Abstract
The influence of intramolecular cross-links on the molecular, structural and functional properties of PEGylated {PEG [poly(ethylene glycol)]-conjugated} haemoglobin has been investigated. The sites and the extent of PEGylation of haemoglobin by reductive alkylation are not influenced by the presence of an αα-fumaryl cross-link at Lys-99(α). The propylated hexaPEGylated cross-linked haemoglobin, (propyl-PEG5K)6-αα-Hb, exhibits a larger molecular radius and lower colloidal osmotic pressure than propylated hexaPEGylated non-cross-linked haemoglobin, (propyl-PEG5K)6-Hb. Perturbation of the haem microenvironment and the α1β2 interface by PEGylation of haemoglobin is reduced by intramolecular cross-linking. Sedimentation velocity analysis established that PEGylation destabilizes the tetrameric structure of haemoglobin. (Propyl-PEG5K)6-Hb and (propyl-PEG5K)6-αα-Hb sediment as stable dimeric and tetrameric molecules, respectively. The ββ-succinimidophenyl PEG-2000 cross-link at Cys-93(β) outside the central cavity also influences the molecular properties of haemoglobin, comparable to that by the αα-fumaryl cross-link within the central cavity. However, the influence of the two cross-links on the oxygen affinity of PEGylated haemoglobin are very distinct, indicating that the high oxygen affinity of PEGylated haemoglobin is not a direct consequence of the dissociation of the haemoglobin tetramers into dimers. αα-Fumaryl cross-linking is preferred to modulate both oxygen affinity and molecular properties of PEGylated haemoglobin, and cross-linking outside the central cavity could only modulate molecular properties of PEGylated haemoglobin. It is suggested that PEGylation induces a hydrodynamic drag on haemoglobin and this plays a role in the microcirculatory properties of PEGylated haemoglobin.
Keywords: cross-link, haemoglobin, PEGylation, reductive alkylation, subunit dissociation
Abbreviations: COP, colloidal osmotic pressure; αα-fumaryl Hb, αα-intramolecular cross-linked haemoglobin at Lys-99(α); ββ-Hb, ββ-intramolecular succinimidophenyl-poly(ethylene glycol) 2000 cross-linked haemoglobin at Cys-93(β); HbA, human adult haemoglobin; IEF, isoelectric focusing; PEG, poly(ethylene glycol); PEG2K, PEG 2000; PEG20K, PEG 20000; PEG5K, PEG 5000; PEG5K aldehyde, ω-methoxy-PEG5K propionaldehyde; PEGylation, conjugation with PEG; (propyl-PEG5K)6-Hb, propylated hexaPEGylated non-cross-linked haemoglobin; (propyl-PEG5K)6-αα-Hb, propylated hexaPEGylated αα-intramolecular cross-linked haemoglobin; (propyl-PEG5K)6-ββ-Hb, propylated hexaPEGylated ββ-intramolecular cross-linked haemoglobin; RP, reverse-phase; SEC, size-exclusion chromatography; (SP-PEG5K)6-Hb, succinimidophenylated hexaPEGylated haemoglobin
INTRODUCTION
Haemoglobin-based blood substitutes are being developed rapidly to overcome the shortage of blood supply [1,2]. The most extensively studied and financed blood substitute is diaspirin cross-linked haemoglobin [3]. Although intramolecular cross-linking of haemoglobin overcame the nephrotoxicity and high oxygen affinity of the acellular haemoglobin [1], the product remained vasoactive [4,5], which has been attributed to the scavenging of NO (nitric oxide) by the extravasated acellular haemoglobin [3]. Enhancing the molecular size of haemoglobin by oligomerization and lowering the affinity of haemoglobin to NO by site-directed mutagenesis are two solutions to overcome the vasoactivity of haemoglobin. Animal studies have shown that both approaches reduce the pressor effect of haemoglobin [6].
Enzon PEGylated {PEG [poly(ethylene glycol)]-conjugated} bovine haemoglobin carries ten copies of the PEG5K (PEG 5000) chain, and was non-hypertensive [7]. Its enhanced molecular volume, high viscosity and high COP (colloidal osmotic pressure) have been attributed as the molecular basis of neutralizing the vasoactivity of acellular haemoglobin [8]. Accordingly, PEGylation of haemoglobin has been considered as a new approach to generate non-hypertensive haemoglobin [9,10]. In an attempt to establish that the neutralization of the vasoactivity is a generalized consequence of PEGylation of haemoglobin, we generated a non-hypertensive succinimidophenylated hexa-PEGylated haemoglobin, (SP-PEG5K)6-Hb, using the extension arm facilitated PEGylation protocol [11,12]. Compared with the Enzon decaPEGylated bovine haemoglobin, (SP-PEG5K)6-Hb has fewer PEG5K chains conjugated, and the positive charge of haemoglobin was not changed upon linking the extension arm or on conjugating the PEG chains through the thiol groups at the distal end of the extension arms (conservative PEGylation) [11,12]. Therefore the results reflect the higher efficiency of conservative PEGylation to neutralize the vasoactivity of haemoglobin.
The new non-hypertensive hexaPEGylated haemoglobin, (SP-PEG5K)6-Hb (generated by thiolation-mediated maleimidechemistry-based PEGylation of haemoglobin with succinimidophenyl-PEG5K) exhibits a very high oxygen affinity, which was considered to be a consequence of the PEGylation at Cys-93(β) of haemoglobin [11]. High oxygen affinity for haemoglobin-based oxygen carriers has been advocated as a desirable property to generate non-hypertensive haemoglobin, as this reduces the propensity of the acellular haemoglobin to offload the oxygen on the arterial side of the circulation [13]. Recently, we generated a propylated hexaPEGylated haemoglobin, (propyl-PEG5K)6-Hb, by reductive alkylation of non-cross-linked haemoglobin by PEG5K aldehyde (ω-methoxy-PEG5K propionaldehyde) [14]. The oxygen affinity of (propyl-PEG5K)6-Hb is comparable with that of (SP-PEG5K)6-Hb, even though Cys-93(β) was unmodified in (propyl-PEG5K)6-Hb.
However, the COP of (propyl-PEG5K)6-Hb was considerably higher than that of (SP-PEG5K)6-Hb. In general, the COP of the protein solution is a correlate of the number of particles (molecules) in the solution. Accordingly, the higher COP of (propyl-PEG5K)6-Hb could be a consequence of a greater number of molecules in the solution than in the solution of (SP-PEG5K)6-Hb at the same protein concentration. Typically, haemoglobin undergoes tetramer–dimer dissociation, which involves cleavage of the non-covalent interactions along the symmetrical interfaces α1β2 and α2β1 [15,16]. Therefore the different COP values between the two PEGylated proteins are possibly due to the fact that the tetramer–dimer dissociation of haemoglobin is enhanced by the two PEGylation protocols at different levels. Besides, if (propyl-PEG5K)6-Hb is predominantly present in dimers, it can lead to the high oxygen affinity of (propyl-PEG5K)6-Hb. Thus we now consider that intramolecular cross-links in haemoglobin could improve the molecular, structural and functional properties of (propyl-PEG5K)6-Hb.
In the present study, we have investigated the influence of the presence of intramolecular cross-links in haemoglobin on the molecular, structural and functional properties of the PEG–haemoglobin conjugate. The reductive-alkylation-chemistry-mediated PEGylation of haemoglobin has been chosen as the method for PEGylation of haemoglobin, as this approach shows a higher level of site selectivity than extension-arm-facilitated PEGylation. The intramolecularly cross-linked haemoglobin at Lys-99(α), αα-fumaryl Hb, has been chosen as the model approach in view of the extensive structural and colligative information available. The molecular, structural and functional properties of PEGylated non-cross-linked and cross-linked haemoglobin were compared. The new information generated here is expected to facilitate the design of novel haemoglobin-based blood substitutes.
EXPERIMENTAL
Reductive alkylation of HbA (human adult haemoglobin) with ω-methoxy-PEG5K propionaldehyde
HbA was purified from human erythrocytes as described previously [17]. αα-Fumaryl Hb was prepared as described previously [18]. ββ-Hb [ββ-intramolecular succinimidophenyl-PEG2K (PEG-2000) cross-linked haemoglobin at Cys-93(β)] was prepared as described by Manjula et al. [19]. HbA, αα-fumaryl Hb and ββ-Hb (0.25 mM tetramer) in 50 mM Bis-Tris acetate buffer (pH 6.5) were reacted with 10 mM PEG5K aldehyde (Shearwater Polymers) in the presence of 50 mM sodium cyanoborohydride (Sigma Chemical Co.) at 4 °C overnight. The reaction mixture was subjected to diafiltration through a 70 kDa membrane against PBS (pH 7.4) using a Minim Tangential Flow Filtration instrument (Pall Corporation) to remove unreacted PEG and other excess reagents. The final product in the retentate was concentrated and was frozen at −80 °C.
Dynamic light scattering
Dynamic light scattering for molecular radius measurement was performed using a DynaPro instrument (Protein Solutions). Samples at a protein concentration of 1 mg/ml were centrifuged at 15700 g for 4 min before analysis.
Analytical methods
SEC (size-exclusion chromatography) of PEGylated proteins were carried out using Superose 12 columns (1 cm×30 cm) (Amersham Biosciences). RP (reverse-phase)-HPLC analysis of globin chains on a Vydac C4 column (4.6 mm×250 mm) and SDS/PAGE (14% polyacrylamide) analysis were carried out as described previously [20,21]. IEF (isoelectric focusing) analysis was operated using pre-cast resolve gels from Isolab and a blend of pH 6–8 resolve ampholytes. Gels were electrofocused for 3 h to completely resolve the components in the sample. The colloidal osmotic pressure and viscosity of PEGylated proteins were measured as described by Hu et al. [14]. Oxygen-binding equilibrium measurements of PEGylated proteins were carried out using a Hem-O-Scan Analyzer at 37 °C as described by Manjula et al. [20].
Tryptic peptide mapping
Tryptic peptide mapping of the PEGylated proteins was carried out by methods described previously [22,23]. The tryptic peptides were analysed by RP-HPLC on a Vydac C18 column (10 mm×250 mm) [14]. Percentage modification of the peptides in the PEGylated proteins was calculated by the ratio of the peak area of each peptide of the PEGylated haemoglobin and PEGylated αα-fumaryl Hb relative to the corresponding peak in the HbA and αα-fumaryl Hb peptide map respectively. The recovery of peptide βT4 was used as an internal standard.
Analytical ultracentrifugation
Sedimentation velocity measurements were conducted in a Beckman XL-I analytical ultracentrifuge in PBS at pH 7.4, 25 °C and 55000 rev./min using a AN-60Ti rotor. Scans (40–50) were collected once the sendimentation boundary cleared the meniscus. Between 14 and 20 scans were typically used for the calculation of the sedimentation parameters. Boundary movement was followed at 405 nm using the centrifuge's absorption optics. For each sample, data were collected at three nominal concentrations (A405=0.1, 0.5 and 1.0). The g(s*) differential apparent sedimentation coefficient distributions were determined using DCDT+ version 2.0.4 (http://www.jphilo.mailway.com) using values of ν of 0.74 ml/g for HbA [24] and 0.806 ml/g for the PEGylated proteins [25] and normalized to standard conditions (S20,w and D20,w) by correcting for buffer density and viscosity.
CD spectroscopy
CD spectra of haemoglobin samples were recorded on a JASCO-720 spectropolarimeter at 25 °C with a 0.2-cm-pathlength cuvette (310 μl). For the spectra from 250 to 200 nm, the haemoglobin concentration was 1.3 μM as tetramer. For the spectra from 480 to 250 nm, the haemoglobin concentration was 26.0 μM as tetramer. All of the haemoglobin samples were in PBS, pH 7.4. The molar ellipticity, θ, is expressed in deg·cm2/dmol on a haem basis.
Front-face fluorescence measurements
Intrinsic front-face fluorescence measurements of haemoglobin samples were performed using a Shimadzu RF-5301 spectrofluorimeter at room temperature (25 °C). The emission spectra were recorded from 300 to 400 nm using an excitation wavelength of 280 nm. Excitation and emission slit widths were both 5 nm. All the samples used were at haemoglobin concentration of 5.7 mg/ml in PBS, pH 7.4. A cuvette with a 1 cm pathlength was used.
RESULTS
Influence of αα-fumaryl intramolecular cross-link on the site-selectivity and extent of PEGylation of haemoglobin
The sites and the extents of PEGylation of αα-fumaryl Hb are presented in Table 1. As can be seen, Val-1(α) and Val-1(β) have been completely modified by PEGylation in αα-fumaryl Hb, the same as in HbA. In addition, four lysine residues also showed modification by PEGylation in αα-fumaryl Hb to some extent and are comparable with those in HbA. Thus the presence of an αα-fumaryl intramolecular cross-link in the central cavity of haemoglobin has essentially little influence on the site-selectivity of PEGylation. Accordingly, PEGylated αα-fumaryl Hb is referred to as (propyl-PEG5K)6-αα-Hb, in conformity with the earlier nomenclature of hexaPEGylated haemoglobin, (propyl-PEG5K)6-Hb [14].
Table 1. Sites of PEGylation in αα-fumaryl Hb.
The sites of PEGylation in the PEGylated proteins are determined by tryptic peptide mapping of their globin chains as described in the Experimental section.
| Modification (%) | ||
|---|---|---|
| Residue modified | (Propyl-PEG5K)6-Hb | (Propyl-PEG5K)6-αα-Hb |
| Val-1(α) | 100 | 100 |
| Val-1(β) | 100 | 100 |
| Lys-8(β) | 23 | 23 |
| Lys-11(α) | 23 | 27 |
| Lys-40(α) | 12 | 11 |
| Lys-56(α) | 17 | 22 |
Electrophoretic analysis of PEGylated proteins
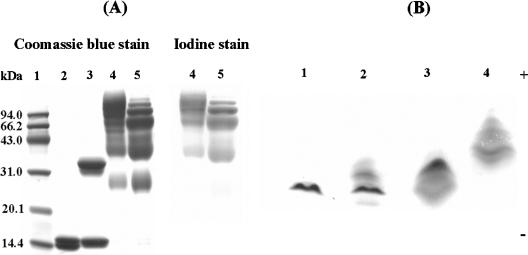
As shown by SDS/PAGE analysis (Figure 1A), the electrophoretic pattern for HbA is a doublet corresponding to the α- and β-chains (lane 2). The α-chain of αα-fumaryl Hb showed a slower mobility as a consequence of cross-linking (lane 3). (Propyl-PEG5K)6-Hb displayed two major and two minor protein bands with lower mobility than the unmodified globin chains (lane 5). For (propyl-PEG5K)6-αα-Hb (lane 4), the two major bands of (propyl-PEG5K)6-Hb became lighter with the concomitant appearance of two new bands with lower mobility upon αα-cross-linking. The detection of PEG chains attached to haemoglobin by iodine staining showed that stain intensity is comparable between the two PEGylated proteins. This suggests that the attached PEG chains are comparable between the two PEGylated proteins.
Figure 1. Characterization of PEGylated proteins by SDS/PAGE and IEF.
SDS/PAGE was carried out on a pre-cast 14% Tris/glycine gel from the Invitrogen Corporation. Lane 1, molecular mass markers; lane 2, HbA; lane 3, αα-fumaryl Hb; lane 4, (propyl-PEG5K)6-αα-Hb; lane 5, (propyl-PEG5K)6-Hb. Proteins were identified by Coomassie Blue staining, and PEG was detected by iodine staining. (Propyl-PEG5K)6-αα-Hb and (propyl-PEG5K)6-Hb were both loaded at the same amount of protein content (12 μg). IEF was operated using pre-cast resolve gels from Isolab and a blend of pH 6–8 resolve ampholytes. Lane 1, HbA; lane 2, αα-fumaryl Hb; lane 3, (propyl-PEG5K)6-Hb; lane 4, (propyl-PEG5K)6-αα-Hb.
The influence of the intramolecular cross-linking on the IEF pattern of the PEGylated proteins is shown in Figure 1(B). The PEGylated proteins do not focus as compact bands, and are distinct from HbA and αα-fumaryl Hb. (Propyl-PEG5K)6-Hb focused slightly beyond HbA, and (propyl-PEG5K)6-αα-Hb focused slightly beyond (propyl-PEG5K)6-Hb. Since the reductive-alkylation-chemistry-based PEGylation of haemoglobin could conserve the positive charge of haemoglobin, the influence of PEGylation on the IEF pattern reflects the molecular shielding influence of the PEG shell on the surface charges of haemoglobin from the bulk solvent [26]. Since HbA and αα-fumaryl Hb exhibit similar isoelectric patterns, the molecular-shielding influence of the PEG shell on the surface charges of (propyl-PEG5K)6-Hb is enhanced as a result of αα-fumaryl intramolecular cross-links.
Hydrodynamic volume of PEGylated proteins determined by SEC
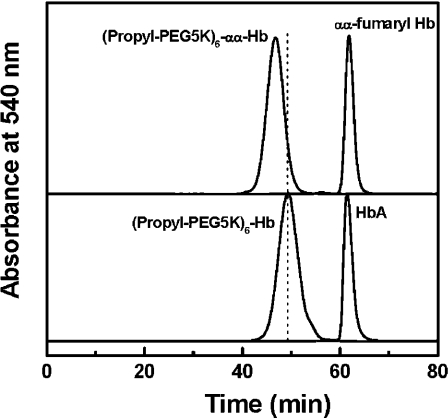
The hydrodynamic volume of PEGylated proteins was measured using SEC. As shown in Figure 2, PEGylation of HbA results in an earlier elution of the protein, reflecting a significant increase in the hydrodynamic volume of haemoglobin. The SEC pattern of HbA is not influenced by the presence of αα-fumaryl intramolecular cross-links. PEGylation of αα-fumaryl Hb results in a larger hydrodynamic volume than that of PEGylation of HbA, as reflected by the earlier elution. Based on the results of the tryptic peptide mapping (Table 1), the increase in the hydrodynamic volume of (propyl-PEG5K)6-αα-Hb is not related to the higher level or an altered site selectivity of PEGylation. Thus αα-fumaryl intramolecular cross-linking in haemoglobin appears to increase the hydrodynamic volume of PEGylated haemoglobin.
Figure 2. Size-exclusion chromatographic analysis of PEGylated haemoglobin samples.
The analysis was carried out at room temperature on two HR10/30 Superose 12 columns connected in series. The columns were eluted with PBS, pH 7.4, at a flow rate of 0.5 ml/min, and the effluent was monitored at 540 nm.
Molecular volume of PEGylated proteins determined by dynamic light scattering
The molecular radii of the PEGylated proteins, as determined by dynamic light scattering, and their calculated molecular volumes are summarized in Table 2. The molecular radius of αα-fumaryl Hb is comparable with that of HbA. (Propyl-PEG5K)6-Hb showed a molecular radius of 5.40 nm, reflecting the enhanced molecular dimensions of HbA upon PEGylation. Interestingly, the molecular radius of (propyl-PEG5K)6-αα-Hb exhibits a further increase as compared with (propyl-PEG5K)6-Hb, and its calculated molecular volume is nearly twice that of (propyl-PEG5K)6-Hb.
Table 2. Molecular radius of hexaPEGylated haemoglobins.
Molecular volume was calculated with the equation V=4πr3/3, where r is the radius of the sample.
| Sample | Radius (nm) | Molecular volume (nm3) |
|---|---|---|
| HbA | 3.14 | 129.6 |
| αα-Fumaryl Hb | 3.16 | 132.1 |
| (Propyl-PEG5K)6-Hb | 5.40 | 659.2 |
| (Propyl-PEG5K)6-αα-Hb | 6.56 | 1181.9 |
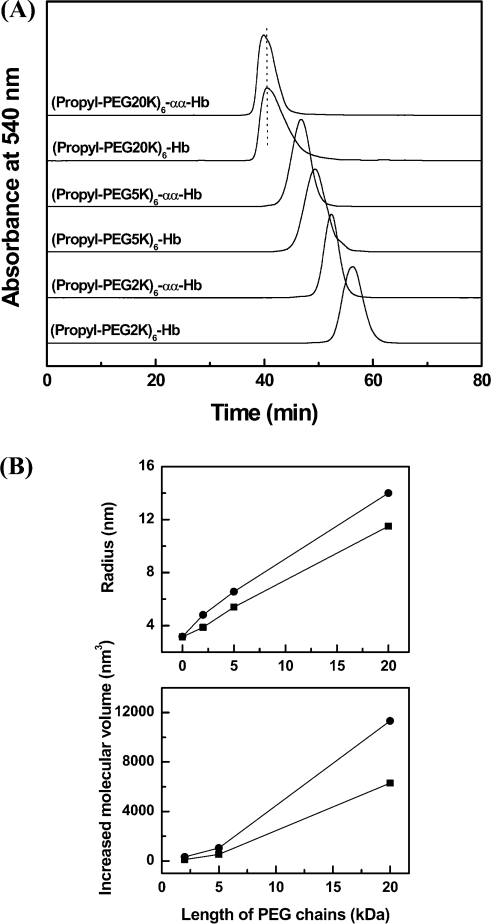
Influence of PEG chain length on the molecular volume of PEGylated proteins
PEG2K and PEG20K (PEG-20000) aldehyde are homologues of the PEG5K aldehyde. To establish the effect of PEG chain length on the hydrodynamic volume of PEGylated proteins, PEG2K, PEG5K and PEG20K aldehyde were used for reductive alkylation of cross-linked and non-cross-linked haemoglobin. The hydrodynamic volumes of PEGylated proteins were compared using SEC (Figure 3A). PEGylation of cross-linked haemoglobin using the three PEG reagents exhibited a larger hydrodynamic volume than the respective PEGylated non-cross-linked haemoglobins.
Figure 3. Influence of PEG chain length on the molecular volume of PEGylated αα-fumaryl Hbs.
(A) Size-exclusion chromatographic analysis of PEGylated protein. The analysis was carried out at room temperature on two HR10/30 Superose 12 columns connected in series. The columns were eluted with PBS, pH 7.4, at a flow rate of 0.5 ml/min. (B) Size enhancement of haemoglobin (■) and αα-fumaryl Hb (●) as a function of the length of attached PEG chains. The curves were made using Origin 6.0 software. Molecular radii were measured by dynamic light scattering at a protein concentration of 1 mg/ml. Increased molecular volume (ΔV) was calculated with an equation ΔV=4π(r3−r03)/3, where r and r0 are the radii of PEGylated haemoglobins and HbA respectively.
Figure 3(B) compares the increase in the molecular radius of haemoglobin on PEGylation as a function of PEG chain length. As the length of the PEG chain is increased, the molecular radius of the PEGylated product is also increased. The molecular volumes of the three PEGylated αα-fumaryl Hbs are nearly twice that of the corresponding PEGylated non-cross-linked haemoglobin. Therefore the influence of the αα-fumaryl intramolecular cross-link on the propensity of PEGylation to enhance the molecular volume of haemoglobin is correlated with the PEG chain length.
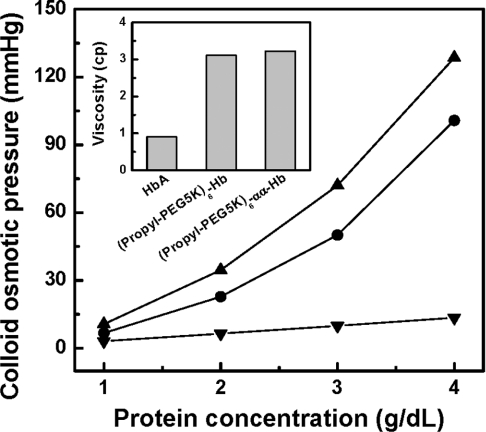
Influence of αα-fumaryl cross-link on the viscosity and COP of (propyl-PEG5K)6-Hb
The influence of αα-fumaryl intramolecular cross-linking on the viscosity and COP of PEGylated haemoglobin is presented in Figure 4. The COP of (propyl-PEG5K)6-αα-Hb exhibits a non-linear dependence on the protein concentration. (Propyl-PEG5K)6-αα-Hb exhibited a lower COP value than (propyl-PEG5K)6-Hb over the entire range of the protein concentration, in spite of its larger molecular volume than (propyl-PEG5K)6-Hb. This result reflects that there are more colloidal particles in (propyl-PEG5K)6-Hb than in (propyl-PEG5K)6-αα-Hb. The viscosity of (propyl-PEG5K)6-αα-Hb at a protein concentration of 4 g/dl has been compared with that of HbA and (propyl-PEG5K)6-Hb, and the results are presented in the inset of Figure 4. The PEGylation-induced increase in the viscosity of haemoglobin is not essentially influenced by the αα-fumaryl intramolecular cross-link. These influences as a consequence of intramolecular cross-links could be due to dissociation of non-cross-linked haemoglobin tetramers to dimers upon PEGylation.
Figure 4. Colloidal osmotic pressures of HbA (■), (propyl-PEG5K)6-Hb (●) and (propyl-PEG5K)6-αα-Hb (▲) as a function of protein concentration.
The curves were made using Origin 6.0 software. A series of concentrations of HbA samples were measured using a Wescor 4420 Colloidal Osmometer in PBS, pH 7.4, at room temperature. The inset shows a comparison of the viscosity of (propyl-PEG5K)6-αα-Hb with that of (propyl-PEG5K)6-Hb at 4 g/dl.
Analytical ultracentrifugation
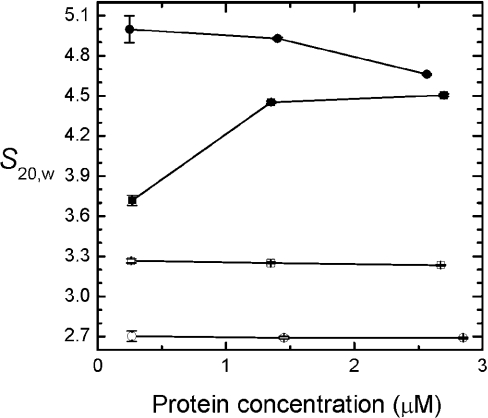
Sedimentation velocity studies of cross-linked and non-cross-linked PEGylated haemoglobin and cross-linked and non-cross-linked haemoglobin were conducted to gain more insight into the effect of PEGylation on the dimer–tetramer association of haemoglobin (Figure 5). Three of the four molecules show a decrease in the sedimentation coefficient (S) with increasing protein concentration, characteristic of monodisperse particles (●, ○, □); only non-cross-linked haemoglobin shows the increase in S with protein concentration characteristic of self-association (■). The molecular mass of (propyl-PEG5K)6-αα-Hb estimated from S020,w/D020,w is ∼90 kDa, consistent with a hexaPEGylated tetramer (○). Cross-linked but otherwise unmodified HbA sediments as a monodisperse particle (●) whose estimated molecular mass of ∼55 kDa is also consistent with a tetramer. The sedimentation rate of (propyl-PEG5K)6-Hb is lower; the molecular mass estimated for this particle is ∼60 kDa, consistent with predominantly PEGylated haemoglobin dimers (□). From these data, we conclude that PEGylation destabilizes the haemoglobin tetramer. The slow sedimentation of (propyl-PEG5K)6-Hb and (propyl-PEG5K)6-αα-Hb relative to the unmodified proteins indicates that PEGylation introduces hydrodynamic drag that can be envisaged as a ‘parachute’ impeding transport of the modified proteins [25]. This conclusion is consistent with the diffusion constants measured for the two cross-linked haemoglobin molecules. D20,w values of 8.1±2.4 and 4.3±2.0 Ficks were measured for αα-fumaryl Hb and (propyl-PEG5K)6-αα-Hb respectively at the highest protein concentrations analysed (Figure 5).
Figure 5. S20,W of PEGylated proteins as a function of haemoglobin concentration.
The curves were made using Origin 6.0 software. Sedimentation velocity measurements of (propyl-PEG5K)6-Hb (□), (propyl-PEG5K)6-αα-fumaryl Hb (○), HbA (■) and αα-fumaryl Hb (●) were conducted in a Beckman XL-I analytical ultracentrifuge in PBS, pH 7.4, at 25 °C and 55000 rev./min. Boundary movement was followed at 405 nm using the centrifuge's absorption optics.
Influence of αα-fumaryl intramolecular cross-link on structural features of (propyl-PEG5K)6-Hb
CD measurements
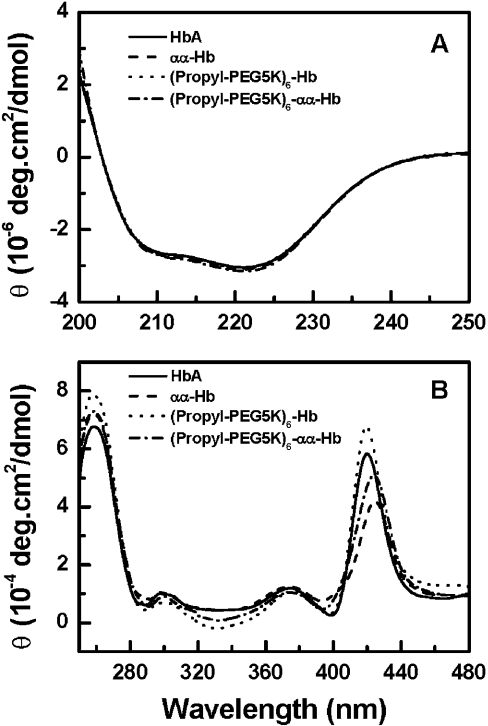
The structural features of (propyl-PEG5K)6-Hb and (propyl-PEG5K)6-αα-Hb have been investigated using CD spectroscopy. The far-UV (absorbance at 200–250 nm) CD spectra for the PEGylated proteins are shown in Figure 6(A). As indicated by the ellipticity values at 222 nm, the α-helical content of HbA was not changed upon the introduction of an αα-fumaryl cross-link and/or PEGylation. Thus the secondary structure of HbA was not influenced by either the αα-fumaryl cross-link or subsequent PEGylation.
Figure 6. CD spectra of PEGylated proteins.
CD spectra of HbA (——), αα-fumaryl Hb (– – –), (propyl-PEG5K)6-Hb (····) and (propyl-PEG5K)6-αα-Hb (·−·−) were recorded at 25 °C with a 0.2-cm-pathlength cuvette (310 μl) in the far-UV region (200–250 nm) (A), and the near-UV and Soret region (250–480 nm) (B). The molar ellipticity, θ, is expressed in deg·cm2/dmol on a haem basis.
In the near-UV CD region (Figure 6B), the L band (centred around 260 nm) is considered to be sensitive to the interactions between the haem and the surrounding globin, being influenced by the attached ligand [27]. PEGylation of HbA induced an increase in the intensity of the L band, while PEGylation of αα-fumaryl Hb showed no change in the ellipticity of the L band. This indicated that the increased intensity of the L band of HbA upon PEGylation was not related to PEGylation itself, but to PEGyl-ation-induced structural changes of HbA, i.e. the dissociation of the haemoglobin tetramer. The region around 285 nm is considered to be indicative of the transition from the R (relaxed) state to the T (tense) state, and correlated to the environment of α42 and β37 aromatic residues in HbA [28]. PEGylation of HbA and αα-fumaryl Hb both induced a decrease in the ellipticity around 285 nm [28], possibly due to the PEGylation-induced conformational change around α42 and β37 that reflects the α1β2 subunit interface contact domain. Thus the PEG shell of the PEGylated haemoglobin appears to reduce the propensity of the molecule to transition from the R state to the T state, consistent with the fact that PEGylation increases the oxygen affinity of haemoglobin [14].
The Soret band of haemoglobin is informative on the interactions of the haem prosthetic group with the surrounding aromatic residues and to modifications in the spatial orientation of these amino acids with respect to haem, affecting porphyrin transitions and π−π* transitions in the surrounding aromatic residues [29]. The presence of the αα-fumaryl intramolecular cross-link reduces the intensity of the Soret band of HbA with a wavelength shift to the red. This represents the presence of deoxy-like features in the αα-fumaryl cross-linked haemoglobin. PEGylation of haemoglobin increases the intensity of the Soret band without noticeable changes in the wavelength. This reflects the fact that the microenvironment of haem is perturbed upon PEGylation [14]. The hexaPEGylation of αα-fumaryl Hb decreases the intensity of the Soret band slightly, but the intensity is significantly lower than that of (propyl-PEG5K)6-Hb. The red shift in the Soret band, induced as a result of the αα-fumaryl cross-linking, is conserved even on PEGylation, which is considered to be a reflection of the lower affinity of haem to oxygen [28].
Front-face fluorescence measurements
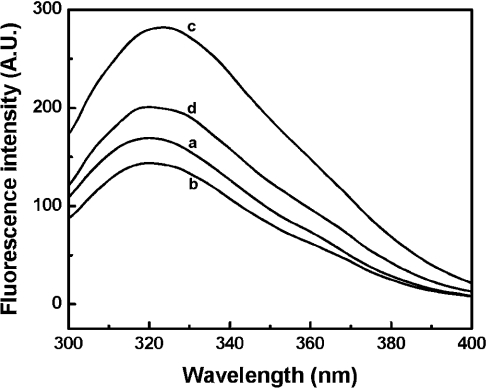
Intrinsic fluorescence of haemoglobin is primarily due to the fluorescence of Trp-37(β) at the α1β2 interface, which reflects the stability of the quaternary structure of haemoglobin [30]. When excited at 280 nm, the fluorescence intensity of HbA decreased as a result of αα-fumaryl intramolecular cross-linking, with a peak position at 320 nm (Figure 7). However, fluorescence intensity of HbA on PEGylation is significantly increased (66.3%) and exhibits a noticeable red-shifted peak position (3 nm), which reflects the perturbation of the quaternary structure of haemoglobin. Compared with (propyl-PEG5K)6-Hb, fluorescence intensity of (propyl-PEG5K)6-αα-Hb decreased (28.7%) with a blue shift to 320 nm, indicating that αα-fumaryl intramolecular cross-linking could reduce the perturbation of the quaternary structure of PEGylated non-cross-linked haemoglobin. In conjunction with the sedimentation velocity studies, this may be considered to be a reflection of the enhanced dissociation of the tetramers (reflection of the presence of dimers), and inhibition of such dissociation by the αα-fumaryl intramolecular cross-link.
Figure 7. Intrinsic fluorescence emission spectra of HbA (a), αα-fumaryl Hb (b), (Propyl-PEG5K)6-Hb (c) and (propyl-PEG5K)6-αα-Hb (d).
The excitation wavelength was 280 nm. The measurements were performed using a Shimadzu spectrofluorimeter at room temperature. All of the samples used were at a haemoglobin concentration of 5.7 mg/ml in PBS, pH 7.4. A.U., arbitrary units.
Influence of engineering a ββ-succinimidophenyl PEG2K intramolecular cross-link on the molecular properties of (propyl-PEG5K)6-Hb
The central cavity of haemoglobin plays a dominant role in dictating the structural stability and functional properties of haemoglobin. The influence of the αα-fumaryl cross-link on the molecular properties of (propyl-PEG5K)6-Hb may be unique as it is an αα-cross-link within the central cavity. In an attempt to establish the fact that the observed influence on the molecular properties of (propyl-PEG5K)6-Hb is a consequence of an intramolecular cross-link, we have asked the question whether a cross-link outside the central cavity of haemoglobin still works. A ββ-succinimidophenyl PEG2K intramolecular cross-link at Cys-93(β) was also engineered into (propyl-PEG5K)6-Hb to provide an answer to this question. As shown in Table 3, modulation of the molecular properties by the presence of ββ-cross-link in (propyl-PEG5K)6-Hb nearly parallels that of αα-fumaryl intramolecular cross-linking. The molecular radius and the molecular volume were increased. Viscosity was influenced to a limited extent, but the COP of (propyl-PEG5K)6-Hb decreased upon ββ-cross-linking. Therefore ββ-cross-linking of (propyl-PEG5K)6-Hb also achieves the same results as the αα-cross-linking, apparently by preventing the PEGylated haemoglobin tetramers from dissociating into dimers.
Table 3. Comparison of the solution properties of hexaPEGylated haemoglobins.
| Radius (nm) | Molecular volume (nm3) | COP (mmHg) | Viscosity (cp) | |
|---|---|---|---|---|
| (Propyl-PEG5K)6-Hb | 5.40 | 659.2 | 128.5 | 3.11 |
| (Propyl-PEG5K)6-αα-Hb | 6.56 | 1181.9 | 100.8 | 3.23 |
| (Propyl-PEG5K)6-ββ-Hb | 6.70 | 1259.2 | 85.2 | 2.97 |
It is surprising that the COP, the molecular radius and hydrodynamic volume of (propyl-PEG5K)6-ββ-Hb (generated by reductive alkylation of ββ-intramolecular cross-linked haemoglobin by PEG5K propionaldehyde) are slightly lower than those of (propyl-PEG5K)6-αα-Hb. This suggests that ββ-cross-linking used here is more efficient in reducing the number of dimers than αα-cross-linking. However, this is not the correct molecular explanation, since ββ- and αα-cross-linked haemoglobins in the present study are essentially homogeneous. Accordingly, the number of PEGylated particles in the two PEGylated cross-linked haemoglobins should be same, and their COPs, molecular radii and hydrodynamic volumes should be comparable with one another's. However, it should be noted that the cross-linker in ββ-cross-linked haemoglobin is long and flexible outside the central cavity of haemoglobin, whereas that in αα-fumaryl Hb is short and rigid within the central cavity of haemoglobin. The hexaPEGylated haemoglobin tetramers with weakened interactions between the dimers can be held together loosely by the ββ-cross-linker, whereas they can be held tightly in αα-cross-linked haemoglobin. Therefore the long and flexible ββ-succinimidophenyl PEG2K linker can increase the molecular radius and hydrodynamic volume of (propyl-PEG5K)6-ββ-Hb, as compared with those of (propyl-PEG5K)6-αα-Hb. The lower COP of (propyl-PEG5K)6-ββ-Hb reflects that the PEG shell around (propyl-PEG5K)6-ββ-Hb is less compact than that around (propyl-PEG5K)6-αα-Hb, and its possible consequences for the packaging of the PEG in the PEG shell. This molecular aspect of the two PEGylated cross-linked haemoglobins needs to be established by further comparative biophysical analysis.
Influence of intramolecular cross-linking on the oxygen affinity of PEGylated haemoglobin
HexaPEGylation of haemoglobin increases its oxygen affinity (lowers the p50), and the p50 decreased from the control value of 11.8 to 6.3 mmHg (1 mmHg=0.133 kPa) (Table 4). The presence of a mid-central cavity αα-fumaryl cross-link decreased the oxygen affinity of the PEGylated haemoglobin. The p50 increased from 6.3 to 16.0 mmHg. Nonetheless, it should be noted that the p50 value of αα-fumaryl Hb is considerably higher (lower oxygen affinity) than that of hexaPEGylated αα-fumaryl Hb. The intrinsic propensity of hexaPEGylation of haemoglobin to increase the oxygen affinity of haemoglobin is seen even with αα-fumaryl Hb. However, the p50 of HbA decreased to 6.3 mmHg from the control value of 11.8 mmHg as a result of ββ-cross-linking at Cys-93(β) of Hba. It may be noted that the Cys-93(β) is outside the central cavity as compared with Lys-99(α), the target site of intramolecular cross-linking using bis-dibromosalicyl fumarate. HexaPEGylation of ββ-Hb decreased its p50 to 5.9 mmHg, indicating that the p50 of ββ-Hb was not significantly influenced upon PEGylation. On the other hand, the Hill coefficients of HbA, αα-fumaryl Hb and ββ-Hb are all decreased upon PEGylation, whereas that of PEGylated αα-fumaryl Hb is the lowest. Therefore the consequence of the two different cross-links on the oxygen affinity is very distinct, even though both influenced the PEGylation-induced molecular properties at the same level.
Table 4. Oxygen-binding properties of PEGylated proteins.
Oxygen equilibrium curves of the samples were measured using Hem-O-Scan at 37 °C in PBS, pH 7.4. h is the Hill coefficient.
| Sample | p50 (mmHg) | h |
|---|---|---|
| HbA | 11.8 | 2.8 |
| αα-Fumaryl Hb | 30.5 | 2.4 |
| ββ-Hb | 6.3 | 2.1 |
| (Propyl-PEG5K)6-Hb | 6.3 | 1.9 |
| (Propyl-PEG5K)6-αα-Hb | 16.0 | 1.5 |
| (Propyl-PEG5K)6-ββ-Hb | 5.9 | 1.9 |
DISCUSSION
PEGylation of haemoglobin overcomes the vasoactivity of acellular haemoglobin by making the vasoconstrictive haemoglobin molecule into a vasodilator. The PEG–haemoglobin conjugate as a vasodilator is essentially a consequence of PEGylation-induced molecular properties of the conjugate [7]. The efficiency of albumin as a plasma volume expander is also significantly enhanced on PEGylation, which induces some new clinical properties to albumin [31]. However, it was seen that PEGylated haemoglobin has a higher COP than does PEGylated albumin, even though their molecular masses were comparable (results not shown). This observation prompted us to investigate whether the introduction of an intramolecular cross-link into haemoglobin affected the molecular, structural and functional properties of PEGylated haemoglobin.
The major finding of the present work is that PEGylated non-cross-linked haemoglobin generated by reductive alkylation chemistry predominantly exits as dimers. When the dimers are held together as tetramers by intramolecular cross-links, the molecular, structural and functional properties of the PEGylated products are significantly changed.
The influence of the cross-links on the molecular properties of the PEGylated haemoglobin is reflected by the significantly enhanced molecular volume and the lower COP of (propyl-PEG5K)6-αα-Hb relative to (propyl-PEG5K)6-Hb, which makes the cross-linked haemoglobin a better substrate in terms of new paradigms for the design of blood substitutes [32]. Owing to the increase in the molecular volume, intramolecular cross-links in the PEGylated haemoglobin will reduce its extravasation rate further. Besides, the lower COP makes it possible to use a higher concentration of haemoglobin, without the possible dilution of the infused haemoglobin by the increase in flow of fluids from the interstitial tissues to the vascular system. This has been the major limitation of the current versions of PEGylated haemoglobins used in attempts to increase the level of tissue oxygenation. The absence of influence of intramolecular cross-links on the viscosity of PEGylated haemoglobin suggests that the viscosity of PEGylated haemoglobin is a direct correlate of the PEG conjugated to protein (protein/PEG ratio).
The influence of the cross-links on the structural properties of the PEGylated haemoglobin is reflected by the CD spectra and fluorescence spectra of the products. The CD measurements reflect the perturbation of the haem environment of haemoglobin upon PEGylation, and an αα-fumaryl cross-link could decrease the perturbation. The fluorescence data suggest perturbation of the α1β2 interface of haemoglobin by PEGylation, and a reduced effect of PEGylation on these structural aspects in the presence of the αα-fumaryl cross-link. Thus, compared with the PEGylated haemoglobin, the PEGylated cross-linked haemoglobin is a better choice to develop for blood substitutes from the structural point of view.
The molecular basis for enhancing the dissociation of haemoglobin tetramers into dimers upon PEGylation is also of interest from a structural point of view. Typically, association of dimers into tetramers is driven primarily by the formation of the α1β2 interface that involves more polar contacts between the C- and N-termini and helices C and FG corners of both subunits [33]. Since the complete modification of N-termini has taken place by reductive alkylation chemistry, this can influence the interactions at both the αα-ends and the ββ-ends of the central cavity. In addition, the association of αβ dimers to tetramers is facilitated by electrostatic attraction between positively charged α subunits and negatively charged β subunits [33]. The new hydrated PEG shell around the protein core can also shield the charge of α and β subunits, which in turn can decrease the intersubunit electrostatic attractions. Further studies will be needed to delineate the molecular basis of the increased dissociation of PEGylated non-cross-linked haemoglobin.
The influence of the cross-links on the functional property of PEGylated haemoglobin has also been investigated in the present study. The mid-central cavity cross-link (αα-fumaryl cross-link) as well as cross-links outside the central cavity have similar influence on the molecular properties of PEGylated haemoglobin. However, they exert very distinct influences on the oxygen affinity of PEGylated haemoglobin. Preventing the dissociation of the PEGylated haemoglobin into dimers does not significantly influence the oxygen affinity of the molecule, as reflected by the cross-link of PEGylated haemoglobin outside the central cavity. On the other hand, αα-fumaryl cross-linking that facilitates the retention of some deoxy-like features of haemoglobin in its oxy conformation helps to reduce the oxygen affinity of PEGylated haemoglobin. Thus, if only the PEGylation-induced molecular properties need to be modulated, one could use the cross-link outside the central cavity of haemoglobin, and if oxygen affinity of the PEGylated haemoglobin also needs to be reduced, besides the modulation of the molecular properties of the PEGylated haemoglobin, αα-fumaryl cross-link will be the choice.
HexaPEGylated haemoglobin generated by the extension-arm-facilitated PEGylation, (SP-PEG5K)6-Hb, exhibits a high oxygen affinity [12,34]. The good flow properties of (SP-PEG5K)6-Hb could be a consequence of the PEGylation-induced molecular properties of the haemoglobin molecule [35] or the high oxygen affinity of the molecule [32] or a synergy of both of these components. The high oxygen affinity of (SP-PEG5K)6-Hb has been attributed to the PEGylation at Cys-93(β). (Propyl-PEG5K)6-Hb has high oxygen affinity, even though Cys-93(β) is not PEGylated [14], and reductive alkylation of HbA by glyceraldehyde, a low-molecular-mass analogue of PEG5K aldehyde, only slightly increased the oxygen affinity of haemoglobin (results not shown). This leads to the suggestion that the high oxygen affinity of PEGylated haemoglobin is induced by the PEG shell surrounding haemoglobin. In addition, PEGyl-ation of recombinant haemoglobin in which Cys-93(β) is mutated to alanine also results in the generation of PEGylated haemoglobin with high oxygen affinity [36]. Thus a common molecular mechanism involved in increasing the oxygen affinity of haemoglobin may be through surface decoration of haemoglobin with PEG chains. The propensity of the αα-fumaryl cross-link to lower the oxygen affinity of (propyl-PEG5K)6-Hb suggests that the high oxygen affinity of PEGylated haemoglobin is a consequence of structural perturbations within the central cavity of haemoglobin, and not a direct consequence of the enhanced dissociation of the PEGylated haemoglobin tetramers into dimers.
The sedimentation velocity of haemoglobin is reduced upon PEGylation, even though the molecular mass of the conjugates is higher than that of the unmodified protein, owing to the contribution of the PEG shell around the protein core. As discussed above, the conjugation of multiple copies of PEG5K chains to haemoglobin results in an enhancement in the molecular volume of the protein disproportionate to the mass of conjugated PEG; the PEG shell has a very low density of atoms relative to the protein core. The low-density PEG shell behaves as a parachute, increasing the hydrodynamic drag and thus lowering the conjugate's sedimentation velocity.
It is conceivable that PEG chains exert a similar influence when the conjugated haemoglobin is introduced into the circulatory system as a blood substitute. In this situation the interaction of the PEG chains with the endothelium at the blood/tissue interface may provide an additional mechanical stimulus distinct from the shear stress developed on the endothelial surface that is a function of the local shear rate and the bulk viscosity of the medium. The potential role of PEGylation in providing an additional mechanism of interaction with the endothelium has important physiological/biological implications, as it would lower the overall viscosity while maintaining the level of mechanical stimulation of the endothelium necessary for mechanotransduction-mediated homoeostasis. A direct practical consequence of theses findings is helpful for the development of these PEGylated proteins as new and effective blood substitutes.
Acknowledgments
We thank Dr Parimala Nacharaju, Dr Ananda and Dr Fantao Meng for critical review of the manuscript. This work was supported by a grant-in-aid from the American Heart Association Heritage Affiliate 9951021T, the National Institutes of Health grants HL58247, HL71064 and the U.S. Army grant PR023085.
References
- 1.Chang T. M. S. Future prospects for artificial blood. Trends Biotechnol. 1999;17:61–67. doi: 10.1016/s0167-7799(98)01242-6. [DOI] [PubMed] [Google Scholar]
- 2.Klein H. G. The prospects for red-cell substitutes. N. Engl. J. Med. 2000;342:1666–1668. doi: 10.1056/NEJM200006013422211. [DOI] [PubMed] [Google Scholar]
- 3.Winslow R. M. Blood substitutes. Adv. Drug Del. Rev. 2000;40:131–142. doi: 10.1016/s0169-409x(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 4.Kramer G. C. Counterintuitive red blood cell substitute: polyethylene glycol-modified human hemoglobin. Crit. Care Med. 2003;31:1882–1883. doi: 10.1097/01.CCM.0000069341.90159.C7. [DOI] [PubMed] [Google Scholar]
- 5.Winslow R. M. αα-Crosslinked hemoglobin: was failure predicted by preclinical testing? Vox Sang. 2000;79:1–20. doi: 10.1159/000031200. [DOI] [PubMed] [Google Scholar]
- 6.Gulati A., Barve A., Sen A. P. Pharmacology of hemoglobin therapeutics. J. Lab. Clin. Med. 1999;133:112–119. doi: 10.1016/s0022-2143(99)90003-3. [DOI] [PubMed] [Google Scholar]
- 7.Conover C. D., Linberg R., Shum K. L., Shorr R. G. The ability of polyethylene glycol conjugated bovine hemoglobin (PEG–Hb) to adequately deliver oxygen in both exchange transfusion and top-loaded rat models. Artif. Cells Blood Substitutes Immobilization Biotechnol. 1999;27:93–107. doi: 10.3109/10731199909117685. [DOI] [PubMed] [Google Scholar]
- 8.Intaglietta M. Whitaker Lecture 1996: microcirculation, biomedical engineering, and artificial blood. Ann. Biomed. Eng. 1997;25:593–603. doi: 10.1007/BF02684838. [DOI] [PubMed] [Google Scholar]
- 9.Rohlfs R. J., Bruner E., Chiu A., Gonzales A., Gonzales M. L., Magde M. D., Vandegriff K. D., Winslow R. M. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J. Biol. Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 10.Winslow R. M., Gonzales A., Gonzales M. L., Magde M., McCarthy M., Rohlfs R. J., Vandegriff K. D. Vascular resistance and the efficacy of red cell substitutes in a rat hemorrhage model. J. Appl. Physiol. 1998;85:993–1003. doi: 10.1152/jappl.1998.85.3.993. [DOI] [PubMed] [Google Scholar]
- 11.Acharya A. S., Intaglietta M., Tsai A. G., Malavalli A., Vandegriff K., Winslow R. M., Smith P. K., Friedman J. M., Manjula B. N. Enhanced molecular volume of conservatively pegylated Hb: (SP-PEG5K)6–HbA is non-hypertensive. Artif. Cells Blood Substitutes Biotechnol. 2005;33:239–255. doi: 10.1081/bio-200066365. [DOI] [PubMed] [Google Scholar]
- 12.Manjula B. N., Tsai A. G., Intaglietta M., Tsai C.-H., Ho C., Smith P. K., Perumalsamy K., Kanika N. D., Friedman J. M., Acharya A. S. Conjugation of multiple copies of polyethylene glycol to hemoglobin facilitated through thiolation: influence on hemoglobin structure and function. Protein J. 2005;42:133–146. doi: 10.1007/s10930-005-7837-2. [DOI] [PubMed] [Google Scholar]
- 13.Vandegriff K. D., Malavalli A., Wooldridge J., Lohman J., Winslow R. M. MP4, a new nonvasoactive PEG–Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu T., Prabhakaran M., Acharya S. A., Manjula B. N. Influence of the chemistry of conjugation of poly(ethylene glycol) to Hb on the oxygen-binding and solution properties of the PEG–Hb conjugate. Biochem. J. 2005;392:555–564. doi: 10.1042/BJ20050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–738. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979;129:175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- 17.Manjula B. N., Acharya A. S. Purification and molecular analysis of hemoglobin by high-performance liquid chromatography. Methods Mol. Med. 2003;82:31–47. doi: 10.1385/1-59259-373-9:031. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee R., Welty E. V., Walder R. Y., Pruitt S. L., Rogers P. L., Arnone A., Walder J. A. Isolation and characterization of a new hemoglobin derivative cross-linked between the α chains (Lysine 99 α1–Lysine 99 α2) J. Biol. Chem. 1986;261:9929–9937. [PubMed] [Google Scholar]
- 19.Manjula B. N., Malavalli A., Smith P. K., Chan N. L., Arnone A., Friedman J. M., Acharya A. S. Cys-93-ββ-succinimidophenyl polyethylene glycol 2000 hemoglobin A: intramolecular cross-bridging of hemoglobin outside the central cavity. J. Biol. Chem. 2000;275:5527–5534. doi: 10.1074/jbc.275.8.5527. [DOI] [PubMed] [Google Scholar]
- 20.Manjula B. N., Tsai A., Upadhya R., Perumalsamy K., Smith P. K., Malavalli A., Vandegriff K. D., Winslow R. M., Intaglietta M., Prabhakaran M., et al. Site-specific PEGylation of hemoglobin at Cys-93(β): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjugate Chem. 2003;14:464–472. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 21.Rao M. J., Schneider K., Chait B. C., Chao T. L., Keller H. L., Anderson S. M., Manjula B. N., Kumar R. A., Acharya A. S. Recombinant hemoglobin A produced in transgenic swine: structural equivalence with human hemoglobin A. Artif. Cells Blood Substitutes Immobilization Biotechnol. 1994;22:695–700. doi: 10.3109/10731199409117900. [DOI] [PubMed] [Google Scholar]
- 22.Lippincott J., Hess E., Apostol I. Mapping of recombinant hemoglobin using immobilized trypsin cartridges. Anal. Biochem. 1997;252:314–325. doi: 10.1006/abio.1997.2334. [DOI] [PubMed] [Google Scholar]
- 23.Doyle M. P., Apostol I., Kerwin B. A. Glutaraldehyde modification of recombinant human hemoglobin alters its hemodynamic properties. J. Biol. Chem. 1999;274:2583–2591. doi: 10.1074/jbc.274.4.2583. [DOI] [PubMed] [Google Scholar]
- 24.Kellett G. L. Dissociation of hemoglobin into subunits: ligand-linked dissociation at neutral pH. J. Mol. Biol. 1971;59:401–424. doi: 10.1016/0022-2836(71)90307-x. [DOI] [PubMed] [Google Scholar]
- 25.Dhalluin C., Ross A., Leuthold L. A., Foser S., Gsell B., Muller F., Senn H. Structural and biophysical characterization of the 40 kDa PEG–interferon-α2a and its individual positional isomers. Bioconjugate Chem. 2005;16:504–517. doi: 10.1021/bc049781+. [DOI] [PubMed] [Google Scholar]
- 26.Li D., Manjula B. N., Acharya A. S. Extension arm facilitated PEGylation of hemoglobin: correlation of the properties with the extent of PEGylation. Protein J. 2006;25:263–274. doi: 10.1007/s10930-006-9010-y. [DOI] [PubMed] [Google Scholar]
- 27.Zentz C., Pin S., Alpert B. Stationary and time-resolved circular dichroism of hemoglobins. Methods Enzymol. 1994;232:247–266. doi: 10.1016/0076-6879(94)32051-9. [DOI] [PubMed] [Google Scholar]
- 28.Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974;13:2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- 29.Hsu M. C., Woody R. W. The origin of the heme Cotton effects in myoglobin and hemoglobin. J. Am. Chem. Soc. 1971;93:3515–3525. doi: 10.1021/ja00743a036. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch R. E. Hemoglobin fluorescence. Methods Mol. Med. 2003;82:133–154. doi: 10.1385/1-59259-373-9:133. [DOI] [PubMed] [Google Scholar]
- 31.Cabrales P., Nacharaju P., Manjula B. N., Tsai A. G., Acharya S. A., Intaglietta M. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol–albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 32.Winslow R. M. Current status of blood substitute research: towards a new paradigm. J. Intern. Med. 2003;253:508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 33.Perutz M. F. Mechanisms regulating the reactions of human hemoglobin with oxygen and carbon monoxide. Q. Rev. Biophys. 1989;22:139–237. doi: 10.1146/annurev.ph.52.030190.000245. [DOI] [PubMed] [Google Scholar]
- 34.Acharya A. S., Manjula B. N., Smith P. K. 5,585,484. Hemoglobin crosslinkers, U.S. Pat. 1996
- 35.Tsai A. G., Cabrales P., Intaglietta M. Increased tissue PO2 and decreased O2 delivery and consumption after 80% exchange transfusion with polymerized hemoglobin. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H320–H330. doi: 10.1152/ajpheart.00654.2004. [DOI] [PubMed] [Google Scholar]
- 36.Li D., Ho N. T., Simplaceanu V., Ho C., Acharya A. S., Manjula B. N. Molecular aspects of the high oxygen affinity of nonhypertensive PEGylated hemoglobin, [(SP-PEG5K)6–Hb]. Artif. Cells Blood Substitutes Biotechnol. 2006 doi: 10.1080/10731190600974376. in the press. [DOI] [PubMed] [Google Scholar]