Abstract
Ns (neuroserpin) is a member of the serpin (serine protease inhibitor) gene family that is primarily expressed within the central nervous system. Its principal target protease is tPA (tissue plasminogen activator), which is thought to contribute to synaptic plasticity and to be secreted in a stimulus-dependent manner. In the present study, we demonstrate in primary neuronal cultures that Ns co-localizes in LDCVs (large dense core vesicles) with the regulated secretory protein chromogranin B. We also show that Ns secretion is regulated and can be specifically induced 4-fold by secretagogue treatment. A novel 13-amino-acid sorting signal located at the C-terminus of Ns is identified that is both necessary and sufficient to target Ns to the regulated secretion pathway. Its deletion renders Ns no longer responsive to secretagogue stimulation, whereas PAI-Ns [Ns (neuroserpin)–PAI-1 (plasminogen activator inhibitor-1) chimaera appending the last 13 residues of Ns sequence to the C-terminus of PAI-1] shifts PAI-1 secretion into a regulated secretory pathway.
Keywords: immunohistochemistry, large dense-core vesicle, neuron, neuroserpin, serpin, tissue plasminogen activator (tPA)
Abbreviations: ANP, atrial natriuretic peptide; BiP, immunoglobulin heavy-chain-binding protein; CCD camera, charge-coupled device camera; CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; DPBS, Dulbecco's PBS; E15, embryonic day 15; ER, endoplasmic reticulum; FBS, fetal bovine serum; HRP, horseradish peroxidase; HSP47, heat-shock protein 47; LDCV, large dense core vesicle; NBM, neurobasal medium; NMDA, N-methyl-D-aspartate; Ns, neuroserpin; PAI-1, plasminogen activator inhibitor-1; PAI-Ns, Ns–PAI-1 chimaera appending the last 13 residues of Ns sequence to the C-terminus of PAI-1; RRX, Rhodamine Red-X; serpin, serine protease inhibitor; tPA, tissue plasminogen activator; wtNs, wild-type Ns
INTRODUCTION
Ns (neuroserpin) is a serine protease inhibitor that is specifically axonally secreted [1]. Immunohistochemical analysis of murine brain reveals Ns protein in the pyramidal neurons of the hippocampus and neurons of the cortex, cerebellum and hypothalamus, where it has been proposed to regulate extracellular proteolysis [2]. The primary target for Ns is thought to be the protease tPA (tissue plasminogen activator), which has been localized to the same regions of the brain as Ns [3].
tPA expression and/or activity has been associated with LTP (long-term potentiation), kindling, seizure, axonal migration and complex-motor learning [3]. Increased levels of tPA are seen in all of these events and it has been suggested that tPA acts by modulating the activity of the NMDA (N-methyl-D-aspartate) receptor, either by direct proteolysis of the receptor or through interactions with the LRP (low-density lipoprotein receptor-related protein) [4–7]. tPA secretion is thought to be regulated and to respond to stimuli [8]. In model systems of regulated secretion, tPA co-localized with corticotropin in LDCVs (large dense core vesicles) [9], and its secretion was stimulated by high potassium, barium chloride, NMDA or cAMP [5,10–13].
tPA is also associated with disease in models of seizure or stroke. tPA−/− mice (mice deficient in tPA) show delayed seizure progression [14] and are resistant to kainic acid-induced neuronal death [15,16]. tPA−/− mice also demonstrate a 50% decrease in stroke volume following middle cerebral artery occlusion [17,18]. Consistent with its role as a regulator of tPA, Ns is up-regulated in animal models of stroke and seizure, and Ns has been shown to be neuroprotective in both disease models [14,19,20]. Mutations in Ns are also linked to a progressive form of myoclonic epilepsy and dementia in humans [21–24]. These mutations lead to inclusion body formation in neurons throughout the cerebral cortex and in some subcortical nuclei. It is not known whether it is the presence of Ns inclusions, or the loss of tPA regulation, or a combination of both that leads to disease [25]. Nonetheless, accumulating evidence suggests that the balance between Ns and tPA in the CNS (central nervous system) is important [3,14]. However, the mechanism that regulates this balance is not known. In the present study, we have begun to address this important question by characterizing the regulation of Ns secretion, and our findings suggest that Ns is targeted to a regulated secretory pathway in neurons via a novel C-terminal sorting sequence.
EXPERIMENTAL
Antibodies against Ns and PAI-1 (plasminogen activator inhibitor-1)
Polyclonal antibodies against Ns and PAI-1 were produced in rabbits by standard procedures as described in [2,26]. The antibody directed against the last 15 amino acid residues of Ns, HPETMNTSGHDFEEL (anti-Ns15 antibody), was generated by immunizing rabbits with the peptide antigen coupled with the carrier, KLH (keyhole-limpet haemocyanin; Biosynthesis, Lewisville, TX, U.S.A.). Murine monoclonal antibodies against human Ns (anti-NsH7) were produced by a standard procedure and purified by Protein A–Sepharose (Amersham Biosciences, Piscataway, NJ, U.S.A.). Biotinylation of polyclonal anti-Ns antibodies was performed using D-biotinoyl-ϵ-aminocaproic acid-N-hydroxysuccinimide ester (biotin-7-NHS; where NHS is N-hydroxysuccinimido; Roche Applied Science, Indianapolis, IN, U.S.A.).
Immunohistochemistry of human hippocampal and cortical tissue slices
Paraffin-embedded normal adult hippocampal and cortical slices were purchased from Novagen (Hybrid-Ready; Novagen, San Diego, CA, U.S.A.). The slips were de-paraffinized and immersed in methanol containing 0.3% H2O2 for 30 min to exhaust endogenous peroxidase activity. The sections were then pre-incubated with 10% (v/v) normal goat serum and 1% (w/v) BSA (Sigma–Aldrich, St. Louis, MO, U.S.A.) in DPBS (Dulbecco's PBS; Invitrogen, Carlsbad, CA, U.S.A.) for 20 min followed by reaction with mouse anti-Ns H7 antibody (1:800) for 1 h. The sections were washed in DPBS and incubated with HRP (horseradish peroxidase)-conjugated anti-mouse IgG (Vectorstain; Vector Labs, Burlingame, CA, U.S.A.), followed by another DPBS wash and developed with chromogen-33-diaminobenzidine tetrahydrochloride (Sigma–Aldrich) for 5 min. The sections were then counterstained with Mayer's haematoxylin for 2 min. Appropriate sections were digitally photographed through a Nikon Eclipse E800 microscope and a CCD camera (charge-coupled device camera; Nikon) at ×100 oil-immersion lens.
Primary cortical cultures
Primary cortical cells were isolated from E15 (embryonic day 15) CD1 mouse embryos as described in [27]. Cortices were triturated with a pipette and the resulting single-cell suspension was plated on to coverslips coated with poly(L-lysine) (0.1 mg/ml; Sigma–Aldrich) in 24-well plates and incubated in NBM (neurobasal medium; Invitrogen) supplemented with B27 nutrients and 10% (v/v) FBS (fetal bovine serum; Fisher Scientific, Pittsburgh, PA, U.S.A.) and penicillin/streptomycin at 37 °C for 1 h. After a brief wash with DPBS, the medium was switched to serum-free NBM supplemented with B27 nutrients. Primary cortical cultures were grown in a 37 °C incubator and half of the medium in each well was replaced with new NBM/B27 medium every 3 days according to the manufacturer's protocol.
Generation of Att-20 cell lines expressing recombinant Ns and PAI-1 variants
Mouse anterior pituitary Att-20 cells (American Type Culture Collection, Manassas, VA, U.S.A.) were grown in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% FBS and penicillin/streptomycin at 37 °C. The human Ns cDNA was subcloned into the mammalian expression vector pcDNA-3.1 (Invitrogen), sequenced and transfected into Att-20 cells [wtNs (wild-type Ns)] using the GenePORTER transfection kit (Gene Therapy Systems, San Diego, CA, U.S.A.). The Ns variants were constructed by PCR, sequenced and subcloned in pcDNA3.1. The human PAI-1 cDNA cloned into pcDNA 3.1-hygro (pcPAI-1; Invitrogen) was a gift from Dr Michael K.K. Wong (Department of Medicine, Division of Hematology–Oncology, University of Pittsburgh Cancer Institute, Pittsburgh, PA, U.S.A.). For making the PAI-Ns (Ns–PAI-1 chimaera appending the last 13 residues of Ns sequence to the C-terminus of PAI-1) chimaera, we used a splicing-by-overlap extension method to attach the cDNA that codes for the last 13 amino acid residues of the Ns C-terminus to the 3′-end of PAI-1 cDNA coding sequence. BamHI and NotI were used to excise the PAI-1 cDNA from the pcPAI-1. For PCR, we used the sense primer 5′-GGTACCGAGCTCGGATCCACTAGTCC-3′ and the anti-sense primer 5′-GCGCGCGCGGCCGCTCATTAAAGTTCCTCAAAGTCATGGCCACTTGTATTCATTGTTTCGGGTTCCATCACTTGG-3′ (Integrated DNA Technology, Coralville, IA, U.S.A.). This latter primer codes for the last seven amino acids of PAI-1 followed by the last 13 amino acids of Ns followed by a stop codon. The PCR products were digested with BamHI and NotI, purified with QIAquick PCR (Qiagen, Valencia, CA, U.S.A.) and ligated back into the pcDNA 3.1-hygro vector. Independent clones were expanded and plasmids coding for the chimaeric PAI-Ns or PAI-1 were transfected into Att-20 cells by GenePORTER2 transfection kit (Gene Therapy Systems). Clones from wtNs and Δ13Ns were selected in the presence of Geneticin (G418-sulphate; 150 μg/ml; Sigma–Aldrich) and grown in 10% FBS DMEM with Geneticin (1.5 μg/ml). Clones from PAI-1 and PAI-Ns were selected by hygromycin B (250 μg/ml; Roche Applied Science) and grown in 10% FBS DMEM with hygromycin B (250 μg/ml).
To verify the protein expression, cells were incubated overnight in DMEM without serum and lysed with cell lysis buffer [50 mM Hepes, 500 mM NaCl, 1% Triton X-100 and 0.05% Tween 20, pH 7.0, supplemented with a Complete™ mini-protease inhibitor cocktail tablet (Roche Applied Science)]. The conditioned media and crude lysates were centrifuged at 2655 g for 10 min at 4 °C and the samples were subjected to SDS/PAGE and Western blotting (see below). wtNs, Δ13Ns, PAI-1 and PAI-Ns all formed SDS stable complexes with tPA in gel shift assays, indicating that all four proteins were functional inhibitory serpins (serine protease inhibitors) (results not shown).
Immunofluorescence and laser-scanning confocal microscopy
Primary cortical cells on coverslips were washed with DPBS and fixed with 3.7% (w/v) formaldehyde for 5 min. Then cells were permeabilized at 37 °C for 1 h with 0.1% saponin (Sigma–Aldrich) in a 5% BSA solution, which was also used in the subsequent steps. After DPBS washes, primary antibodies were incubated overnight at 4 °C. Mouse anti-NsH7, rabbit anti-human chromogranin B (QED Biosciences, San Diego, CA, U.S.A.), mouse anti-BiP (immunoglobulin heavy-chain-binding protein)/GRP78 and mouse anti-GM130 (BD Biosciences, Franklin Lakes, NJ, U.S.A.) and mouse anti-NeuN (Chemicon, Temecula, CA, U.S.A.) antibodies were used at 1:200, 1:800, 1:250, 1:250 and 1:500 dilutions respectively. In some cases, rabbit anti-human Ns antibodies were used at 1:400 dilutions. For controls, purified normal mouse or rabbit IgG (Sigma–Aldrich) at the same concentrations as primary antibodies were used. F(ab′)2-fragmented secondary anti-mouse or rabbit antibody [Alexa Fluor® 488 or 546; Invitrogen; RRX (Rhodamine Red-X); Jackson ImmunoResearch Laboratories, West Glove, PA, U.S.A.] at 1:200–1:400 dilution were used to label the appropriate primary antibodies for 1 h at 37 °C. In some cases, Alexa Fluor® 668-conjugated Nissl stain (Invitrogen) was used at 1:25 to label neurons. For additional controls, only secondary antibody was used in the absence of primary antibodies and in double staining experiments secondary antibodies were cross-reacted with the competing primary antibody to rule out cross-reactivity. After DPBS washes, some slips were also labelled briefly with either DAPI (4′,6-diamidino-2-phenylindole) or TO-PRO-3 nuclear stain (Invitrogen). Antifading agent (Invitrogen) was added and the coverslips were placed on to slips and sealed with nail polish. All slips were kept at 4 °C in the dark.
For fluorescence microscopy, images were digitally acquired through a Nikon Eclipse E800 microscope (Nikon) with a CCD camera using the image acquisition software SPOT advanced version 3.0.4 (Diagnostic Instruments, Sterling Heights, MI, U.S.A.). Each image from a range of different wavelengths was registered sequentially based on an optimized exposure time calculated by the software. If necessary, appropriate filters were used to lessen the mercury light source to reduce the excitation power. All the samples were exposed to the appropriate range of wavelengths for times much less than the time required to detect an appropriate negative control slip, stained with either non-immune IgG serum or secondary fluorescent antibodies only. Typical exposure times were 30–60 s for retrieving signals from the negative control slips, and milliseconds to approx. 5 s for detecting specific staining.
For laser-scanning confocal microscopy, images were acquired using a Radiance 2100 laser-scanning system (Bio-Rad, Hercules, CA, U.S.A.) using the Laser Sharp 2000 version 4.1 software. The argon–krypton lasers and the red diode laser excite at 488, 568 and 637 nm. All confocal images were acquired under the Kalman mode with a minimum laser power set based on negative control slips. The following are settings were used for calibration and during experiments, as necessary: ×100 oil-immersion objective lens, 8-bit, 1024×1024 pixel resolution of the CCD camera, LOT (Look-Out Table) set to green for 488 nm emission, red for 568 nm emission and blue for 668 nm (far-red) emission. The dichroic mirrors (Chroma Technology Corp., Rockingham, VT, U.S.A.) were set to 560 DCLPXR (dichroic long-pass mirror, extended reflection) for green, 650 DCLPXR for red and none for far-red. The emission filters were set to HD515/30 for green, HQ600/40 for red and HQ660 long-pass for far red. Finally, laser-scanning speed was set to 160 lines per second, with sequential recording of three channels. After image acquisition, reconstruction and deconvolution of registered images were performed using the Volocity program version 2.6 (Improvision) as indicated. No special effect or filter (i.e. reduce noise, blur, sharpen etc.) was used.
Pulse–chase metabolic labelling
Att-20 cell lines were grown at the density of 1×105/well in 6-well plates for 3 days prior to experiments. Cells were briefly washed with DPBS and DMEM without L-methionine (Biofluid, Rockville, MD, U.S.A.) three times. Cells were pulse-labelled for 1 h with 80 μCi/ml of [35S]methionine (37 MBq; Amersham Biosciences) in DMEM without L-methionine at 37 °C. The medium was then switched to regular DMEM and incubated for 1–8 h. For secretagogue-induced secretion experiments, [35S]methionine-labelled proteins were chased for 1 h with regular DMEM in the presence or absence of 2 mM BaCl2. The conditioned media were collected and cells were lysed in ice-cold cell lysis buffer (as described above). The conditioned media and cell lysates were centrifuged at 3500 and 9500 g respectively for 10 min at 4 °C. The supernatants for both conditioned media and cell lysates were kept on ice and the protein concentrations were determined using a standard BCA (bicinchoninic acid) assay (Pierce Biotechnology, Rockford, IL, U.S.A.).
Immunoprecipitation and immunoblotting
A 50% mixture of Protein G–Sepharose (Protein G–Sepharose 4 Fast Flow; Amersham) in DPBS was incubated with an equal volume of rabbit anti-human Ns, anti-PAI-1 or anti-Ns13 antibodies at 4 °C for 2 h. The mixture was centrifuged at a low speed (50 g) for 5 min at 4 °C and washed with DPBS and the cell lysis buffer. Then 40 μl of the 50% mixture of Protein G–Sepharose bound to an appropriate antibody was added to the volume equivalent to 100 μg of cell lysate or to an equal volume of the conditioned media and incubated for 2 h at 4 °C. After washing with the cell lysis buffer and DPBS, the final mixture was centrifuged at 20 g for 1 min at 4 °C, and the supernatant was discarded. Then, 20 μl of 2× SDS/PAGE loading buffer was added and boiled for 5 min. Samples were then centrifuged at 20 g for 1 min and cooled down to room temperature (23 °C) and loaded on to Tris-glycine gels (4–20, 4–12 or 10%; Invitrogen), followed by Coomassie Brilliant Blue staining. The gels were dried overnight in a gel dryer and exposed to a phosphor screen for 3 days. Then, the screen was scanned with a STORM PhosphorImager system (Molecular Dynamics) and labelled proteins were quantified by ImageQuant version 5.2 software for analysis.
For immunoblotting samples were subjected to SDS/PAGE (4–12 or 4–20% Tris-glycine gradient gels; Invitrogen) followed by transfer to nitrocellulose membranes for 2 h. The membranes were then blocked [5% (w/v) milk and 0.05% Tween 20 in Tris-buffered saline (50 mM Tris and 140 mM NaCl, pH 7.5)] after which they were probed for 1 h with either rabbit anti-Ns (1:10000), biotinylated rabbit anti-Ns (1:100), rabbit anti-Ns13 (1:5000) or rabbit anti-PAI-1 (1:20000). After washing, the membranes were reacted with HRP-conjugated streptavidin (1:2000) or anti-rabbit secondary antibody (1:10000) for 1 h, washed, then briefly incubated (5 min) with SuperSignal West Pico chemiluminescent substrate (Pierce) and exposed to an X-ray film (Kodak).
Statistical analysis
All the analysis was carried out using one-tail Student's t test and values were considered significant at P<0.05.
RESULTS
Detection of Ns in human brain and in primary murine neurons
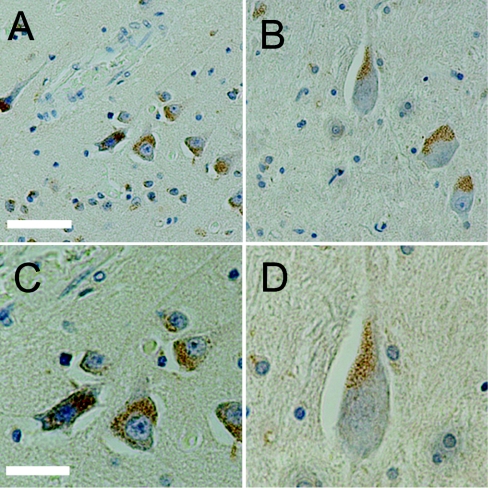
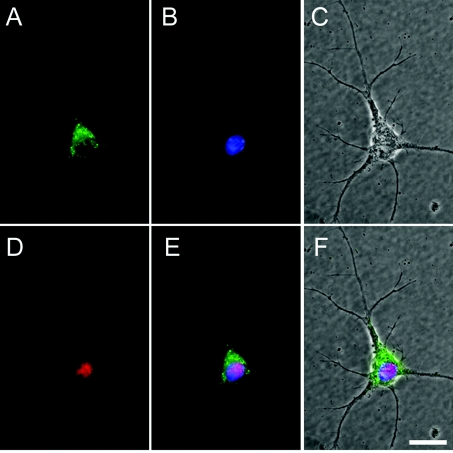
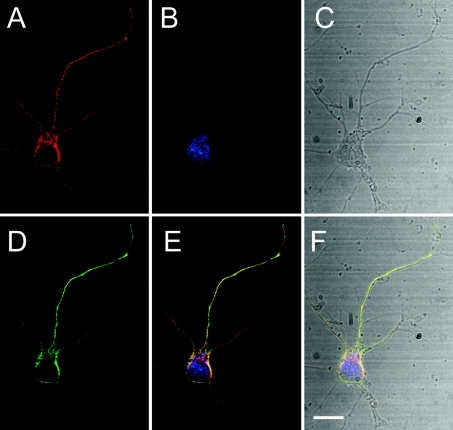
In the mouse, Ns is expressed in neurons of highly plastic areas, where it is believed to control the activity of tPA [2]. To see if Ns distributes similarly in human brain tissue, immunohistochemistry was performed on normal adult human hippocampal and cortical sections with monoclonal anti-Ns antibodies. These results revealed a perinuclear punctate staining pattern, which suggested an intracellular storage pool of endogenous Ns (Figure 1). Ns was predominantly present in pyramidal neurons, in which it appeared to be localized primarily in a region proximal to the axon hillock but distal from the nucleus. In addition, weak Ns staining was also detected in some axons as well as in interneurons. This localization and the punctate appearance of the Ns staining led us to hypothesize that Ns may normally be stored in intracellular vesicles where it can be rapidly released in response to stimulus. To test this hypothesis, we first characterized the subcellular localization of Ns in murine E15 primary cortical cultures. The cells were isolated from CD1 mice and analysed by immunofluorescent microscopy and laser-scanning confocal microscopy. Similar to the results obtained with immunohistochemistry of human brain tissues, a punctate perinuclear staining pattern was observed for Ns in primary neurons (Figure 2). The polarized staining pattern was distinct from both the ER (endoplasmic reticulum) and the Golgi, and was prominent in the larger neurite extensions (Figure 3), which have been identified as axons (results not shown). These results are consistent with the earlier suggestion that Ns in cultured cells is axonally secreted [1]. Further confocal microscopy analysis indicated that Ns largely co-localizes with chromogranin B (Figure 4), a protein known to be packaged into LDCVs and secreted via a regulated secretory pathway [28,29]. These results suggest that Ns is packaged into LDCVs and targeted to a regulated secretory pathway where it may be released at sites of neuronal activity. These results are also consistent with the recent findings that Ns antigen can be detected in purified secretory-granule fractions from bovine pituitary and adrenal tissues [30].
Figure 1. Immunohistochemistry of endogenous Ns in adult human brain sections.
Ns (brown) forms a punctate pattern that is localized within neurons of the cortex (A, C) and hippocampus (B, D). Ns is primarily detected in the soma, proximal to the main axon; (C) and (D) (×40) are magnified images of (A) and (B) (×20) respectively. The sections were counterstained with Mayer's haematoxylin. Scale bars: (A, B) 10 μm (×20) and (C, D) 5 μm (×40).
Figure 2. Immunofluorescence microscopy of endogenous Ns in E15 primary mouse cortical cultures.
Ns (green) is detected in a representative neuron (red) and forms a punctate pattern similar to that observed in human neurons in Figure 1. (A) Polyclonal anti-Ns antibodies; (B) DAPI nuclear stain (blue); (C) a phase contrast image of the same cell; (D) a monoclonal antibody of the neuronal specific marker NeuN; (E) a three-colour merged image of Ns, DAPI and NeuN; (F) a merged image of (C) and (E). The secondary antibodies used were anti-rabbit Alexa Fluor® 488 for Ns and anti-mouse RRX for NeuN, and the original magnification was with a ×100 oil-immersion objective lens. Scale bar, 5 μm.
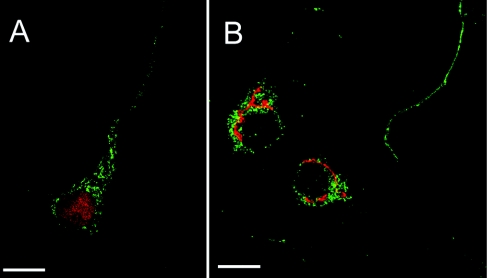
Figure 3. Laser-scanning confocal microscopy of Ns and markers for the ER and the Golgi.
Ns (green) in each panel is co-stained with either the ER marker BiP (red in A) and Golgi marker GM130 (red in B). Ns is not detected in the ER or Golgi. Note the punctate stain of Ns in the axons. Secondary antibodies used for fluorescence were anti-rabbit Alexa Fluor® 488 and anti-mouse RRX. The confocal images were deconvolved iteratively by using Volocity computer software. The scale bar shows 5 μm, and a ×100 oil-immersion objective lens was used.
Figure 4. Ns partially co-localizes with chromogranin B.
Confocal microscopy of a primary mouse cortical neuron shows that the endogenous Ns in red (A) and chromogranin B in green (D) are in a neuron in blue (B). Transmission (grey) in (C) shows that this neuron is a single isolated cell. (E) The merged image of (A), (B) and (D). (F) The merged image of (A), (B), (C) and (D). Monoclonal anti-Ns antibody, polyclonal anti-chromogranin B antibodies and fluorescent Nissl stain were used to detect Ns, chromogranin B and neurons respectively. Secondary antibodies anti-rabbit Alexa Fluor® 488 and anti-mouse Alexa Fluor® 568 were used for fluorescence. All the images were deconvolved iteratively by using Volocity computer software. The scale bar shows 5 μm, and a ×100 oil-immersion objective lens was used.
Novel sorting signal
To identify a putative sorting signal on Ns that directs it to the LDCVs, a BLAST sequence alignment of the human Ns amino acid sequence was performed against more than 500 serpins in the NCBI (National Center for Biotechnology Information) database. This analysis indicated that in contrast with the vast majority of serpins, which end within four residues of a conserved proline residue, Ns has a nearly unique 13-amino-acid C-terminal extension (Figure 5). Only three other serpins were identified in this search with similar C-terminal extensions (Table 1). The most similar was present on the pancreatic protein ZG-46p, also called pancpin, which has been reported to be packaged into the zymogen granules of the pancreas [31,32]. The other two serpins identified with similar extensions were the Drosophila protein Spn4.1, which lacks the conserved proline residue, but which has a 13-residue extension, and the chaperone, HSP47 (heat-shock protein 47) or collagen-binding protein, which has a nine-residue extension [33,34]. These latter two serpins both appeared to have C-terminal ER retention signals similar to a KDEL sequence, and HSP47 has been shown to localize to the ER. Based on these findings, we decided to test the hypothesis that the C-terminal extension on Ns may contain a sorting sequence that directs Ns to LDCVs.
Figure 5. Amino acid sequence of Ns.
The N-terminal signal sequence (positions 1–20) is shown in boldface italics, the conserved C-terminal proline residue is double underlined, and the 13-residue C-terminal extension is shown in underlined boldface text.
Table 1. Comparison of serpin C-terminal sequences.
Comparison of the C-terminal residues of Ns, pancpin, Spn4.1 and HSP47. The sequence number of the first residue shown is given in parentheses and the conserved proline residue is underlined in each sequence.
| Serpin | C-terminal residues | Localization |
|---|---|---|
| Ns | (395) ∼MHPETMNTSGHDFEEL | − |
| Pancpin/ZG-46p | (390) ∼TNPDTQEIKGRDLDSL | Zymogen granule |
| Spn4.1 | (410) ∼RL-EENTFASSEHDEL | ER |
| HSP47 | (406) ∼VRPK-GD–-KMRDEL | ER |
Since sorting to secretory vesicles most likely does not occur until a protein has folded and passed through ER, then if the C-terminal 13-amino-acid extension on Ns functions as a vesicular targeting sequence it must be available on the surface of the folded Ns protein. The structure of recombinant Ns has been solved; however, the form of Ns used for crystallization lacked the last 13 amino acids and thus the location of the C-terminal extension in the tertiary structure of Ns is not known [35]. Therefore, to determine the accessibility of the C-terminal 13 residues of Ns, the cDNA for full-length human Ns (wtNs), and a variant of Ns with the sequence coding for the C-terminal 13 amino acids removed (Δ13Ns) were constructed in the mammalian expression vector pCDNA-3 and stably transfected into the mouse anterior pituitary cell line Att-20 (Table 2). Antibodies were also raised to a synthetic peptide representing the last 15 amino acid residues of Ns, HPETMNTSGHDFEEL. These antibodies (anti-Ns15) were then used in immunoprecipitation experiments of native Ns to determine the surface accessibility of this peptide sequence in undenatured wtNs. These results are shown in Figure 6 and they indicate that the anti-Ns15 antibody can effectively immunoprecipitate native wtNs from the conditioned media of Att-20 cells but cannot immunoprecipitate the mutant Ns lacking the C-terminal 13 residues. This suggests that the C-terminal 13 residue extension on Ns is exposed on the surface of native wtNs protein.
Table 2. Serpin variants stably expressed in Att-20 cells.
Comparison of the C-terminal residues of wtNs with Δ13Ns, wtPAI-1 and the PAI-1-Ns chimaera, which contains the full sequence of wtPAI-1 with the last 13 residues of Ns appended to the C-terminus. The conserved proline residue is underlined in each sequence.
| Serpin variants | C-terminal residues |
|---|---|
| wtNs | ∼MHPETMNTSGHDFEEL |
| Δ13Ns | ∼MHP |
| wtPAI-1 | ∼MEP |
| PAI-Ns | ∼MEPETMNTSGHDFEEL |
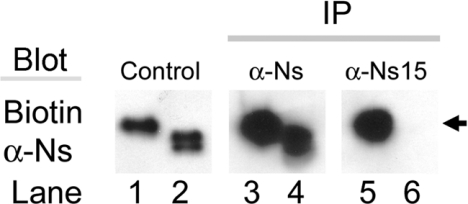
Figure 6. The Ns C-terminus is exposed on the surface of the molecule.
Conditioned media from wtNs (lanes 1, 3 and 5) or Δ13Ns (lanes 2, 4 and 6) was immunoprecipitated with either polyclonal anti-Ns (α-Ns; lanes 3 and 4) or with anti-Ns15 antibodies (α-Ns15; lanes 5 and 6), which detects only the Ns C-terminus, followed by immunoblotting with biotinylated rabbit anti-Ns. Lanes 1 and 2 are starting material not subjected to immunoprecipitation. Notice that anti-Ns15 antibody precipitated wtNs (lane 5) but not Δ13Ns (lane 6). Arrows show the position of a 50 kDa marker.
Secretagogue-mediated release of Ns
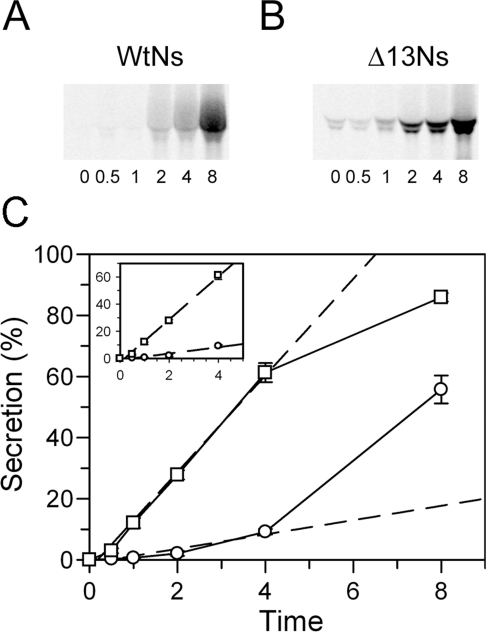
To characterize the role of the C-terminal extension in regulating Ns secretion, the stably transfected Att-20 cells expressing wtNs or the Δ13Ns variant were subjected to metabolic pulse labelling with [35S]methionine for 1 h followed by incubation with non-radioactive methionine for various times. Immunoprecipitation of [35S]methionine-labelled Ns from the medium and cell lysates showed that very little secreted wtNs was detected prior to 2 h of chase (Figure 7A), whereas secretion of Δ13Ns was significantly faster and could be easily detected in the media of pulse-labelled cells at the beginning of chase (Figure 7B). The doublet band of Ns seen is due to differences in glycosylation and both forms of Ns are active inhibitors of tPA (results not shown). By plotting the percentage of the total expressed Ns that was secreted against time, it was found that even following an 8 h chase period approx. 50% of the labelled wtNs still remained within the cells (Figure 7C). This relatively slow release of Ns supports the suggestion that it is retained intracellularly in a regulated secretory pathway. In contrast, the relatively rapid release of the Δ13Ns variant suggests that it may not be retained within the cells and instead may be targeted to a constitutive secretion pathway. Quantitative analysis of the initial rates of Ns secretion calculated from the first 4 h of the chase period demonstrated that the initial rate of Δ13Ns secretion was more than 6-fold faster than that of the wtNs, suggesting that removal of the C-terminal 13 residues results in an Ns protein that is constitutively secreted (Figure 7C).
Figure 7. Secretion rate is lower for wtNs than the variant.
Pulse–chase metabolic labelling was performed in Att-20 cell lines. Proteins were metabolically pulse-labelled with [35S]methionine for 1 h followed by a chase of non-radioactive methionine for the times indicated. (A, B) Ns immunoprecipitated from conditioned media were subjected to SDS/PAGE and visualized by STORM imager. The number located at the bottom of each lane indicates the chase time in hours. Representative gels are shown. (C) Quantification of Ns secretion (circle, wtNs; square, Δ13Ns). The broken line is a linear fit of the basal level of secretion calculated from the first 4 h of data. The molecular band quantification was performed with the ImageQuant computer software. The percentage of total secretion is calculated by the amount of Ns in the media divided by the total amount of Ns in both media and cell lysates. The data show the results of two independent experiments performed in duplicate. P<0.05 using Student's t test was obtained for all time points of wtNs compared with the Δ13Ns.
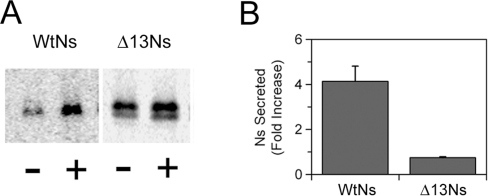
To test the hypothesis that Ns secretion is not only targeted to the LDCVs but that its secretion is also regulated, we examined if Ns secretion could be induced in response to secretagogue stimulation. To test this hypothesis the stably transfected Att-20 cells were subjected to metabolic pulse labelling with [35S]methionine for 1 h as above except that the cells were treated with or without the potent secretagogue BaCl2 at the start of the chase. These studies demonstrated that following 1 h of chase, there was an approx. 4-fold increase in wtNs secretion in the presence of BaCl2 (Figure 8). Similar results were also obtained with the secretagogue cAMP (results not shown). In contrast, BaCl2 treatment had no effect on the release of the Δ13Ns protein. This demonstrates that wtNs can be secreted in a stimulus-dependent manner, but that the secretion of the Δ13Ns is no longer regulated. Taken together, the results suggest that the C-terminal 13 amino acid residues constitute a sorting sequence on Ns for a regulated secretory pathway.
Figure 8. Secretion of Ns is regulated.
Pulse–chase metabolic labelling was performed in Att-20 cell lines as in Figure 7 except that during the 1 h of chase period, the cells were treated with (+) or without (−) 2 mM BaCl2. After immunoprecipitation with polyclonal anti-Ns antibody, samples were subjected to SDS/PAGE and STORM imager analysis. (A) A representative scan of the SDS/PAGE. (B) Quantification of the fold increase in Ns secretion by barium stimulation. The data show the results of three independent experiments performed in triplicate with wtNs and two independent experiments performed in triplicate with Δ13Ns. P<0.05 using Student's t test was obtained for stimulated secretion of wtNs compared with the control in the absence of barium. There was no significant increase in secretion with the Δ13Ns.
Targeting of PAI-1 to a regulated secretion pathway
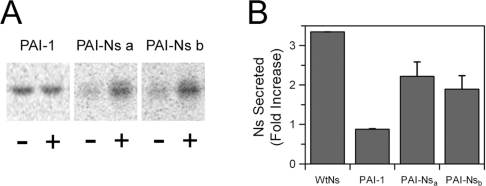
Next, we determined whether the role of the C-terminal extension in secretory vesicle targeting is unique to Ns or whether this peptide is capable of sorting another protein to a regulated secretion pathway. For these experiments the 13-residue extension from Ns was added to the C-terminus of a structurally similar serpin PAI-1, the primary plasma inhibitor of tPA. Unlike Ns, PAI-1 ends with the conserved proline residue at the C-terminus and does not have the 13-amino-acid extension; however, similar to Ns, PAI-1 also inhibits tPA with high specificity [36]. For these studies the PAI-1 cDNA was cloned in pCDNA-3 and stably transfected into Att-20 cells to determine whether its secretion was also regulated. In contrast with wtNs, a significant amount of PAI-1 was secreted into the culture medium following [35S]methionine labelling and incubation with non-radioactive methionine for only 1 h (Figure 9). Furthermore, stimulation by 2 mM BaCl2 during the chase had no effect on the secretion of PAI-1, suggesting that PAI-1 is constitutively secreted. Next, a PAI-1 variant containing the C-terminal 13 amino acids from Ns (PAI-Ns) was constructed and stably expressed in Att-20 cells (Table 2). Metabolic pulse–chase labelling experiments demonstrated that PAI-Ns was secreted significantly more slowly than wild-type PAI-1 with only 1–2% of the PAI-Ns protein being secreted into the culture supernatant during the 1 h chase compared with 20% of the total wtPAI-1 protein being secreted in 1 h (results not shown). Furthermore, when the secretagogue was included during the chase, the level of PAI-Ns in the medium increased by more than 2-fold, indicating that appending the C-terminal 13 amino acids from Ns to PAI shifted PAI-1 into a stimulus-dependent secretion pathway (Figure 9).
Figure 9. Secretion of the PAI-Ns chimaeric protein is regulated.
Pulse–chase metabolic labelling was performed in Att-20 cell lines as in Figure 8 with (+) or without (−) 2 mM BaCl2 during the 1 h chase period. Cells expressing wtNs, wtPAI-1 and two independent clones of the PAI-Ns chimaera were examined. Following immunoprecipitation with either polyclonal anti-PAI-1 (wtPAI-1 and PAI-Ns) or anti-Ns (wtNs) antibodies, samples were subjected to SDS/PAGE and STORM imager analysis. (A) Representative scan of the SDS/PAGE. (B) Quantification of the fold increase in Ns or PAI-1 secretion induced by barium stimulation. The data show the results of two independent experiments performed in triplicate for each protein. P<0.05 using Student's t test was obtained for stimulated secretion of wtNs and the two chimaera compared with the controls in the absence of barium. There was no significant increase in secretion with the wtPAI-1.
DISCUSSION
In earlier in vivo studies, we showed that within 10 min of kainate injection into the amygdala of mice there was local release of both tPA and Ns within the tissues of the amygdala [14]. Based on these results, we postulated that the increase in extracellular Ns was likely in response to increased local levels of tPA activity [14]. Such a rapid rise in extracellular Ns suggested that there must be a mechanism to regulate the release of stored Ns, since within this short 10 min time frame there was unlikely to be sufficient time to respond to the increased extracellular tPA levels by increasing Ns synthesis and its transport through the constitutive secretory pathway. Therefore the study presented here was undertaken to test the hypothesis that Ns is targeted to a regulated secretory pathway where it can be rapidly released upon stimulation, and to identify the signal on Ns that directs it into this pathway.
The localization of Ns by immunohistochemistry and immunofluorescence microscopy is consistent with a vesicular distribution for Ns. Furthermore, expression of Ns in Att-20 cells indicates that its secretion is regulated since spontaneous secretion of Ns is relatively slow with less than 1% being secreted in 1 h, while upon stimulation Ns secretion is increased approx. 4-fold. In contrast, nearly 20% of the homologous serpin, PAI-1, the primary tPA inhibitor in plasma, is secreted in 1 h, and PAI-1 secretion is not increased further in response to BaCl2. These results indicate that Ns, but not PAI-1, is specifically targeted to a regulated secretion pathway. The sequence responsible for this targeting appears to lie within the last 13 residues of Ns since their deletion both increased the basal rate of Ns secretion and made Ns secretion no longer responsive to stimulus, while addition of these 13 residues to PAI-1 both reduced its rate of basal secretion and made it responsive to BaCl2 stimulation. Together, these results indicate that Ns secretion is regulated and that the last 13 amino acid residues of Ns contain sufficient information to function as the sorting sequence for this regulated secretory pathway.
Unlike Ns, most serpins are either constitutively secreted or localized to the cytoplasm [37,38]. Ns, pancpin and the ER-localized serpins are exceptions to this general pattern, and it is interesting to note that both Ns and the ER-localized serpins appear to contain their targeting sequences at their C-termini. Thus we speculate that the 13-residue C-terminal extension on pancpin may also be responsible for targeting that serpin to the zymogen granules of the pancreas. The targeting of Ns is also very likely to be cell-specific, and in cells lacking LDCVs Ns targeting will necessarily be different. This has been shown by studies where Ns was expressed in Cos-7 cells. These studies found that wtNs was secreted relatively slowly from Cos-7 cells and immunofluorescence analysis localized the intracellular Ns to the ER and Golgi compartments [39]. It could be that since these cells lack LDCVs, Ns may accumulate in compartments that Ns normally only transits through in cells that naturally express Ns such as neurons, and we show here that in neurons Ns does not localize to either the ER or Golgi. PAI-1 provides another example of cell-specific serpin targeting. PAI-1 is thought to be constitutively secreted from most cell types including endothelial cells, smooth-muscle cells and many tumour cells, and in the present study we show that PAI-1 is constitutively secreted from Att-20 cells. However, PAI-1 is also synthesized by megakaryocytes, the precursor cell of platelets, and PAI-1 is stored within the α granules of platelets [40]. The mechanism for this localization of PAI-1 is not known, but suggests that protein targeting can be highly cell-specific.
In general, the mechanisms of secretory pathway sorting are not well established. Based on studies of endocrine and neuroendocrine cells, it has been suggested that proteins can enter a regulated secretory pathway by a number of processes. These include bivalent ion interactions and low-pH-induced protein aggregation, and/or interaction with specific receptor cargo proteins, which may guide a protein to LDCVs [41]. Both of these putative mechanisms are thought to involve a surface-exposed sorting sequence that is unique to a particular protein or a class of proteins. Our results suggest that the 13 residues at the C-terminus of Ns are exposed on the surface of native Ns, permitting interaction with other proteins or ions, and a portion of this C-terminal sequence shows significant similarity to a sequence identified on another protein whose secretion is regulated, ANP (atrial natriuretic peptide). ANP is targeted to LDCVs and co-localizes with chromogranin B [42]. ANP also contains the sequence motif, DHLEEK, which is remarkably similar to the last six residues of Ns, HDFEEL. The DHLEEK sequence in ANP has been suggested to act as a calcium-binding sorting signal for regulated secretion [43]. In studies where the two glutamate residues of this sequence were replaced with glutamine residues, there was a shift in ANP secretion from a regulated pathway into a constitutive secretory pathway. The mechanism of targeting to the regulated pathway was suggested to involve ANP aggregation in the presence of calcium and reduced pH, conditions which are comparable with the environment found in immature granules. Interestingly, chromogranin B has been shown to facilitate calcium influx into LDCVs and to play a key role in the LDCV biogenesis [44,45]. Chromogranin B is also known to function as a helper protein that targets other proteins to the LDCVs [46]. Taken together, these studies and our results suggest that the two glutamate residues at the C-terminus of Ns may form part of the Ns sorting sequence, and may act by mediating calcium-dependent aggregation necessary for vesicular packaging and targeting to a regulated secretion pathway. In addition, the co-localization of chromogranin B with Ns suggests that chromogranin B may help Ns to enter the secretory pathway.
A mechanism for regulating Ns secretion that entails induced protein aggregation during packaging into LDCVs might also explain the susceptibility of Ns to pathological mutations that result in polymerization and inclusion body formation [25]. Pathological serpin polymerization is a concentration-dependent process [47], and other than Ns, the only serpin proteins that are known to undergo this type of loop-sheet polymerization are all highly expressed proteins with normal plasma levels greater than 1 μM (α1-antitrypsin, α1-antichymotrypsin, antithrombin III and C1-inhibitor) [48]. The Ns concentration in the CNS is unknown; however, even relatively low levels of Ns expression could achieve locally high concentrations during packaging if this process involved induced aggregation, and in the context of a mutant Ns, this aggregation could promote pathological loop-sheet polymerization. Thus understanding the mechanisms that regulate Ns secretion may also further our understanding of how and where mutant Ns aggregates, potentially leading to new ways to treat the devastating dementia caused by pathological Ns polymerization.
To study the regulated release of Ns we have used barium as a stimulus because it is a potent secretagogue that has been used extensively in model systems of regulated protein secretion, and because it has been shown to specifically induce the release of tPA from the LDCVs in PC-12 cells [11]. Barium chloride is thought to stimulate secretion by blocking voltage-sensitive potassium channels, and thereby inducing membrane depolarization, which causes voltage-sensitive calcium channels to open [29]. Barium is also thought to act as a substitute for intercellular calcium upon entering through the calcium channels. Interestingly, a recent study has shown that barium appears to preferentially stimulate a reserve vesicular pool rather than a readily releasable vesicular pool [49]. Our results from the immunohistochemistry in adult human brain show that Ns staining is abundant in a region proximal to the axon hillock. This localization might suggest that in vivo Ns may be preferentially stored in a reserve pool that accumulates at the axon hillock. Moreover, the regulation of Ns secretion may be linked to the regulated release of tPA, which has been suggested to occur at synaptic sites in an activity-dependent manner [8,12]. It may be that the mobilization and secretion of Ns from a reserve pool are mediated by retrograde signalling from a synaptic site that is triggered in response to increased levels of tPA activity at the synapse. Although such a co-ordinated secretion mechanism still remains to be elucidated, it would allow a window of time for tPA to act during synaptic plastic events before Ns were released and tPA's action inhibited.
Future studies will clarify the mechanism whereby the C-terminal residues of Ns target Ns to a regulated secretion pathway. In addition, studies aimed at understanding the signals within the CNS that regulate the release of Ns will undoubtedly add to our understanding of the role of Ns in regulating tPA in the brain.
Acknowledgments
This work was supported by grants HL55374, HL55747 and HL54710 to D. A. L. from the National Institutes of Health.
References
- 1.Osterwalder T., Contartese J., Stoeckli E. T., Kuhn T. B., Sonderegger P. Neuroserpin, an axonally secreted serine protease inhibitor. EMBO J. 1996;15:2944–2953. [PMC free article] [PubMed] [Google Scholar]
- 2.Hastings G. A., Coleman T. A., Haudenschild C. C., Stefansson S., Smith E. P., Barthlow R., Cherry S., Sandkvist M., Lawrence D. A. Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons: implications for the regulation of motor learning and neuronal survival. J. Biol. Chem. 1997;272:33062–33067. doi: 10.1074/jbc.272.52.33062. [DOI] [PubMed] [Google Scholar]
- 3.Yepes M., Lawrence D. A. Tissue-type plasminogen activator and neuroserpin: a well-balanced act in the nervous system? Trends Cardiovasc. Med. 2004;14:173–180. doi: 10.1016/j.tcm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo M., Holtzman D. M., Li Y., Osaka H., DeMaro J., Jacquin M., Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E. T., Vivien D., Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 6.Yepes M., Sandkvist M., Moore E. G., Bugge T. H., Strickland D. K., Lawrence D. A. Tissue-type plasminogen activator induces opening of the blood–brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlak R., Melchor J. P., Matys T., Skrzypiec A. E., Strickland S. Ethanol-withdrawal seizures are controlled by tissue plasminogen activator via modulation of NR2B-containing NMDA receptors. Proc. Natl. Acad. Sci. U.S.A. 2005;102:443–448. doi: 10.1073/pnas.0406454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lochner J. E., Honigman L. S., Grant W. F., Gessford S. K., Hansen A. B., Silverman M. A., Scalettar B. A. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J. Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- 9.Santell L., Marotti K. R., Levin E. G. Targeting of tissue plasminogen activator into the regulated secretory pathway of neuroendocrine cells. Brain Res. 1999;816:258–265. doi: 10.1016/s0006-8993(98)01054-3. [DOI] [PubMed] [Google Scholar]
- 10.Gualandris A., Jones T. E., Strickland S., Tsirka S. E. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmer R. J., Mahata M., Mahata S., Sebald M. T., O'Connor D. T., Miles L. A. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway: catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J. Biol. Chem. 1997;272:1976–1982. doi: 10.1074/jbc.272.3.1976. [DOI] [PubMed] [Google Scholar]
- 12.Baranes D., Lederfein D., Huang Y. Y., Chen M., Bailey C. H., Kandel E. R. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Monreal M., Lopez-Atalaya J. P., Benchenane K., Leveille F., Cacquevel M., Plawinski L., MacKenzie E. T., Bu G., Buisson A., Vivien D. Is tissue-type plasminogen activator a neuromodulator? Mol. Cell Neurosci. 2004;25:594–601. doi: 10.1016/j.mcn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Yepes M., Sandkvist M., Coleman T. A., Moore E., Wu J. Y., Mitola D., Bugge T. H., Lawrence D. A. Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J. Clin. Invest. 2002;109:1571–1578. doi: 10.1172/JCI14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsirka S. E., Gualandris A., Amaral D. G., Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 16.Tsirka S. E. Clinical implications of the involvement of tPA in neuronal cell death. J. Mol. Med. 1997;75:341–347. doi: 10.1007/s001090050119. [DOI] [PubMed] [Google Scholar]
- 17.Nagai N., De Mol M., Lijnen H. R., Carmeliet P., Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y. F., Tsirka S. E., Strickland S., Stieg P. E., Soriano S. G., Lipton S. A. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat. Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 19.Yepes M., Sandkvist M., Wong M. K., Coleman T. A., Smith E., Cohan S. L., Lawrence D. A. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- 20.Cinelli P., Madani R., Tsuzuki N., Vallet P., Arras M., Zhao C. N., Osterwalder T., Rulicke T., Sonderegger P. Neuroserpin, a neuroprotective factor in focal ischemic stroke. Mol. Cell. Neurosci. 2001;18:443–457. doi: 10.1006/mcne.2001.1028. [DOI] [PubMed] [Google Scholar]
- 21.Davis R. L., Holohan P. D., Shrimpton A. E., Tatum A. H., Daucher J., Collins G. H., Todd R., Bradshaw C., Kent P., Feiglin D., et al. Familial encephalopathy with neuroserpin inclusion bodies. Am. J. Pathol. 1999;155:1901–1913. doi: 10.1016/S0002-9440(10)65510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis R. L., Shrimpton A. E., Holohan P. D., Bradshaw C., Feiglin D., Collins G. H., Sonderegger P., Kinter J., Becker L. M., Lacbawan F., et al. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- 23.Takao M., Benson M. D., Murrell J. R., Yazaki M., Piccardo P., Unverzagt F. W., Davis R. L., Holohan P. D., Lawrence D. A., Richardson R., et al. Neuroserpin mutation S52R causes neuroserpin accumulation in neurons and is associated with progressive myoclonus epilepsy. J. Neuropathol. Exp. Neurol. 2000;59:1070–1086. doi: 10.1093/jnen/59.12.1070. [DOI] [PubMed] [Google Scholar]
- 24.Yazaki M., Liepnieks J. J., Murrell J. R., Takao M., Guenther B., Piccardo P., Farlow M. R., Ghetti B., Benson M. D. Biochemical characterization of a neuroserpin variant associated with hereditary dementia. Am. J. Pathol. 2001;158:227–233. doi: 10.1016/S0002-9440(10)63961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis R. L., Shrimpton A. E., Carrell R. W., Lomas D. A., Gerhard L., Baumann B., Lawrence D. A., Yepes M., Kim T. S., Ghetti B., et al. Association between conformational mutations in neuroserpin and onset and severity of dementia. Lancet. 2002;359:2242–2247. doi: 10.1016/S0140-6736(02)09293-0. [DOI] [PubMed] [Google Scholar]
- 26.Bijnens A. P., Ngo T. H., Gils A., Dewaele J., Knockaert I., Stassen J. M., Declerck P. J. Elucidation of the binding regions of PAI-1 neutralizing antibodies using chimeric variants of human and rat PAI-1. Thromb. Haemostasis. 2001;85:866–874. [PubMed] [Google Scholar]
- 27.Bacskai B. J., Xia M. Q., Strickland D. K., Rebeck G. W., Hyman B. T. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang T., Wacker I., Steyer J., Kaether C., Wunderlich I., Soldati T., Gerdes H. H., Almers W. Ca2+-triggered peptide secretion in single cells imaged with green fluorescent protein and evanescent-wave microscopy. Neuron. 1997;18:857–863. doi: 10.1016/s0896-6273(00)80325-6. [DOI] [PubMed] [Google Scholar]
- 29.Kaether C., Salm T., Glombik M., Almers W., Gerdes H. H. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur. J. Cell Biol. 1997;74:133–142. [PubMed] [Google Scholar]
- 30.Hill R. M., Coates L. C., Parmar P. K., Mezey E., Pearson J. F., Birch N. P. Expression and functional characterization of the serine protease inhibitor neuroserpin in endocrine cells. Ann. N.Y. Acad. Sci. 2002;971:406–415. doi: 10.1111/j.1749-6632.2002.tb04503.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen C. Y., Cronshagen U., Kern H. F. A novel pancreas-specific serpin (ZG-46p) localizes to the soluble and membrane fraction of the Golgi complex and the zymogen granules of acinar cells. Eur. J. Cell Biol. 1997;73:205–214. [PubMed] [Google Scholar]
- 32.Ozaki K., Nagata M., Suzuki M., Fujiwara T., Miyoshi Y., Ishikawa O., Ohigashi H., Imaoka S., Takahashi E., Nakamura Y. Isolation and characterization of a novel human pancreas-specific gene, pancpin, that is down-regulated in pancreatic cancer cells. Genes Chromosomes Cancer. 1998;22:179–185. [PubMed] [Google Scholar]
- 33.Clarke E. P., Cates G. A., Ball E. H., Sanwal B. D. A collagen-binding protein in the endoplasmic reticulum of myoblasts exhibits relationship with serine protease inhibitors. J. Biol. Chem. 1991;266:17230–17235. [PubMed] [Google Scholar]
- 34.Osterwalder T., Kuhnen A., Leiserson W. M., Kim Y. S., Keshishian H. Drosophila serpin 4 functions as a neuroserpin-like inhibitor of subtilisin-like proprotein convertases. J. Neurosci. 2004;24:5482–5491. doi: 10.1523/JNEUROSCI.5577-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briand C., Kozlov S. V., Sonderegger P., Grutter M. G. Crystal structure of neuroserpin: a neuronal serpin involved in a conformational disease. FEBS Lett. 2001;505:18–22. doi: 10.1016/s0014-5793(01)02764-8. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence D. A., Strandberg L., Ericson J., Ny T. Structure–function studies of the SERPIN plasminogen activator inhibitor type 1. Analysis of chimeric strained loop mutants. J. Biol. Chem. 1990;265:20293–20301. [PubMed] [Google Scholar]
- 37.Gettins P. G. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 38.Silverman G. A., Whisstock J. C., Askew D. J., Pak S. C., Luke C. J., Cataltepe S., Irving J. A., Bird P. I. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell. Mol. Life Sci. 2004;61:301–325. doi: 10.1007/s00018-003-3240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda E., Romisch K., Lomas D. A. Mutants of neuroserpin that cause dementia accumulate as polymers within the endoplasmic reticulum. J. Biol. Chem. 2004;279:28283–28291. doi: 10.1074/jbc.M313166200. [DOI] [PubMed] [Google Scholar]
- 40.Yepes M., Loskutoff D. J., Lawrence D. A. Plasminogen Activator Inhibitor-1. In: Colman R. W., Marder V. J., Clowes A. W., George J. N., Goldhaber S. Z., editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 41.Dannies P. S. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr. Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 42.Taupenot L., Harper K. L., O'Connor D. T. The chromogranin–secretogranin family. N. Engl. J. Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 43.Canaff L., Brechler V., Reudelhuber T. L., Thibault G. Secretory granule targeting of atrial natriuretic peptide correlates with its calcium-mediated aggregation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9483–9487. doi: 10.1073/pnas.93.18.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo S. H., So S. H., Kweon H. S., Lee J. S., Kang M. K., Jeon C. J. Coupling of the inositol 1,4,5-trisphosphate receptor and chromogranins A and B in secretory granules. J. Biol. Chem. 2000;275:12553–12559. doi: 10.1074/jbc.275.17.12553. [DOI] [PubMed] [Google Scholar]
- 45.Huh Y. H., Jeon S. H., Yoo S. H. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J. Biol. Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- 46.Huttner W. B., Natori S. Regulated secretion: helper proteins for neuroendocrine secretion. Curr. Biol. 1995;5:242–245. doi: 10.1016/s0960-9822(95)00049-2. [DOI] [PubMed] [Google Scholar]
- 47.Crowther D. C., Serpell L. C., Dafforn T. R., Gooptu B., Lomas D. A. Nucleation of α1-antichymotrypsin polymerization. Biochemistry. 2003;42:2355–2363. doi: 10.1021/bi0259305. [DOI] [PubMed] [Google Scholar]
- 48.Lomas D. A., Belorgey D., Mallya M., Miranda E., Kinghorn K. J., Sharp L. K., Phillips R. L., Page R., Robertson A. S., Crowther D. C. Molecular mousetraps and the serpinopathies. Biochem. Soc. Trans. 2005;33:321–330. doi: 10.1042/BST0330321. [DOI] [PubMed] [Google Scholar]
- 49.Duncan R. R., Greaves J., Wiegand U. K., Matskevich I., Bodammer G., Apps D. K., Shipston M. J., Chow R. H. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]