Abstract
During the acute-phase reaction, SAA (serum amyloid A) replaces apoA-I (apolipoprotein A-I) as the major HDL (high-density lipoprotein)-associated apolipoprotein. A remarkable portion of SAA exists in a lipid-free/lipid-poor form and promotes ABCA1 (ATP-binding cassette transporter A1)-dependent cellular cholesterol efflux. In contrast with lipid-free apoA-I and apoE, lipid-free SAA was recently reported to mobilize SR-BI (scavenger receptor class B, type I)-dependent cellular cholesterol efflux [Van der Westhuyzen, Cai, de Beer and de Beer (2005) J. Biol. Chem. 280, 35890–35895]. This unique property could strongly affect cellular cholesterol mobilization during inflammation. However, in the present study, we show that overexpression of SR-BI in HEK-293 cells (human embryonic kidney cells) (devoid of ABCA1) failed to mobilize cholesterol to lipid-free or lipid-poor SAA. Only reconstituted vesicles containing phospholipids and SAA promoted SR-BI-mediated cholesterol efflux. Cholesterol efflux from HEK-293 and HEK-293[SR-BI] cells to lipid-free and lipid-poor SAA was minimal, while efficient efflux was observed from fibroblasts and CHO cells (Chinese-hamster ovary cells) both expressing functional ABCA1. Overexpression of SR-BI in CHO cells strongly attenuated cholesterol efflux to lipid-free SAA even in the presence of an SR-BI-blocking IgG. This implies that SR-BI attenuates ABCA1-mediated cholesterol efflux in a way that is not dependent on SR-BI-mediated re-uptake of cholesterol. The present in vitro experiments demonstrate that the lipidation status of SAA is a critical factor governing cholesterol acceptor properties of this amphipathic apolipoprotein. In addition, we demonstrate that SAA mediates cellular cholesterol efflux via the ABCA1 and/or SR-BI pathway in a similar way to apoA-I.
Keywords: apolipoprotein, ATP-binding cassette transporter A1 (ABCA1), cholesterol, phospholipid, scavenger receptor, serum amyloid A
Abbreviations: ABCA1, ATP-binding cassette transporter A1; apoA-I, apolipoprotein A-I; apoE, apolipoprotein E; CE, cholesteryl ester; CHO cell, Chinese-hamster ovary cell; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; β-gal, β-galactosidase; HDL, high-density lipoprotein; HDL3, HDL particles subclass 3; HEK-293 cell, human embryonic kidney cell; IEF, isoelectric focusing; MOI, multiplicity of infection; PC, phosphatidylcholine; SAA, serum amyloid A; SR-BI, scavenger receptor class B, type I; hSR-BI, human SR-BI; mSR-BI, murine SR-BI; TBS, Tris-buffered saline
INTRODUCTION
The SAA (serum amyloid A) family of proteins is encoded by multiple genes, which display allelic variation and a high degree of homology in mammals [1]. In humans, the highly homologous SAA1 and SAA2 genes code for the corresponding non-glycosylated acute-phase SAA1 and SAA2 proteins, both commonly referred to as SAA. Triggered by inflammation through stimulation of hepatocytes by lymphokine-mediated processes, the concentrations of SAA may increase during the acute-phase reaction to levels 500–2000-fold greater than that found in the non-inflammatory state (20–50 μg/ml). Besides its role as a major acute-phase reactant [2], SAA acts as the precursor protein during secondary reactive amyloidosis and as an apolipoprotein [3,4]. SAA associates with lipoproteins of the high-density range [HDL (high-density lipoprotein)], in particular with lipid-rich α-migrating HDL3 (HDL particles subclass 3). Coupled with an inflammation-related decrease in apoA-I (apolipoprotein A-I; the major HDL-associated apolipoprotein under non-inflammatory conditions), an increased content of SAA (up to 87% of total HDL-protein content [5]) modulates the metabolic properties of its physiological carrier during inflammation. A major function of HDL is its role during reverse cholesterol transport. However, HDL-mediated cholesterol efflux capacity from peripheral tissue as well as HDL-mediated cholesterol/CE (cholesteryl ester) delivery to the liver and/or steroidogenic tissues is drastically altered during the acute-phase response. Both a decreased capacity of acute-phase (SAA-enriched) HDL to acquire cellular cholesterol from macrophages [6–8] and a decreased capacity to deliver CEs to hepatic cells [9] have been reported. Besides changes in the apolipoprotein composition, changes in the lipid composition may lead to an increased particle diameter of acute-phase HDL [10–12] that may be considered another determinant modulating the turnover of HDL particles and its constituents.
The only receptor able to mediate effective bidirectional lipid/cholesterol flux is SR-BI (scavenger receptor class B, type I). SR-BI, a multiligand scavenger receptor, can bind a variety of ligands including native and modified lipoprotein particles [13]. Evidence from genetically engineered mouse models highlights its physiological role as HDL receptor. SR-BI binds discoidal reconstituted HDL containing apoA-I and other apolipoproteins, as well as spherical HDL particles. A structural motif recognized by SR-BI is an amphipathic helix present in all HDL-associated apolipoproteins [14].
Another important sterol transporter contributing to cholesterol efflux and HDL assembly/remodelling is ABCA1 (ATP-binding cassette transporter A1) [15]. ABCA1 mediates the transfer of cellular phospholipids and cholesterol to extracellular lipid-free apoA-I or pre-β-migrating HDL particles. Through a series of intermediate steps, lipidated apoA-I then proceeds to discoidal HDL particles that are converted into spherical lipoprotein particles by lecithin:cholesterol acyltransferase, generating a CE-rich hydrophobic core within the lipoprotein particle. The resulting β-migrating HDL3 particles can interact with SR-BI or undergo conversion into large HDL2-like particles and pre-β-HDL [16]. Previous observations have suggested that lipid-free SAA, following internalization by macrophages, can inhibit intracellular acylCoA:cholesterol acyltransferase but may activate neutral cholesterol hydrolase [17]. This imbalance could promote a shift in the cellular cholesterol pool towards free cholesterol that in turn can be mobilized via the ABCA1 pathway [17]. Lipid-free SAA may bind cholesterol and modulate cholesterol flux [18], apparently by acting as a cholesterol acceptor via the ABCA1 pathway [19,20].
Recent findings provide evidence that SR-BI acts as a candidate receptor for SAA- and/or SAA-containing lipoproteins [21,22]. Lipid-free SAA has further been reported to promote SR-BI-dependent cholesterol efflux [23]. This observation is of importance as a remarkable portion (up to 15% [24]) of total plasma SAA concentrations (ranging up to 100 mg/dl during the acute-phase reaction [2]) exists in a lipid-free (non-lipid-associated) or lipid-poor form and may contribute to cholesterol homoeostasis as reported for other apolipoproteins. However, lipid-free apoA-I (although acting as a ligand for SR-BI [25]) and apoE (another HDL-associated apolipoprotein) failed to promote SR-BI-dependent cellular cholesterol efflux. Only lipidation of these apolipoproteins promotes cellular cholesterol efflux via SR-BI [25,26].
Therefore the goal of the present study was to reveal whether the extent of lipidation of SAA governs SR-BI/ABCA1-dependent cholesterol efflux to this apolipoprotein.
MATERIALS AND METHODS
Materials
Radiochemicals were purchased from DuPont/NEN. Ham's-F12K medium was from Gibco (Life Technologies) and FBS (fetal bovine serum) was from Boehringer Ingelheim Bioproducts. Plastic-ware used for tissue culture was obtained from Costar. BLT-1 (2-hexyl-1-cyclopentanone thiosemicarbazone) was from ChemBridge and Probucol was from Sigma. All other chemicals were obtained from Merck, except where indicated.
Preparation of HDL, apoA-I and SAA
ApoE-free HDL3 (d=1.125–1.21 g/ml) was prepared by discontinuous density ultracentrifugation of plasma obtained from normolipidaemic blood donors [27].
For preparation of human apoA-I, HDL3 was delipidated, apolipoproteins were redissolved in 50 mM glycine/0.5 M NaCl/6 M urea (pH 8.8) and separated by size-exclusion chromatography on a Sephacryl S-200 column [28]. The fractions containing the major peak were pooled, dialysed against 50 mM NH4HCO3 and freeze-dried. The product was homogeneous as assessed by SDS/PAGE, reverse-phase HPLC and amino acid analysis [28].
SAA1, the most abundant SAA isoform during inflammation (accounting up to 90% of total SAA [29]), was isolated from human plasma obtained from patients undergoing plasmapheresis for therapeutic purposes. Briefly, SAA was isolated by hydrophobic interaction-FPLC on octylsepharose followed by fast protein liquid-gel permeation chromatography on a Superdex TM75 column exactly as described in [30]. Further purification of SAA from co-eluting apoCs and apoA-II was achieved by preparative IEF (isoelectric focusing) technique by a Rotofor™ IEF cell between pH 5 and 7 [30]. Purity of SAA1 was checked by immunochemical techniques and amino acid analysis. Cholesterol and phospholipid content in SAA1 and apoA-I preparations (estimated as described in [7]) was below the detection limits.
Generation of reconstituted particles
Complexes comprising PC (phosphatidylcholine; Sigma) with apolipoproteins were prepared using the sodium cholate dialysis method as described in [31]. PC was suspended in 10 mM Tris/HCl (pH 8, 150 mM NaCl and 0.01% EDTA) by vortex-mixing. The mixture was held on ice for 1 h followed by the addition of sodium cholate (the final cholate/PC molar ratio was 1). SAA or apoA-I was added and the mixture was incubated for a further 20 h at 4 °C. Subsequently, sodium cholate was removed by extensive dialysis against PBS at 4 °C. The PC–apolipoprotein complexes were prepared using a weight ratio of 2.71:1.
Labelling procedures
SAA labelling with 125I-sodium was performed as described in [7] using N-bromosuccinimide as the coupling agent. Routinely, 100 μCi of 125I-sodium was used to label 200 μg of protein. This procedure resulted in specific radioactivities of between 500 and 800 c.p.m./ng of protein.
HDL3 was labelled with [cholesteryl-1,2,6,7-3H]palmitate by CE-transfer protein-catalysed transfer from donor liposomes [27]. Briefly, 200 μCi of the corresponding label and 100 μg of egg yolk lecithin were dried under argon, followed by the addition of 1 ml of 10 mM PBS. The mixture was shaken for 2 min at 37 °C and sonicated. Lipoproteins (1 ml, containing 3–6 mg of protein), 1 ml of lipoprotein-deficient serum (as a source of CE-transfer protein) and 1 ml of PBS were added. The mixture was incubated under argon at 37 °C in a shaking water bath overnight. Subsequently, the labelled HDL3 fractions were reisolated in a TLX120 bench top ultracentrifuge in a TLA100.4 rotor (Beckman). The HDL3 band (d=1.125–1.21 g/ml) was aspirated and dialysed against 10 mM PBS (pH 7.4). This labelling procedure resulted in specific radioactivities of 5–9 c.p.m./ng of protein.
Cell culture
CHO (Chinese-hamster ovary cells)
ldlA cells (clone 7, an LDL-receptor-deficient CHO cell line) were cultured in Ham's-F12K medium containing 5% (v/v) FBS, 2 mM glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin [32]. Stable transfectants expressing mSR-BI (murine SR-BI) (ldlA[mSR-BI]) were maintained in a medium containing 0.5 mg/ml G418.
Fibroblasts
Human skin fibroblasts were from explants of skin biopsies obtained from healthy volunteers [33] and maintained in DMEM (Dulbecco's modified Eagle's medium) containing 10% FBS, 2 mM glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin.
HEK-293 cells (human embryonic kidney cells)
HEK-293 cells were maintained in DMEM containing 10% FBS, 2 mM glutamine, 50 units/ml penicillin and 50 μg/ml streptomycin.
Construction of recombinant hSR-BI (human SR-BI) adenovirus and generation of HEK-293 cells transiently expressing hSR-BI
The adenoviral plasmid shuttle vector (pAvCvSv) and pJM17 vector were kindly supplied by Dr L. Chan (Baylor College of Medicine, Houston, TX, U.S.A.). The hSR-BI cDNA [kindly supplied by Dr H. Hauser, ETH (Eldgenössische Technische Hochschule), Zürich, Switzerland], which was originally inserted into pcEXV-3 vector, was partially restricted with EcoRI and the 2.5 kb band was eluted from gel. This band was subcloned into pBluescript using EcoRI site, amplified, restricted with KpnI and this fragment was finally partially restricted with BamHI. Plasmid shuttle vector was opened using KpnI and BglII and KpnI/BamHI-restricted hSR-BI cDNA was inserted. These modifications were necessary to enable insertion of hSR-BI cDNA under the control of the CMV (cytomegalovirus) promoter in the plasmid shuttle vector. To obtain transiently infected cells, adenovirus infection of HEK-293 cells with hSR-BI (termed HEK-293[hSR-BI]) or β-gal (β-galactosidase; termed HEK-293[β-gal]) as control was performed as described in [34,35]. No cell toxicity was observed under these experimental conditions.
HEK-293 cells were cultivated in 12-well culture dishes. At a density of 5×104 cells/cm2 they were rinsed once with PBS and infected with recombinant adenovirus [MOI (multiplicity of infection)=1–10 plaque-forming units) in 250 μl of infection medium (DMEM containing 50 units/ml penicillin and 50 μg/ml streptomycin) for 2 h.
Efflux experiments
Efflux of [3H]cholesterol from cells was measured by appearance of [3H]cholesterol in the cellular supernatant. Cells were incubated in the presence of [3H]cholesterol (0.5 μCi/ml) for 24 h as described in [36]. After cholesterol loading, cells were rinsed twice with TBS (Tris-buffered saline) [containing 5% (w/v) BSA] and twice with TBS and incubated for further 2 h in DMEM containing 0.1% BSA. In some experiments, Probucol was added at a final concentration of 20 μM. Subsequently, the cells were rinsed again with TBS and efflux experiments were initiated by the addition of SAA, apoA-I or HDL3. In some experiments, BLT-1 was added at 10 μM final concentration. In other experiments using ldlA7 and ldlA[mSR-BI] cells, cholesterol efflux was studied in the presence of a polyclonal rabbit anti-mSR-BI IgG, shown to block both CE association and cellular cholesterol efflux [36,37]. After 2–4 h, the culture medium was removed and the cells were lysed to estimate cell-associated radioactivity and radioactivity present in the medium. Efflux of [3H]cholesterol to culture medium was calculated as a percentage of the total by subtracting cell-associated radioactivity+radioactivity present in culture medium from radioactivity present in culture medium×100.
Association studies
Association studies of [3H]CE-labelled HDL3 to HEK-293 cells were performed at 37 °C for 2 h with 20 μg/ml [3H]CE-labelled HDL3 in the absence (total association) or presence of 1 mg of protein/ml (unspecific association) of unlabelled autologous lipoprotein [27]. Subsequently, the medium was aspirated, and the cells were rinsed twice with TBS [containing 5% (w/v) BSA] followed by two washes with TBS. Cells were then lysed with 0.3 M NaOH. The radioactivity and protein content of the cell lysate were measured in the same aliquot. Specific cell association was calculated as the difference between the total and non-specific cell association.
SDS/PAGE and immunoblotting
The samples were separated by SDS/PAGE on 7% linear gels and blotted on to nitrocellulose membranes [7]. Subsequently, blots were incubated with a sequence-specific rabbit anti-SR-BI peptide (496–509, dilution 1:1500) antibody (Abcam), a rabbit polyclonal anti-ABCA1 antibody (Genosphere Biotechnologies, dilution 1:1000), or a rabbit polyclonal anti-ABCG1 antibody (Novus, dilution 1:1500). After incubation with peroxidase-conjugated secondary antibodies (1:1000), visualization of immunoreactive bands (84 kDa for SR-BI; 250 kDa for ABCA1; 63 kDa for ABCG1) was achieved using the ECL® (enhanced chemiluminescence; Amersham) technique.
RESULTS
Effect of SR-BI expression on cholesterol efflux to lipid-free SAA
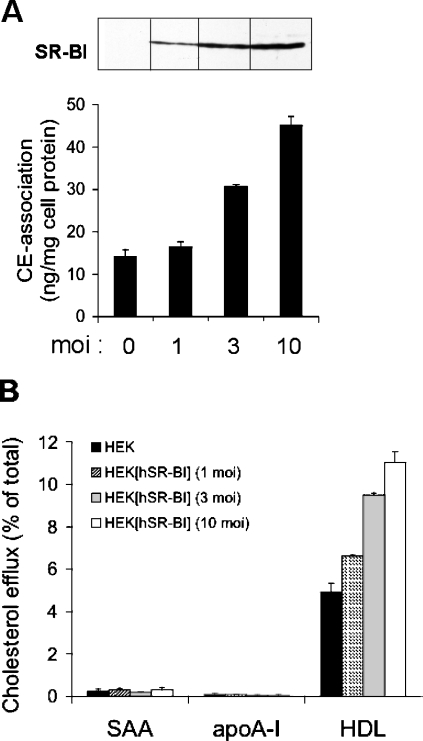
We first tested whether SR-BI affects the ability of lipid-free SAA to promote cholesterol efflux. To perform these experiments, HEK-293 cells overexpressing human SR-BI were used. Expression and functionality of SR-BI were confirmed by Western-blot experiments and by measuring CE uptake. As expected, an increased expression of SR-BI protein is paralleled by an increased cell association of [3H]CE-labelled HDL3 to HEK- 293[hSR-BI] cells (Figure 1A). Next, the ability of lipid-free apolipoproteins to promote cholesterol efflux from [3H]cholesterol-loaded HEK-293 and HEK-293[hSR-BI] cells was tested. Figure 1(B) shows that increasing SR-BI expression was without effect on cholesterol efflux to lipid-free SAA. As anticipated, lipid-free apoA-I also failed to promote cholesterol efflux from HEK-293[hSR-BI] cells. As HEK-293 cells in general do not express detectable levels of ABCA1, ABCA1-mediated lipidation of lipid-free SAA or apoA-I during the incubation period of cholesterol efflux experiments is negligible. Only HDL3-induced cholesterol efflux correlated with SR-BI expression levels. Therefore lipid-free SAA, in a similar manner as lipid-free apoA-I, is unable to induce SR-BI-mediated cholesterol efflux.
Figure 1. Cholesterol mobilization to lipid-free SAA or apoA-I occurs independently of SR-BI expression levels.
HEK-293 cells were infected with hSR-BI at the indicated MOI as described in the Materials and methods section. (A) SR-BI expression was verified by Western-blot analysis using sequence-specific rabbit anti-SR-BI peptide antibody. Association of [3H]CE-labelled HDL3 with HEK-293 cells and hSR-BI-infected HEK-293 cells was measured following incubation of cells for 2 h with 20 μg/ml [3H]CE-labelled HDL3. Cell-associated radioactivity was determined as described in the Materials and methods section. (B) HEK-293 and HEK-293[hSR-BI] cells were examined for their ability to efflux [3H]cholesterol to indicated acceptors. The cells were labelled with [3H]cholesterol as described in the Materials and methods section and incubated with lipid-free SAA (10 μg/ml), lipid-free apoA-I (10 μg/ml) or HDL3 (50 μg/ml) at 37 °C for 4 h. Media and cells were separately collected, and the radioactivity was measured by liquid-scintillation counting. Efflux was determined as a fraction of the c.p.m. in media over the total (c.p.m. in media plus c.p.m. in cells). Specific cholesterol efflux was calculated by subtracting efflux in the absence of acceptors from efflux in the presence of acceptors. Results represent mean values for triplicate determinations from one representative experiment out of three, and error bars represent ±S.D.
Effect of SR-BI expression on cholesterol efflux to lipid-poor SAA
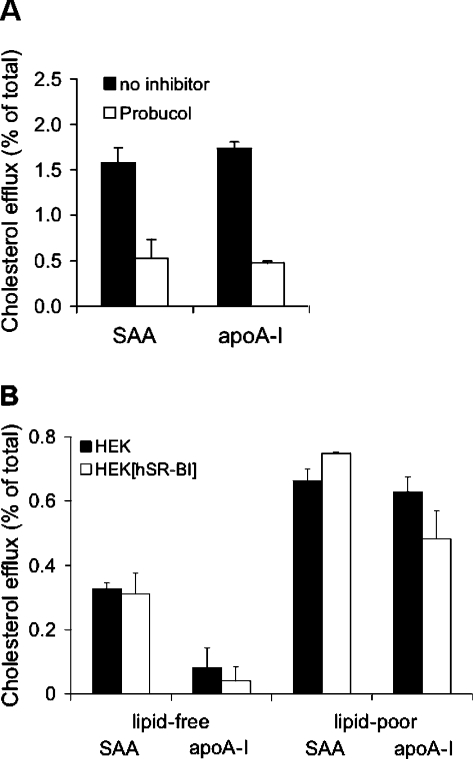
Next, we tested whether fibroblasts could lipidate SAA and apoA- I in an ABCA1-dependent manner. For these experiments, [3H]cholesterol-loaded fibroblasts were incubated with lipid-free SAA or apoA-I and cholesterol efflux was measured. As shown in Figure 2(A), both lipid-free apolipoproteins promoted cellular cholesterol efflux from 1.6% (SAA) to 1.8% (apoA-I) of total radioactivity (measured after 4 h). This apolipoprotein-mediated cholesterol efflux was mainly mediated by ABCA1 as approx. 70% inhibition of cholesterol efflux was achieved by the specific ABCA1 inhibitor Probucol [38]. As fibroblasts do not express detectable SR-BI protein levels [39], a contribution of this receptor during cholesterol efflux can be excluded.
Figure 2. Cholesterol mobilization to lipid-poor SAA or apoA-I occurs independently of SR-BI expression levels.
(A) [3H]Cholesterol-loaded fibroblast cells were incubated with lipid-free SAA or apoA-I (10 μg/ml) at 37 °C for 2 h. Where indicated, cells were pre-incubated with Probucol (20 μM) as described in the Materials and methods section. (B) Lipid-poor SAA or apoA-I was obtained by pre-incubating SAA or apoA-I (10 μg/ml) in DMEM with fibroblasts for 4 h. The lipid content of lipid-poor SAA or apoA-I was in the range 4.6–6.8 ng of cholesterol/μg of protein and 9.3–12.6 ng of PC/μg of protein and was measured as described in [7]. After the incubation period, the medium containing SAA or apoA-I was collected and added to [3H]cholesterol-labelled HEK-293 or HEK-293[hSR-BI] cells and incubated for a further 4 h at 37 °C. In parallel, lipid-free SAA or apoA-I (10 μg/ml) was added to cells. Subsequently, radioactivity in media and cells was measured and cholesterol efflux was determined. Results represent mean values for duplicate determinations from one representative experiment out of two, and error bars represent the ranges of the measurements.
The next set of experiments aimed to study whether SR-BI could mediate cellular cholesterol efflux to lipid-poor SAA or apoA-I. To generate lipid-poor (minimally lipidated) apolipoproteins, fibroblasts were incubated with lipid-free SAA or apoA-I for 4 h. After this pre-incubation period, the cellular supernatant (containing lipid-poor apolipoproteins generated via ABCA1-dependent mechanisms of fibroblasts) was transferred to [3H]cholesterol-loaded HEK-293[hSR-BI] cells. Figure 2(B) shows that cholesterol efflux to lipid-free apolipoproteins is lower (0.1% for apoA-I and 0.3% for SAA), as observed with lipid-poor apolipoproteins (∼0.6% of total cell-associated radioactivity for SAA or apoA-I). No difference became apparent between HEK-293 and HEK-293[hSR-BI] cells. Based on these findings (Figure 2), we conclude that SR-BI does not contribute to lipid-free or lipid-poor SAA- or apoA-I-induced cholesterol efflux.
Inhibition of ABCA1-mediated cholesterol efflux by SR-BI
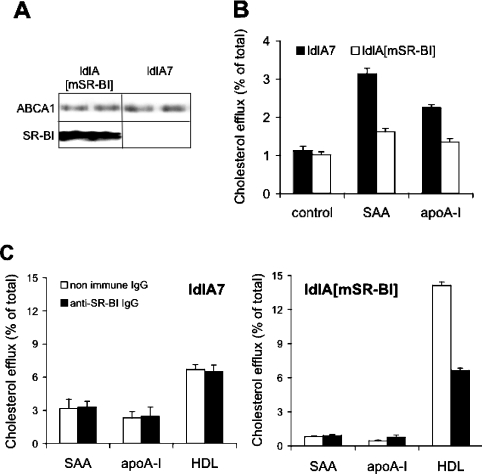
To confirm findings obtained above (Figure 2B) and to test whether the presence of ABCA1 besides SR-BI modulates the ability of SAA to mobilize cholesterol, experiments were performed with ldlA7 and ldlA[mSR-BI] cells [32]. Both cell lines express ABCA1 at similar levels (Figure 3A). High expression of SR-BI is only present in ldlA[mSR-BI] cells, while endogenous SR-BI expression is very low in ldlA7 cells. Both cell lines were loaded with [3H]cholesterol and efflux experiments were performed with lipid-free SAA or apoA-I as acceptors. Of note is the observation that ldlA[mSR-BI] cells (compared with ldlA7 cells) showed a decreased capacity for cholesterol efflux to SAA (Figure 3B). Similar data were obtained using apoA-I as cholesterol acceptor. This observation is in line with a previous report demonstrating an inhibitory effect of SR-BI on ABCA1-mediated cholesterol efflux [40]. Chen et al. [40] suggested that SR-BI could re-uptake cholesterol effluxed by ABCA1. To test this assumption, experiments were performed in the presence of a specific anti-SR-BI IgG (Figure 3C). Using ldlA[mSR-BI] cells, cholesterol efflux to SAA was not affected by anti-SR-BI IgG, while cholesterol efflux to apoA-I was slightly increased (0.45±0.06% versus 0.79±0.15%). As anticipated, the anti-SR-BI IgG blocked SR-BI-dependent cholesterol efflux from ldlA[mSR-BI] cells to HDL3. When the same experiments were performed with control ldlA7 cells, no difference in cellular cholesterol efflux to lipid-free SAA or apoA-I and HDL was observed after pre-incubating the cells with either non-immune IgG or anti-SR-BI IgG. From these experiments we conclude that the inhibitory activity of SR-BI on ABCA1-dependent cholesterol flux to SAA is not mediated by re-uptake of cholesterol via SR-BI.
Figure 3. SAA- and apoA-I-induced cholesterol efflux from ldlA[mSR-BI] and ldlA7 cells.
(A) SR-BI and ABCA1 expression by ldlA[mSR-BI] and ldlA7 cells (controls) was analysed by Western-blot analysis using sequence-specific rabbit anti-SR-BI peptide antibody or polyclonal anti-ABCA1 antibody. (B) [3H]Cholesterol-labelled ldlA7 and ldlA[mSR-BI] cells were incubated in the absence (control) or presence of lipid-free SAA or apoA-I (10 μg/ml, 4 h, 37 °C) or (C) in the presence of lipid-free SAA or apoA-I (10 μg/ml) or HDL3 (50 μg/ml) at 37 °C for 4 h in the presence of anti-SR-BI IgG or non-immune IgG. Subsequently, radioactivity in media and cells was measured and cholesterol efflux was determined. Results represent mean values for triplicate determinations from one representative experiment out of four, and error bars represent ±S.D.
Reconstituted phospholipid:apolipoprotein vesicles induce SR-BI-mediated cholesterol efflux
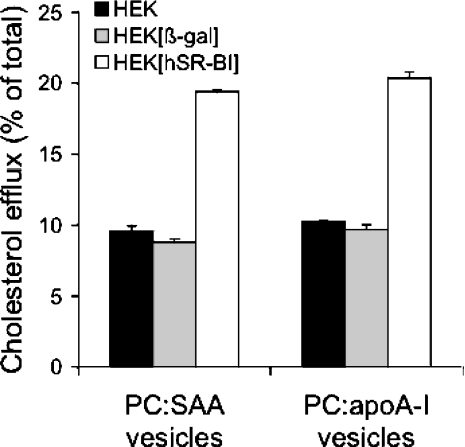
Since lipid-free and lipid-poor SAA failed to promote SR-BI-mediated cholesterol efflux (Figure 1–3), we tested whether more extensive lipidation of SAA favours SR-BI-dependent cholesterol efflux. Recent findings demonstrated that reconstituted vesicles containing lipids and apoA-I or apoE effectively promoted SR-BI-mediated cholesterol efflux [26,41,42]. HEK-293[hSR-BI] cells were used for these experiments. To avoid bidirectional cholesterol transfer, the reconstituted phospholipid vesicles used as cholesterol acceptors contained only PC and the corresponding apolipoprotein. As shown in Figure 4, vesicles containing SAA or apoA-I effectively promoted SR-BI-dependent cholesterol efflux from HEK-293[hSR-BI] cells when compared with HEK-293 and HEK-293[β-gal] cells. From these results (Figures 1–4), we conclude that sufficient lipidation of SAA, similar to that observed with apoA-I (Figure 3, [25]) and apoE [26], is a prerequisite to mobilize SR-BI-dependent cholesterol efflux.
Figure 4. Effect of SR-BI expression on cholesterol efflux to SAA:phospholipid vesicles.
HEK-293 (control), HEK-293[β-gal] (MOI=10) (virus control) and HEK-293[hSR-BI] cells (MOI=10) were examined for their ability to efflux [3H]cholesterol to PC vesicles (generated as described in the Materials and methods section) containing SAA or apoA-I (25 μg of protein/ml). The PC–apolipoprotein complexes were prepared using a weight ratio of 2.71:1. Cells were incubated for 4 h at 37 °C in the presence of indicated acceptors and cholesterol efflux was determined as described in the text. Results represent mean values for duplicate determinations from one representative experiment out of two, and error bars represent the ranges of the measurements.
The role of BLT-1 in cholesterol efflux
Recent data [23] demonstrated an inhibition of cholesterol efflux from hepatoma cells to lipid-free SAA by BLT-1, an inhibitor of SR-BI-mediated cholesterol transport [43]. As we could show that cellular cholesterol efflux to lipid-free or lipid-poor SAA (Figures 1–3) occurs independently of SR-BI, we were interested to assess the specificity of BLT-1 by using three different cell models. The first set of experiments was performed with HEK-293 (controls) and HEK-293[hSR-BI] cells that do not express ABCA1. Figure 5(A) shows that BLT-1 specifically inhibited SR-BI-mediated cholesterol efflux to HDL3. No inhibitory effect of BLT-1 was found in controls and HEK-293[β-gal]-infected cells. Probucol had no effect on SR-BI-mediated cellular cholesterol efflux from HEK-293, HEK-293[β-gal] and HEK-293[hSR-BI] cells.
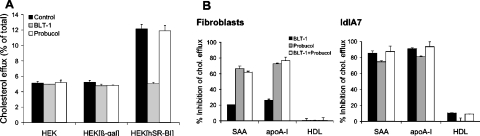
Figure 5. Effect of BLT-1 and Probucol on lipid-free SAA- or apoA-I-induced cholesterol efflux.
(A) [3H]cholesterol-labelled HEK-293 (control), HEK-293[β-gal] (MOI=10) (virus control) and HEK-293[hSR-BI] (MOI=10) cells were incubated with HDL3 (20 μg/ml), and (B) fibroblasts or ldlA7 cells were incubated with lipid-free SAA or apoA-I (10 μg/ml) or HDL3 (20 μg/ml) for 2 h in the absence or presence of BLT-1 (10 μM). Probucol (20 μM) was added to the cells 2 h prior to cell experiments as described in the Materials and methods section. Cholesterol efflux was determined as described. Inhibition of cholesterol efflux (expressed in percentage) was calculated as the difference of efflux from cells without inhibitors compared with cells in the presence of respective inhibitors. Results represent mean values for duplicate determinations from one representative experiment out of three, and error bars represent the ranges of the measurements.
Next we tested whether BLT-1 interferes with ABCA1-dependent cholesterol efflux. Therefore cholesterol efflux studies were performed with fibroblasts and ldlA7 cells that express high levels of functional ABCA1; fibroblasts do not express SR-BI [39], while low expression of SR-BI is observed on ldlA7 cells (Figure 3). When experiments were performed with fibroblasts (Figure 5B), BLT-1 inhibited apolipoprotein-mediated cellular cholesterol efflux from 20% (SAA) to 26% (apoA-I). Probucol was more effective (80% inhibition) (Figure 5B). No additive effects were observed when BLT-1 and Probucol were used in combination. Cholesterol efflux to HDL3 was not affected by BLT-1, data in line with the fact that fibroblasts do not express detectable levels of SR-BI [39]. However, when the same experiments were performed with ldlA7 cells, cholesterol efflux to lipid-free SAA or apoA-I was inhibited by BLT-1 up to 90%. A similar inhibitory activity was observed when Probucol was used (Figure 5B). Again, BLT-1 and Probucol in combination did not further alter cellular cholesterol efflux. From this set of experiments, we conclude that BLT-1 inhibits almost completely ABCA1-dependent cholesterol efflux in ldlA7 cells. However, BLT-1 showed only minimal inhibitory activity on HDL3-promoted cholesterol efflux (Figure 5B), data in line with low levels of endogenous SR-BI expression in ldlA7 cells (Figure 3A).
Our findings suggest that BLT-1 apparently interferes with ABCA1-mediated cholesterol efflux to lipid-free apolipoproteins. This observation could explain observations by others that BLT-1 may inhibit cholesterol efflux to lipid-free or lipid-poor SAA [23].
DISCUSSION
ApoA-I levels decrease during acute and chronic inflammatory conditions, while SAA, a positive acute-phase reactant, becomes the predominant apolipoprotein by modulating properties of HDL as well as enzyme activities involved in HDL remodelling [3]. A decreased activity of lecithin:cholesterol acyltransferase [8] and an increased activity of phospholipid transfer protein [11,12,44] during the acute-phase are determining factors for decreased plasma concentrations of HDL but increased concentrations of lipid-free/lipid-poor apoA-I. The latter particles act as acceptors for cellular cholesterol via ABCA1-dependent mechanisms under physiological (non-inflammatory) conditions. Accumulating evidence suggests that lipid-free/lipid-poor SAA (when abundantly available under inflammatory conditions) also contributes to cholesterol efflux via ABCA1-dependent mechanisms ([19]; Figures 2A and 5). This pathway could contribute to prevention of local cholesterol accumulation at sites of tissue destruction [45]. Alternatively, SAA – when present on HDL – could contribute to delivery of acute-phase HDL-associated cholesterol to cells involved in tissue repair at sites of inflammation [46].
The fact that lipid-associated SAA acts as a specific ligand for SR-BI is apparently related to the secondary structure of the protein. SAA contains two α-helical regions, and the N-terminal portion contains a short amphipathic α-helix that is likely to be involved in lipid binding [47,48]. Both apoA-I and synthetic amphipathic peptides containing a structural α-helical motif, effectively competed for SAA binding to hSR-BI-transfected HeLa and THP-1 cells [21]; this indicates that one or more α-helices of SAA are recognized by SR-BI.
The fact that neither lipid-free nor lipid-poor SAA acquires cellular cholesterol via SR-BI-dependent mechanisms (Figures 1B and 2B) is a major finding of the present study; an adequate lipidation status of SAA (as shown here with PC:SAA-containing vesicles) facilitates cholesterol efflux of this apolipoprotein via the SR-BI pathway. From these data, we conclude that under in vivo conditions, SAA (as commonly known for other apolipoproteins, e.g. apoA-I) has to be lipidated through a series of intermediate steps to form spherical SAA-enriched HDL particles that in turn may mobilize cholesterol via SR-BI. Our results, using purified human SAA1, are in contrast with previous findings [23]. Van der Westhuyzen et al. [23] showed supporting evidence for SR-BI-mediated cholesterol efflux to lipid-free or lipid-poor SAA; in that study BLT-1, an inhibitor of SR-BI [43], was found to inhibit cholesterol efflux from hepatoma cells [23]. However, we provide evidence that BLT-1 may interfere with ABCA1-mediated cholesterol efflux (Figure 5). In line with a recent study [49], we found that BLT-1 inhibited cholesterol efflux from human fibroblasts (cells that do not express SR-BI [39]) to lipid-free apoA-I by approx. 30%. Of note is the finding that cholesterol efflux from ldlA7 cells to lipid-free SAA or apoA-I was inhibited by BLT-1 or Probucol to a similar extent (∼90%). Thus BLT-1 apparently inhibits ABCA1-dependent efflux from ldlA7 cells almost completely.
Another important observation of the present study is that SR-BI inhibits ABCA1-mediated cholesterol efflux to lipid-free SAA. From our experiments, we conclude that the inhibitory activity of SR-BI on ABCA1-mediated cholesterol efflux cannot be sufficiently explained by re-uptake of ABCA1-effluxed cholesterol via SR-BI, as suggested in [40], as anti-SR-BI IgG failed to increase cholesterol flux from ldlA[mSR-BI] cells to SAA. Accordingly, other, not yet identified, mechanism(s) may contribute [19]. Direct binding of cholesterol to SR-BI may lead to a decreased ABCA1-accessible cholesterol pool [50]. Alternatively, SR-BI could alter membrane dynamics and properties (as already reported [37,51,52]) and these alterations may inactivate ABCA1.
In line with a recent study [20] we found that lipid-free SAA or apoA-I only minimally releases cholesterol from ABCA1-deficient HEK-293 cells. Compared with results obtained with lipid-free SAA, minimal lipidation of SAA (resulting in lipid-poor SAA) moderately enhanced the ability to induce cholesterol efflux (Figure 2B, 0.3% versus 0.7% of total radioactivity). A possible role of ABCG1 mediating sequential lipidation of ABCA1-lipidated SAA – as shown for apoA-I [53] – could be excluded as we were unable to detect ABCG1 on HEK-293 cells by Western blot experiments (results not shown); data in line with others [54].
Summarizing, we show that ABCA1- and SR-BI-dependent pathways are operative for cholesterol removal by SAA. ABCA1 is the major player mediating cholesterol flux to lipid-free SAA. This is of importance during inflammatory conditions where expression of ABCA1 in macrophages is significantly altered [8]. Most importantly, SR-BI-mediated cholesterol efflux is only operative following extensive lipidation of SAA, a phenomenon similar to that observed with other amphipathic apolipoproteins [25,26].
Acknowledgments
We are grateful to Monty Krieger (Massachusetts Institute of Technology, Cambridge, MA, U.S.A.) for providing ldlA7 and ldlA[mSR-BI] cells. We thank Dr Vadon (Department of Blood Transfusion, Medical University of Graz, Graz, Austria) for providing human plasma, and Monika Sundl for technical assistance. This work was supported by grants from the Austrian Science Fund (FWF; P17013-B05 and P19074-B05), the Austrian National Bank (OENB 9962), the Austrian Science Foundation (Bridge 810994) and the Austrian Nanoinitiative (N251-NAN) to W. S. and E. M.
References
- 1.Uhlar C. M., Burgess C. J., Sharp P. M., Whitehead A. S. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994;15:228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- 2.Malle E., De Beer F. C. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur. J. Clin. Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 3.Malle E., Steinmetz A., Raynes J. G. Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis. 1993;102:131–146. doi: 10.1016/0021-9150(93)90155-n. [DOI] [PubMed] [Google Scholar]
- 4.Uhlar C. M., Whitehead A. S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 5.Clifton P. M., Mackinnon A. M., Barter P. J. Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J. Lipid Res. 1985;26:1389–1398. [PubMed] [Google Scholar]
- 6.Banka C. L., Yuan T., de Beer M. C., Kindy M., Curtiss L. K., de Beer F. C. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J. Lipid Res. 1995;36:1058–1065. [PubMed] [Google Scholar]
- 7.Artl A., Marsche G., Lestavel S., Sattler W., Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler. Thromb. Vasc. Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- 8.Khovidhunkit W., Moser A. H., Shigenaga J. K., Grunfeld C., Feingold K. R. Regulation of scavenger receptor class B type I in hamster liver and Hep3B cells by endotoxin and cytokines. J. Lipid Res. 2001;42:1636–1644. [PubMed] [Google Scholar]
- 9.Artl A., Marsche G., Pussinen P., Knipping G., Sattler W., Malle E. Impaired capacity of acute-phase high density lipoprotein particles to deliver cholesteryl ester to the human HUH-7 hepatoma cell line. Int. J. Biochem. Cell Biol. 2002;34:370–381. doi: 10.1016/s1357-2725(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 10.Cabana V. G., Lukens J. R., Rice K. S., Hawkins T. J., Getz G. S. HDL content and composition in acute phase response in three species: triglyceride enrichment of HDL a factor in its decrease. J. Lipid Res. 1996;37:2662–2674. [PubMed] [Google Scholar]
- 11.Pussinen P. J., Malle E., Metso J., Sattler W., Raynes J. G., Jauhiainen M. Acute-phase HDL in phospholipid transfer protein (PLTP)-mediated HDL conversion. Atherosclerosis. 2001;155:297–305. doi: 10.1016/s0021-9150(00)00568-2. [DOI] [PubMed] [Google Scholar]
- 12.Pussinen P. J., Metso J., Malle E., Barlage S., Palosuo T., Sattler W., Schmitz G., Jauhiainen M. The role of plasma phospholipid transfer protein (PLTP) in HDL remodeling in acute-phase patients. Biochim. Biophys. Acta. 2001;1533:153–163. doi: 10.1016/s1388-1981(01)00153-6. [DOI] [PubMed] [Google Scholar]
- 13.Krieger M. Charting the fate of the ‘good cholesterol’: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 14.Zannis V. I., Chroni A., Krieger M. Role of apoA-I, ABCA1, LCAT and SR-BI in the biogenesis of HDL. J. Mol. Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 15.Oram J. F., Heinecke J. W. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 16.von Eckardstein A., Nofer J. R., Assmann G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Tam S. P., Flexman A., Hulme J., Kisilevsky R. Promoting export of macrophage cholesterol: the physiological role of a major acute-phase protein, serum amyloid A 2.1. J. Lipid. Res. 2002;43:1410–1420. doi: 10.1194/jlr.m100388-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Liang J. S., Sipe J. D. Recombinant human serum amyloid A (apoSAAp) binds cholesterol and modulates cholesterol flux. J. Lipid Res. 1995;36:37–46. [PubMed] [Google Scholar]
- 19.Stonik J. A., Remaley A. T., Demosky S. J., Neufeld E. B., Bocharov A., Brewer H. B. Serum amyloid A promotes ABCA1-dependent and ABCA1-independent lipid efflux from cells. Biochem. Biophys. Res. Commun. 2004;321:936–941. doi: 10.1016/j.bbrc.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 20.Abe-Dohmae S., Kato K. H., Kumon Y., Hu W., Ishigami H., Iwamoto N., Okazaki M., Wu C. A., Tsujita M., Ueda K., et al. Serum amyloid A generates high density lipoprotein with cellular lipid in an ABCA1- or ABCA7-dependent manner. J. Lipid Res. 2006;47:1542–1550. doi: 10.1194/jlr.M600145-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Baranova I. N., Vishnyakova T. G., Bocharov A. V., Kurlander R., Chen Z., Kimelman M. L., Remaley A. T., Csako G., Thomas F., Eggerman T. L., et al. Serum amyloid A binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J. Biol. Chem. 2005;280:8031–8040. doi: 10.1074/jbc.M405009200. [DOI] [PubMed] [Google Scholar]
- 22.Cai L., de Beer M. C., de Beer F. C., van der Westhuyzen D. R. Serum amyloid A is a ligand for scavenger receptor class B type I and inhibits high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 2005;280:2954–2961. doi: 10.1074/jbc.M411555200. [DOI] [PubMed] [Google Scholar]
- 23.van der Westhuyzen D. R., Cai L., de Beer M. C., de Beer F. C. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J. Biol. Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 24.Cabana V. G., Feng N., Reardon C. A., Lukens J., Webb N. R., de Beer F. C., Getz G. S. Influence of apoA-I and apoE on the formation of serum amyloid A-containing lipoproteins in vivo and in vitro. J. Lipid Res. 2004;45:317–325. doi: 10.1194/jlr.M300414-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y., Jian B., Wang N., Sun Y., Moya M. L., Phillips M. C., Rothblat G. H., Swaney J. B., Tall A. R. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 26.Chroni A., Nieland T. J., Kypreos K. E., Krieger M., Zannis V. I. SR-BI mediates cholesterol efflux via its interactions with lipid-bound ApoE. Structural mutations in SR-BI diminish cholesterol efflux. Biochemistry. 2005;44:1242–1254. doi: 10.1021/bi051029o. [DOI] [PubMed] [Google Scholar]
- 27.Marsche G., Levak-Frank S., Quehenberger O., Heller R., Sattler W., Malle E. Identification of the human analog of SR-BI and LOX-1 as receptors for hypochlorite-modified high density lipoprotein on human umbilical venous endothelial cells. FASEB J. 2001;15:1095–1097. doi: 10.1096/fj.00-0532fje. [DOI] [PubMed] [Google Scholar]
- 28.Bergt C., Marsche G., Panzenboeck U., Heinecke J. W., Malle E., Sattler W. Human neutrophils employ the myeloperoxidase/hydrogen peroxide/chloride system to oxidatively damage apolipoprotein A-I. Eur. J. Biochem. 2001;268:3523–3531. doi: 10.1046/j.1432-1327.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 29.Raynes J. G., McAdam K. P. Serum amyloid A isoforms in inflammation. Scand. J. Immunol. 1991;33:657–666. doi: 10.1111/j.1365-3083.1991.tb02538.x. [DOI] [PubMed] [Google Scholar]
- 30.Malle E., Hess H., Münscher G., Knipping G., Steinmetz A. Purification of serum amyloid A and its isoforms from human plasma by hydrophobic interaction chromatography and preparative isoelectric focusing. Electrophoresis. 1992;13:422–428. doi: 10.1002/elps.1150130189. [DOI] [PubMed] [Google Scholar]
- 31.Matz C. E., Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 32.Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 33.Malle E., Oettl K., Sattler W., Hoefler G., Kostner G. M. Cholesterol biosynthesis in dermal fibroblasts from patients with metabolic disorders of peroxisomal origin. Eur. J. Clin. Invest. 1995;25:59–67. doi: 10.1111/j.1365-2362.1995.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 34.Goti D., Hrzenjak A., Levak-Frank S., Frank S., van der Westhuyzen D. R., Malle E., Sattler W. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J. Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 35.Wadsack C., Hrzenjak A., Hammer A., Hirschmugl B., Levak-Frank S., Desoye G., Sattler W., Malle E. Trophoblast-like human choriocarcinoma cells serve as a suitable in vitro model for selective cholesteryl ester uptake from high density lipoproteins. Eur. J. Biochem. 2003;270:451–462. doi: 10.1046/j.1432-1033.2003.03394.x. [DOI] [PubMed] [Google Scholar]
- 36.Marsche G., Hammer A., Oskolkova O., Kozarsky K. F., Sattler W., Malle E. Hypochlorite-modified high density lipoprotein, a high affinity ligand to scavenger receptor class B, type I, impairs high density lipoprotein-dependent selective lipid uptake and reverse cholesterol transport. J. Biol. Chem. 2002;277:32172–32179. doi: 10.1074/jbc.M200503200. [DOI] [PubMed] [Google Scholar]
- 37.Gu X., Kozarsky K., Krieger M. Scavenger receptor class B, type I-mediated [3H]cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor. J. Biol. Chem. 2000;275:29993–30001. doi: 10.1074/jbc.275.39.29993. [DOI] [PubMed] [Google Scholar]
- 38.Favari E., Zanotti I., Zimetti F., Ronda N., Bernini F., Rothblat G. H. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 2004;12:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell B. J., Denis M., Genest J. Cellular physiology of cholesterol efflux in vascular endothelial cells. Circulation. 2004;110:2881–2888. doi: 10.1161/01.CIR.0000146333.20727.2B. [DOI] [PubMed] [Google Scholar]
- 40.Chen W., Silver D. L., Smith J. D., Tall A. R. Scavenger receptor-BI inhibits ATP-binding cassette transporter 1-mediated cholesterol efflux in macrophages. J. Biol. Chem. 2000;275:30794–30800. doi: 10.1074/jbc.M004552200. [DOI] [PubMed] [Google Scholar]
- 41.Liu T., Krieger M., Kan H. Y., Zannis V. I. The effects of mutations in helices 4 and 6 of ApoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. doi: 10.1074/jbc.M112103200. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Kan H. Y., Lavrentiadou S., Krieger M., Zannis V. Reconstituted discoidal ApoE-phospholipid particles are ligands for the scavenger receptor BI. The amino-terminal 1–165 domain of ApoE suffices for receptor binding. J. Biol. Chem. 2002;277:21149–21157. doi: 10.1074/jbc.M200658200. [DOI] [PubMed] [Google Scholar]
- 43.Nieland T. J., Penman M., Dori L., Krieger M., Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miida T., Yamada T., Yamadera T., Ozaki K., Inano K., Okada M. Serum amyloid A protein generates preβ1 high-density lipoprotein from α-migrating high-density lipoprotein. Biochemistry. 1999;38:16958–16962. doi: 10.1021/bi9913045. [DOI] [PubMed] [Google Scholar]
- 45.Kisilevsky R., Lindhorst E., Ancsin J. B., Young D., Bagshaw W. Acute phase serum amyloid A (SAA) and cholesterol transport during acute inflammation: A hypothesis. Amyloid: Int. J. Exp. Clin. Invest. 1996;3:252–260. [Google Scholar]
- 46.Gonnerman W. A., Lim M., Sipe J. D., Hayes K. C., Cathcart E. S. The acute phase response in Syrian hamsters elevates apolipoprotein serum amyloid A (apoSAA) and disrupts lipoprotein metabolism. Amyloid: Int. J. Exp. Clin. Invest. 1996;3:261–269. [Google Scholar]
- 47.Turnell W., Sarra R., Glover I. D., Baum J. O. Secondary structure prediction of human SAA1. Presumptive identification of calcium and lipid binding sites. Mol. Biol. Med. 1986;3:387–407. [PubMed] [Google Scholar]
- 48.Stevens J. F. Hypothetical structure of human serum amyloid A protein. J. Protein Folding Disord. 2004;11:71–80. doi: 10.1080/13506120412331272296. [DOI] [PubMed] [Google Scholar]
- 49.Duong M., Collins H. L., Jin W., Zanotti I., Favari E., Rothblat G. H. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler. Thromb. Vasc. Biol. 2006;26:541–547. doi: 10.1161/01.ATV.0000203515.25574.19. [DOI] [PubMed] [Google Scholar]
- 50.Sun Y., Hao M., Luo Y., Liang C. P., Silver D. L., Cheng C., Maxfield F. R., Tall A. R. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J. Biol. Chem. 2003;278:5813–5820. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 51.Parathath S., Connelly M. A., Rieger R. A., Klein S. M., Abumrad N. A., De La Llera-Moya M., Iden C. R., Rothblat G. H., Williams D. L. Changes in plasma membrane properties and phosphatidylcholine subspecies of insect Sf9 cells due to expression of scavenger receptor class B, type I, and CD36. J. Biol. Chem. 2004;279:41310–41318. doi: 10.1074/jbc.M404952200. [DOI] [PubMed] [Google Scholar]
- 52.Kellner-Weibel G., de La Llera-Moya M., Connelly M. A., Stoudt G., Christian A. E., Haynes M. P., Williams D. L., Rothblat G. H. Expression of scavenger receptor BI in COS-7 cells alters cholesterol content and distribution. Biochemistry. 2000;39:221–229. doi: 10.1021/bi991666c. [DOI] [PubMed] [Google Scholar]
- 53.Vaughan A. M., Oram J. F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi A., Takanezawa Y., Hirata T., Shimizu Y., Misasa K., Kioka N., Arai H., Ueda K., Matsuo M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]