Abstract
Major depression and suicide are associated with altered concentrations of specific noradrenergic proteins in the human locus coeruleus (LC). Based on experimental studies that can reproduce these LC abnormalities in laboratory animals, we hypothesized that noradrenergic pathobiology in depression is a result of over-activity of the LC. LC activity is under the control of both excitatory and inhibitory inputs. A major inhibitory input to the LC is GABAergic, arising from the nucleus prepositus hypoglossi. Numerous studies demonstrating low levels of GABA in the CSF and plasma of subjects with major depressive disorder (MDD) raise the possibility that LC over-activity in depression may be secondary to reduced GABAergic input to the LC. Here, GABAergic input to the LC in depression was evaluated by studying the binding of [3H]flunitrazepam to GABAA receptors at three anatomically-defined levels of the human postmortem LC. LC tissues were collected from subjects with MDD, subjects with depressive disorders including MDD that died as a result of suicide, and psychiatrically normal control subjects. A modest rostral-caudal gradient of GABAA receptor binding density was observed amongst all subjects. No significant differences in the amount of binding to GABAA receptors were observed between control subjects (n=21) and MDD subjects (n=9) or depressed suicide victims (n=17). These results demonstrate that GABAA receptor binding in the LC measured with [3H]flunitrazepam is not altered in subjects with depressive illnesses.
Keywords: postmortem brain; major depressive disorder; suicide, norepinephrine; GABAA receptor; flunitrazepam; locus coeruleus
1. Introduction
The locus coeruleus (LC) is the largest noradrenergic nucleus in the brain projecting extensively to a number of cortical and subcortical regions [57]. Norepinephrine (NE) released from LC neurons plays a crucial role in the regulation of emotional activation, vigilance, sleep-wake cycle, and mood. Disruptions of noradrenergic transmission have long been believed to underlie certain psychiatric disorders, particularly depression. Converging evidence from studies of the LC from postmortem depressed subjects and from catecholamine-depletion studies in living depressed subjects has provided compelling evidence that depression is associated with a deficit in noradrenergic transmission [12,36,39]
Previous observations from postmortem studies have revealed that major depressive disorder (MDD) and suicide are associated with altered concentrations of several noradrenergic proteins in the LC. For example, elevated levels of tyrosine hydroxylase [42,69], increased agonist binding to α2 –adrenergic receptors [40,43] and reduced levels of norepinephrine transporters [20] have been reported in the LC from MDD subjects and/or from suicide victims. Studies utilizing laboratory animals provide interesting insights into the possible basis for these postmortem findings. For example, depletion of NE or repeated stress in rats increases tyrosine hydroxylase expression, increases binding to α2 –adrenergic receptors, and decreases binding to the norepinephrine transporter [10,23,30,61,63,64,66]. Together, these findings are highly suggestive of dysfunctional noradrenergic neurotransmission in depression, possibly through sustained stress-induced LC activation and the ultimate depletion of NE.
The possibility that there is an elevated LC activity in depression brings attention to the possibility of depression-associated deficits in inputs to the LC from neurons that regulate LC activity. Recently, abnormalities in excitatory inputs (corticotropin releasing factor, glutamate) to the LC in depression and/or suicide have been reported [2,7,17,32]. It is reasonable to speculate that elevated LC activity in depression may be simultaneously associated with diminished inhibitory input to the LC. GABAergic fibers, originating in the nucleus prepositus hypoglossi, project to the LC and inhibit LC firing [1]. Receptors responding to GABA in the LC include GABAA receptors [45,67], which are ligand-gated chloride channels [for review, see 3,35]. Interestingly, there is a wealth of information suggesting that there is a deficit of inhibitory neurotransmitter, GABA, in depression [8,26,31,54,55,56,62].
Given the putative role of GABA in depressive disorders and GABA-modulation of the noradrenergic LC, the present study investigated the distribution of the GABAA receptors along the rostral-caudal axis of the human LC and potential abnormalities in the concentrations of GABAA binding sites in the LC that might occur in MDD and suicide. MDD and suicide subjects were matched with control subjects for age, sex, cigarette smoking history and postmortem interval as closely as possible. Brain tissue was collected from carefully screened subjects (post-mortem) who were diagnosed retrospectively with depressive disorders at the time of death, and from control subjects who lacked major (Axis I) psychiatric disorders, except as indicated for nicotine dependence.
2. Materials and Methods
Tissue collection and psychiatric autopsy
Human brain tissue was obtained at autopsy at the Cuyahoga County Coroner's Office, Ohio, in accordance with an approved Institutional Review Board protocol. The description of the tissue collection process has been described previously [20]. Briefly, causes of death were determined by the coroner and information on lifetime and latest psychiatric status, psychotropic medication, and possible illicit drug use of all subjects was obtained in structured clinical interviews by a trained interviewer with the next of kin. The interviewer used the Schedule for Affective Disorders and Schizophrenia-Lifetime version (SADS-L) [14] supplemented by questions from the Diagnostic Interview Schedule (DIS-III-R) [28]. Responses from the depressed subjects evaluated with the SADS-L were also recorded with the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) [15] and these subjects met criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; American Psychiatric Association, 1994) for major depressive disorder, dysthymia, adjustment disorder with depressed mood or alcohol induced mood disorder. A total of 17 subjects who had a depressive disorder at the time of death were studied (Table 1), 9 of which had a diagnosis of MDD. Evaluations of drug and alcohol abuse and dependency were assessed using the DIS-III-R. Axis I diagnoses of MDD were made by experienced psychiatrists and a clinical psychologist, based on the data gathered from the structured interviews and, when available, hospital and physician's records. Control subjects were sudden death victims, free of any psychiatric diagnoses at the time of death (Table 2), except nicotine dependence where noted. A toxicological screen of blood, bile, and urine from all subjects was performed by the Cuyahoga County Coroner's Office as described previously. No antidepressant drugs were detected in any of the subjects selected for the study. Control subjects were matched as closely as possible to MDD and suicide subjects based on age, brain pH value, gender, postmortem interval and cigarette smoking. Past histories of cigarette smoking were not considered in the comparison between smokers and non-smokers.
Table 1.
Demographic data of major depressive and suicide subjects, including disorders evident at the time of death.
| Subject | Brain pH |
PMIa (h) |
Toxicology | Depressive Disorder Diagnosis |
Other Diagnoses |
|---|---|---|---|---|---|
| TT | 6.52 | 24 | NDDb | MDDc (Sd) | None |
| DD | 6.48 | 17 | COe | MDD (S) dysthymic disorder |
None |
| KS8 | 6.68 | 44 | NDD | MDD (S) | None |
| JJ | 6.24 | 23 | CO, phenytoin, phenobarbital |
MDD (S) | None |
| GG | 6.91 | 18 | ethanol | MDD (S) | nicotine dependence |
| KS 12 | 6.24 | 19 | chlorpheniramine | MDD (Nf) | nicotine dependence |
| KS 17 | 6.84 | 27 | NDD | MDD (Ng) | alcohol abuse, nicotine dependence |
| KS 24 | 6.85 | 26 | ethanol, phenyl- propanolamine |
MDD (S) | nicotine dependence |
| KS 28 | 6.78 | 33 | NDD | MDD (S) | nicotine dependence |
| KS 2 | 6.52 | 24 | NDD | adjustment disorder with depressed mood (S) |
None |
| KS 5 | 6.31 | 20 | ethanol, propoxyphene acetaminophen |
dysthymic disorder (S) | None |
| KS 16 | 6.60 | 5 | ethanol, CO | adjustment disorder with depressed mood (S) |
None |
| KS 1 | 6.74 | 22 | NDD | dysthymia (S) | alcohol abuse |
| KS 25 | 6.15 | 27 | NDD | adjustment disorder with depressed mood (S) |
None |
| KS 29 | 6.87 | 16 | propoxyphene, lidocaine | adjustment disorder w/ depressed mood (S) |
None |
| KS 3 | 6.89 | 12 | ethanol | dysthymic disorder (S) | alcohol dependence, nicotine dependence |
| KS 4 | 7.07 | 22 | ethanol | alcohol induced mood disorder (depressed) (S) |
alcohol dependence, nicotine dependence |
| KS 13 | 6.47 | 18 | ethanol | adjustment disorder with depressed mood (S) |
alcohol dependence, pathological gambling, nicotine dependence |
| KS 9 | 6.945 | 6 | NDD | Schizoaffective (S) | nicotine dependence |
PMI, postmortem interval
NDD, no drugs detected
MDD, major depressive disorder
S, suicide
CO, carbon monoxide
N, natural death – hypertrophic cardiomyopathy
N, natural death – pulmonary thromboemboli
Table 2.
Demographic data of control subjects.
| Subject | Brain pH |
PMIa (h) |
Toxicology | Cause of death | Diagnosis |
|---|---|---|---|---|---|
| RR | 6.47 | 17 | NDDb | acute hemorrhagic pancreatitis |
no diagnosis |
| BB | 6.28 | 17 | NDD | heart | no diagnosis |
| KS6 | 6.42 | 22 | NDD | crushing impact | no diagnosis |
| KS15 | 5.99 | 17 | CO | asphyxiation due to CO | no diagnosis |
| UU | 6.71 | 16 | NDD | Heart | no diagnosis |
| EE | 7.09 | 29 | NDD | Heart | no diagnosis |
| KS11 | 6.58 | 26 | meperidine, promethazine, morphine |
acute gastric ulcer | no diagnosis |
| KS22 | 6.9 | 20 | ethanol, quinine | cardiomyopathy | no diagnosis |
| KS20 | 6.32 | 24 | NDD | homicide | no diagnosis |
| HH | 6.87 | 17 | brompheniramine | heart disease | no diagnosis |
| KS26 | 6.7 | 26 | NDD | heart disease | no diagnosis |
| FF | 6.88 | 17 | NDD | homicide | nicotine dependence |
| KS21 | 6.98 | 9 | NDD | heart disease | nicotine dependence |
| KS23 | 6.78 | 21 | ethanol | heart disease | nicotine dependence |
| KS27 | 6.62 | 21 | NDD | abdominal aortic aneurysm | nicotine dependence |
| SS | 6.46 | 11 | NDD | hypertensive cardiomyopathy |
nicotine dependence |
| II | 6.71 | 23 | NDD | aortic aneurism | nicotine dependence |
| CC | 6.85 | 26 | NDD | myocardial infarction | nicotine dependence |
| KS7 | 6.65 | 28 | NDD | atherosclerosis/ myocardial infarction |
nicotine dependence |
| KS14 | 6.71 | 10 | CO | asphyxiation due to CO | nicotine dependence |
| KS19 | 6.86 | 9 | NDD | heart disease | nicotine dependence |
PMI, postmortem interval
NDD, no drugs detected
CO, carbon monoxide
Dissection
Details of the dissection of tissue blocks of human dorsal pons containing the LC have been described previously [20]. Briefly, blocks containing the LC were bisected at the midline and only right side LC nuclei were studied. Left side nuclei were used for other experiments. We have previously found no differences in right and left side biochemical or cellular data (37,41,42). Tissue blocks were then sectioned sequentially using a cryostat microtome (− 16°C) in a transverse plane perpendicular to the floor of the fourth ventricle throughout the entire length of the LC beginning near its rostral end, collecting all tissue sections. The LC had its rostral border defined as a point where at least 25 ± 5 neuromelanin-containing cells were identified (at the frenulum). The caudal border was defined near the caudal end at a point where 25 ± 5 or less neuromelanin-containing cells were present. Equivalent anatomical levels of the LC were identified in Nissl-stained sections by computing 3 levels that represented 25% (rostral), 50% (middle), and 75% (caudal) of the total rostrocaudal length of the LC (as define above). After confirming anatomically equivalent levels in each subject, four adjacent sections (20 μm each) from each level (totally 12 sections from each subject) were chosen for these experiments. After dissection, sections were dried overnight at 4 °C and placed into the freezer (−80°C) until assayed.
Immunohistochemical staining
Nissl staining and tyrosine hydroxylase (TH) immunostaining of sections were used to create anatomical templates for measuring the densities of radioligand binding by quantitative autoradiography. For Nissl staining, the same sections used for the radioligand autoradiography were stained after film exposure with cresyl violet for the identification of LC cell bodies. For tyrosine hydroxylase immunostaining, sections were fixed in 4% paraformaldehyde in 0.05 M phosphate-buffered saline (PBS) for 1 h at 21°C. Sections were then pre-incubated in 5% normal horse serum in 0.05 M PBS (containing 0.1 % Triton X-100 and 0.005 % sodium azide) for 30 min followed by incubating for 24 h at 4°C in the same solution containing mouse anti-tryosine hydroxylase monoclonal antibody (1:500; Chemicon, Temecula, CA, USA). Sections were subsequently washed in 0.05 M PBS, and incubated for 4 h at 21°C in biotinylated horse anti-mouse IgG (1:200) in 0.05 M PBS (Vector, Burlingame, CA, USA). The sections were then processed using the Vectastain ABC immunoperoxidase kit (Vector, Burlingame, CA, USA) for 24 h at 4°C and the antibody distribution was detected using 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO, USA). Quantitative autoradiography. The autoradiographic method used for [3H]flunitrazepam binding was a modification of that reported by Benes et al. [5]. At the time of assay, tissue sections were first thawed at 4°C for 1 h and then incubated with 1 nM [3H]flunitrazepam (84.5 Ci/mmol; PerkinElmer Life Sciences, Inc. Boston, MA) in 0.17 M Tris-HCl, pH 7.4, at 21°C for 40 min in the presence (non-specific binding, in duplicate) or absence (total binding, in duplicate) of 1 μM diazepam (Sigma Chemical Co., St. Louis, MO). The slide-mounted sections were washed in two changes of ice-cold Tris-HCl for 1 min each, and then dipped rapidly in ice-cold distilled water. Slides were dried at 21°C overnight and then apposed, along with [3H]standards calibrated in μCi of 3H (American Radiolabeled Chemicals, Inc., St. Louis, MO), to 3H-hyperfilm (Amersham, Arlington Hts. IL) for one month. Densitometric measurements of autoradiograms were made using a computer-aided imaging system (MCID Elite, Imaging Research, St. Catherine, Ontario, Canada). LC autoradiograms were analyzed by simultaneously overlaying the image of the autoradiogram with the template created by Nissl staining of the same section and with a template created by the tyrosine hydroxylase immunostaining of an adjacent section. The cell-dense region of the LC was outlined and binding density was computed. The reported binding density is the average of radioactivity, determined from the standard, measured across hundreds of pixels located within a defined area of the film (representing the LC). The specific binding or [3H]flunitrazepam to GABAA receptors was determined by subtracting non-specific binding (computed in adjacent sections) from the total binding.
Statistical analysis
Mean values of the specific binding of [3H]flunitrazepam to GABAA receptors in the LC for groups of MDD, suicide, and control subjects were compared statistically using a one-way ANOVA followed by Bonferroni post tests. Comparisons between smokers and non-smokers were made using the Student's t-test. A p value < 0.05 was considered statistically significant. All data are presented as means ± S.E.M. Pearson correlation analysis was used to compute potential relationships between binding densities and age, postmortem interval, and brain pH.
3. Results
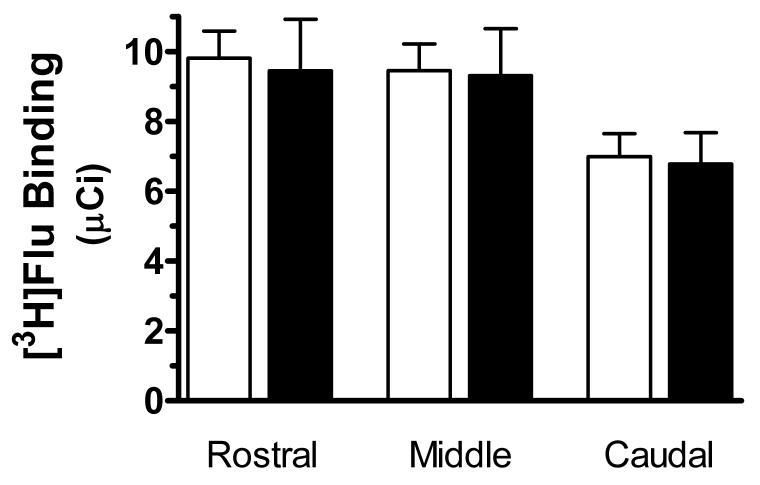
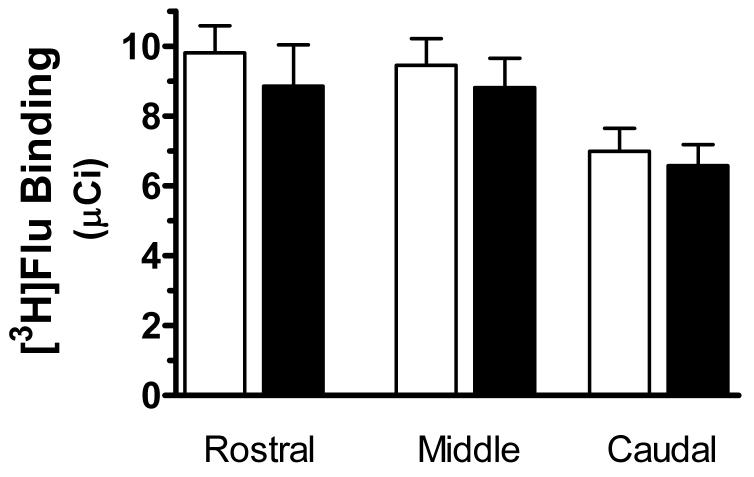
A representative autoradiogram of the binding of [3H]flunitrazepam to GABAA receptors in the middle LC from a control subject is shown in Figure 1. The amount of specific binding of [3H]flunitrazepam to GABAA receptors was measured in the immediate region of the compact neuromelanin-containing cell body region of the LC, shown by Nissl stains of same section. The specific binding of [3H]flunitrazepam to GABAA receptors in the entire region of the LC was low relative to the density of binding in the cerebellum, as observed in the upper left corner of Figure 1. Both the compact cellular region of the LC and the area of the dendritic expanse of the LC demonstrated relatively low [3H]flunitrazepam binding levels. There was an uneven distribution of [3H]flunitrazepam binding along the rostrocaudal axis of the LC (F=10.742,20, p<0.0001), with the density of binding in caudal portion of the LC being significantly lower than that in the rostral and medial portions of the LC (p<0.001) The rostrocaudal gradient of binding density did not correlate with the uneven distribution of noradrenergic cell number along the rostrocaudal axis of the LC (data not shown, see ref. 20). No significant differences in the amount of [3H]flunitrazepam binding to GABAA receptors were observed between MDD and control subjects at any anatomical level of the LC (Figure 2). Likewise, no differences in binding were observed comparing suicide victims to control subjects (Figure 3).
Figure 1.
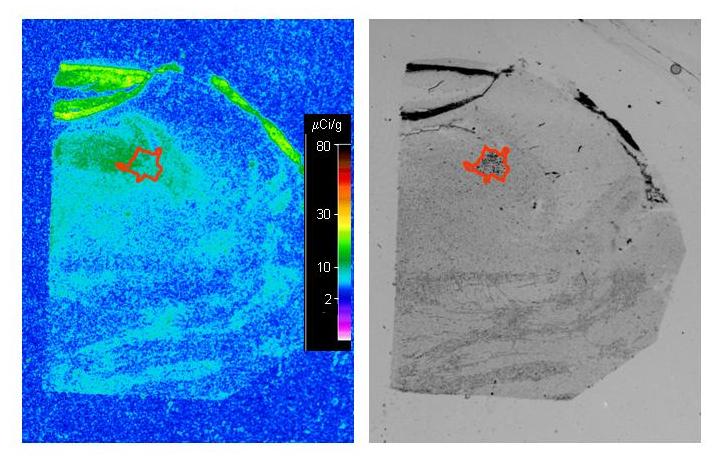
Digital images of an autoradiogram depicting the specific binding of [3H]flunitrazepam to GABAA receptors (left panel) and the Nissl staining of same section (right panel) at the middle level of a representative control subject. The image of specific binding was generated by digitally subtracting the image of non-specific binding from that of total binding using autoradiograms generated from adjacent tissue sections. The drawing on image of the Nissl stained section, overlaid onto the autoradiogram, depicts the region of the compact LC cell group that was measured.
Figure 2.
The specific binding of [3H]flunitrazepam ([3H]Flu) to GABAA receptors at rostral (R), medial (M), and caudal (C) levels of the LC from normal control subjects (open bars, n = 21) and subjects with MDD (filled bars, n = 9). Data shown were generated by measuring the cell-dense region of the LC. No significant differences between the two study groups were observed.
Figure 3.
The specific binding of [3H]flunitrazepam ([3H]Flu) to GABAA receptors at rostral (R), medial (M), and caudal (C) levels of the LC from normal control subjects (open bars, n = 21) and suicide subjects (filled bars, n = 17). Data shown were generated by measuring the cell-dense region of the LC. No significant differences between the two study groups were observed.
The possibility was considered that [3H]flunitrazepam binding over the cell dense region, as described above, might be different in density from [3H]flunitrazepam binding to the dendritic field of the LC, and might be differentially affected in depression. Tyrosine hydroxylase immunostains could not be used as a guide to analyze binding on autoradiograms in the dendritic region because of the confounding issue of increased tyrosine hydroxylase immunoreactivity in the LC of MDD subjects [42,69]. Therefore, the binding of [3H]flunitrazepam in the dendritic field of the LC was estimated using two concentric circles centered over the cellular region of the LC as observed in stained tissue sections (overlaid onto the binding autoradiogram). The inner concentric circle (area of 5 mm2) encompassed the compact cellular region of the LC while the outer concentric circle (total area of 10 mm2) defined an area (5 mm2) that laid immediately outside the compact cellular region of the LC, i.e the immediate dendritic region of the LC. There was significantly lower binding in the dendritic region of the LC as compared to the compact cellular region in all three study groups (e.g. for controls at the middle level: cellular region was 8.1 ± 0.7 μCi/g; dendritic region was 6.4 ± 0.6 μCi/g; p < 0.0001, paired Student's t-test). However, there were no significant differences in the amount of [3H]flunitrazepam binding to GABAA receptors in the dendritic region of the LC comparing the different study groups (data not shown).
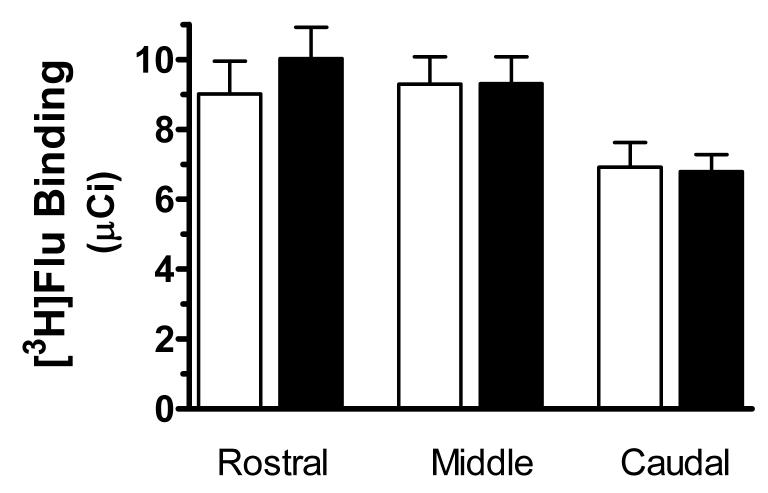
Table 3 summarizes the demographic information regarding all study subjects. There were no significant differences in ages or brain pH between control and MDD subjects or between control and suicide subjects. Postmortem intervals of the control group were significantly lower than the MDD group (p = 0.04), but not the suicide group. Given that there were no significant differences in binding between control and MDD and control and suicide groups, data from all subjects were merged to examine possible correlations between binding and demographic variables. No significant correlations were found between PMI values and binding at any individual level (rostral, middle and caudal, respectively). In addition, there were no significant correlations between brain pH and [3H]flunitrazepam binding in the LC. There was a very modest correlation between age and [3H]flunitrazepam binding in the caudal portion of the LC (r2 = 0.10, p = 0.04), but other levels of the LC did not demonstrate this correlation. Smokers and nonsmokers were matched across groups as closely as possible, primarily because we have previously observed differences in the biochemistry of the LC between these groups [21]. Given a lack of effect of depression or suicide, all smokers were compared to all nonsmokers (regardless of other diagnoses) and no significant difference in [3H]flunitrazepam binding to GABAA receptors was observed between these two groups at any level of the LC (Figure 4).
Table 3.
Summary of vital statistics of study subjects.
| Group | n | M/F | Age | pH | Postmortem interval |
No. of smokers |
|---|---|---|---|---|---|---|
| Control | 21 | 17/4 | 49 ± 3 | 6.66 ± 0.06 | 19.3 ± 1.3 | 10 |
| Major depressive disorder |
9 | 9/2 | 50 ± 5 | 6.61 ± 0.09 | 25.5 ± 2.8* | 5 |
| Suicide1 | 17 | 15/2 | 49 ± 4 | 6.65 ± 0.07 | 21.2 ± 2.5 | 7 |
This group includes a number of different affective disorders including 7 of the 9 major depressive disorder subjects (see Table 1).
p < 0.05 compared to control subjects (t-test)
Figure 4.
The specific binding of [3H]flunitrazepam ([3H]Flu) to GABAA receptors at rostral (R), medial (M), and caudal (C) levels of the LC from non-smokers (open bars, n = 21) and smokers (filled bars, n = 19). Data shown were generated by measuring the cell-dense region of the LC. No significant differences between the two study groups were observed.
4. Discussion
The present study is the first examination of [3H]flunitrazepam binding to GABAA receptors in the human locus coeruleus of depressed subjects. This report demonstrates that the amount of [3H]flunitrazepam binding is not different in major depressive subjects as compared to psychiatrically normal controls. Additionally, suicide is not associated with altered benzodiazepine binding to GABAA receptors in the LC. These findings do not provide support for the hypothesis that altered biochemistry in the LC associated with depression and suicide [20,40,42,43,69] results from disrupted GABAergic input to the LC.
There is considerable preclinical and clinical evidence that depression is associated with reduced GABA function [26,47]. For example, plasma GABA levels have been reported to be low in patients with major depression [48,49,50]. Using magnetic resonance spectroscopy, Sanacora et al. [54,56] demonstrated low GABA levels in the occipital cortex of depressed patients. A significant decrease in 3α, 5α-tetrahydroprogesterone(3α, 5α-THP) and 3α, 5β-THP concentrations, both of which are neuroactive steroids and positive modulators of the GABAA receptor, was observed in MDD patients and this abnormality could be corrected by treatment with antidepressant fluoxetine [53]. Interestingly, GABA agonists demonstrate activity in animal models useful for identifying antidepressant compounds [4,24,25,70]. There is also evidence that facilitation of GABAergic transmission produces antidepressant effects in humans [34]. Together, these findings suggest that depression is associated with a GABA deficiency and that correcting this deficiency may be beneficial to depressed patients.
If GABA neurotransmission is deficient in depression, then a consequence of this could be an adaptive change in the density of GABA receptors. The regulation of the GABAA receptor is complex, typical of receptor-operated channels [51,65,68]. Nevertheless, chronic GABA agonist exposure to rats induces down-regulation and allosteric uncoupling of the GABAA receptor [51], demonstrating that GABAA receptors can adapt to changes in activation. However, studies of the GABAA receptor binding in postmortem tissues from suicide and depressed subjects have not consistently demonstrated GABAA receptor alterations. An increase in the percentage of type I benzodiazepine binding sites was observed in the hippocampus of suicide subjects [29]. A modest decrease in the affinity of [3H]flunitrazepam for GABAA receptors in several hippocampal layers of violent suicide victims compared with matched controls has been reported [52]. An elevated density of GABAA/benzodiazepine receptors has been reported in the cerebral cortex (Brodmann's area 10) [46] and frontal cortex [9] of depressed suicide victims. However, Stocks and coworkers [60] reported that the density and affinity of benzodiazepine binding sites did not differ significantly in the hippocampus and amygdala between depressed suicide victims and controls. Similarly, Kugaya et al. [22] also reported no differences between patients with major depression and healthy control subjects in GABAA receptor binding measured in vivo with a benzodiazepine radioligand. In addition, Merali and coworkers [31] reported low levels of mRNAs encoding several alpha subunits of the GABAA receptor in the frontal cortex of suicide victims. Overall, postmortem studies of radioligand binding to GABAA receptors, including the present study, have not provided strong support for GABA dysregulation as a pathological marker of depression. There are many possible reasons why postmortem studies have failed to consistently identify GABA receptor abnormalities in depression and/or suicide and several reviews have addressed in detail common shortcomings of postmortem research [38,59].
In the present study, 5 of the depressed subjects (1 MDD and 4 depressed suicide) had an alcohol use disorder (see Table 1). Since GABAA receptors are targets of ethanol action in the brain [11], the potential effect of ethanol on [3H]flunitrazepam binding to GABAA was carefully considered. The average binding levels of these 5 subjects was not significantly different from the depressed suicide and MDD non-drinkers, or from the control group (data not shown). Hence, it is unlikely that ethanol exposure influenced the findings of this study.
GABA neurons projecting to the LC originate largely in the nucleus prepositus hypoglossi [13]. There are apparently no GABA cell bodies intrinsic to the LC, but glutamic acid decarboxylase immunoreactive nerve terminals are present, closely juxtaposed to noradrenergic cell bodies and dendrites [6]. GABA inhibits the firing of LC neurons primarily by activation of GABAA receptors [44], but compared to other brain regions, the human LC has a low density of GABAA receptors. Benzodiazepine binding to the GABAA receptor in the LC is displaced by the triazolopyridazine Cl 218872, indicating type I benzodiazepine binding sites [18,67]. The findings of the present study confirmed relatively low binding density of GABAA receptors in the human LC and further demonstrated an uneven distribution of the binding of [3H]flunitrazepam to GABAA receptors along the rostral-caudal axis of the human LC. Although there is a modest density gradient of GABAA receptor binding in the LC, this gradient did not correlate with the number of neuromelanin-containing (noradrenergic) neurons along the LC axis. These findings indicate that the majority of [3H]flunitrazepam binding to GABAA receptors within the region of the LC does not likely occur on the cell bodies of noradrenergic neurons in the LC, since other proteins (e.g. monoamine oxidase A, norepinephrine transporter, α2A adrenoceptors) known to reside on the noradrenergic cell bodies demonstrate high correlations of binding density relative to cell density along the axis of the LC [19,37,41]. Since LC neurons do express GABAA subunit mRNAs [16,27,33], it is likely that they do indeed have receptors on their cell bodies or dendrites, though the level of expression may be low, and that other cells (e.g. glia) or terminals of neurons projecting to the LC (serotonin, glutamate etc) likely also express GABAA receptors. Despite the low levels of benzodiazepine binding to the GABAA receptor, infusion of benzodiazepines into the rat LC reduces spontaneous firing of LC neurons, demonstrating functional benzodiazepine binding sites in this region [58].
Numerous pathological alterations in the LC from depressed suicide victims have been described [20,40,42,43,69], and many of these are consistent with the hypothesis that depression is associated with a stress-related over-activity of the LC, and a concomitant deficiency of norepinephrine [36]. One possible mediator of an overactive LC could be reduced GABAergic input to this cell group, based on considerable evidence of deficient GABAergic transmission in depression as described above. The present data demonstrate that benzodiazepine binding to the GABAA receptor is normal in depression and do not provide support for altered GABAergic input to the LC in depression. However, given the subunit complexity of the GABAA receptor, the present study is insufficient to conclude that there is no abnormality of this receptor in the LC in depression, or that GABA input to the LC is not disrupted in depression. Several other tools can be used to evaluate GABAergic input to the LC in postmortem tissues from depressed subjects, including but not limited to receptor subunit immunoreactivities, GABA transporter immunoreactivities, GABA levels, and synthetic enzyme immunoreactivities.
Acknowledgements
We gratefully acknowledge the work of Drs. James C. Overholser, Herbert Y. Meltzer, Bryan L. Roth, George Jurjus, and Ginny Dilley, Lisa Konick, and Lesa Dieter in the retrospective psychiatric diagnoses. The excellent assistance of the Cuyahoga County Coroner's Office, Cleveland, OH is greatly appreciated. This project was supported by grants from the National Institute of Mental Health (MH63187, MH46692, MH 67996, and MH02031) and the National Center for Research Resources (RR17701).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- 1.Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- 2.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol. Psychiatry. 2003;8:324–32. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 4.Bartholini G. Experimental basis for the antidepressant action of the GABA receptor agonist progabide. Neurosci Lett. 1984;47:351–355. doi: 10.1016/0304-3940(84)90538-x. [DOI] [PubMed] [Google Scholar]
- 5.Benes FM, Wickramasinghe R, Vincent SL, Khan Y, Todtenkopf M. Uncoupling of GABA(A) and benzodiazepine receptor binding activity in the hippocampal formation of schizophrenic brain. Brain Res. 1997;755:121–9. doi: 10.1016/s0006-8993(97)00113-3. [DOI] [PubMed] [Google Scholar]
- 6.Berod A, Chat M, Paut L, Tappaz M. Catecholaminergic and GABAergic anatomical relationship in the rat substantia nigra, locus coeruleus, and hypothalamic median eminence: immunocytochemical visualization of biosynthetic enzymes on serial semithin plastic-embedded sections. J Histochem Cytochem. 1984;32:1331–1338. doi: 10.1177/32.12.6150057. [DOI] [PubMed] [Google Scholar]
- 7.Bissette G, Klimek V, Pan J, Stockmeier CA, Ordway GA. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28:1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 8.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cheetham SC, Crompton MR, Katona CL, Parker SJ, Horton RW. Brain GABAA/benzodiazepine binding sites and glutamic acid decarboxylase activity in depressed suicide victims. Brain Res. 1988;460:114–23. doi: 10.1016/0006-8993(88)91211-5. [DOI] [PubMed] [Google Scholar]
- 10.Cubells JF, Kim KS, Baker H, Volpe BT, Chung Y, Houpt TA, Wessel TC, Joh TH. Differential in vivo regulation of mRNA encoding the norepinephrine transporter and tyrosine hydroxylase in rat adrenal medulla and locus ceruleus. J Neurochem. 1995;65:502–509. doi: 10.1046/j.1471-4159.1995.65020502.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci. 2003;28:263–74. [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado PL, Coconcea C. Norepinephrine in mood disorders. In: Ordway GA, Schwartz MA, Frazer A, editors. Brain Norepinephrine: Neurobiology and Therapeutics. Cambridge University Press; London: in press. [Google Scholar]
- 13.Ennis M, Aston-Jones G. GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J Neurosci. 1989;9:2973–2981. doi: 10.1523/JNEUROSCI.09-08-02973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endicott J, Spitzer RL RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 16.Heikkila AT, Echenko O, Uusi-Oukari M, Sinkkonen ST, Korpi ER. Morphine withdrawal increases expression of GABA(A) receptor epsilon subunit mRNA in locus coeruleus neurons. Neuroreport. 2001;12:2981–5. doi: 10.1097/00001756-200109170-00045. 2001. [DOI] [PubMed] [Google Scholar]
- 17.Karolewicz B, Stockmeier CA, Ordway GA. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology. 2005;30:1557–1567. doi: 10.1038/sj.npp.1300781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klepner CA, Lippa AS, Benson DI, Sano MC, Beer B. Resolution of two biochemically and pharmacologically distinct benzodiazepine receptors. Pharmacol. Biochem. Behav. 1979;11:457–62. doi: 10.1016/0091-3057(79)90125-4. [DOI] [PubMed] [Google Scholar]
- 19.Klimek V, Ordway GA. Distribution of alpha2-adrenoceptors in the human locus coeruleus. Brain Res. 1996;741:263–274. doi: 10.1016/s0006-8993(96)00937-7. [DOI] [PubMed] [Google Scholar]
- 20.Klimek V, Stockmeier CA, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J. Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimek V, Zhu M-Y, Dilley G, Konick L, Overholser J, Meltzer HY, May WL, Stockmeier CA, Ordway GA. Effects of long-term cigarette smoking on the human locus coeruleus. Arch. Gen. Psychiatry. 2001;58:821–827. doi: 10.1001/archpsyc.58.9.821. [DOI] [PubMed] [Google Scholar]
- 22.Kugaya A, Sanacora G, Verhoeff NP, Fujita M, Mason GF, Seneca NM, Bozkurt A, Khan SA, Anand A, Degen K, Charney DS, Zoghbi SS, Baldwin RM, Seibyl JP, Innis RB. Cerebral benzodiazepine receptors in depressed patients measured with [123I]iomazenil SPECT. Biol. Psychiatry. 2003;54:792–9. doi: 10.1016/s0006-3223(02)01788-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee C-M, Javitch JA, Snyder SH. Recognition sites for norepinephrine uptake: regulation by neurotransmitter. Science. 1983;220:626–629. doi: 10.1126/science.6301013. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd KG, Morselli PL, Depoortere H, Fournier V, Zivkovic B, Scatton B, Broekkamp C, Worms P, Bartholini G. The potential use of GABA agonists in psychiatric disorders: evidence from studies with progabide in animal models and clinical trials. Pharmacol Biochem Behav. 1983;18:957–66. doi: 10.1016/s0091-3057(83)80021-5. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd KG, Zivkovic B, Sanger D, Depoortere H, Bartholini G. Fengabine, a novel antidepressant GABAergic agent. I. Activity in models for antidepressant drugs and psychopharmacological profile. J. Pharmacol. Exp. Ther. 1987;241:245–50. [PubMed] [Google Scholar]
- 26.Lloyd KG, Zivkovic B, Scatton B, Morselli PL, Bartholini G. The GABAergic hypothesis of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:341–51. doi: 10.1016/0278-5846(89)90123-1. [DOI] [PubMed] [Google Scholar]
- 27.Luque JM, Malherbe P, Richards JG. Localization of GABAA receptor subunit mRNAs in the rat locus coeruleus. Mol. Brain Res. 1994;24:219–26. doi: 10.1016/0169-328x(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 28.Malgady RG, Rogler LH, Tryon WW. Issues of validity in the Diagnostic Interview Schedule. J. Psychiatr. Res. 1992;26:59–67. doi: 10.1016/0022-3956(92)90016-h. [DOI] [PubMed] [Google Scholar]
- 29.Manchon M, Kopp N, Rouzioux JJ, Lecestre D, Deluermoz S, Miachon S. Benzodiazepine receptor and neurotransmitter studies in the brain of suicides. Life Sci. 1987;41:2623–2630. doi: 10.1016/0024-3205(87)90276-1. [DOI] [PubMed] [Google Scholar]
- 30.Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J. Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 31.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–85. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bedard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol. Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Moragues N, Ciofi P, Tramu G, Garret M. Localisation of GABA(A) receptor epsilon-subunit in cholinergic and aminergic neurones and evidence for co-distribution with the theta-subunit in rat brain. Neuroscience. 2002;111:657–69. doi: 10.1016/s0306-4522(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen NP, Cesana B, Zizolfi S, Ascalone V, Priore P, Morselli PL. Therapeutic effects of fengabine, a new GABAergic agent, in depressed outpatients: a double-blind study versus clomipramine. Acta Psychiatr. Scand. 1990;82:366–71. doi: 10.1111/j.1600-0447.1990.tb01402.x. [DOI] [PubMed] [Google Scholar]
- 35.Bureau M, Endo S, Smith G, Deng L, Sapp D, Tobin AJ. GABAA-benzodiazepine receptors: demonstration of pharmacological subtypes in the brain. Adv Exp Med Biol. 1991;287:355–64. doi: 10.1007/978-1-4684-5907-4_30. [DOI] [PubMed] [Google Scholar]
- 36.Ordway GA. Neuropathology of central norepinephrine in psychiatric disorders: Postmortem research. In: Ordway GA, Schwartz MA, Frazer A, editors. Brain Norepinephrine: Neurobiology and Therapeutics. Cambridge University Press; London: in press. [Google Scholar]
- 37.Ordway GA, Farley JT, Dilley GE, Overholser JC, Meltzer HY, Balraj EK, Stockmeier CA, Klimek V. Quantitative distribution of monoamine oxidase A in brainstem monoamine nuclei is normal in major depression. Brain Res. 1999;847:71–79. doi: 10.1016/s0006-8993(99)02043-0. [DOI] [PubMed] [Google Scholar]
- 38.Ordway GA, Klimek V. Noradrenergic pathology in psychiatric disorders: postmortem studies. CNS Spectr. 2001;6:697–703. doi: 10.1017/s1092852900001395. [DOI] [PubMed] [Google Scholar]
- 39.Ordway G.A. GA, Klimek V, Mann JJ. Neurocircuitry of mood disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Psychopharmacology. The Fifth Generation of Progress. Lippincott-Williams & Wilken; Philadelphia: 2002. pp. 1051–1064. [Google Scholar]
- 40.Ordway GA, Schenk J, Meltzer HY, Overholser JC, Stockmeier CA, Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol. Psychiatry. 2003;53:315–323. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- 41.Ordway GA, Stockmeier CA, Cason GW, Klimek V. Pharmacology and distribution of norepinephrine transporters in the human locus coeruleus and raphe nuclei. J. Neurosci. 1997;17:1710–1719. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ordway GA, Smith K.Streator, Haycock JW. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J. Neurochem. 1994;62:680–685. doi: 10.1046/j.1471-4159.1994.62020680.x. [DOI] [PubMed] [Google Scholar]
- 43.Ordway GA, Widdowson PS, Smith K, Halaris AE. Agonist binding to alpha2 adrenoceptors is elevated in the locus coeruleus from victims of suicide. J. Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- 44.Osmanovic SS, Shefner SA. gamma-Aminobutyric acid responses in rat locus coeruleus neurones in vitro: a current-clamp and voltage-clamp study. J Physiol (Lond) 1990;421:151–170. doi: 10.1113/jphysiol.1990.sp017938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palacios JM, Wamsley JK, Kuhar MJ. High affinity GABA receptors-autoradiographic localization. Brain Res. 1981;222:285–307. doi: 10.1016/0006-8993(81)91034-9. [DOI] [PubMed] [Google Scholar]
- 46.Pandey GN, Conley RR, Pandey SC, Goel S, Roberts RC, Tamminga CA, Chute D, Smialek J. Benzodiazepine receptors in the post-mortem brain of suicide victims and schizophrenic subjects. Psychiatry Res. 1997;71:137–49. doi: 10.1016/s0165-1781(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 47.Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect. Disord. 1995;34:275–81. doi: 10.1016/0165-0327(95)00025-i. [DOI] [PubMed] [Google Scholar]
- 48.Petty F, Kramer GL, Gullion CM, Rush AJ. Low plasma gamma-aminobutyric acid levels in male patients with depression. Biol. Psychiatry. 1992;32:354–363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 49.Petty F, Schlesser MA. Plasma GABA in affective illness. A preliminary investigation. J. Affect. Disord. 1981;3:339–343. doi: 10.1016/0165-0327(81)90003-3. [DOI] [PubMed] [Google Scholar]
- 50.Petty F, Sherman AD. Plasma GABA levels in psychiatric illness. J Affect Disord. 1984;6:131–138. doi: 10.1016/0165-0327(84)90018-1. [DOI] [PubMed] [Google Scholar]
- 51.Roca DJ, Rozenberg I, Farrant M, Farb DH. Chronic agonist exposure induces down-regulation and allosteric uncoupling of the gamma-aminobutyric acid/benzodiazepine receptor complex. Mol. Pharmacol. 1990;37:37–43. [PubMed] [Google Scholar]
- 52.Rochet T, Kopp N, Vedrinne J, Deluermoz S, Debilly G, Miachon S. Benzodiazepine binding sites and their modulators in hippocampus of violent suicide victims. Biol. Psychiatry. 1992;32:922–31. doi: 10.1016/0006-3223(92)90181-x. [DOI] [PubMed] [Google Scholar]
- 53.Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 54.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 55.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 56.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61:705–13. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 57.Simpson KL, Lin RCS. Neuroanatomical and chemical organization of the locus coeurlues. In: Ordway GA, Schwartz MA, Frazer A, editors. Brain Norepinephrine: Neurobiology and Therapeutics. Cambridge University Press; London: in press. [Google Scholar]
- 58.Simson PE, Weiss JM. Peripheral, but not local or intracerebroventricular, administration of benzodiazepines attenuates evoked activity of locus coeruleus neurons. Brain Res. 1989;490:236–42. doi: 10.1016/0006-8993(89)90241-2. [DOI] [PubMed] [Google Scholar]
- 59.Stockmeier CA, Jurjus G. Monoamine receptors in postmortem brain tissue in suicide. Kluwer Academic Publishers. In: Agam G, Everall I, Belmaker RH, editors. The Postmortem Brain in Psychiatric Research. Kluwer Academic Publishers; Boston: 2002. pp. 363–385. [Google Scholar]
- 60.Stocks GM, Cheetham SC, Crompton MR, Katona CL, Horton RW. Benzodiazepine binding sites in amygdala and hippocampus of depressed suicide victims. J. Affect. Disord. 1990;18:11–5. doi: 10.1016/0165-0327(90)90112-l. [DOI] [PubMed] [Google Scholar]
- 61.Torda T, Kvetnansky R, Petrikova M. Effect of repeated immobilization stress on central and peripheral adrenoceptors in rats. Endocrinol. Exp. 1985;19:157–63. [PubMed] [Google Scholar]
- 62.Tunnicliff G, Malatynska E. Central GABAergic systems and depressive illness. Neurochem. Res. 2003;28:965–76. doi: 10.1023/a:1023287729363. [DOI] [PubMed] [Google Scholar]
- 63.Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol. Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- 64.Wang P, Kitayama I, Nomura J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998;62:2083–2092. doi: 10.1016/s0024-3205(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 65.Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res. Mol. Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]
- 66.Zafar HM, Pare WP, Tejani-Butt SM. Effect of acute or repeated stress on behavior and brain norepinephrine system in Wistar-Kyoto (WKY) rats. Brain Res. Bull. 1997;44:289–95. doi: 10.1016/s0361-9230(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 67.Zezula J, Cortes R, Probst A, Palacios JM. Benzodiazepine receptor sites in the human brain: autoradiographic mapping. Neuroscience. 1988;25:771–95. doi: 10.1016/0306-4522(88)90036-x. [DOI] [PubMed] [Google Scholar]
- 68.Zheng TM, Caruncho HJ, Zhu WJ, Vicini S, Ikonomovic S, Grayson DR, Costa E. Chronic flumazenil alters GABA(A) receptor subunit mRNA expression, translation product assembly and channel function in neuronal cultures. J. Pharmacol. Exp. Ther. 1996;277:525–533. [PubMed] [Google Scholar]
- 69.Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier CA, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol. Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 70.Zivkovic B, Scatton B, Worms P, Lloyd KG, Bartholini G. Pharmacological and therapeutic actions of GABA receptor agonists. J. Neural. Transm. Suppl. 1983;18:319–326. [PubMed] [Google Scholar]