Abstract
Glycoproteins from honey-bee (Apis mellifera), such as phospholipase A2 and hyaluronidase, are well-known major bee-venom allergens. They carry N-linked oligosaccharide structures with two types of α1,3-fucosylation: the modification by α1,3-fucose of the innermost core GlcNAc, which constitutes an epitope recognized by IgE from some bee-venom-allergic patients, and an antennal Lewis-like GalNAcβ1,4(Fucα1,3)GlcNAc moiety. We now report the cloning and expression of two cDNAs encoding the relevant active α1,3-FucTs (α1,3-fucosyltransferases). The first sequence, closest to that of fruitfly (Drosophila melanogaster) FucTA, was found to be a core α1,3-FucT (EC 2.4.1.214), as judged by several enzyme and biochemical assays. The second cDNA encoded an enzyme, most related to Drosophila FucTC, that was shown to be capable of generating the Lex [Galβ1-4(Fucα1-3)GlcNAc] epitope in vitro and is the first Lewis-type α1,3-FucT (EC 2.4.1.152) to be described in insects. The transcription levels of these two genes in various tissues were examined: FucTA was found to be predominantly expressed in the brain tissue and venom glands, whereas FucTC transcripts were detected at highest levels in venom and hypopharyngeal glands. Very low expression of a third homologue of unknown function, FucTB, was also observed in various tissues. The characterization of these honey-bee gene products not only accounts for the observed α1,3-fucosylation of bee-venom glycoproteins, but is expected to aid the identification and subsequent down-regulation of the FucTs in insect cell lines of biotechnological importance.
Keywords: allergy, anti-(horseradish peroxidase), fucosyltransferase (FucT), honey-bee (Apis mellifera), Lewis X, N-glycan
Abbreviations: AMPD, 2-amino-2-methyl-1,3-propanediol; BCIP, 5-bromo-4-chloroindol-3-yl phosphate; EST, expressed sequence tag; FucT, fucosyltransferase; GalGal, Galβ1-4GlcNAcβ1-2Manα1-6(Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-Asn; βGNβGN, GalNAcβ1-4GlcNAcβ1-2Manα1-6(GalNAcβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-Asn; GnGn, GlcNAcβ1-2Manα1-6(GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-Asn; GnGnF6, GlcNAcβ1-2Manα1-6(GlcNAcβ1-2Manα1-3)Manβ1-4(Fucα1-6)GlcNAcβ1-Asn; MM, Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-Asn; HRP, horseradish peroxidase; anti-HRP, antiserum raised against HRP; LacdiNAc, N-acetylgalactosaminyl-β1,4-N-acetylglucosamine (GalNAcβ1-4GlcNAc); LacNAc, galactosyl-β1,4-N-acetylglucosamine (Galβ1-4GlcNAc); lacto-N-fucopentaose III, Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glc-PA; Lex, Galβ1-4(Fucα1-3)GlcNAc; MALDI–TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight MS; NBT, Nitro Blue Tetrazolium; ORF, open reading frame; RP-HPLC, reverse-phase HPLC; RT, reverse transcriptase; pyridylaminated lacto-N-neo-tetraose, Galβ1-4GlcNAcβ1-3Galβ1-4Glc-PA; pyridylaminated lacto-N-tetraose, Galβ1-3GlcNAcβ1-3Galβ1-4Glc-PA
INTRODUCTION
The glycosylation potential of insect species is increasingly being investigated not only to understand the wide range of glycotypes in nature, but also to determine the origin of immunogenic and allergenic cross-reactive carbohydrate determinants and to refine the use of insect-based expression systems in the production of pharmaceutically relevant glycoproteins. In both these cases, the non-mammalian features of insect N-glycans are relevant: whereas many mammalian glycoproteins carry sialic acids on so-called ‘complex’ N-glycans, insect glycoproteins have distinct features that are ‘foreign’ to mammalian immune systems (Figures 1A and 1B). However, the ‘humanization’ of glycans of insect cell expression systems may aid the manufacture of glycoprotein drugs, such as erythropoietin or clotting factors, which have otherwise to be produced at considerable cost from mammalian cell lines or with the risk of using natural sources contaminated with viruses or prions. From the biotechnology perspective, the complete lack (or very low levels) of terminal sialylation [1–6] and the processing of the oligosaccharides to ‘paucimannosidic’ forms (such as structures in Figure 1B) are a particular problem when using insect cell lines [7–10], since such glycoforms may be cleared quickly from the mammalian circulation by lectin-dependent mechanisms. Moreover, the presence of α1,3-fucose bound to the proximal N-acetylglucosamine of an N-glycan (i.e. core α1,3-fucose; see Figures 1B and 1C), as also found in plants including on pollen and food allergens, is of concern for both allergologists and biotechnologists because of its immunogenicity [11,12]. Indeed, core α1,3-fucose constitutes a major epitope recognized by antisera raised in rabbits against insect and plant glycoproteins, such as bee (Apis mellifera) venom or HRP (horseradish peroxidase) respectively [13,14]. Furthermore, in one study, the sera of approx. 25% of patients with bee-venom allergy were found to contain IgE cross-reacting with plant N-glycans, an observation explained by the presence of core α1,3-fucose in both plants and insects [11]. Similar findings have been reported for patients allergic to fire ant [15] and wasp [16] venoms.
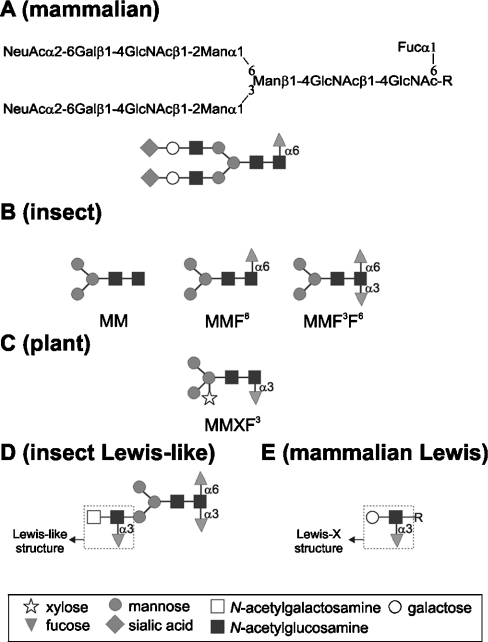
Figure 1. Comparison of N-linked oligosaccharides from mammals, insects and plants.
(A) Typical mammalian ‘complex’ N-glycan structure containing terminal sialic acids and α1,6-fucose linked to the proximal N-acetylglucosamine. (B) N-Glycan structures characteristic for insects. The MM and MMF6 structures are abundant, whereas the immunogenic MMF3F6 structure accounts for 1% of the N-glycans from Drosophila. (C) Typical structure found in plants. The plant N-glycans share some features with insect N-glycans, especially the α1,3-linked fucose. (D) Most complex N-glycan structure found on bee-venom glycoproteins; apart from the immunogenic α1,3-fucose linked to the proximal N-acetylglucosamine, this structure contains a second α1,3-fucose linked to the distal N-acetylglucosamine, creating a Lewis-like structure. As shown in the present study, the synthesis of these structures is catalysed by FucTA and FucTC respectively. (E) Blood-group-related Lewis X structure present in mammals. ‘-R’ represents an oligosaccharide backbone of a glycoconjugate. The glycans are depicted following the glycan nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org) with the mammalian structure also displayed in an extended IUPAC/IUBMB-type form.
Detailed analysis of the N-glycans of two components of bee venom, phospholipase A2 and hyaluronidase (designated respectively as allergens Api m 1 and Api m 2) showed that these glycoproteins possess a number of non-mammalian features (see Figure 1D for the most complex structure observed). Besides the core α1,3-fucosylation mentioned above, some glycoforms of both these proteins carry a Lewis-type fucosylated LacdiNAc [N-acetylgalactosaminyl-β1,4-N-acetylglucosamine (GalNAcβ1-4GlcNAc)] structure [17,18], which resembles the Lewis X structure {Lex [Galβ1-4(Fucα1-3)GlcNAc]; Figure 1E}, except that a GalNAc is present rather than a galactose. Fucosylated LacdiNAc is also present in parasitic trematode and nematode worms [particularly Schistosoma spp. (blood fluke) and Haemonchus contortus (the barber's-pole worm)] and is a major epitope for antibodies from infected individuals or animals [19,20]. In humans, Lewis-type epitopes containing galactose constitute a set of carbohydrate histo-blood group antigens. These moieties are often modified by sialic acids and sulphate groups, and as such play numerous roles including ones in inflammation and cancer [21–23].
On the basis of the aforementioned data, we hypothesized that at least two α1,3-FucTs (α1,3-fucosyltransferases) would be encoded by the honey-bee genome. To date, all enzymes required for addition of α1,3-linked fucose residues to glycans have been found to be members of one enzyme family, CAZy glycosyltransferase family 10 [24], which includes both core α1,3-FucTs from plants, nematodes and insects as well as enzymes from bacteria and plants and enzymes of mammals capable of generating Lewis blood group antigens. Previously, in insects, the native enzyme activity responsible for the acquisition of the core α1,3-fucose epitope has been described in bee venom glands [25] and in a cell line [26]. However, the only data previously obtained on recombinant insect FucTs pertains to Drosophila melanogaster: its genome encodes four α1,3-FucT homologues (DmFucTA, DmFucTB, DmFucTC and DmFucTD). Of these, only DmFucTA, a core α1,3-FucT with orthologues in all other insect genomes examined, has a defined enzymatic activity [1,27]. On the other hand, no Lewis-type enzyme is expected to be encoded by the fruitfly genome since no relevant epitopes have been described in Drosophila to date; thus it is to be assumed that any honey-bee enzyme with a Lewis-type FucT may not be so closely related to the three ‘orphan’ FucTs (FucTB, FucTC and FucTD) from the fruitfly.
To test the hypothesis that a honey-bee FucT homologue least akin to DmFucTA may indeed be a Lewis-type enzyme able to fucosylate LacdiNAc, whereas putative orthologues of DmFucTA are solely core-type enzymes, we searched the draft honey-bee genome and EST (expressed sequence tag) databases for α1,3-FucT homologues. Of three FucT homologues identified, two were indeed expressed as active proteins in both insect cells and yeast and were subject to a variety of biochemical assays in order to define their enzymatic function.
EXPERIMENTAL
Cloning of FucT cDNAs from the honey-bee
Male honey-bee larvae were collected from a hive in Schiltern near Krems in Lower Austria and cDNA was prepared by TRIzol® (Invitrogen) extraction and subsequent reverse transcription (Superscript III, Invitrogen) with oligo(dT)18. A fragment with a partial reading frame of the FucTA homologue was amplified using the primers BBFT3 (GAGATATGGCACCGTATGC) and BBFT2/XbaI (GCTCTAGACCTAGGCAGAAAGTTTTTC) with Expand polymerase mix (Roche), and then cloned into the pGEM-T vector (Promega). The full-length form was reconstructed by ligating a BstAPI/KpnI fragment from the pGEM-T/FucTA plasmid into the corresponding sites of the EST clone (GenBank® BI503295, clone ID BB170010A20D04) in pT7T3-Pac encoding only the 5′- and the 3′-ends of honey-bee FucTA. This reconstructed form was used as a template for PCR using KOD polymerase (Novagen) with the primers BBFT1/NotI (ATAAGAATGCGGCCGCATGGGTCTGCCGCG) and BBFT2/XbaI; the resulting fragment was then digested with NotI and XbaI and ligated into the pIZT-V5/His vector (Invitrogen).
The complete FucTB and FucTC ORFs (open reading frames) were prepared by PCR using, respectively, the primers BFTB1/KpnI (GGGGTACCGATGCTCGGAATTTCACAA) with BFTB2/XbaI (GCTCTAGAGTCAGAAGGTACAATCTCGT) and BFTC1/KpnI (CGGGGTACCATGAGATTATGGATACTGGAAA) with BFTC2/XbaI (GCTCTAGATCAGTCTTTTAAAACGGAACC) in combination with Expand polymerase mix and the fragments were ligated also into the pIZT-V5/His vector. Partial reading frames corresponding to the catalytic and stem regions of FucTA, FucTB and FucTC were isolated using the primers BBFT3/PstI (AACTGCAGGAAATCTAGTGCAGGACGAG) with BBFT2/XbaI, BFTB3/PstI (AAACTGCAGTTTATAAAGATGTATTTAT) with BFTB2/XbaI or BFTC3/PstI (AAACTGCAGGTACCCCTCGACATATAATAC) with BFTC2/XbaI and ligated into a form of the pPICZαB vector (Invitrogen) modified to encode a FLAG tag.
Expression of honey-bee putative FucTs
Constructs in the pIZT-V5/His and pPICZα-type vectors were transformed respectively into insect Sf9 [Spodoptera frugiperda (fall armyworm)] and Pichia pastoris GS115 cells as previously described [27,28]. In P. pastoris, a methylotrophic yeast, the recombinant enzymes were expressed at 16 °C. Various strategies, as described below, were used to verify enzyme activities. Briefly, core α1,3-FucT activity was tested either by Western blotting of transfected Sf9 cells using rabbit anti-HRP (antiserum raised against HRP) or in vitro using various dansyl- and dabsyl-glycopeptides as substrates as used in previous studies [28,29]. The Lewis-type FucT was also assayed with dabsyl-glycopeptides as well as with pyridylaminated lacto-N-neo-tetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc-PA) and pyridylaminated lacto-N-tetraose (Galβ1-3GlcNAcβ1-3Galβ1-4Glc-PA). Where stated, Pichia supernatants were concentrated 10-fold using UltraFree centrifugal concentration devices (Millipore; relative molecular-mass cutoff 30000).
MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS) FucT assay
For analysis by MALDI–TOF-MS, the activity of recombinant α1,3-FucTs from concentrated Pichia supernatants was measured using 40 mM Mes buffer (pH 6.5), 10 mM MnCl2, 1 mM GDP-fucose and 80 μM each of dabsyl-MM [Manα1-6(Manα1-3)-Manβ1-4GlcNAcβ1-Asn], -GnGn [GlcNAcβ1-2Manα1-6-(GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-Asn], -GalGal [Galβ1-4GlcNAcβ1-2Manα1-6(Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-Asn] and -βGNβGN [GalNAcβ1-4GlcNAcβ1-2Manα1-6(GalNAcβ1-4GlcNAcβ1-2Manα1-3)-Manβ1-4GlcNAcβ1-Asn] glycopeptides derived from bovine fibrin, and possessing the common peptide sequence GENR (Gly-Glu-Asn-Arg) (see Figure 2 for oligosaccharide structures and [28]). Reaction tubes were incubated at 30 °C and analysed after 12 h. Following incubation, 0.5 μl of a 1:10 dilution of the reaction mixture was mixed with 0.5 μl of 1% α-cyano-4-hydroxycinnamic acid (Fluka; in 70% acetonitrile) on a MALDI plate. An increase in glycopeptide m/z by 146.1 Da indicated the transfer of one fucose residue.
Figure 2. Synthesis and subsequent modifications of glycan structures used as substrates in enzymatic assays.
The various substrates used in the present study, except the dabsylated MM-glycopeptide, were acceptors for either FucTA or FucTC. Products of these enzymes are shaded; glycans are depicted following the glycan nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org). (A) Dabsylated glycopeptides (MM, GnGn and βGNβGN) were synthesized from the dabsylated GalGal-glycopeptide using relevant enzymes. β1,4- and β1,3-galactosidases were used to remove galactose residues, whereas jack-bean (Canavalia ensiformis) hexosaminidase was used to remove the GlcNAc residues. Bovine β1,4-galactosyltransferase I, an enzyme known to also utilize UDP-GalNAc, was used to the synthesize the dabsylated βGNβGN-glycopeptide. (B) Dansyl-GnGnF6, produced from an asialoagalacto-IgG glycopeptide, has been previously shown to be a substrate for core α1,3-FucTs from plants and insects [1,32]. (C) Lacto-N-neo-tetraose is a Lewis-type FucT substrate.
RP (reverse-phase)-HPLC-based FucT assays
For analysis by HPLC, the activity of concentrated supernatants of Pichia expressing honey-bee FucTA was measured using 40 mM AMPD (2-amino-2-methyl-1,3-propanediol) and Mes buffer (pH 6.5–9.5) (Mes buffer, pH 7.0, for temperature and cation dependency), 10 mM MnCl2, 1 mM GDP-fucose and 25 μM dansylated GnGnF6 [GlcNAcβ1-2Manα1-6(GlcNAcβ1-2Manα1-3)Manβ1-4(Fucα1-6)GlcNAcβ1-Asn] glycopeptide derived from human IgG, with NS (Asn-Ser) as the peptide sequence (see Figure 2 for oligosaccharide structure). Reaction tubes were incubated at 23 °C and analysed after 2 h. Following incubation, an aliquot of the reaction mixture was analysed on an RP-HPLC system (Hypersil™ ODS) under isocratic (at constant concentration) conditions [9.5% (v/v) acetonitrile and 0.045% trifluoroacetic acid].
The activity of unconcentrated supernatants of Pichia expressing honey-bee FucTC was measured using 50 mM AMPD and Mes buffer (pH 6.5–9.5) (Mes buffer, pH 7.5, for temperature and cation dependency), 100 μg/μl BSA, 12.5 mM MnCl2, 1.25 mM GDP-fucose and 125 μM pyridylaminated lacto-N-neo-tetraose. Reaction tubes were incubated at 23 °C and analysed after 2 h. Following incubation, an aliquot of the reaction mixture was analysed on an RP-HPLC system (Nucleosil C18) under isocratic conditions (0.094 M ammonium-formate, pH 4.0, 1.8% methanol).
Western blotting
GnGn, GalGal and βGNβGN isoforms of human apo-transferrin were prepared by enzymatic remodelling using neuraminidase from the bacterium Clostridium perfringens (yielding GalGal-transferrin), β-galactosidase from the ascomycetous fungus Aspergillus oryzae (yielding GnGn- from GalGal-transferrin) and bovine β1,4-galactosyltransferase I (yielding βGNβGN- from GnGn-transferrin, since the enzyme also utilizes UDP-GalNAc as a sugar donor [28,30]). Recombinant honey-bee FucTA (concentrated) and FucTC (unconcentrated) were incubated with 20 μg of the transferrin neoglycoforms in 40 μM Mes (pH 7.0), 10 mM MnCl2, 1 mM GDP-Fuc and 1 mM PMSF in a total volume of 5 μl. The reactions were performed at 23 °C for 48 h. One-fifth of each incubation was mixed with an equal volume of 2× Laemmli buffer (a 10× concentrate contains 0.25 M Tris, 1.92 M glycine and 1 % SDS in aqueous solution) and analysed by Western blotting using 1:10000 dilutions of either rabbit anti-HRP or rat anti-Lex (L5) as primary antibodies and 1:2000 dilutions of the relevant secondary alkaline phosphatase-conjugated antibodies followed by development using SigmaFAST BCIP (5-bromo-4-chloroindol-3-yl phosphate)/NBT (Nitro Blue Tetrazolium) solution.
Sf9 cells were maintained, transfected and lysed as previously described [27]. Briefly, pIZT/V5-His vectors carrying complete ORFs of all three A. mellifera α1,3-FucT homologues with the native stop codon were used to transfect Sf9 cells [maintained in IPL-41 insect medium (Sigma–Aldrich) supplemented with 3% (v/v) foetal-calf serum] using Cellfectin reagent (Invitrogen) following the manufacturer's protocol for insect cells. A small aliquot of the cells was analysed by confocal laser scanning microscopy with a UV-light source to confirm the presence of green fluorescent protein fluorescence within the cells as indication of a successful transfection. Cells were collected 48 h post-transfection, washed once with PBS and stored at −80 °C. Cells were lysed using 50 mM Tris (pH 7.5), 150 mM NaCl and 0.5% Triton X-100 supplemented with Complete™-Mini protease inhibitor cocktail without EDTA (Roche), or His-tag protease inhibitor cocktail (Sigma) for 10 min at 23 °C and 20 min on ice, followed by removal of insoluble material by centrifugation (14100 g, 4 °C and 30 min). Different volumes of the buffer were used to normalize the number of cells per volume (to 1.25×104 cells/μl). Cell lysates (2.5 μl per lane) were mixed with 2× Laemmli loading buffer and analysed by Western blotting using a 1:20000 dilution of rabbit anti-HRP as primary antibody and a 1:2000 dilution of alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) as secondary antibody, followed by development using SigmaFAST BCIP/NBT solution. Prior to blocking, the nitrocellulose sheets were reversibly stained with 0.5% Ponceau S (in 1% acetic acid) to verify that equal amounts of proteins were present in every lane.
RT (reverse transcriptase)–PCR analyses
Carniolan honey-bees (A. mellifera ssp. carnica) were obtained from one colony at a private apiary located near Bratislava (Slovak Republic). Nurses were collected on a comb taken from the hive in the region containing cells with 1–3-day-old larvae. Foragers were caught in an insect net while trying to fly into or out of the hive. After collection, tissues and glands were dissected from distinct numbers of honey-bees [thorax muscle (two individuals), brains (ten individuals), hypopharyngeal glands (12 individuals) and venom glands (17 individuals)] into RNAlater (Ambion, Inc., Austin, TX, U.S.A.) solution and stored at −20 °C. Total RNA from honey-bee tissues and glands was prepared with SV Total RNA isolation system (Promega) after Ultra-Turrax® (IKA Labortechnik, Staufen, Germany) homogenization in a denaturing solution. The quality and the spectrophotometrically estimated concentration of the preparations were tested by agarose gel electrophoresis. RT–PCR assays were performed with the one-tube, two-enzyme Access RT–PCR system (Promega) using 40–160 ng (FucTA, FucTC and actin) or 100–400 ng (FucTB) of RNAs in a volume of 25 μl. The primers used in the RT–PCRs had the same Tm (‘melting’ temperature) for the specific sequences (58 °C) and also worked with the same efficiencies in PCR using plasmid DNAs containing the corresponding FucT cDNAs. Primers were designed so that products would span at least one intron/exon boundary to ensure that cDNA amplicons could be distinguished from genomic DNA amplicons. The following primers were used: FucTA: BBFT3/PstI and BBFT2; FucTB: BFTB/5 (ATAGAACATCGAAAGCAAACG) and BFTB2/XbaI; FucTC: BFTC3/PstI and BFTC2/XbaI; actin: Actinfor (MTCCGGIATGTGCAARGCCG) and Actinrev (CCTGCTCRAAGTCMAGIGC). The primer concentration in the reaction mixture was 0.8 μM. The concentration of Mg2+ was 1.5 mM except for the amplification of FucTB, where the optimal concentration was 1.2 mM. Reverse transcription was performed at 48 °C for 1 h. PCR after the initial denaturation for 2 min at 94 °C was performed for 30, 34 or 50 cycles (30 s at 94 °C, 60 s at 54 °C and 150 s at 68 °C) followed by 7 min at 68 °C. PCR products were analysed on 1% agarose gels.
RESULTS
Cloning of FucT cDNAs from the honey-bee
The first target was to identify which gene is responsible in the honey-bee for the occurrence of core α1,3-fucose on bee-venom glycoproteins; it seemed reasonable that the encoded protein would display homology to other core α1,3-FucTs, including the known D. melanogaster FucTA. During initial searches of the EST databanks in 2001, two partial bee brain cDNA sequences from the honey-bee with homology to Drosophila FucTA were noted. The two relevant clones were ordered, but both were found to contain DNA encoding protein sequences with homology to only the N- and C-terminal regions of the fruitfly FucTA. These EST DNA sequences were then used to design specific primers in order to clone the full ORF as well as an ORF fragment encoding a form lacking the putative cytosolic and transmembrane regions. Only the latter fragment was actually isolated from honey-bee cDNA, but due to the presence of a BstAPI site in the cDNA sequence and a KpnI site in both the cloning and EST vectors, it was possible to reconstruct the full-length honey-bee FucTA sequence encoding a protein of 452 amino acids (Mr=53000), with 52% identity over 439 residues to Drosophila FucTA with a shorter putative stem region than the fly protein. The significance of the homology at the DNA level was also shown by the finding that a Drosophila FucTA probe could recognize the honey-bee FucTA PCR product, but not that of fruitfly FucTB, at low stringency [2× SSC (1×SSC is 0.15 M NaCl/0.015 M sodium citrate) and 0.1% SDS, 1 h, 55 °C; results not shown). The determined honey-bee FucTA cDNA sequence was identical with that later released for the predicted mRNA [accession no. XM_392653; NCBI (National Center for Biotechnology Information) Refseq database] based on that of honey-bee Contig1747; a comparison of the cDNA and genomic sequences also showed that the honey-bee FucTA is encoded by three exons.
Considering the presence of fucosylated LacdiNAc on bee-venom glycoproteins, we also sought a second FucT gene. Indeed, when using the fruitfly FucTC sequence to search the now available honey-bee genome, another predicted FucT was found (XM_394138; honey-bee Contig655); the confirmed cDNA sequence encodes a protein of 399 residues (Mr=47000), which we designate as FucTC. Furthermore, using the amino acid sequence of a Glossinia morsitans (tsetse fly) putative FucT (GenBank® entry AJ582622), another potential homologous sequence was found on honey-bee Contig4553; although this was not a predicted mRNA, a corresponding cDNA designated FucTB was then successfully cloned. This sequence encodes a protein of 431 amino acids (Mr=51000), which, instead of the DYI/VTEK (one-letter amino acid code) motif typical of animal α1,3-FucTs, has an EYVTEK sequence. The three honey-bee FucTs display between 28 and 34% identity to each other over approx. 300 residues (Figure 3A).
Figure 3. Homologies of honey-bee α1,3-FucTs.
(A) Alignment of the conserved regions of honey-bee α1,3-FucT homologues. Residues identical in all sequences are highlighted. The amino acid numbering starts from the putative first methionine residue and potential N-glycosylation sites are underlined. The motifs I and II defined by Oriol et al. [39] are also shown. The ‘core’ motif, containing the sequence SNCXXRN, appears to be present in those core α1,3-FucTs that require the prior action of N-acetylglucosaminyltransferase I (i.e. all plant and insect core α1,3-FucTs described to date, but not Caenorhabditis elegans FUT-1, which does not accept substrates with an α1,3-antennal terminal GlcNAc residue). (B) Phylogenetic tree of insect and mammalian α1,3-FucT homologues. The following protein sequences were analysed using ClustalW and NJPlot programs (http://www.ebi.ac.uk/clustalw/): AmA–C: A. mellifera FucTs A, B and C; DmA–D: D. melanogaster FucTs A, B, C and D; HsIII–XI: Homo sapiens FucTs III–VII and IX–XI; MmX–XI: Mus musculus (house mouse) FucTs X and XI; BmA–C5: B. mori manually assembled FucT homologues A (AADK01016505), B1 (AADK01001074), B2 (AADK01006340), C1 (AADK01004147), C2 (AADK01000551), C3 (AADK01017942), C4 (AADK01001565) and C5 (BAAB01004223); AeA/C: Aedes aegypti manually assembled FucT homologues A (AAGE02021501) and C (AAGE02000340, AAGE02000341); AgA/C: Anopheles gambiae manually assembled FucT homologues A (AAAB01008879) and C (AAAB01008846); GmC: G. morsitans FucT homologue C (AJ582622); TcA–C2: T. castaneum manually assembled FucT homologue A (AAJJ01000602), B1 (AAJJ01000605), B2 (AAJJ01000026), C1 (AAJJ01000008) and C2 (AAJJ01000008). GenBank® accession numbers of genomic DNA sequences that were used to manually assemble sequences are in brackets. Proteins, for which activity was demonstrated in vitro and/or in vivo, are appropriately labelled as core or Lewis-like enzymes.
Phylogenetic analysis (Figure 3B) groups the honey-bee and fruitfly FucTAs together, whereas those designated as FucTB homologues appear to form two distinct groups: one group is more related to the human Lewis-type FucTs III–VII and IX, while the other is closer to the mammalian FucT homologues X and XI. The honey-bee FucTC appears to be closely related to a number of insect sequences; however, a branching into two distinct FucTC groups cannot be excluded. Indeed, the occurrence of two FucTC groups would be in agreement with the apparent inability of fruitfly FucTC to utilize various N-glycan substrates [1,27], whereas, as shown below, honey-bee FucTC does accept such glycans.
Expression of honey-bee FucTs in Pichia
Constructs encoding FLAG-tagged soluble forms of the three honey-bee FucT homologues were prepared for expression in the yeast P. pastoris. Culture supernatants were collected and concentrated prior to assaying specific FucT activities with various dabsylated N-glycopeptides (MM, GnGn, GalGal and βGNβGN; see Figure 2 for structures). MM and GnGn were used as known core FucT substrates, whereas GalGal and βGNβGN are substrates for Lewis-type enzymes [31]. As shown in Figure 4, Pichia transformed with the FucTA vector converted GnGn into a fucosylated form, whereas that transformed with FucTC vector converted GalGal and βGNβGN into difucosylated forms. Upon longer incubation (36 h), the honey-bee FucTA expressed in Pichia also converted GalGal and βGNβGN into monofucosylated forms (results not shown). FucTB-transformed yeast showed no obvious activity in the initial screening; thus all further experiments were performed on FucTA and FucTC.
Figure 4. Activity of honey-bee FucTs expressed in Pichia analysed by MALDI–TOF-MS.
Culture supernatants of yeast transformed with either FucTA (A), FucTB (B) or FucTC (C) were assayed using four dabsylated glycopeptides (MM, GnGn, GalGal and βGNβGN) in the presence of GDP-Fuc. The glycans are depicted following the glycan nomenclature of the Consortium for Functional Glycomics (http://www.functionalglycomics.org), i.e. mannose residues are shown as grey circles, galactose residues as white circles, glucose residues as black circles, N-acetylglucosamines as black squares, N-acetylgalactosamines as white squares and fucose residues as grey triangles (as shown in Figure 2). Structures appearing due to enzymatic activity are underlined. Peaks resulting from laser-induced cleavage of a part of the dabsyl group are labelled with an asterisk.
FucTA activity was also measured using a HPLC-based assay with the fluorescently labelled dansylated-GnGnF6 glycopeptide as the substrate. In agreement with previous studies on core α1,3-FucTs [32], the addition of a fucose by FucTA lead to reduction of the substrate glycan retention time (Figure 5). Since FucTC appeared to be a Lewis-type enzyme, we performed HPLC-based assays using pyridylaminated forms of the tetrasaccharides lacto-N-neo-tetraose and lacto-N-tetraose as substrates, since these have been successfully used with Lewis-type FucTs forming, respectively, α1,3 and α1,4-fucosyl linkages. Previous studies with a plant Lewis-type α1,3/4-fucosyltransferase (tomato FucTC) showed that fucosylation of these substrates results in a shift to earlier elution times [33]. In the present study with honey-bee FucTC, only in the case of lacto-N-neo-tetraose was there an alteration in the retention time, resulting from formation of lacto-N-fucopentaose III [Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glc-PA] (Figure 5); no such shift was observed for yeast transformed with FucTA or FucTB nor when lacto-N-tetraose was incubated with FucTC (results not shown). These results, therefore, show that the enzyme solely acts as an α1,3-FucT. The determined activity of FucTC towards both LacNAc [galactosyl-β1,4-N-acetylglucosamine (Galβ1-4GlcNAc)]- and LacdiNAc-containing substrates is comparable with results showing that the mammalian FucTs III, IV and IX have varying levels of activity in vitro towards both LacNAc (in order to generate Lex) and LacdiNAc, whereas FucT VII displays no activity towards LacdiNAc [34].
Figure 5. Activity of honey-bee FucTs expressed in Pichia analysed by RP-HPLC.
Culture supernatants of yeast transformed with either FucTA or FucTC were assayed using either dansylated-GnGnF6 glycopeptide (FucTA) or lacto-N-neo-tetraose (FucTC) in the presence or absence of GDP-Fuc. The FucTC was also tested using lacto-N-tetraose but did not display any activity towards that substrate (results not shown).
Enzymatic properties of honey-bee FucTA and FucTC
The basic enzymological parameters of FucTA and FucTC were investigated using the aforementioned HPLC-based assays. Honey-bee FucTA is strongly activated by Mn2+ as compared with no-cation controls, a property common to many glycosyltransferases. On the other hand, EDTA, as well as Cu2+ and Zn2+ ions, completely abolish this enzyme's activity. The enzyme shows maximal activity in mildly alkaline conditions (pH 7.5–9.0) at room temperature (23 °C). In contrast with FucTA, FucTC is not inactivated by EDTA, although the enzyme seems to prefer Mn2+ and Mg2+ over other cations. The temperature and pH optima for the FucTC appear to be very broad; the enzyme shows 90% of maximal activity at 4 °C and over 40% of maximal activity at 50 °C (Figure 6).
Figure 6. Properties of recombinant honey-bee FucTA and FucTC.
Cation dependency, pH and temperature optima were determined using either 10-fold concentrated (FucTA) or unconcentrated (FucTC) honey-bee enzymes expressed in Pichia. Relative activity was normalized based on the condition giving the highest rate of fucose transfer.
The honey-bee FucTA and FucTC can utilize protein substrates
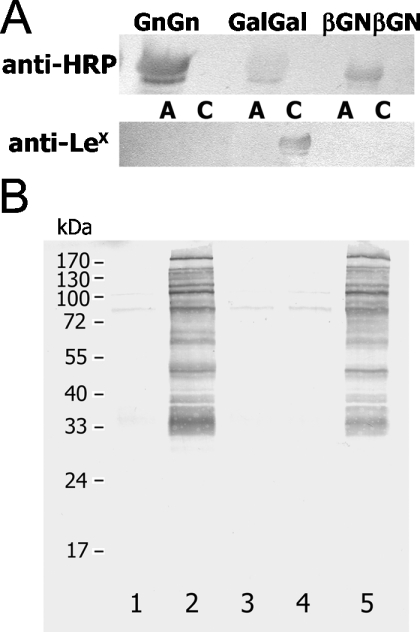
The assays employed to measure the activity of both core and Lewis-like α1,3-FucT utilize substrates containing predominantly carbohydrates, with little or no peptide backbone as compared with ‘real’ glycoproteins. Furthermore, these assays primarily indicate transfer of fucose but do not give information about the epitopes formed. To address this major difference between utilized substrates and the ones present in vivo, we made use of the human glycoprotein apo-transferrin whose glycans were modified to be potential substrates for the two recombinant enzymes. After incubation with GDP-fucose and Pichia culture supernatants containing either FucTA or FucTC, the glycoprotein preparations were separated on a standard SDS/polyacrylamide gel, transferred on to a nitrocellulose membrane and probed with anti-HRP or anti-Lex antibodies. Whereas anti-HRP is known to bind core α1,3-linked fucose as found on many plant and insect glycoproteins [35], the L5 monoclonal antibody is apparently the only antibody capable of recognizing the Lex epitope when attached to N-glycans [36]. The transferrin neoglycoforms carrying N-glycans predominantly terminating with β1,2-GlcNAc (i.e. GnGn-), β1,4-Gal (i.e. GalGal-) and β1,4-GalNAc (i.e. βGNβGN-transferrin) residues incubated with FucTA were recognized by anti-HRP antibodies, demonstrating that transfer of a fucose resulted in generation of the same core α1,3-fucose epitope as found on, e.g. HRP and bee-venom phospholipase. On the other hand, only GalGal-transferrin incubated with FucTC was recognized by antibodies directed against the Lex structure, thus strongly indicating that the fucose residues were attached in α1,3-linkage to the antennal N-acetylglucosamine residues (Figure 7A). Even though FucTC can transfer fucose to βGNβGN, the specificity of the L5 antibody is obviously absolute for Lex and therefore this antibody does not recognize fucosylated LacdiNAc.
Figure 7. Epitope formation by honey-bee FucTA and FucTC.
(A) Honey-bee FucTA and FucTC expressed in Pichia confer anti-HRP and anti-Lex staining on glycoproteins. Recombinant FucTA and FucTC were incubated with modified transferrin carrying N-glycans predominantly terminating with β1,2-GlcNAc (i.e. GnGn-), β1,4-Gal (i.e. GalGal-) or β1,4-GalNAc (i.e. βGNβGN-transferrin) residues. FucTA confers anti-HRP staining on the used transferrin glycoforms (upper panel). FucTC but not FucTA confers anti-Lex (anti-L5) staining only on GalGal-transferrin (lower panel). (B) Expression of honey-bee FucTs in Sf9 insect cells. Cells transfected with either an empty pIZTV5/His vector (1), honey-bee FucTA (2), honey-bee FucTB (3), honey-bee FucTC (4) or Drosophila FucTA (5) were lysed and subjected to SDS/PAGE and Western blotting with anti-HRP.
Analysis of honey-bee FucT activity in vivo
In previous studies, we have used insect cells as hosts for core α1,3-FucT expression in order to perform screening for the ability of an ORF to confer anti-HRP binding in vivo, i.e. to prove the specific modification of glycoproteins of cells transfected with core α1,3-FucTs [27,29]. In the present study, we chose Sf9 cells (a typical biotechnological lepidopteran cell line), which naturally display only a low degree of binding to anti-HRP. Induction of core α1,3-fucosylation upon transfection of relevant ORFs in this cell line is expected to result in the creation of the anti-HRP epitope, which can then be detected by Western blotting. We therefore transfected these cells with the honey-bee α1,3-FucT homologues and employed Drosophila FucTA as a positive control; vectors containing the entire ORF encoding each enzyme, with no tag, were used. As shown in Figure 7(B), of the honey-bee FucTs, only the FucTA conferred the ability to bind anti-HRP to glycoproteins of Sf9 cells. The lanes with the cells transfected with the honey-bee FucTB and FucTC showed no difference as compared with the empty vector control, corroborating the other results suggesting that they are not core FucTs. Furthermore, in vitro assays also showed that only the FucTA-transfected cells had an activity capable of transferring a fucose to GnGnF6, thereby generating a difucosylated structure, above the otherwise very low background levels displayed by control transfectants (results not shown). The overall conclusion is that, as judged by results indicating that this enzyme can form the anti-HRP epitope in vitro and in vivo, the fucose transferred by FucTA is α1,3-linked to the proximal GlcNAc of N-glycans.
Expression of FucTs in different honey-bee tissues
To appraise whether the tissue-specific expression of α1,3-FucT homologues in A. mellifera is compatible with the synthesis of the previously observed N-glycans containing both core and antennal α1,3-linked fucose [17], total RNA from several honey-bee tissues was prepared. Following RNA normalization against actin transcripts, expression pattern of the FucT homologues in honey-bees was estimated by RT–PCR using gene-specific primers. As expected, the expression panel indicates that there is a very strong presence of FucTA transcripts in venom glands, followed by strong expression of FucTA in bee brain and weaker expression in the other analysed samples (Figure 8). FucTC, on the other hand, appears to be expressed more equally in various samples, with an apparently higher expression rate in venom and hypopharyngeal glands. The reproducible appearance of the triplet band in FucTC RT–PCR reactions for most tissues could indicate alternative splicing of the FucTC transcripts. Indeed TA cloning and subsequent sequencing confirmed the existence of two alternatively spliced forms of FucTC: one corresponded to that expressed in Pichia, whereas the second form is 90 nt shorter and has a premature stop codon. Finally, the third α1,3-FucT homologue, FucTB, appears to be weakly expressed in venom glands, foragers' brain tissue and in larvae; it should be noted that it was necessary to increase the number of RT–PCR cycles to 50 and use increased amounts of RNA in order to detect FucTB transcripts in honey-bee RNA preparations. Overall, the expression panel data indicating the presence of both the core-modifying FucTA and the Lewis-like FucTC in venom glands is in accordance with the previous studies indicating the presence of both core and antennal α1,3-fucosylated N-glycan structures on bee-venom glycoproteins.
Figure 8. Expression patterns of FucTs in different tissues and glands of A. mellifera.
Total RNA was isolated from the whole larva (L) as well as from thorax muscles (M), brains (B), venom glands (Vg) and hypopharyngeal glands (Hg) of nurse honey-bees or of foragers from the same colony. RT–PCR was performed with the primers specific for individual FucTs (see the Experimental procedures) using different numbers of cycles. In the case of FucTB, the conditions of reaction were partially distinct from those used for FucTA, FucTC and actin. The amplified fragments of FucTA, FucTB, FucTC and actin with the respective sizes of 1350, 1160, 1200 and 600 bp are shown.
DISCUSSION
The honey-bee is an object of scientific study for a number of reasons; in particular, the search for genetic clues as to its social behaviour has led to the recent sequencing of its genome and the examination of bee brain EST sequences [37], and also the anaphylaxis induced in some individuals, as an allergenic reaction to bee stings, is a focus of allergological interest [11]. The present study has its origins in previous enzymological and structural analyses related to bee-venom glycoproteins [17], which then showed, for the first time, the presence of both core α1,3-fucose and α1,3-fucosylated LacdiNAc structures on an insect glycoprotein. Thus we expected to find at least two genes in the honey-bee encoding enzymes able to synthesize these structures.
In actual fact, the A. mellifera genome encodes three α1,3-FucT homologues, which include the consensus sequence common to all α1,3-FucTs [38,39]. As indicated by the predicted amino acid sequences, one of the honey-bee sequences (FucTA) is phylogenetically close to Drosophila and other proven or putative N-glycan core α1,3-FucTs (Figure 3B). Indeed, when expressed in P. pastoris and insect cells, this honey-bee sequence encodes an active core α1,3-FucT with a substrate specificity similar to that of the recombinant Drosophila FucTA [1], the native honey-bee venom gland and Mamestra brassicae (cabbage moth) MB-0503 cell line core α1,3-FucTs [25,26] and various plant N-glycan core α1,3-FucTs [40–42]. The observed transfer of fucose to GalGal, in addition to GnGn, has also been found with the fruitfly FucTA [1]. On the other hand, no transfer was seen towards MM by either insect FucTA, which contrasts with the preference of the Caenorhabditis (nematode worm) FUT-1 for this substrate [29]. This suggests that the substrate specificity of invertebrate core α1,3-FucTs (i.e. FucTA and FUT-1) is somewhat more variable and flexible than that of invertebrate core α1,6-fucosyltransferases, which require a terminal unsubstituted GlcNAc residue [31].
The second homologue found in the present study (FucTC) resembles a number of other related sequences from various insects, including the Drosophila FucTC sequence. Unlike DmFucTC [27], the honey-bee FucTC encodes an active Lewis α1,3-FucT and is indeed the first such enzyme to be characterized from an insect; it is very likely that this enzyme is involved in synthesis of fucosylated LacdiNAc N-glycans found on bee-venom glycoproteins [17]. It appears that, the FucTC homologues form two distinct subgroups: the honey-bee FucTC seems to be more related to Bombyx mori (silk moth) and Tribolium castaneum (red flour beetle) sequences, whereas the Drosophila FucTC is more similar to Anopheles gambiae (the major African malaria mosquito), Aedes aegypti (the yellow-fever mosquito) and G. morsitans sequences. However, it remains to be determined whether this genetic ‘split’ is generally reflected in the presence or absence of fucosylated LacdiNAc.
Apart from demonstrating that both honey-bee FucTA and FucTC can act on glycoproteins and that FucTA is able to confer fucose-dependent anti-HRP staining in an in vivo system, albeit heterologous, we sought to strengthen the link between the honey-bee α1,3-FucT homologues and fucosylated N-glycan structures found on bee-venom glycoproteins [17,18] by performing expression studies of all three honey-bee α1,3-FucT homologues in different bee tissues. According to the RT–PCR expression panel, FucTA appears to be strongly expressed in venom glands, a result that correlates with the presence of the core α1,3-linked fucose on the N-glycans of bee-venom glycoproteins. The occurrence of FucTA transcripts in bee brain tissue is also in agreement with expression of DmFucTA in Drosophila heads and in a Drosophila neuronal cell line [27] and in the use of anti-HRP as a neuronal marker in insects [43]. The honey-bee FucTA also appears to be expressed in hypopharyngeal glands that produce the royal jelly proteins in honey-bee nurses [44–46] and carbohydrate-metabolizing enzymes after the bees adopt the forager role [47–49]. This result is in contrast to the apparent lack of fucosylated N-glycan structures on major royal jelly glycoproteins [50]. The explanation could be that transcripts detected in muscles and the hypopharyngeal gland might originate from neighbouring neuronal tissue, which is expected to express the FucTA. On the other hand, antibodies detecting N-glycan core α1,3-linked fucose appear also to bind other tissues in Drosophila (e.g. the garland gland) [43,51,52], indicating that α1,3-linked fucosylated N-glycan structures might occur in insect tissues other than, as generally accepted, neurons [1,27] and venom glands [11,17,18].
Honey-bee FucTC appears to be expressed in all tissues analysed, and the reproducible appearance of the triplet band in FucTC RT–PCR reactions (where the largest band is of the expected size) for most tissues could indicate alternative splicing of transcripts of this gene. The higher expression rate of FucTC in venom glands correlates well with the occurrence of LacdiNAc structures present on bee-venom glycoproteins. However, even though honey-bee FucTC is expressed to a high degree in the hypopharyngeal glands, the N-glycans of major royal jelly glycoproteins appear, as mentioned above, not to be fucosylated. It was also observed that the expression of FucTC is similar in both nurse's and forager's glands that produce different proteins and show morphological and ultrastructural differences [53]. On the other hand, the dual in vitro activities of FucTC, towards both LacNAc and LacdiNAc structures, may not have relevance in vivo, since only fucosylated forms of the latter have been previously described on honey-bee glycoproteins. It is indeed probable that the honey-bee only expresses a β1,4-N-acetylgalactosaminyltransferase, akin to those previously described from Trichoplusia ni (cabbage looper) [54] and D. melanogaster [55], and lacks a β1,4-galactosyltransferase of the type found in mammals. Therefore presumably only the substrate with terminal GalNAc, required to generate a fucosylated LacdiNAc, is present in honey-bees in vivo.
The expression panel suggests that honey-bee FucTB is very weakly expressed in larvae, venom glands and foragers' brain tissue but not at all in nurses' brain tissue; indeed, its expression is several orders of magnitude lower than that of honey-bee FucTA and FucTC. Interestingly, honey-bee FucTB appears to be phylogenetically closer to known N-glycan core α1,3-FucTs like Drosophila FucTA and honey-bee FucTA than to the Drosophila FucTB and the putative human and murine FucTs X and XI. However, extensive attempts with both fruitfly and honey-bee FucTB homologues to demonstrate an activity towards potential substrates (MM, GnGn, GalGal, βGNβGN, lacto-N-neo-tetraose and lacto-N-tetraose) were not successful. This, in conjunction with the fact that we were able to account for the major fucosylated structures present in N-glycans on bee-venom and fruitfly glycoproteins, still leaves an open question as regards the in vivo function of FucTB proteins. However, any results with an insect FucTB might prove valuable for the understanding of the function of the homologous mammalian FucT X and XI, for which also, to date, no activity has been detected [56]. Indeed, it may be that the assumption, that standard ‘glycoprotein’ substrates are applicable, is false.
In conclusion, we have identified a core α1,3-FucT, from an insect other than Drosophila, as well as the first insect Lewis-type FucT. These enzymes account for the N-glycan α1,3-fucosylation events previously hypothesized on the basis of N-glycan analysis of honey-bee glycoproteins and both generate products associated with immune responses to glycans from invertebrates [11,13,19,20]. Despite both being members of the same enzyme family, honey-bee FucTA and FucTC have different substrate specificities; indeed, as with other members of CAZy glycosyltransferase family 10, the only feature in common is the transfer of fucose, from GDP-Fuc, to GlcNAc residues. The wider significance of our findings is, however, in connection with those commonly used baculovirus hosts such as T. ni (HiFive®) and Sf21 cells [57,58] known to express core α1,3-fucosylated N-glycans, but for which we lack relevant DNA sequence information. Indeed, of the various non-Drosophila insect species for which such data are currently publicly available, only B. mori is of direct biotechnological importance, with silkworm BmN cells sometimes being used for baculovirus-based expression. However, the relatively high homology between the fruitfly and honey-bee FucTA core α1,3-FucT sequences, and the observed ability of these sequences to cross-hybridize, may be useful in screening cDNA libraries from baculovirus-host species for which no genomic data is currently available. Identification of the core α1,3-FucTs from such cell lines would pave the way for knocking-out/down the corresponding gene, thus resulting in abolition/reduction of the expression of the immunogenic core α1,3-fucose moiety. This approach would complement other efforts to re-engineer insect cell lines [9,59] in order to generate ‘human-like’ glycans on proteins of pharmaceutical interest.
Acknowledgments
We thank Dr Hubert Paschinger for the gift of male honey-bee larvae, Dr Melitta Schachner and Dr Gabriele Loers (Zentrum fur Molekulare Neurobiologie Hamburg, Universität Hamburg, Hamburg, Germany) for the L5 anti-Lex monoclonal antibody, Dr Jozef Juršík for honey-bees, Dr Gene Robinson (University of Illinois Honey Bee Research Group, Department of Entomology, University of Illinois at Urbana-Champaign, Urbana, IL, U.S.A.) for the bee brain EST clones, and Thomas Iskratsch for preparing the transferrin neoglycoforms. This work was supported by grants from the Fonds zur Förderung der wissenschaftlichen Forschung (P17681) to I.B.H.W., and by a grant from the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences VEGA 2/5074/25 to Dr Ján Mucha (Department of Glycobiology, Slovak Academy of Sciences).
References
- 1.Fabini G., Freilinger A., Altmann F., Wilson I. B. H. Identification of core α1,3-fucosylated glycans and the requisite fucosyltransferase in Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- 2.Butters T. D., Hughes R. C., Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunciamycin. Biochim. Biophys. Acta. 1981;640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- 3.Roth J., Kempf A., Reuter G., Schauer R., Gehring W. J. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- 4.Hooker A. D., Green N. H., Baines A. J., Bull A. T., Jenkins N., Strange P. G., James D. C. Constraints on the transport and glycosylation of recombinant IFN-γ in Chinese hamster ovary and insect cells. Biotechnol. Bioeng. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Lopez M., Tetaert D., Juliant S., Gazon M., Cerutti M., Verbert A., Delannoy P. O-glycosylation potential of lepidopteran insect cell lines. Biochim. Biophys. Acta. 1999;1427:49–61. doi: 10.1016/s0304-4165(98)00176-7. [DOI] [PubMed] [Google Scholar]
- 6.North S. J., Koles K., Hembd C., Morris H. R., Dell A., Panin V. M., Haslam S. M. Glycomic studies of Drosophila melanogaster embryos. Glycoconjug. J. 2006;23:345–354. doi: 10.1007/s10719-006-6693-4. [DOI] [PubMed] [Google Scholar]
- 7.Wagner R., Liedtke S., Kretzschmar E., Geyer H., Geyer R., Klenk H. D. Elongation of the N-glycans of fowl plague virus hemagglutinin expressed in Spodoptera frugiperda (Sf9) cells by coexpression of human β1,2-N-acetylglucosaminyltransferase I. Glycobiology. 1996;6:165–175. doi: 10.1093/glycob/6.2.165. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson M., Wood H. A., Kulakosky P. C., Shuler M. L. Use of mannosamine for inducing the addition of outer arm N-acetylglucosamine onto N-linked oligosaccharides of recombinant proteins in insect cells. Biotechnol. Prog. 1999;15:168–173. doi: 10.1021/bp9900211. [DOI] [PubMed] [Google Scholar]
- 9.Hollister J., Grabenhorst E., Nimtz M., Conradt H., Jarvis D. L. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Léonard R., Rendić D., Rabouille C., Wilson I. B. H., Préat T., Altmann F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J. Biol. Chem. 2006;281:4867–4875. doi: 10.1074/jbc.M511023200. [DOI] [PubMed] [Google Scholar]
- 11.Tretter V., Altmann F., Kubelka V., März L., Becker W. M. Fucose α1,3-linked to the core region of glycoprotein N-glycans creates an important epitope for IgE from honey-bee venom allergic individuals. Int. Arch. Allergy Immunol. 1993;102:259–266. doi: 10.1159/000236534. [DOI] [PubMed] [Google Scholar]
- 12.Paschinger K., Fabini G., Schuster D., Rendić D., Wilson I. B. H. Definition of immunogenic carbohydrate epitopes. Acta Biochim. Pol. 2005;52:629–632. [PubMed] [Google Scholar]
- 13.Prenner C., Mach L., Glössl J., März L. The antigenicity of the carbohydrate moiety of an insect glycoprotein, honey-bee (Apis mellifera) venom phospholipase A2. The role of α1,3-fucosylation of the asparagine-bound N-acetylglucosamine. Biochem. J. 1992;284:377–380. doi: 10.1042/bj2840377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosaka A., Yano A., Itoh N., Kuroda Y., Nakagawa T., Kawasaki T. The structure of a neural specific carbohydrate epitope of horseradish peroxidase recognized by anti-horseradish peroxidase antiserum. J. Biol. Chem. 1991;266:4168–4172. [PubMed] [Google Scholar]
- 15.Hoffman D. R., Sakell R. H., Schmidt M. Sol i 1, the phospholipase allergen of imported fire ant venom. J. Allergy Clin. Immunol. 2005;115:611–616. doi: 10.1016/j.jaci.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Hemmer W., Focke M., Kolarich D., Wilson I. B. H., Altmann F., Wöhrl S., Götz M., Jarisch R. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honey-bee and yellow jacket venom in patients with stinging-insect allergy. J. Allergy Clin. Immunol. 2001;108:1045–1052. doi: 10.1067/mai.2001.120013. [DOI] [PubMed] [Google Scholar]
- 17.Kubelka V., Altmann F., Staudacher E., Tretter V., März L., Hård K., Kamerling J. P., Vliegenthart J. F. G. Primary structures of the N-linked carbohydrate chains from honey-bee venom phospholipase A2. Eur. J. Biochem. 1993;213:1193–1204. doi: 10.1111/j.1432-1033.1993.tb17870.x. [DOI] [PubMed] [Google Scholar]
- 18.Kubelka V., Altmann F., März L. The asparagine-linked carbohydrate of honey-bee venom hyaluronidase. Glycoconjug. J. 1995;12:77–83. doi: 10.1007/BF00731872. [DOI] [PubMed] [Google Scholar]
- 19.Naus C. W. A., van Remoortere A., Ouma J. H., Kimani G., Dunne D. W., Kamerling J. P., Deelder A. M., Hokke C. H. Specific antibody responses to three schistosome-related carbohydrate structures in recently exposed immigrants and established residents in an area of Schistosoma mansoni endemicity. Infect. Immun. 2003;71:5676–5681. doi: 10.1128/IAI.71.10.5676-5681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vervelde L., Bakker N., Kooyman F. N. J., Cornelissen A. W. C. A., Bank C. M. C., Nyame A. K., Cummings R. D., van Die I. Vaccination-induced protection of lambs against the parasitic nematode Haemonchus contortus correlates with high IgG antibody responses to the LDNF glycan antigen. Glycobiology. 2003;13:795–804. doi: 10.1093/glycob/cwg107. [DOI] [PubMed] [Google Scholar]
- 21.Becker D. J., Lowe J. B. Fucose: biosynthesis and biological function in mammals. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto J., Furukawa K., Cordon-Cardo C., Yin B. W., Rettig W. J., Oettgen H. F., Old L. J., Lloyd K. O. Expression of Lewisa, Lewisb, X, and Y blood group antigens in human colonic tumors and normal tissue and in human tumor-derived cell lines. Cancer Res. 1986;46:1553–1561. [PubMed] [Google Scholar]
- 23.Kannagi R., Izawa M., Koike T., Miyazaki K., Kimura N. Carbohydratemediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coutinho P. M., Deleury E., Davies G. J., Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 25.Staudacher E., Altmann F., Glössl J., März L., Schachter H., Kamerling J. P., Hård K., Vliegenthart J. F. G. GDP-fucose: β-N-acetylglucosamine (Fuc to (Fucα1→6GlcNAc)-Asn-peptide) α1→3-fucosyltransferase activity in honey-bee (Apis mellifera) venom glands. The difucosylation of asparagine-bound N-acetylglucosamine. Eur. J. Biochem. 1991;199:745–751. doi: 10.1111/j.1432-1033.1991.tb16179.x. [DOI] [PubMed] [Google Scholar]
- 26.Staudacher E., Kubelka V., März L. Distinct N-glycan fucosylation potentials of three lepidopteran cell lines. Eur. J. Biochem. 1992;207:987–993. doi: 10.1111/j.1432-1033.1992.tb17134.x. [DOI] [PubMed] [Google Scholar]
- 27.Rendić D., Linder A., Paschinger K., Borth N., Wilson I. B. H., Fabini G. Modulation of neural carbohydrate epitope expression in Drosophila melanogaster cells. J. Biol. Chem. 2006;281:3343–3353. doi: 10.1074/jbc.M508334200. [DOI] [PubMed] [Google Scholar]
- 28.Mucha J., Domlatil J., Lochnit G., Rendić D., Paschinger K., Hinterkörner G., Hofinger A., Kosma P., Wilson I. B. H. The Drosophila melanogaster homologue of the humna histo-blood group Pk gene encodes a glycolipid-modifying α1,4-N-acetylgalactosaminyltransferase. Biochem. J. 2004;382:67–74. doi: 10.1042/BJ20040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paschinger K., Rendić D., Lochnit G., Jantsch V., Wilson I. B. H. Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J. Biol. Chem. 2004;279:49588–49598. doi: 10.1074/jbc.M408978200. [DOI] [PubMed] [Google Scholar]
- 30.Palcic M. M., Hindsgaul O. Flexibility in the donor substrate specificity of β 1,4-galactosyltransferase: application in the synthesis of complex carbohydrates. Glycobiology. 1991;1:205–209. doi: 10.1093/glycob/1.2.205. [DOI] [PubMed] [Google Scholar]
- 31.Paschinger K., Staudacher E., Stemmer U., Fabini G., Wilson I. B. H. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology. 2005;15:463–474. doi: 10.1093/glycob/cwi028. [DOI] [PubMed] [Google Scholar]
- 32.Roitinger A., Leiter H., Staudacher E., Altmann F. HPLC method for the determination of Fuc to Asn-linked GlcNAc fucosyltransferases. Glycoconjug. J. 1998;15:89–91. doi: 10.1023/a:1006951802623. [DOI] [PubMed] [Google Scholar]
- 33.Wilson I. B. H. Identification of a cDNA encoding a plant Lewis-type α1,4-fucosyltransferase. Glycoconjug. J. 2001;18:439–447. doi: 10.1023/a:1016030000527. [DOI] [PubMed] [Google Scholar]
- 34.Cailleau-Thomas A., Coullin P., Candelier J. J., Balanzino L., Mennesson B., Oriol R., Mollicone R. FUT4 and FUT9 genes are expressed early in human embryogenesis. Glycobiology. 2000;10:789–802. doi: 10.1093/glycob/10.8.789. [DOI] [PubMed] [Google Scholar]
- 35.Wilson I. B. H., Harthill J. E., Mullin N. P., Ashford D. A., Altmann F. Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8:651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- 36.Lucka L., Fernando M., Grunow D., Kannicht C., Horst A. K., Nollau P., Wagener C. Identification of Lewis x structures of the cell adhesion molecule CEACAM1 from human granulocytes. Glycobiology. 2005;15:87–100. doi: 10.1093/glycob/cwh139. [DOI] [PubMed] [Google Scholar]
- 37.Whitfield C. W., Band M. R., Bonaldo M. F., Kumar C. G., Liu L., Pardinas J. R., Robertson H. M., Soares M. B., Robinson G. E. Annotated expressed sequence tags and cDNA microarrays for studies of brain and behavior in the honey bee. Genome Res. 2002;12:555–566. doi: 10.1101/gr.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breton C., Oriol R., Imberty A. Conserved structural features in eukaryotic and prokaryotic fucosyltransferases. Glycobiology. 1998;8:87–94. doi: 10.1093/glycob/8.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Oriol R., Mollicone R., Cailleau A., Balanzino L., Breton C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology. 1999;9:323–334. doi: 10.1093/glycob/9.4.323. [DOI] [PubMed] [Google Scholar]
- 40.Léonard R., Kolarich D., Paschinger K., Altmann F., Wilson I. B. H. A genetic and structural analysis of the N-glycosylation capabilities of rice and other monocotyledons. Plant Mol. Biol. 2004;55:631–644. doi: 10.1007/s11103-004-1558-3. [DOI] [PubMed] [Google Scholar]
- 41.Leiter H., Mucha J., Staudacher E., Grimm R., Glössl J., Altmann F. Purification, cDNA cloning, and expression of GDP-L-Fuc:Asn- linked GlcNAc α1,3-fucosyltransferase from mung beans. J. Biol. Chem. 1999;274:21830–21839. doi: 10.1074/jbc.274.31.21830. [DOI] [PubMed] [Google Scholar]
- 42.Wilson I. B. H., Rendić D., Dumić J., Freilinger A., Altmann F., Mucha J., Müller S., Hauser M.-T. Cloning and expression of α1,3-fucosyltransferase homologues from Arabidopsis thaliana. Biochim. Biophys. Acta. 2001;1527:88–96. doi: 10.1016/s0304-4165(01)00151-9. [DOI] [PubMed] [Google Scholar]
- 43.Jan L. Y., Jan Y. N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. U.S.A. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knecht D., Kaatz H. H. Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie. 1990;21:457–468. [Google Scholar]
- 45.Ohashi K., Natori S., Kubo T. Change in the mode of gene expression of the hypopharyngeal gland cells with an age-dependent role change of the worker honey-bee Apis mellifera L. Eur. J. Biochem. 1997;249:797–802. doi: 10.1111/j.1432-1033.1997.t01-1-00797.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmitzova J., Klaudiny J., Albert S., Schroder W., Schreckengost W., Hanes J., Judova J., Simuth J. A family of major royal jelly proteins of the honey-bee Apis mellifera L. Cell Mol. Life Sci. 1998;54:1020–1030. doi: 10.1007/s000180050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo T., Sasaki M., Nakamura J., Sasagawa H., Ohashi K., Takeuchi H., Natori S. Change in the expression of hypopharyngeal-gland proteins of the worker honey-bees (Apis mellifera L.) with age and/or role. J. Biochem. (Tokyo) 1996;119:291–295. doi: 10.1093/oxfordjournals.jbchem.a021237. [DOI] [PubMed] [Google Scholar]
- 48.Ohashi K., Natori S., Kubo T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honey-bee (Apis mellifera L.) Eur. J. Biochem. 1999;265:127–133. doi: 10.1046/j.1432-1327.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- 49.Simpson J., Riedel I. B. M., Wilding M. Invertase in the hypopharyngeal glands of the honey-bee. J. Apic. Res. 1968;7:127–133. [Google Scholar]
- 50.Kimura Y., Miyagi C., Kimura M., Nitoda T., Kawai N., Sugimoto H. Structural features of N-glycans linked to royal jelly glycoproteins: structures of high-mannose type, hybrid type, and biantennary type glycans. Biosci. Biotechnol. Biochem. 2000;64:2109–2120. doi: 10.1271/bbb.64.2109. [DOI] [PubMed] [Google Scholar]
- 51.Snow P. M., Patel N. H., Harrelson A. L., Goodman C. S. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J. Neurosci. 1987;7:4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seppo A., Matani P., Sharrow M., Tiemeyer M. Induction of neuron-specific glycosylation by Tollo/Toll-8, a Drosophila Toll-like receptor expressed in non-neural cells. Development. 2003;130:1439–1448. doi: 10.1242/dev.00347. [DOI] [PubMed] [Google Scholar]
- 53.Deseyn J., Billen J. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae) Apidologie. 2005;36:49–57. [Google Scholar]
- 54.Vadaie N., Jarvis D. L. Molecular cloning and functional characterization of a Lepidopteran insect β4-N-acetylgalactosaminyltransferase with broad substrate specificity, a functional role in glycoprotein biosynthesis, and a potential functional role in glycolipid biosynthesis. J. Biol. Chem. 2004;279:33501–33508. doi: 10.1074/jbc.M404925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haines N., Irvine K. D. Functional analysis of Drosophila β1,4-N-acetlygalactosaminyltransferases. Glycobiology. 2005;15:335–346. doi: 10.1093/glycob/cwi017. [DOI] [PubMed] [Google Scholar]
- 56.Baboval T., Smith F. I. Comparison of human and mouse Fuc-TX and Fuc-TXI genes, and expression studies in the mouse. Mamm. Genome. 2002;13:538–541. doi: 10.1007/s00335-001-2152-5. [DOI] [PubMed] [Google Scholar]
- 57.Hsu T. A., Takahashi N., Tsukamoto Y., Kato K., Shimada I., Masuda K., Whiteley E. M., Fan J. Q., Lee Y. C., Betenbaugh M. J. Differential N-glycan patterns of secreted and intracellular IgG produced in Trichoplusia ni cells. J. Biol. Chem. 1997;272:9062–9070. doi: 10.1074/jbc.272.14.9062. [DOI] [PubMed] [Google Scholar]
- 58.Kubelka V., Altmann F., Kornfeld G., März L. Structures of the N-linked oligosaccharides of the membrane glycoproteins from three lepidopteran cell lines (Sf-21, IZD-Mb-0503, Bm-N) Arch. Biochem. Biophys. 1994;308:148–157. doi: 10.1006/abbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- 59.Hill D. R., Aumiller J. J., Shi X., Jarvis D. L. Isolation and analysis of a baculovirus vector that supports recombinant glycoprotein sialylation by SfSWT-1 cells cultured in serum-free medium. Biotechnol. Bioeng. 2006;95:37–47. doi: 10.1002/bit.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]