Abstract
Cp (ceruloplasmin), a copper containing plasma protein, mainly synthesized in the liver, is known to be functional between the interface of iron and copper metabolism. We have reported previously that Cp is regulated by cellular iron status, but the process of the regulation of Cp by copper still remains a subject for investigation. In the present paper, we show that PDTC (pyrrolidine dithiocarbamate), a thiol compound widely known to increase intracellular redox copper, regulates Cp expression in hepatic cells by a copper-dependent transcriptional mechanism. To find out the mechanism of induction, chimeric constructs of the Cp 5′-flanking region driving luciferase were transfected into human hepatic cells. Deletion and mutational analyses showed the requirement of a novel APRE [AP-1 (activator protein-1) responsive element] present about 3.7 kb upstream of the translation initiation site. The role of AP-1 was confirmed by electrophoretic mobility-shift analysis. Western blot and overexpression studies detected the AP-1 as a heterodimer of c-jun and c-fos proteins. The activation of AP-1 was found to be copper-dependent as a specific extracellular chelator bathocuproine disulfonic acid blocked PDTC-mediated induction of AP-1–DNA binding and increased reporter gene activity. Whereas, in a copper-free medium, PDTC failed to activate either AP-1 or Cp synthesis, supplementation of copper could reverse AP-1 activation and Cp synthesis. Our finding is not only the first demonstration of regulation of Cp by redox copper but may also explain previous findings of increased Cp expression in cancers like hepatocarcinoma, where the intracellular copper level is higher in a redox compromised environment.
Keywords: activator protein-1 (AP-1), ceruloplasmin, copper, gene regulation, luciferase, transcription
Abbreviations: AP-1, activator protein-1; APRE, AP-1 responsive element; BCEC, bovine cerebral endothelial cells; BCS, bathocuproine disulfonic acid; Cp, ceruloplasmin; DMEM/F12, Dulbecco's modified Eagle's F12 medium; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift assay; HIF-1, hypoxia-inducible factor-1; HRE, hypoxia response element; NFκB, nuclear factor κB; PDTC, pyrrolidine dithiocarbamate
INTRODUCTION
Cp (ceruloplasmin) is a copper containing 132 kDa acute phase protein primarily of hepatic origin and accounts for 95% of the total circulating copper in healthy adults. The suggested physiological functions of Cp include a role in copper transport, coagulation [1,2], angiogenesis [3], defence against oxidant stress by scavenging superoxide radicals [4] and sequestering free copper ions [5], as well as in oxidation of low-density lipoproteins [6–9]. Cp also plays an important role in iron homoeostasis as a ferroxidase by catalysing the conversion of Fe2+ into Fe3+ for binding to apo-transferrin [10]. The role of Cp in iron homoeostasis is supported in patients with hereditary Cp deficiency [11] and in mice with targeted disruption of the Cp gene [12]. These findings, together with early organ culture and animal studies [10,13], suggest that Cp is required for efficient iron release from cells and tissues. In contrast, Cp has been shown to increase iron uptake by iron-deficient cells of hepatic and erythroid origin [14,15] and by glioblastoma cells [16,17]. Accordingly, we have shown that iron deficiency and hypoxia regulate Cp synthesis by a transcriptional mechanism involving HIF-1 (hypoxia-inducible factor-1) [18]. In contrast, the regulation of Cp synthesis by copper is still the subject of much speculation. Very few studies have reported the relationship between cellular copper content and Cp synthesis, among them are a study of Cp synthesis in copper-deficient rats [19] and an investigation of increased Cp expression in liver cells [20] by a non-physiological high level of free copper (>100 μM).
Copper in its metallic form is transported in to the cell by the copper transporter CTR1 [21] and regulates MT (metallothionein) to avoid copper-mediated toxicity [22]. But, when copper binds to a thiol compound such as PDTC (pyrrolidine dithiocarbamate) it can be redox active and influence several cellular processes such as apoptosis [23] and down regulation of p53 DNA binding activity [24,25] by increasing intracellular redox-active copper. PDTC has also been shown to inhibit the oxidative activation of the transcription factor NFκB (nuclear factor κB) [26] by altering the cellular content of transition metals. PDTC may also exert either anti-oxidant or pro-oxidant effects by its ability to bind transition metals like copper, zinc, iron and manganese by its two thiol moieties [27].
The role of Cp in regulating oxidative stress and iron homoeostasis makes it a strong candidate for regulation by alteration of cellular redox balance, but surprisingly this has not so far been studied. Since it is well known that PDTC alters cellular redox by increasing intracellular copper, we have chosen this compound to help us understand the regulation of Cp by redox copper. In the present study, we have demonstrated that treatment of the hepatic cell lines HepG2 or Hep3B with PDTC increased Cp synthesis by a transcriptional mechanism. PDTC treatment activates AP-1 (activator protein-1) by a copper-dependent mechanism to bind to a novel AP-1 site in the Cp 5′-flanking region and transactivates the Cp gene. The present study is the first to demonstrate the regulation of Cp expression by alteration of cellular redox involving copper, which may explain the mechanism of increased Cp expression in cancers [3,28].
EXPERIMENTAL
Reagents
Human Cp was purchased from Calbiochem. Rabbit polyclonal anti-human Cp IgG and peroxidase-conjugated anti-rabbit IgG were obtained from Accurate Chemical and BioRad respectively. Other reagents were obtained from Sigma unless otherwise indicated.
Cell lines and culture conditions
Human hepatocarcinoma HepG2 and Hep3B cells were obtained from American Type Culture Collection. The cells were cultured in DMEM/F12 (Dulbecco's modified Eagle's F12 medium; Sigma D8437; containing 0.0000013 g/l of CuSO4·5H2O, or approx. 20 nM of copper) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 100 units/ml penicillin and 100 mg/ml streptomycin. Cells at 50–60% confluence were used in all experiments. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C in a chamber (Forma Scientific).
Immunoblot analyses of Cp, c-jun, c-fos and α-actin
To immunoblot Cp, conditioned medium from HepG2 or Hep3B cells was subjected to SDS/PAGE (7% gels) and transferred to Immobilon-P membrane (Millipore). The membrane was incubated with anti-human Cp IgG (1:10000) as the primary antibody, and then with peroxidase-conjugated secondary antibody (1:5000). Cp was detected by chemiluminescence using ECL® reagent (Amersham). The blot was subsequently incubated with Coomassie Blue to verify uniform loading of all samples. To analyse the cellular content of c-jun, c-fos and α-actin, hepatic cell extracts were prepared as described previously [29]. Cell extracts (40 μg) were subjected to SDS/PAGE (10% gels) and transferred to Immobilon-P membrane. The membranes were incubated with anti-human c-jun (Cell Signaling), anti-human c-fos (Cell Signaling) and anti-human α-actin (Santa Cruz) antibodies at 1:2500 dilutions, and then with a peroxidase-conjugated secondary antibody (1:5000). The levels of expression of c-jun, c-fos and α-actin were detected by chemiluminescence.
RNA blot analysis
RNA blot analysis was performed using total RNA (20 μg) isolated from HepG2 and Hep3B cells by TriPure reagent (Roche) as described previously [18,30].
Construction of vectors containing the Cp promoter and enhancer segments
Cp 5′-flanking region/promoter constructs, engineered to contain SacI and XhoI restriction sites, were made by PCR amplification using Pfu polymerase (Stratagene), from a 4774 bp fragment of the 5′-flanking region of the human Cp gene ligated into the pGL3basic vector (Promega) [18,30]. To make the long constructs, the PCR products from two separate amplification reactions were ligated to form a single construct. In brief, a proximal construct was made from −2389 (just upstream of an EcoRI site) to −1. Several distal constructs were PCR-amplified from 5′-termini at −4774, −3701 and −3639 to the 3′-terminus at −2325. The proximal and distal products were ligated at the EcoRI site and then into the 5′-SacI and 3′-XhoI sites upstream of luciferase in the pGL3basic vector. Site-directed mutagenesis of the Cp-APRE [where APRE is the AP-1 (activator protein-1) responsive element] site (−3684 to −3677) was performed using the megaprimer method [18,30]. All of the constructs were verified by sequencing. The c-jun and c-fos expression vector under the CMV promoter were used as previously described [31].
Transient transfection of cells and reporter gene assays
To measure transcriptional efficiency of Cp constructs, HepG2 or Hep3B cells at approx. 50% confluence in 35 mm dishes were transiently transfected for 6 h with a reporter plasmid (2 μg) using Lipofectamine™ 2000 (Invitrogen). To monitor transfection efficiency, a reporter gene construct (0.25 μg) containing β-galactosidase behind an SV40 promoter was co-transfected. Transfected cells were allowed to recover for 6 h in DMEM/F12 containing 10% fetal bovine serum and then were incubated with PDTC in serum-free medium for 16 h. Luciferase (Promega) and β-galactosidase (Invitrogen) activities in cell extracts were determined as described in the manufacturer's protocol.
Preparation of nuclear extracts
Nuclear extracts were prepared from HepG2 or Hep3B cells as described previously [29]. Briefly, 1×108 cells were washed twice with ice-cold PBS and once with Buffer A [20 mM Hepes (pH 7.9), 20% glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT (dithiothreitol), 0.1% Nonidet P40 and protease inhibitors (PMSF, leupeptin, aprotenin and pepstatin)]. After incubation with Buffer A on ice for 10 min, the cells were lysed using an homogenizer and the homogenates were centrifuged at 2500 g at 4 °C for 2 min. The nuclear pellet was resuspended in five volumes of Buffer B [20 mM Hepes (pH 7.9), 20% glycerol, 350 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 0.1% Nonidet P40 and protease inhibitors (PMSF, leupeptin, aprotenin and pepstatin)]. The nuclei were lysed by incubation for 1 h on ice with intermittent tapping. Homogenates were then centrifuged at 10000 g at 4 °C for 15 min, and aliquots of supernatants were snap frozen at −80 °C until use.
EMSA (electrophoretic mobility-shift assay)
Sequences of the sense strands of the oligonucleotide probes used for EMSA were as follows: 5′-GAC CTG ACT GAT AGG GGA GAA TAG-3′ (Cp-APRE), 5′-GAC CAG ACA GAT AGG GGA GAA TAG-3′ (mutated Cp-APRE), 5′-GAT CAA AGC ATG AGT CAG ACA CCT-3′ (collagenase-1), 5′-TCT GTA CGT GAC CAC ACT CAC CTC-3′ [Cp-HRE (hypoxia response element)]. The sense and antisense strands were annealed, gel-purified and end-labelled with [γ-32P]ATP (NEN Life Science Products) using T4- polynucleotide kinase (Promega). Unincorporated nucleotide was removed by gel filtration using G-25 Sephadex columns. To measure the DNA–protein interaction, 2–5×104 c.p.m. of oligonucleotide probe was incubated with 5 μg of nuclear extract and 0.5 μg of sonicated, denatured salmon sperm DNA (Invitrogen) in 10 mM Tris/HCl (pH 7.8), 50 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 5 mM DTT and 5% glycerol, for 20 min at 4 °C in a total volume of 25 μl. The reaction mixture was subjected to electrophoresis (200 V in 0.3× Tris-buffered EDTA solution at 4 °C) using 5% non-denaturing polyacrylamide gels. Dried gels were subjected to autoradiography for up to 24 h. For competition experiments, a 100-fold molar excess of unlabelled, annealed oligonucleotide was pre-mixed with radiolabelled probe before addition to the binding reaction. For gel supershift analysis, 2 μl of a rabbit polyclonal antibody against c-jun or HIF-1α (Novus Biological) was pre-incubated with nuclear extracts before addition of the 32P-labelled probes.
Statistics
All experiments were performed at least three times with similar results, and representative experiments are shown. Densitometric results are normalized with respect to internal controls and are expressed relative to the results in untreated controls. Results are expressed as means±S.D. To test significance the Student's t test was performed using Sigma Plot version 8. P values <0.05, 0.01 and 0.001 are represented as *, **, and *** respectively.
RESULTS
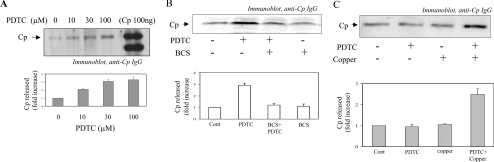
Human hepatic cell lines HepG2 and Hep3B are well-established cellular models to study Cp regulation [14,18]. To find out whether PDTC had any influence on the expression of Cp, HepG2 cells were treated with PDTC (10–100 μM) for 16 h and an immunoblot analysis of the conditioned medium was performed. The steady-state synthesis and secretion of the Cp was increased by addition of up to 100 μM PDTC (Figure 1A). Densitometric analysis revealed an approx. 3-fold increase in Cp in the conditioned medium with 100 μM PDTC treatment. To find out whether PDTC also regulated Cp expression in Hep3B, cells were treated with PDTC (100 μM) for 16 h. A similar extent of increase in Cp expression (approx. 2.9-fold) as HepG2 was detected by Western blot analysis in conditioned medium (Figure 1B), indicating that the regulation may apply to hepatic cells in general. Since the increase in Cp expression by PDTC was found in both HepG2 and Hep3B cells, we have reported further experiments either in HepG2 or Hep3B cells, although all of the experiments were performed in both cell lines with similar results. To understand the role of copper, Hep3B cells were pretreated with the non-permeable cuprous ion chelator BCS (bathocuproine disulfonic acid) 30 min before the addition of PDTC. BCS addition almost completely blocked the PDTC-induced Cp expression, suggesting a role of copper in the process (Figure 1B). RPMI-1640 is well known as a metal-free medium [6,32]. To further confirm the role of copper, HepG2 cells were treated with PDTC in metal-free RPMI-1640 (Sigma, R7388). PDTC failed to increase the synthesis of Cp (Figure 1C), but when 20 nM of copper sulfate (a similar concentration present in DMEM/F12) was added along with PDTC in RPMI-1640, Cp expression was found to increase approx. 2.5-fold (Figure 1C). The addition of copper alone in the absence of PDTC had no effect on Cp synthesis (Figure 1C, lane 3) strongly suggesting the role of redox-active copper in Cp expression.
Figure 1. Copper-dependent regulation of Cp expression by PDTC in hepatic cells.
(A) HepG2 cells were incubated with PDTC (0–100 μM). After 16 h of incubation conditioned medium were subjected to immunoblot analysis of Cp. (B) Hep3B cells were incubated with PDTC (100 μM). BCS (50 μM) was added 30 min before the addition of PDTC. After 16 h, immunoblot analysis of Cp was performed with the conditioned medium. (C) HepG2 cells were grown in DMEM/F12 with 10% fetal bovine serum and then incubated in RPMI-1640 before treatment with PDTC (100 μM), PDTC (100 μM)+CuSO4 (20 nM), CuSO4 (20 nM) alone or no treatment. Conditioned medium was collected and subjected to Western blot analysis as described previously. The results are representative of a minimum of three separate experiments.
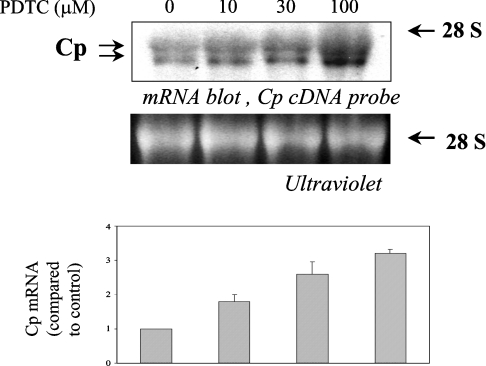
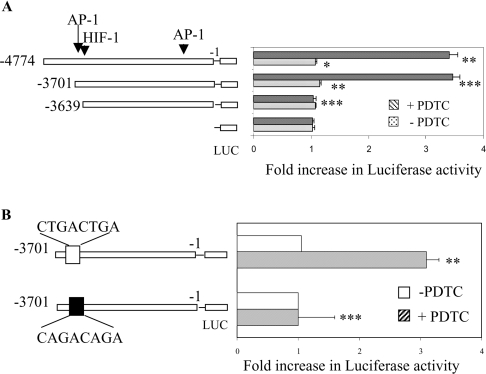
Northern blot analysis was performed to investigate whether the increase in Cp synthesis was due to an increase in Cp mRNA. Treatment of Hep3B cells with increasing concentrations of PDTC showed a maximum 3.2-fold increase in Cp mRNA with 100 μM of PDTC treatment (Figure 2). A similar result was also obtained with HepG2 cells (results not shown). Since we have shown previously that the half-life of Cp mRNA in hepatic cells is more than 10 h [14], we assumed that the increase in Cp mRNA by PDTC was due to transcriptional mechanisms. To confirm this and to find out the regulatory sequence(s) responsive to PDTC, a 4774 bp 5′-flanking fragment cloned into the pGL3basic vector was transfected into HepG2 cells and tested for PDTC-induced activation of luciferase activity. A more than 3-fold increase in luciferase activity by PDTC treatment was detected compared with untreated controls (Figure 3A). This result indicates the presence of cis-acting sequence(s) that confer sensitivity to PDTC within the 4774 bp 5′-flanking region of human Cp. To identify the responsive site(s), a series of fragments was constructed with progressively larger 5′-deletions. HepG2 cells were transfected with these constructs and incubated for 16 h with PDTC. Transactivation of the entire 4774 bp construct was increased approx. 3.3-fold, consistent with the relative increases in Cp protein and Cp mRNA levels. Similarly, PDTC transactivated a chimaeric construct (Cp−3701,−1 Luc) approx. 3.4-fold. However, the activation by PDTC was completely abrogated in a construct consisting of 3639 bp (Cp−3639, −1 Luc) (Figure 3A). These results suggest that the increase in Cp synthesis is due to transcriptional mechanisms and that one or more element(s) may be present in this region of the Cp 5′-flanking region (−3701 to −3639) that is responsible for PDTC-induced transactivation of the Cp gene.
Figure 2. Increase in Cp mRNA expression by PDTC in Hep3B.
Hep3B cells were treated with PDTC (0–100 μM) for 16 h. Total RNA was isolated and Cp mRNA expression was determined by Northern blot analysis (upper panel). UV detection of the 28 S ribosomal subunit served as a loading control (middle panel). In the bottom panel densitometric analysis of the experiment is provided.
Figure 3. Determination of the PDTC-responsive element of the Cp gene 5′-flanking region by deletion and mutation analysis.
(A) Chimaeric pGL3-basic vectors containing the proximal 4774, 3701 or 3639 bp (upstream of the translation initiation site) of the Cp gene 5′-flanking region were transiently transfected into HepG2 cells (with a plasmid containing β-galactosidase to correct for transfection efficiency) using Lipofectamine™ 2000. After recovery, the transfected cells were incubated with 100 μM PDTC (striped bars) for 16 h or were untreated (dotted bars). Luciferase (LUC) activity in cell extracts was measured and normalized for β-galactosidase activity. Shown are the means±S.D. n=3, *P<0.05, **P<0.01 and ***P<0.001. (B) The Cp 5′-flanking region containing the putative Cp-APRE was mutated. The constructs were transiently transfected (with a β-galactosidase plasmid) into Hep3B cells. After recovery, cells were incubated with 100 μM PDTC (striped bars) for 16 h or were left untreated (open bars). Luciferase (LUC) activity in cell extracts was measured and normalized for β-galactosidase activity. Shown are the means±S.D. n=3, **P<0.01 and ***P<0.001.
Previously, PDTC has been shown to activate AP-1 [33], thus we considered the possibility of the involvement of AP-1 in the activation of Cp. A search for a consensus AP-1 element revealed one putative AP-1 binding site in this region (5′-CTGACTGA-3′). To discover the involvement of this APRE we mutated the APRE (Cp-APRE) and transfected it into Hep3B cells. In comparison with a more than 3-fold increase for the wild-type, the increase in luciferase activity in the mutated Cp-APRE construct was almost completely blocked, indicating an essential role of this site in PDTC-mediated activation of Cp.
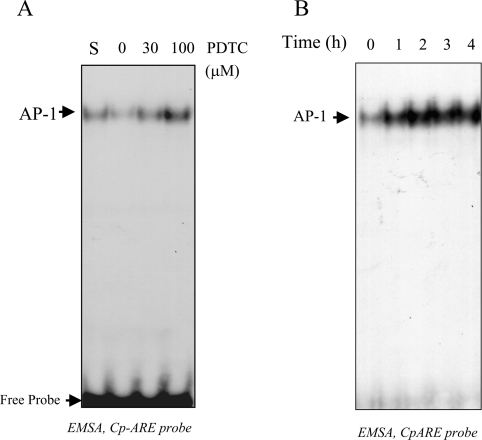
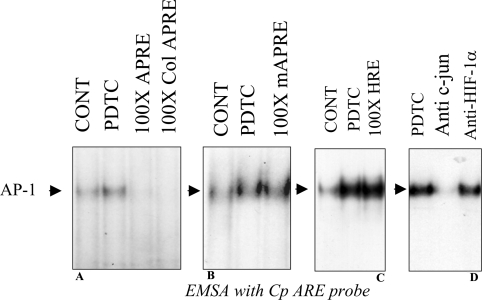
EMSAs were performed to identify the transcription factor complex that binds to the functional AP-1 binding site. Nuclear extracts prepared from PDTC-treated Hep3B or HepG2 cells were incubated with a radiolabelled 24 bp probe containing the Cp-APRE site of human Cp. In response to increased concentrations of PDTC, specific formation of a radiolabelled complex with increased intensity was observed (Figure 4A). Addition of 10% fetal bovine serum also increased the binding of the complex that had the same electrophoretic mobility as that found by PDTC treatment, indicating that AP-1 might be involved in this process (Figure 4A). The induction of a putative AP-1 complex was detected as maximal between 1–2 h (Figure 4B) and remained induced up to at least 4 h after treatment. Competition experiments were performed to show the specificity of binding of the complex to the Cp-APRE probe. The binding of the radiolabelled Cp-APRE probe to the putative AP-1 complex in nuclear extracts from PDTC-treated HepG2 cells was effectively competed by a 100-fold molar excess of unlabelled probe as well as a classical unlabelled AP-1 probe, collagenase-1 (Figure 5A). Interestingly, the complex formed by the nuclear extracts from untreated cells was also competed out, indicating the presence of a basal level of AP-1 in the hepatic cells. However, unlabelled competitor probe containing a mutation in the Cp-APRE binding site was ineffective even at 100-fold molar excess (Figure 5B). Similarly, when 100-fold molar excess of unlabelled Cp-HRE probe was used as a non-specific competitor, no reduction of AP-1 binding was detected, indicating the specificity of the binding (Figure 5C). To further confirm the complex as AP-1, a supershift analysis was performed. A rabbit polyclonal anti-c-jun antibody blocked the formation of the complex but it was not blocked by an anti-HIF-1α antibody (Figure 5D). These results confirmed that a previously unreported active AP-1 binding site is present in the distal 5′-flanking region of the Cp gene, which binds AP-1 in response to PDTC in hepatic cells.
Figure 4. Specific binding of a PDTC-stimulated transcription factor complex to Cp-APRE.
EMSAs were carried out to determine the Cp-APRE complexes. (A) HepG2 cells were treated for 2 h with increasing concentrations of PDTC. S denotes treatment with 10% fetal bovine serum. (B) Hep3B cells were treated with 100 μM PDTC for different time periods. In both (A) and (B), after the treatment, nuclear extracts were prepared and incubated with a 32P-labelled double-stranded 24-mer probe containing the Cp-APRE. Probe-bound complexes were resolved by 5% nondenaturing PAGE and visualized by autoradiography.
Figure 5. Identification of the PDTC-induced transcription factor as AP-1.
HepG2 cells were treated with 100 μM PDTC for 2 h and nuclear extracts were prepared. The radiolabelled double-stranded 24-mer Cp-APRE probe was pre-mixed with unlabelled, annealed, 24-mer oligonucleotide competitors at 100-fold molar excess before addition to the nuclear extracts. The competitor probes were wild-type CpAPRE and the collagenase-1 APRE (Col APRE) (A), mutated Cp APRE (mAPRE) (B), and Cp-HRE as the non-specific probe in (C). (D) For supershift analysis, before subjecting nuclear extracts to electrophoresis, the mixture containing the 32P-labelled double-stranded 24-mer probe containing the Cp-APRE was incubated with 2 μl of either anti-c-jun or anti-HIF-1α and probe-bound complexes were identified by autoradiography. CONT, control.
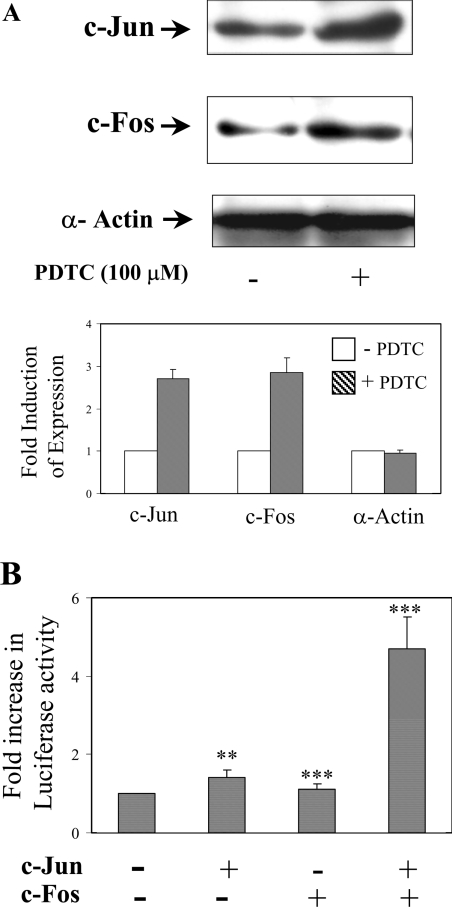
The increase in AP-1 by PDTC treatment in hepatic cells might be due to an increase in its components like c-jun and/or c-fos. To investigate whether any of the two components were increased, HepG2 cells were treated with 100 μM of PDTC for 2 h and the expression of c-jun and c-fos in the cell extracts was tested by Western blot analysis. The protein levels of c-jun and c-fos were found to be elevated (2.7- and 2.85-fold respectively) when compared with untreated cells (Figure 6A). Similarly, the overexpression of c-jun and c-fos along with Cp −3701,−1 Luc, increased luciferase activity approx. 4.5-fold, even without treatment with PDTC (Figure 6B), confirming the contribution of PDTC in the increase of c-jun and c-fos expression. Overexpression of c-jun elevated the luciferase activity marginally, whereas, c-fos alone failed to have an effect on the luciferase activity. These experiments strongly suggest that PDTC induces c-jun and c-fos to form the active AP-1 to regulate the expression of Cp.
Figure 6. PDTC-induced activation of AP-1 involves c-jun and c-fos.
(A) Cell extracts (40 μg) from 100 μM PDTC-treated HepG2 cells (2 h) were subjected to SDS/PAGE (10% gels) followed by immunoblot analysis using polyclonal rabbit anti-human c-jun, c-fos and α-actin IgG. (B) Overexpression of c-jun and c-fos increased the transactivation of the AP-1 containing Cp 5′-flanking region. Hep3B cells were transiently transfected with a chimaeric construct containing the proximal 3701 bp of the Cp gene 5′-flanking region driving luciferase in pGL3-basic vectors along with cDNAs of c-jun and c-fos driven by a CMV promoter. All cells were co-transfected with a β-galactosidase plasmid to correct for transfection efficiency. After recovery, the luciferase activity in cell extracts of transfected cells was measured by chemiluminescence and normalized for β-galactosidase activity. Shown are the means±S.D. n=3, **P<0.01 and ***P<0.001.
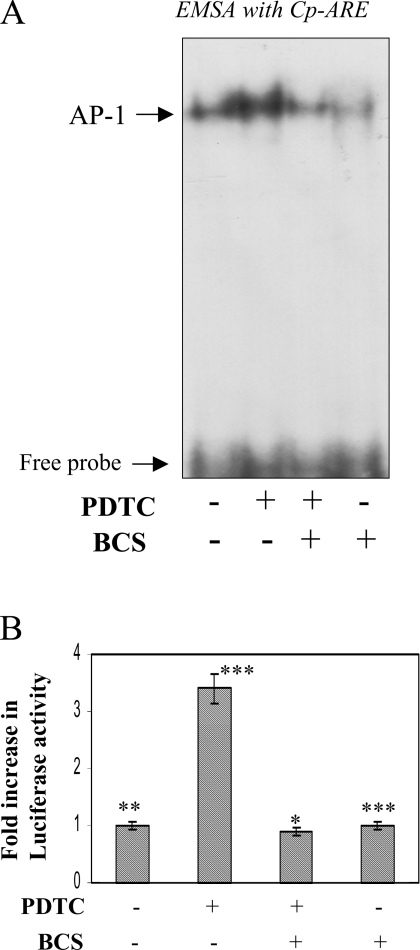
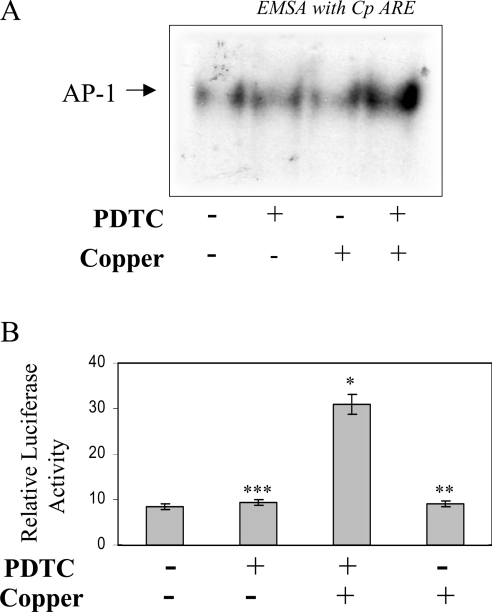
To verify that redox-active copper is actually required for AP-1 activation, Hep3B cells were incubated with 50 μM of BCS prior to PDTC treatment. Presumably, BCS can compete with PDTC for extracellular copper and reduce the influx of copper ions mediated by PDTC. The addition of BCS blocked the PDTC-mediated activation of AP-1, as found by EMSA using the Cp-APRE probe (Figure 7A). Similarly, when HepG2 cells transfected with the Cp −3701,−1 Luc construct were pretreated with BCS before PDTC treatment, the increase in luciferase activity was blocked almost completely (Figure 7B). To further prove the role of copper in PDTC-induced activation of AP-1, we took advantage of copper-free medium RPMI-1640 [6,32] as described previously for Figure 1(C). When HepG2 cells were treated with PDTC in RPMI-1640, no activation of AP-1 was detected either by EMSA or by luciferase activity in Cp −3701,−1 Luc-transfected cells (Figures 8A and 8B respectively). The activation of AP-1 can be restored by the addition of 20 nM copper in the presence of PDTC (Figures 8A and 8B) but not by copper alone. In contrast, addition of up to 100 nM of zinc sulfate failed to influence the activity (results not shown), indicating the specificity of copper in the activation of AP-1 by PDTC in hepatic cells.
Figure 7. The extracellular copper chelator BCS blocks PDTC-induced activation of AP-1.
(A) Hep3B cells were pretreated with BCS (50 μM) 30 min before PDTC (100 μM) treatment. Nuclear extracts were prepared after 2 h of PDTC treatment and mixed with a radiolabelled Cp-APRE probe to perform EMSA. (B) HepG2 cells were transiently transfected with a chimaeric construct containing the proximal 3701 bp of the Cp gene along with β-galactosidase. After recovery, the cells were pretreated with 50 μM of BCS, 30 min before PDTC (100 μM) treatment. After 16 h, luciferase activity was determined by chemiluminescence and normalized for β-galactosidase activity. Means±S.D. n=3, *P<0.05, **P<0.01 and ***P<0.001.
Figure 8. Activation of AP-1 by PDTC in a copper-free medium by the addition of copper salt.
(A) HepG2 cells were originally grown in DMEM/F12. Before treatment with 100 μM PDTC, cells were transferred in to copper-free RPMI-1640 medium without serum. Copper sulfate (20 nM) was added along with PDTC. After 2 h, nuclear extracts were prepared and mixed with a 32P-labelled Cp-APRE probe to perform EMSA. (B) Subconfluent Hep3B cells were transiently transfected with a chimaeric construct containing the proximal 3701 bp of the Cp gene 5′-flanking driving luciferase along with β-galactosidase. The cells were recovered in DMEM/F12 with 10% fetal bovine serum. After recovery, the cells were transferred into RPMI-1640 medium and treated similarly with 100 μM PDTC and 20 nM CuSO4. After 16 h luciferase activity was assayed and normalized with β-galactosidase. Means±S.D. n=3, *P<0.05, **P<0.01 and ***P<0.001.
DISCUSSION
Dithiocarbamates, particularly PDTC, have been shown to increase intracellular copper by virtue of its binding with the thiol moiety [23–25,27]. In the present study we have demonstrated that PDTC increased the expression of Cp in the human hepatic cell lines HepG2 and Hep3B by a copper-dependent mechanism. The PDTC-mediated intracellular influx of copper activates the redox sensitive transcription factor AP-1. Subsequently, AP-1 binds to an active APRE about 3.7 kb 5′ upstream of the translation start site and stimulates Cp expression. The presence of this particular AP-1 site has never been reported before. To show that copper is critically involved in this process, we used the membrane impermeable copper chelator BCS to block both the induction of AP-1 and Cp expression. To further establish the role of copper we used RPMI-1640 medium, which is devoid of transition metals such as iron, copper, zinc or manganese [6,32]. Treatment of HepG2 or Hep3B cells by PDTC in RPMI-1640 medium failed to activate AP-1 or increase Cp expression, but simultaneous addition of copper effectively activated AP-1 and increased Cp expression. The maximal activation of AP-1 in these conditions was achieved by 20 nM copper, whereas zinc, the other transition metal shown to regulate NFκB along with PDTC, was ineffective even at 100 nM concentrations. Our study is the first to show that the physiological concentration of copper may have a significant role in regulating Cp expression, particularly when it becomes redox-active.
The action of PDTC as a copper ionophore has been shown to modulate several cellular functions. PDTC treatment modulates p53 activity by increasing intracellular copper, which leads to oxidation of p53 cysteine residues, resulting in alteration of p53 DNA-binding activity [24,25]. Similarly, in thymocytes and the breast cancer cell line MCF-7, the PDTC-mediated increase in intracellular copper results in apoptotic cell shrinkage and chromatin fragmentation [23]. Pax-8 and thyroperoxide expression was affected in thyroid follicular cells by an increase in intracellular copper by PDTC treatment [34]. PDTC has been shown to modulate NF-κB and AP-1 activities. Whereas the inhibition of NF-κB activity by PDTC was found to be zinc-dependent [35,36], with the exception of one study [33], no other attempt has been made to understand the contribution of transition metals in PDTC-mediated AP-1 activation. Kim et al. [33] have shown that PDTC activates AP-1 in the presence of zinc (200 μM) in BCEC (bovine cerebral endothelial cells). In BCEC, PDTC-mediated maximal activation of AP-1 was found at 6 h compared with 2 h in the present study. Interestingly, in the previous study [33] the role of copper was not considered. In the present study, addition of zinc up to 100 nM was found to be ineffective, whereas copper activated AP-1 even at 20 nM concentrations. The discrepancy in these findings may be attributed to cell specificity. The recent demonstration of activation of JNK (c-Jun N-terminal kinase) by PDTC and/or hydrogen peroxide in a copper-dependent mechanism [37] in several types of cell also supports the current finding of copper-dependent activation of AP-1.
A recent study has shown the role of AP-1 in the regulation of Cp [38]. The study demonstrated the presence of one constitutively active AP-1 binding site in the position −248 to −240 from the translation initiation site in ovarian cancer cells, but was not found active in Chang liver cells. In the present study, we checked the activity of the same AP-1 site using a construct containing 300 nt of the Cp promoter coupled with the pGL3 vector but found it was not inducible either by PMA (a positive regulator of AP-1) or PDTC (results not shown), confirming the previous finding that the particular AP-1 site is not active in hepatic cells. Interestingly, the previous study [38] reported that the increase in Cp expression in ovarian cancer cells was 79-fold compared with normal cells whereas the increase in promoter activity was found to be far less. The variability in the magnitude of Cp mRNA expression with the promoter study in ovarian cancer cells could be explained by considering the active presence of the novel AP-1 site reported in the present study. Given the crucial role played by AP-1 in cellular growth, differentiation and tumorigenesis [39,40], the finding of AP-1-mediated regulation of Cp may explain the previous reports of increased Cp synthesis in hepatic, breast and several other cancers [3,28]. The present results of copper-mediated activation of AP-1 and the subsequent increase in Cp expression could also explain increased Cp expression in hepatic cancers, where copper levels were found to be elevated from 11.43±4.74 μg/g tissue to 15.53±5.90 μg/g in cirrhotic tissue [41] in a redox compromised environment.
Acknowledgments
The work is supported by a research grant from the Department of Biotechnology, India (to C.K.M.) and by a fellowship from the Council of Scientific and Industrial Research, India (to D.D. and N.T.).
References
- 1.Rydén L. Ceruloplasmin. In: Copper Proteins and Copper Enzymes, Volume III. Lontie R., editor. Boca Raton, U.S.A.: CRC Press; 1984. pp. 37–100. [Google Scholar]
- 2.Saenko E. L., Yaropolov A. I., Harris E. D. Biological functions of ceruloplasmin expressed through copper-binding sites and a cellular receptor. J. Trace Elem. Exp. Med. 1994;7:69–88. [Google Scholar]
- 3.Raju K. S., Alesandrii G., Zinche M., Gullino P. M. Ceruloplasmin, copper ions, and angiogenesis. J. Natl. Cancer Inst. 1982;69:1183–1182. [PubMed] [Google Scholar]
- 4.Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin: a scavenger of superoxide anion radicals. J. Biol. Chem. 1979;254:4040–4045. [PubMed] [Google Scholar]
- 5.Gutteridge J. M. C., Richmond R., Halliwell B. Oxygen free-radicals and lipid peroxidation: inhibition by the protein ceruloplasmin. FEBS Lett. 1980;112:269–272. [Google Scholar]
- 6.Mukhopadhyay C. K., Ehrenwald E., Fox P. L. Ceruloplasmin enhances smooth muscle cell- and endothelial cell-mediated low density lipoprotein oxidation by a superoxide-dependent mechanism. J. Biol. Chem. 1996;271:14773–14778. doi: 10.1074/jbc.271.25.14773. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay C. K., Mazumder B., Lindley P. F., Fox P. L. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11546–11551. doi: 10.1073/pnas.94.21.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukhopadhyay C. K., Fox P. L. Ceruloplasmin copper induces oxidant damage by a redox process utilizing cell-derived superoxide as reductant. Biochemistry. 1998;37:14222–14229. doi: 10.1021/bi981137t. [DOI] [PubMed] [Google Scholar]
- 9.Fox P. L., Mazumder B., Ehrenwald E., Mukhopadhyay C. K. Ceruloplasmin and cardiovascular disease. Free Radical Biol. Med. 2000;28:1735–1744. doi: 10.1016/s0891-5849(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 10.Osaki S., Johnson D. A. Mobilization of liver iron by ferroxidase (ceruloplasmin) J. Biol. Chem. 1969;244:5757–5765. [PubMed] [Google Scholar]
- 11.Miyajima H., Nishimura Y., Mizuguchi K., Sakamoto M., Shimizu T., Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987;37:761–767. doi: 10.1212/wnl.37.5.761. [DOI] [PubMed] [Google Scholar]
- 12.Harris Z. L., Durley A. P., Man T. K., Gitlin J. D. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragan H. A., Nacht S., Lee G. R., Bishop C. R., Cartwright G. E. Effect of ceruloplasmin on plasma iron in copper-deficient swine. Am. J. Physiol. 1969;217:1320–1323. doi: 10.1152/ajplegacy.1969.217.5.1320. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay C. K., Attieh Z. K., Fox P. L. Role of ceruloplasmin in cellular iron uptake. Science. 1998;279:714–717. doi: 10.1126/science.279.5351.714. [DOI] [PubMed] [Google Scholar]
- 15.Attieh Z. K., Mukhopadhyay C. K., Seshadri V., Tripoulas N. A., Fox P. L. Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J. Biol. Chem. 1999;274:1116–1123. doi: 10.1074/jbc.274.2.1116. [DOI] [PubMed] [Google Scholar]
- 16.Qian Z. M., Tsoi Y. K., Ke Y., Wong M. S. Ceruloplasmin promotes iron uptake rather than release in BT325 cells. Exp. Brain Res. 2001;140:369–374. doi: 10.1007/s002210100831. [DOI] [PubMed] [Google Scholar]
- 17.Xie J. X., Tsoi Y. K., Ke Y., Qian Z. M. Effects of ferroxidase activity and species on ceruloplasmin mediated iron uptake by BT325 cells. Mol. Brain Res. 2002;99:12–16. doi: 10.1016/s0169-328x(01)00336-9. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay C. K., Mazumder B., Fox P. L. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency J. Biol. Chem. 2000;275:21048–21054. doi: 10.1074/jbc.M000636200. [DOI] [PubMed] [Google Scholar]
- 19.Linder M. C., Houle P. A., Isaacs E., Moor J. R., Scott L. E. Copper regulation of ceruloplasmin in copper-deficient rats. Enzyme. 1979;24:23–35. doi: 10.1159/000458625. [DOI] [PubMed] [Google Scholar]
- 20.Martin F., Linden T., Katschinski D. M., Oehme F., Flamme I., Mukhopadhyay C. K., Eckhardt K., Tröger J., Barth S., Camenisch G., Wenger R. H. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105:4613–4619. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- 21.Eisses J. F., Kaplan J. H. Molecular characterization of hCTR1, the human copper uptake protein. J. Biol. Chem. 2002;277:29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 22.Tapia L., Gonzalez-Aguero M., Cisternas M. F., Suazo M., Cambiazo V., Uauy R., Gonzalez M. Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem. J. 2004;378:617–624. doi: 10.1042/BJ20031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobel C. I., Kimland M., Lind B., Orrenius S., Slater A. F. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J. Biol. Chem. 1995;270:26202–26208. doi: 10.1074/jbc.270.44.26202. [DOI] [PubMed] [Google Scholar]
- 24.Verhaegh G. W., Richard M. J., Hainaut P. Regulation of p53 by metal ions and by antioxidants: dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol. Cell Biol. 1997;17:5699–5706. doi: 10.1128/mcb.17.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H. H., Mommad J. Pyrrolidine dithiocarbamate prevents p53 activation and promotes p53 cysteine residue oxidation. J. Biol. Chem. 1998;273:18898–18905. doi: 10.1074/jbc.273.30.18898. [DOI] [PubMed] [Google Scholar]
- 26.Schreck R., Meier B., Mannel D. N., Droge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J. Exp. Med. 1992;175:1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orrenius S., Nobel C. S. I., van den Dobbelsteen D. J., Burkitt M. J., Slater A. F. G. Dithiocarbamates and the redox regulation of cell death. Biochem. Soc. Trans. 1996;24:1032–1038. doi: 10.1042/bst0241032. [DOI] [PubMed] [Google Scholar]
- 28.Kunapuli S. P., Singh H., Singh P., Kumar A. Ceruloplasmin gene expression in human cancer cells. Life Sci. 1987;40:2225–2228. doi: 10.1016/0024-3205(87)90057-9. [DOI] [PubMed] [Google Scholar]
- 29.Sindhu K. V., Rani V., Gupta M. K., Ghaskadbi S., Choudhury D., Goswami S. K. Isolation of a library of target-sites for sequence specific DNA binding proteins from chick embryonic heart: a potential tool for identifying novel transcriptional regulators involved in embryonic development. Biochem. Biophys. Res. Commun. 2004;323:912–919. doi: 10.1016/j.bbrc.2004.08.157. [DOI] [PubMed] [Google Scholar]
- 30.Seshadri V., Fox P. L., Mukhopadhyay C. K. Dual role of insulin in transcriptional regulation of the acute phase reactant ceruloplasmin. J. Biol. Chem. 2002;277:27903–27911. doi: 10.1074/jbc.M203610200. [DOI] [PubMed] [Google Scholar]
- 31.Goswami S. K., Shafiq S., Siddiqui M. A. Modulation of MLC-2v gene expression by AP-1: complex regulatory role of Jun in cardiac myocytes. Mol. Cell. Biochem. 2001;217:13–20. doi: 10.1023/a:1007296330181. [DOI] [PubMed] [Google Scholar]
- 32.Ehrenwald E., Fox P. L. Role of endogenous ceruloplasmin in low density lipoprotein oxidation by human U937 monocytic cells. J. Clin. Invest. 1996;97:884–890. doi: 10.1172/JCI118491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim C. H., Kim J. H., Lee J., Hsu C. Y., Young S. A. Thiol antioxidant reversal of pyrrolidine dithiocarbamate-induced reciprocal regulation of AP-1 and NF-κB. Biol. Chem. 2003;384:143–150. doi: 10.1515/BC.2003.015. [DOI] [PubMed] [Google Scholar]
- 34.Iseki A., Kambe F., Okumura K., Hayakawa T., Seo H. Regulation of thyroid follicular cell function by intracellular redox-active copper. Endocrinology. 2000;141:4373–4382. doi: 10.1210/endo.141.12.7835. [DOI] [PubMed] [Google Scholar]
- 35.Kim C. H., Kim J. H., Moon S. J., Chung K. C., Hsu C. Y., Seo J. T., Ahn Y. S. Pyrithione, a zinc ionophore, inhibits NF-κB activation. Biochem. Biophys. Res. Commun. 1999;259:505–509. doi: 10.1006/bbrc.1999.0814. [DOI] [PubMed] [Google Scholar]
- 36.Kim C. H., Kim J. H., Xu J., Hsu C. Y., Ahn Y. S. Pyrrolidine dithiocarbamate induces bovine cerebral endothelial cell death by increasing the intracellular zinc level. J. Neurochem. 1999;72:1586–1592. doi: 10.1046/j.1471-4159.1999.721586.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y. R., Shrivastava A., Tan T. H. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- 38.Lee C. M., Lo H. W., Shao R.-P., Wang S.-C., Xia W., Gershenson D. M., Hung M. C. Selective activation of ceruloplasmin promoter in ovarian tumors: potential use for gene therapy. Cancer Res. 2004;64:1788–1793. doi: 10.1158/0008-5472.can-03-2551. [DOI] [PubMed] [Google Scholar]
- 39.Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 40.Eferl R., Wagner E. F. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 41.Liaw K. Y., Lee P. H., Wu F. C., Tsai J. C., Lin-Shiau S. Y. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am. J. Gastroenterol. 1997;92:2260–2263. [PubMed] [Google Scholar]