reciprocal) prior, inverse Gamma priors with means 0.1 and 0.01km. The inverse Gamma with mean 0.1 was used as the default. Houses with GPS positions less than 10m apart were treated as a single unit. Bayesian inference was implemented via a Markov chain Monte Carlo algorithm. Specification of prior distributions for the model parameters was done along the lines of the work described by Alexander et al. (2000). The Markov chain was run for 50,000 iterations, of which every 10th was kept for analysis, after an initial ‘burn-in’ of 10,000 iterations. Models were fitted separately to the urban and rural areas, using a program custom-written in the C language.
3. Results
For the current study, 1,496 patients provided consent, with 1,332 (89% of total population) individuals meeting the inclusion/exclusion criteria and 1,249 (83% of total population) individuals, aged 0-86 years old, who could be unambiguously defined as permanent residents of a single household: 543 from the urban municipality area and 706 from rural areas.
Prevalence and intensity of infection
Overall, 81.9% of individuals were infected with at least one helminth species and over half (50.3%) harboured multiple species infections. The overall prevalence of hookworm was 66.9%, the prevalence of S. mansoni was 44.9%, and the prevalence of A. lumbricoides was 48.8%. Prevalence of other helminth species was negligible: Trichuris trichiura (1.1%); Enterobius vermicularis (0.8%); Hymenolepis nana (0.2%); and Taenia sp. (0.2%).
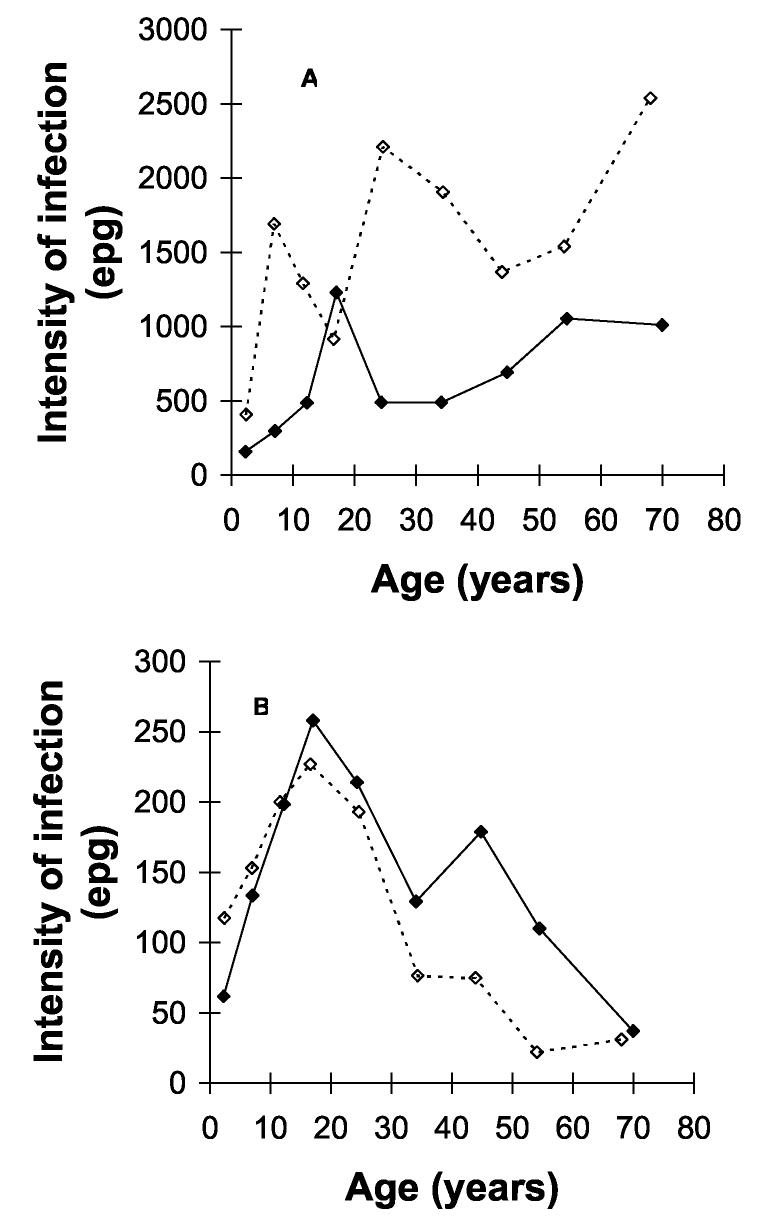
The arithmetic mean intensity of hookworm infection was 1096 epg, with intensity significantly lower in individuals living in urban areas than those in rural areas (611 epg vs. 1469 epg, p<0.001). The arithmetic mean intensity of S. mansoni was 130.9 epg and did not differ significantly between urban and rural areas (p=0.45). The relationship between age and intensity of infection of each species by area is shown in Figure 1. This indicates a similar age profile in each area: hookworm intensity rose steadily with age (Fig. 1A) and for S. mansoni, age-intensity profiles were convex in each area, with maximum intensity at 15-19 years (Fig. 1B).
Fig. 1.
Age-intensity profiles of (a) hookworm and (b) S. mansoni in rural (solid lines) and urban (dashed lines) areas of Americaninhas, southeastern Brazil. Although different levels, a similar age profile was observed in rural and urban areas, with hookworm intensity steadily rising with age and S. mansoni intensity convex in form.
According to WHO classifications (WHO, 2002), 7.9%, 8.3% and 18.2% of individuals harboured heavy infections with hookworm, S. mansoni, and A. lumbricoides, respectively. The prevalence of heavy hookworm infection was significantly higher in rural areas (12.0% vs. 4.0%, p<0.0001). No significant differences between areas were observed for heavy infection with S. mansoni or A. lumbricoides infection.
Within household clustering of infection
The study population was distributed between 242 households; 87 households (mean persons per household was 3.6, range 1-9) in the urban area and 155 households (mean persons per household was 4.5, range 1-11) in rural areas. Significant levels of household clustering of heavy infection, as indicated by the proportion of variance of heavy infections explained by the household random effect, were identified for heavy hookworm infection in each area but only for heavy S. mansoni infection in rural areas (Table 1). The highest degree of household clustering was observed for S. mansoni in rural areas (0.56). The proportion of variance of heavy infections explained by household clustering for hookworm was similar in both urban and rural areas (0.32 vs. 0.33); whereas, less clustering was observed for heavy A. lumbricoides urban areas than in rural areas (0.21 vs. 0.39).
Table 1.
Household and spatial clustering of helminth infection in rural and urban households in Americaninhas, Brazil.
| Species | Variance explained by household clustering† |
Spatial half-distances (m)* |
||
|---|---|---|---|---|
| Urban area | Rural areas | Urban areas | Rural areas | |
| Hookworm | 0.32 (0.10-0.66), p=0.005 | 0.33 (0.18-0.53), p<0.001 | 12 (7-56) | 28 (7-84) |
| S. mansoni | 0.08 (0.01-0.42), p=0.132 | 0.56 (0.38-0.73), p<0.001 | ‡ | 700 (247-1,875) |
| A. lumbricoides | 0.21 (0.09-0.40), p<0.001 | 0.39 (0.26-0.55), p<0.001 | - | - |
Proportion of variance explained by household clustering based on the random effect logistic regression, adjusted for age and sex. 95% confidence intervals in parentheses.
95% confidence intervals in parentheses. For S. mansoni in urban areas, results depend on choice of prior distribution, suggesting a lack of spatial clustering.
Analysis was not done for A. lumbricoides because its egg counts were truncated at 500 per slid
Spatial heterogeneity of infection
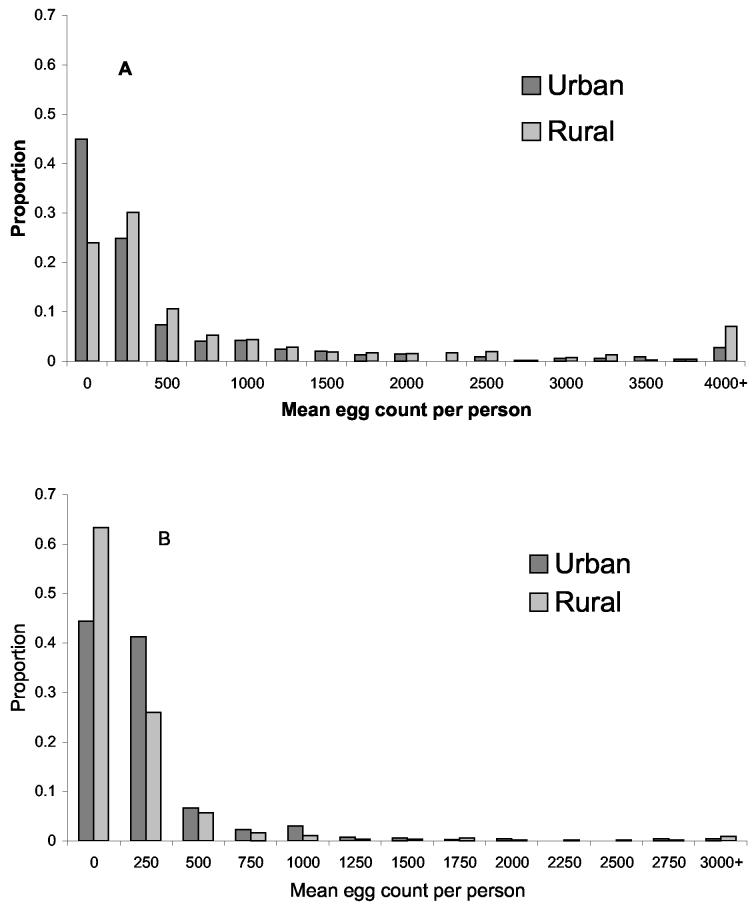
In both rural and urban areas, egg counts of both hookworm and S. mansoni were highly aggregated (Fig. 2). The estimate of k for hookworm egg counts, as derived from the spatial model, was 0.147 in urban areas and 0.284 in rural areas. The corresponding estimates for S. mansoni egg counts were 0.223 and 0.216.
Fig. 2.
Frequency distribution of egg counts (eggs/gram faeces) per person in urban and rural areas of Americaninhas, southeastern Brazil. (A) hookworm and (B) S. mansoni. Eggs counts in both areas were highly aggregated.
Figure 3 shows the spatial variation in infection intensity for hookworm and S. mansoni, estimated from the spatial model, taking into account age and sex effects. High infection intensities of both hookworm and, to a lesser extent, S. mansoni are evident in the northeast corner of the urban area. In rural areas, high infection intensities of S. mansoni occur in the northeast corner. Consistent with the logistic regression analysis of household clustering, S. mansoni in the urban area did not show clear evidence of spatial clustering, with the estimated spatial variance (1/ϕ) being small. Moreover, these results were highly dependent on the choice of prior, which was not the case for the other three analyses (not shown). By contrast, S. mansoni in the rural area, and hookworm in both areas, did show clear evidence of spatial clustering. The spatial correlation of S. mansoni was estimated to reduce by half over a distance of 700m in the rural area (Table 1), and a wide variation in SPR (inter-quartile range 0.4-5, Fig. 3D). Rural hookworm had a much smaller half-distance (28m) and a narrower inter-quartile range in SPR (0.9-3.1). Urban hookworm showed an even smaller half-distance (12m), and a still narrower inter-quartile range of SPR (1-1.3).
Fig. 3.
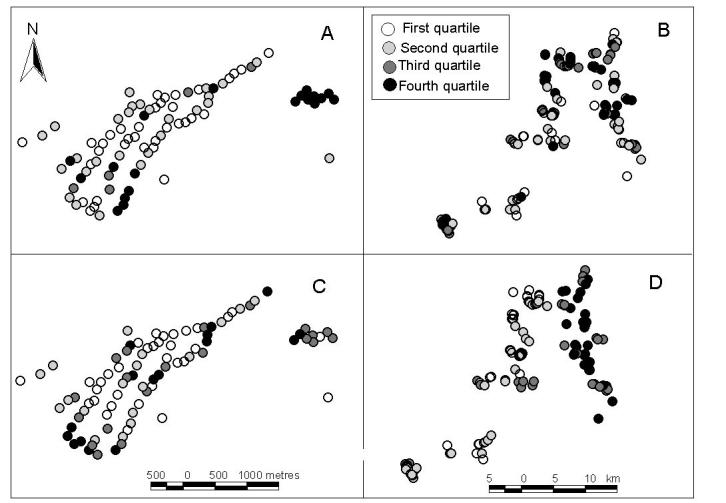
Maps of small-scale spatial heterogeneity of the intensity of infection in rural and urban areas of Americaninhas, southeastern Brazil. Each dot represents one household, with the shading showing the quartile of the standardized parasite density ratio as derived by the Bayesian spatial model. North is the top of the page. (A) Hookworm in urban areas, where the quartiles are 1, 1.1 and 1.3; (B) Hookworm in rural areas where the quartiles are 0.9, 1.6 and 3.1; (C) S. mansoni in urban areas where the quartiles are 1, 1.1 and 1.2; and (D) S. mansoni in rural areas where the quartiles are 0.4, 1.2 and 5. For each species, marked spatial heterogeneity was evident in both areas. High infection intensities of both hookworm and, to a lesser extent, S. mansoni are evident in the northeast corner of the urban area. In rural areas, high infection intensities of S. mansoni occur in the northeast corner.
4. Discussion
We have investigated both household clustering and spatial heterogeneity (and clustering) of hookworm and S. mansoni in an endemic region of Brazil. This was made possible using accurate global positioning systems and statistical techniques applicable to highly aggregated epidemiological data such as parasite counts. We highlight important species-specific differences in the extent of both household and spatial clustering between rural and urban areas. Previous studies, some extending back to the 1930s, have established that presence of household clustering for A. lumbricoides and T. trichiura and to a lesser extent hookworm (Otto et al., 1931; Forrester et al., 1988; Chan et al., 1994; Behnke et al., 2000; Bethony et al., 2001; Sharpiro et al., 2004). None of these studies, however, investigated how clustering of different parasite species varies between different environments. We found evidence of household clustering of heavy S. mansoni in rural areas but not in urban areas, whereas the extent of clustering of heavy hookworm infection was similar in both areas but greater than clustering of S. mansoni in urban areas.
We also found evidence of spatial clustering of S. mansoni intensity in rural areas but no evidence of spatial clustering in urban areas; similar degrees of spatial clustering of hookworm intensity were evident in both types of area. The evidence of spatial clustering of both hookworm and S. mansoni infection in rural areas contrasts with findings from a recent study of schoolchildren in rural Côte d'Ivoire which found a random spatial distribution of both hookworm and S. mansoni (Utzinger et al., 2003). A potential shortcoming of this study, acknowledged by the authors, was the difficulty of linking schoolchildren to an unambiguous household, limiting generalization of findings. Our collection of samples on a community-wide basis by household overcomes this problem, and our findings are consistent with results reported from household studies of urinary schistosomiasis (Clennon et al., 2004), filariasis (Alexander et al., 2003) and malaria (Snow et al., 1993; Brooker et al., 2004). Our study had the particular advantage of taking into account the typically highly skewed distribution of parasite counts and for controlling for age and sex differences in infection intensity.
The observed parasite-specific differences in household and spatial clustering by area are likely to reflect either variation in exposure and/or host factors (immunological, genetic or physiological) (Warren, 1973; Anderson, 1987; Bundy and Medley, 1992). Insight can be obtained through an understanding of the life cycle and transmission of each parasite species. Hookworm transmission occurs through exposure to soil contaminated with hookworm larvae and is influenced by several factors, including micro-climatic suitability, sanitation and hygiene, and environmental contamination with human excreta. Previous studies have suggested that exposure to infective hookworm larvae commonly occurs outside the household, for example in areas where agricultural practices are carried out or in defined defecation sites away from the household (Schad et al., 1983). However, the similar degrees of household and spatial clustering in both rural and urban areas along with the relatively small estimated half-distances (12m in urban areas and 28 m in rural areas) suggests that exposure to hookworm in our study area is concentrated in the peri-domiciliary environment in both rural and urban areas. The transmission of A. lumbricoides predominantly occurs among young children within households (Bundy and Medley, 1992), consistent with the significant household clustering observed.
Schistosomiasis, in contrast, is a water-borne infection and transmission is based on infective water bodies and human water contact. Recent study of S. haematobium in coastal Kenya suggest that proximity of a household to infected water bodies are significant factors in explaining small-scale spatial variation in infection (Clennon et al., 2004). Elsewhere in Kenya, a recent analysis suggested clustering of S. mansoni egg counts in relation to a local river (Booth et al., 2004). In our study, we found quite a large spatial correlation (700m) in rural areas, with the northeastern part of the study area having the highest infection intensities. Studies are underway to examine possible explanations for this spatial variation.
Interestingly, we found no evidence of either household or spatial clustering for S. mansoni in urban areas but high levels of both types of clustering in rural areas. Previous studies have highlighted the importance of water contact patterns which are typically shared among household members (Cairncross et al., 1996; Watts et al., 1998; Bethony et al., 2004), resulting in a household effect determining schistosome infection intensities (Bethony et al., 2001, 2004). The reason for such high degree of clustering in rural areas may be associated with proximity of specific households to water bodies and the reproduction of hygiene behaviours among members of the same household, which can result in household members sharing both the same infective sites and water contact activities (Watts et al., 1998). The relatively dispersed distribution of rural households compared to urban households mean that household members are more likely to share water contact patterns in the same water body. In urban areas, however, individuals from several different households share a single water source equally close to most households.
Considered in isolation, the observed species-specific differences in household and spatial clustering between rural and urban areas suggest that variation in exposure is sufficient to explain observed patterns; it is unlikely that genetic factors differed in a systematic fashion between rural and urban areas. Such a conclusion however needs to be viewed in relation to the increasingly available evidence of genetic susceptibility to infection (reviewed in Quinnell 2003). Quantitative genetic analyses of helminth infection within human populations suggest that that both household and genetic factors both play important roles in determining variation in infection patterns. The investigation of hookworm by Bethony et al. (unpublished) in northeastern Brazil, for example, estimated 26% genetic heritability of the variance of faecal egg counts when modeled with genetic and household effects. Similar analyses of hookworm (Williams-Blangero et al., 1997), A. lumbricoides (Williams-Blangero et al., 1999) and S. mansoni (Bethony et al., 2002) reveal a similar genetic component in heterogeneity in infection patterns.
What is less clear however is the relative importance of genetics and exposure. Analysis of A. lumbricoides worm burdens using the natural family unit rather than households after successive rounds of treatment has found no consistent pattern in clustering, suggesting that household-specific factors, such as the peri-domiciliary environment and family-specific practices and behaviours overwhelm any genetic basis for clustering (Chan et al., 1992; Bundy and Michael, 2001). A more complicated picture is provided however in a recent study by Wahyuni et al. (2004) in Indonesia of clustering of filarial infection. This study showed that behavioural and environmental factors tended to override any genetic predisposition in adults but that in children significant genetic effects were detected, due in part to the greater homogeneity of exposure among children. Adding to this complexity, our data suggest that the predominance of exposure factors over any genetic component will differ according to the environment, either rural or urban.
Helminth control is typically implemented at the school or community level. As such, it is very unlikely that chemotherapy will be targeted at specific households or groups of households; focalised snail control may however be targeted at specific water bodies (Clennon et al., 2004). We suggest therefore that from a perspective of helminth control, further study of household and spatial clustering and heterogeneity of infection has limited operational relevance. Rather we encourage investigation of small-scale heterogeneities, especially in relation to immunological studies (Booth and Dunne, 2004), to help shed additional light on the genetics, immunology and ecology of infection. The evidence of genetic factors play a role in determining the variation in helminth egg counts has been consistently made in murine and human models (Quinnell, 2003), most likely acting through genetic control of the immune response to the infection. This control may work directly, making individuals either more immunologically susceptible or resistant to different levels of infection. To help to investigate these issues further there is a need to develop robust quantitative frameworks that incorporate both spatial and genetic covariates and to conduct detailed immunoepidemiological studies which measure both genetic and spatial factors. From this scientific perspective, the integrated use of spatial analysis with statistical methods which partition genetic from household and environmental factors may help to better understand the determinants of infection.
Acknowledgements
We are very grateful to inhabitants of Americaninhas who kindly participated in the study. We are also most appreciative of those who were responsible for carrying out the fieldwork, which made this analysis possible, and deserve many thanks. Fieldwork was financially supported by the Human Hookworm Vaccine Initiative (HHVI) of the Sabin Vaccine Institute and the Bill and Melinda Gates Foundation. SB is supported by a Wellcome Trust Advanced Training Fellowship (073656), JB is supported by an International Research Scientist Development Award (IRSDA) (K01 TW00009) from the John E. Fogarty International Center, NIH, and FF was supported by a Chadwick Trust travel award.
References
- Alexander N, Moyeed R, Stander J. Spatial modelling of individual-level parasite counts using the negative binomial distribution. Biostatistics. 2000;1:453–463. doi: 10.1093/biostatistics/1.4.453. [DOI] [PubMed] [Google Scholar]
- Alexander ND, Moyeed RA, Hyun PJ, Dimber ZB, Bockarie MJ, Stander J, Grenfell BT, Kazura JW, Alpers MP. Spatial variation of Anopheles-transmitted Wuchereria bancrofti and Plasmodium falciparum infection densities in Papua New Guinea. Filaria J. 2003;2:14. doi: 10.1186/1475-2883-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM. Determinants of infection in human schistosomiasis. In: Mahmound AF, editor. Balillière's Clinical Tropical Medicine and Communicable Disease. 2nd edn. Balillière Tindall; London: 1987. pp. 279–300. [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: dynamics and control. Oxford University Press; Oxford: 1991. [Google Scholar]
- Anderson RM. Epidemiology. In: Cox F.E.G. Cox., editor. Modern Parasitology. Blackwell; Oxford: 1991. pp. 75–116. [Google Scholar]
- Behnke JM, De Clercq D, Sacko M, Gilbert FS, Ouattara DB, Vercruysse J. The epidemiology of human hookworm infections in the southern region of Mali. Trop. Med. Int. Hlth. 2000;5:343–354. doi: 10.1046/j.1365-3156.2000.00553.x. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Kloos H, Blangero J, Alves-Fraga L, Germaine B, Michalek A, Williams-Blangero S, LoVerde P, Correa-Olivera R, Gazzinelli A. Exposure to Schistosoma mansoni infection in a rural area in Brazil: Part 1: household risk factors. Trop. Med. Int. Hlth. 2001;6:136–145. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Brooker S, Gazzinelli A, LoVerde PT, Correa-Oliveira R, Kloos H. Exposure to Schistosoma mansoni infection in a rural area in Brazil III: the household aggregation of human water contact behavior. Trop. Med. Int. Hlth. 2004;9:381–389. doi: 10.1111/j.1365-3156.2004.01203.x. [DOI] [PubMed] [Google Scholar]
- Bethony J, Williams JT, Blangero J, Kloos H, Gazzinelli A, Soares-Filho B, Coelho L, Alves-Fraga L, Williams-Blangero S, Loverde PT, Correa-Oliveira R. Additive host genetic factors influence fecal egg excretion rates during Schistosoma mansoni infection in a rural area in Brazil. Am. J. Trop. Med. & Hyg. 2002;67:336–343. doi: 10.4269/ajtmh.2002.67.336. [DOI] [PubMed] [Google Scholar]
- Bernardinelli L, Montomoli C. Empirical Bayes versus fully Bayesian analysis of geographic variation in disease risk. Stat. Med. 1992;11:983–1007. doi: 10.1002/sim.4780110802. [DOI] [PubMed] [Google Scholar]
- Bliss CA, Fisher RA. Fitting the negative binomial to biological data and a note on the efficient fitting of the negative binomial. Biometrics. 1953;9:176–200. [Google Scholar]
- Booth M, Vennervald BJ, Kenty L, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Mwatha JK, Otedo A, Ouma JH, Muchiri E, Dunne DW. Micro-geographical variation in exposure to Schistosoma mansoni and malaria, and exacerbation of splenomegaly in Kenyan school-aged children. BMC Infect. Dis. 2004;4:13. doi: 10.1186/1471-2334-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M, Dunne DW. Spatial awareness in parasite immuno-epidemiology. Parasit. Immunol. 2004;26:499–507. doi: 10.1111/j.0141-9838.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Brooker S, Michael E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv. Parasitol. 2000;47:245–288. doi: 10.1016/s0065-308x(00)47011-9. [DOI] [PubMed] [Google Scholar]
- Brooker S, Beasley NMR, Ndinaromtan M, Madjiouroum EM, Baboguel M, Djenguinabe E, Hay SI, Bundy DAP. Use of remote sensing and a geographical information system in a national helminth control programme in Chad. Bull. WHO. 2002;80:783–789. [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Kabatereine NB, Tukahebwa EM, Kazibwe F. Spatial analysis of the distribution of intestinal nematode infections in Uganda. Epi & Infect. 2004;132:1065–1071. doi: 10.1017/s0950268804003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Clarke S, Njagi K, Polack S, Benbolt M, Estambale B, Muchiri E, Magnussen P, Cox J. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Trop. Med. Int. Hlth. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Brooker S, Clements ACA, Bundy DAP. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv. Parasitol. 2006;62:221–261. doi: 10.1016/S0065-308X(05)62007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy DAP, Cooper ES, Thompson DE, Didier JM, Simmons I. Epidemiology and population dynamics of Ascaris lumbricoides and Trichuris trichiura infection in the same community. Trans. Roy. Soc. Trop. Med. Hyg. 1987;81:987–993. doi: 10.1016/0035-9203(87)90372-5. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Kan SP, Rose R. Age-related prevalence, intensity and frequency distribution of gastrointestinal helminth infection in urban slum children from Kuala Lumpur, Malaysia. Trans. Roy. Soc. Trop. Med. Hyg. 1988;82:298–294. doi: 10.1016/0035-9203(88)90450-6. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Medley GF. Immunoepidemiology of human geohelminthiasis: ecological and immunological determinants of worm burden. Parasitology. 1992;104:S105–S119. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Michael E. Parasite Epidemiology. In: Gillespie SHR, Pearson D, editors. Principles and Practice of Clinical Parasitology. John Wiley & Sons; London: 2001. pp. 21–52. [Google Scholar]
- Hess G, Randolph SE, Arneberg P. Spatial aspects of disease dynamics. In: Hudson PJ, Rizzoli AP, Grenfell BT, Heesterbeek JAP, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford University Press; Oxford: 2001. pp. 102–118. [Google Scholar]
- Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop. Med. Int. Hlth. 1996;1:27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- Chan L, Bundy DAP, Kan SP. Aggregation and predisposition to Ascaris lumbricoides and Trichuris trichiura at the familial level. Trans. Roy. Soc. Trop. Med. & Hyg. 1994;88:46–48. doi: 10.1016/0035-9203(94)90492-8. [DOI] [PubMed] [Google Scholar]
- Clennon JA, King CH, Muchiri EM, Kariuki HC, Ouma JH, Mungai P, Kitron U. Spatial patterns of urinary schistosomiasis in a highly endemic area of coastal Kenya. Am. J. Trop. Med. & Hyg. 2004;70:443–448. [PubMed] [Google Scholar]
- Cornell SJ, Isham VS, Grenfell BT. Stochastic and spatial dynamics of nematode parasistes in farmed ruminants. Proc. Roy. Soc. London B. 2003;271:1243–1250. doi: 10.1098/rspb.2004.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernould JC, Kaman A, Labbo R, Couret D, Chippaux JP. Recent urban growth and urinary schistosomiasis in Niamey, Niger. Trop. Med. Int. Hlth. 2000;5:431–37. doi: 10.1046/j.1365-3156.2000.00577.x. [DOI] [PubMed] [Google Scholar]
- Forrester JE, Scott ME, Bundy DAP, Golden MHN. Clustering of Ascaris lumbricoides and Trichuris trichiura infections within households. Trans. Roy. Soc. Trop. Med. & Hyg. 1988;82:282–288. doi: 10.1016/0035-9203(88)90448-8. [DOI] [PubMed] [Google Scholar]
- Grtseels B. Morbidity due to infection with Schistosoma manosni. Trop. Geogr. Med. 1992;44:189–200. [PubMed] [Google Scholar]
- Handzel T, Karanja DM, Addiss DG, Hightower AW, Rosen DH, Colley DG, Andove J, Slutsker L, Secor WE. Geographic distribution of schistosomiasis and soil-transmitted helminths in Western Kenya: implications for anthelminthic mass treatment. Am. J. Trop. Med. & Hyg. 2003;69:318–323. [PubMed] [Google Scholar]
- Haswell-Elkins M, Elkins D, Anderson RM. The influence of individual, social group and household factors on the distribution of Ascaris lumbricoides within a community and implications for control strategies. Parasitology. 1989;98:125–34. doi: 10.1017/s003118200005976x. [DOI] [PubMed] [Google Scholar]
- Hay SI, Randolph SE, Rogers DJ, editors. Academic Press; London: 2000. Remote Sensing and Geographical Information Systems in Epidemiology. [Google Scholar]
- Hess G, Randolph SE, Arneberg P. Spatial aspects of disease dynamics. In: Hudson PJ, Rizzoli AP, B.T. Grenfell, Heesterbeek JAP, Dobson AP, editors. The Ecology of Wildlife Diseases. Oxford University Press; Oxford: 2001. pp. 102–118. [Google Scholar]
- Kardorff R, Gabone RM, Mugashe C, Obiga D, Ramarokoto CE, Mahlert C, Spannbrucker N, Lang A, Gunzler V, Gryseels B, Ehrich JH, Doehring E. Schistosoma mansoni-related morbidity on Ukerewe Island, Tanzania: clinical, ultrasonographical and biochemical parameters. Trop Med Int Health. 1997;2:230–239. doi: 10.1046/j.1365-3156.1997.d01-269.x. [DOI] [PubMed] [Google Scholar]
- Kardorff R, Stelma FF, Vocke AK, Yazdanpanah Y, Thomas AK, Mbaye A, Talla I, Niang M, Ehrich JH, Doehring E, Gryseels B. Ultrasonography in a Senegalese community recently exposed to Schistosoma mansoni infection. Am J Trop Med Hyg. 1996;54:586–590. doi: 10.4269/ajtmh.1996.54.586. [DOI] [PubMed] [Google Scholar]
- King CH, Blanton RE, Muchiri EM, Ouma JH, Kariuki HC, Mungai P, Magak P, Kadzo H, Ireri E, Koech DK. Low heritable component of risk for infection intensity and infection-associated disease in urinary schistosomiasis among Wadigo village populations in Coast Province, Kenya. Am. J. Trop. Med. Hyg. 2004;70:57–62. [PubMed] [Google Scholar]
- Kloos H, Gazzinelli A, Van Zuyle P. Microgeographical patterns of schistosomiasis and water contact behaviour; examples from Africa and Brazil. Memorias do Instituto Oswaldo Cruz. 1998;93(Suppl. 1.):37–50. doi: 10.1590/s0074-02761998000700006. [DOI] [PubMed] [Google Scholar]
- Otto GF, Cort WW, Keller AE. Environmental studies of families in Tennessee infested with Ascaris, Trichuris, and hookworm. Am. J. Hyg. 1933;1:156–193. [Google Scholar]
- Quinnell RJ. Genetics of susceptibility to human helminth infection. Int. J. Parasitol. 2003;33:1219–1231. doi: 10.1016/s0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Raso G, Matthys B, N'Goran EK, Tanner M, Vounatsou P, Utzinger J. Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Cote d'Ivoire. Parasitology. 2005;131:97–108. doi: 10.1017/s0031182005007432. [DOI] [PubMed] [Google Scholar]
- Raso G, Vounatsou P, Gosoniu L, Tanner M, N'goran EK, Utzinger J. Risk factors and spatial patterns of hookworm infection among schoolchildren in a rural area of western Cote d'Ivoire. Int. J. Parasitol. 2006 doi: 10.1016/j.ijpara.2005.09.003. in press. [DOI] [PubMed] [Google Scholar]
- Saathoff E, Olsen A, Sharp B, Kvalsvig JD, Appleton CC, Kleinschmidt I. Ecologic covariates of hookworm infection and reinfection in rural KwaZulu-Natal/South Africa: a geographic information system-based study. Am. J. Trop. Med. & Hyg. 2005;72:384–391. [PubMed] [Google Scholar]
- Schad GA, Nawalinski TA, Kochar V, Cross JH. Human ecology and the distribution and abundance of hookworm populations. In: Croll NA, Cross JH, editors. Human ecology and infectious diseases. Academic Press; New York: 1983. pp. 187–223. [Google Scholar]
- Shapiro S, Tukahebwa EM, Katsen J, Magnussen P, Olsen A, Clarke S, Kabatereine NB, Ndyomugyenyi R, Brooker S. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans. Roy. Soc. Trop. Med. & Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shaw DJ, Dobson AP. Patterns of macro-parasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology. 1995;111:S111–S133. doi: 10.1017/s0031182000075855. [DOI] [PubMed] [Google Scholar]
- Snow RW, Schellenberg JR, Peshu N, Forster D, Newton CR, Winstanley PA, Mwangi I, Waruiru C, Warn PA, Newbold C. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Trans. Roy. Soc. Trop. Med. & Hyg. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Albonico M, Chwaya HM, Savioli L, Tielsch JM, Schulze KJ,, Yip R. Hemoquant determination of hookworm-related blood loss and its role in iron deficiency in African children. Am. J. Trop. Med. & Hyg. 1996;55:399–404. doi: 10.4269/ajtmh.1996.55.399. [DOI] [PubMed] [Google Scholar]
- Terhell AJ, Houwing-Duistermaat JJ, Ruiterman Y, Haarbrink M, Abadi K, Yazdanbakhsh M. Clustering of Brugia malayi infection in a community in South-Sulawesi, Indonesia. Parasitology. 2000;120:23–29. doi: 10.1017/s0031182099005247. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Muller I, Vounatsou P, Singer BH, N'Goran EK, Tanner M. Random spatial distribution of Schistosoma mansoni and hookworm infections among school children within a single village. J. Parasitol. 2003;89:686–692. doi: 10.1645/GE-75R. [DOI] [PubMed] [Google Scholar]
- Wahyuni S, Houwing-Duistermaat JJ, Syafruddin, Supali T, Yazdanbakhsh M, Sartono E. Clustering of filarial infection in an age-graded study: genetics, household and environmental influences. Parasitology. 2004;128:315–321. doi: 10.1017/s0031182003004487. [DOI] [PubMed] [Google Scholar]
- Warren KS. Regulation of the prevalence and intensity of schistosomiasis in man: immunology or ecology? J. Infect. Dis. 1973;127:595–609. doi: 10.1093/infdis/127.5.595. [DOI] [PubMed] [Google Scholar]
- Watts S, Khallaayoune K, Bensefia R, Laamrani H, Gryseels B. The study of human behaviour and schistosomiasis transmission in an irigation area in Morocco. Soc. Sci. & Med. 1998;46:755–765. doi: 10.1016/s0277-9536(97)00171-8. [DOI] [PubMed] [Google Scholar]
- Werneck GL, Costa CHN, Walker AM, David JR, Wand M, Maguire JH. The urban spread of visceral leishmaniasis: clues from spatial analysis. Epidemiology. 2002;13:364–367. doi: 10.1097/00001648-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Williams-Blangero S, Blangero J, Bradley M. Quantitative genetic analysis of susceptibility to hookworm infection in a population from rural Zimbabwe. Hum. Biol. 1997;69:201–208. [PubMed] [Google Scholar]
- Williams-Blangero S, Subedi J, Upadhayay RP, Manral DB, Rai DR, Jha B, Robinson ES, Blangero J. Genetic analysis of susceptibility to infection with Ascaris lumbricoides. Am. J. Trop. Med. & Hyg. 1999;60:921–926. doi: 10.4269/ajtmh.1999.60.921. [DOI] [PubMed] [Google Scholar]
- Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl. Acad. Sci. 1997;7:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. Geneva: 2002. (WHO Technical Report Series 912). [PubMed] [Google Scholar]