Abstract
PrPC (cellular prion protein) is located at the surface of neuronal cells in detergent-insoluble lipid rafts, yet is internalized by clathrin-dependent endocytosis. As PrPC is glycosyl-phosphatidylinositol-anchored, it requires a transmembrane adaptor protein to connect it to the clathrin endocytosis machinery. Using receptor-associated protein and small interfering RNA against particular LDL (low-density lipoprotein) family members, in combination with immunofluorescence microscopy and surface biotinylation assays, we show that the transmembrane LRP1 (LDL receptor-related protein 1) is required for the Cu2+-mediated endocytosis of PrPC in neuronal cells. We show also that another LRP1 ligand that can cause neurodegenerative disease, the Alzheimer's amyloid precursor protein, does not modulate the endocytosis of PrPC.
Keywords: amyloid precursor protein (APP), clathrin, Cu2+-dependent endocytosis, low-density lipoprotein receptor-related protein-1 (LRP1), prion, receptor-associated protein (RAP)
Abbreviations: APP, amyloid precursor protein; GPI, glycosyl-phosphatidylinositol; HRP, horseradish peroxidase; HSPG, heparin sulfate proteoglycan; KPI, Kunitz-type protease inhibitor; LDL, low-density lipoprotein; LRP, LDL receptor-related protein; PAI-1, plasminogen activator inhibitor-1; PrPC, cellular form of the prion protein; PrPSc, infectious form of the prion protein; RAP, receptor-associated protein; siRNA, small interfering RNA; uPA, urokinase-type plasminogen activator; uPAR, uPA receptor
INTRODUCTION
The prion protein (PrP) is the principal agent responsible for the transmissible spongiform encephalopathies, a group of fatal neurodegenerative diseases including Creutzfeldt–Jakob disease in humans, scrapie in sheep and bovine spongiform encephalopathy in cattle [1]. In these prion diseases the normal cellular form of the prion protein, PrPC, undergoes a conformational change to the infectious form, PrPSc. PrPC is transported through the secretory pathway to the cell surface, where it is attached to the outer leaflet of the plasma membrane via a GPI (glycosyl-phosphatidylinositol) anchor and localized in cholesterol- and glycosphingolipid-rich lipid rafts [2,3]. The N-terminal half of PrPC contains four octapeptide repeats (PHGG(G/S)WGQ; residues 59–90) that bind Cu2+ ions. The physiological importance of Cu2+ binding to the octarepeats is evidenced by the finding that exposure of neuronal cells to concentrations (50–100 μM) of Cu2+, similar to that in the extracellular spaces of the brain [4,5], results in the rapid internalization of PrPC [6–8]. This metal-dependent endocytosis of PrPC was abrogated by deletion of the octapeptide repeats or by an insertional mutation within the repeats, which is associated with an inherited form of human prion disease [7].
Electron microscopy studies [9,10], as well as selective pharmacological and molecular disruption experiments [11], have shown that in neuronal cells PrPC is endocytosed by a clathrin-mediated mechanism. PrPC moves laterally out of detergent-insoluble lipid rafts into detergent-soluble regions of the plasma membrane prior to its endocytosis via clathrin-coated pits [10,11]. As PrPC is GPI-anchored and lacks a cytoplasmic domain, it cannot interact directly with the clathrin endocytic machinery on the cytoplasmic face of the plasma membrane. Rather, its internalization requires it to ‘piggy-back’ on an integral transmembrane protein. The existence of such a transmembrane adaptor protein was first postulated over 10 years ago [9], yet its identity remains unknown. Using mutants of PrPC that lacked either the octapeptide repeats or the polybasic KKRP tetrapeptide at the N-terminus of the mature protein [11], we showed that copper binding to the octapeptide repeats promotes dissociation of PrPC from lipid rafts, whereas the N-terminal polybasic region mediated its interaction with a transmembrane adaptor protein which engages the clathrin endocytic machinery [11].
The LDL (low-density lipoprotein) receptor family is a group of cell-surface transmembrane proteins that bind a variety of ligands and internalize via clathrin-coated pits [12–14]. LRP1 (LDL receptor-related protein 1), along with LRP1B and LRP2 (megalin) are the largest members of this family of endocytic receptors with multiple ligand binding sites, although only LRP1 and LRP1B are highly expressed in neuronal cells [13]. LRP1 is a 600 kDa transmembrane glycoprotein that is cleaved in the trans-Golgi network by furin to generate a 515 kDa α- and an 85 kDa β-subunit, which remain non-covalently associated. Several ligands, including apolipoprotein E, APP (amyloid precursor protein) and α2-macroglobulin, bind to the α-subunit of LRP1 [15,16].
We hypothesized that LRP1 or LRP1B may be candidates for the transmembrane adaptor protein required by PrPC to endocytose via clathrin-coated pits in neuronal cells. By selectively blocking the interaction of LDL family members with their ligands using soluble RAP (receptor-associated protein) and by the use of siRNA (small interfering RNA), we show that LRP1, but not LRP1B, is required to mediate the Cu2+-stimulated endocytosis of PrPC in human neuroblastoma SH-SY5Y cells.
EXPERIMENTAL
PrP constructs and cell culture
Insertion of the coding sequence of murine PrP containing a 3F4 epitope tag into pIRESneo (BD Biosciences) and the generation of SH-SY5Y cells stably expressing the protein have been reported previously [7]. SH-SY5Y cells expressing PrPC were also stably transfected with a pIREShyg vector containing the cDNA encoding the KPI (Kunitz-type protease inhibitor) domain containing isoform of APP, APP751. Cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% (v/v) foetal bovine serum, 50 units/ml penicillin and 0.1 mg/ml streptomycin. Cells were maintained in a humidified incubator at 37 °C in a 5% CO2/95% air atmosphere.
RNA interference studies
SH-SY5Y cells expressing PrPC were seeded into T25 flasks at 70% confluence and incubated with 500 pmol of a 2 μM Smartpool siRNA solution against LRP1, LRP1B or APP (Dharmacon) in complex with DharmaFECT-1 transfection regent (Dharmacon) in serum-free medium. Mock-transfectants were incubated in the presence of DharmaFECT-1 only. After 2 h, serum was added to 10% (v/v). Cells were incubated for a further 28 h (LRP1 and LRP1B) or 46 h (APP) prior to experimentation.
Cell-surface biotinylation endocytosis assay and immunoprecipitation
The cell-surface biotinylation endocytosis assay was performed as described previously [7,11]. Cells were co-incubated with 20 μg/ml recombinant RAP (Merck Biosciences) during copper treatment, where indicated. Immunoprecipitation was carried out as described previously [11].
SDS/PAGE and Western blot analysis
Immunoprecipitated biotinylated complexes were mixed with dissociation buffer [125 mM Tris/HCl, pH6.8, 2% (w/v) SDS, 20% (v/v) glycerol, 100 mM dithiothreitol and 0.02% Bromophenol Blue] and boiled for 5 min. Proteins were resolved by SDS/PAGE [14.5% (v/v) gels] and then transferred to Hybond-P PVDF membranes. The membranes were blocked for 1 h in PBS (1.5 mM KH2PO4, 2.7 mM Na2HPO4, 150 mM NaCl, pH 7.4) containing 5% (w/v) dried milk powder and 0.1% Tween-20, followed by incubation with HRP (horseradish peroxidase)-conjugated streptavidin (1:1000 dilution in PBS containing 0.1% Tween-20) for 1 h. Bound HRP conjugates were visualized using the ECL® detection system (Amersham Biosciences). PrPC, APP and β-actin were detected using antibodies 3F4, 22C11 and AC15 respectively, with an HRP-conjugated rabbit anti-mouse secondary antibody (Sigma–Aldrich). For detection of LRP1 and LRP1B, cell lysate samples were prepared in non-reducing dissociation buffer. LRP1 was detected with a monoclonal antibody 5A6 (Merck Biosciences) and LRP1B was detected using a rabbit polyclonal antibody (kindly given by Dr Guojun Bu, Washington University School of Medicine, St. Louis, U.S.A.).
Immunofluorescence microscopy
Cells were seeded on to coverslips and grown to 50% confluence. The fate of cell-surface PrPC was monitored by pre-labelling cells with antibody 3F4 for 30 min at 4 °C. For endocytosis experiments, cells were incubated for 20 min at 37 °C in OptiMEM in the presence or absence of 100 μM CuSO4 presented as a histidine chelate [7]. Cells were then fixed with 4% (v/v) paraformaldehyde/0.1% (v/v) glutaraldehyde in PBS for 15 min, permeabilized in PBS containing 0.1% Triton X-100, and then blocked for 1 h in PBS containing 3% (v/v) goat serum or 5% (v/v) fish skin gelatin (Sigma–Aldrich). Coverslips were then incubated overnight at 4 °C with either a goat polyclonal antibody directed against LRP1 (N-20; Santa Cruz Biotechnology) or the rabbit polyclonal antibody against LRP1B. Finally, coverslips were incubated with the appropriate fluorescent probe-conjugated secondary antibodies (Molecular Probes) for 1 h and mounted on slides using fluoromount G mounting medium (SouthernBiotech). Cells were visualized using a DeltaVision Optical Restoration Microscopy System (Applied Precision). Data were collected from 30–40 0.1 μm-thick optical sections, and three-dimensional datasets were deconvolved using the softWoRx program (Applied Precision). The presented images represent individual z-slices taken from the middle of the cell. Co-localization of endocytosed PrPC with LDL-family members was performed using Imaris 4.0 (Bitplane AG).
RESULTS
RAP reduces the Cu2+-mediated endocytosis of PrPC
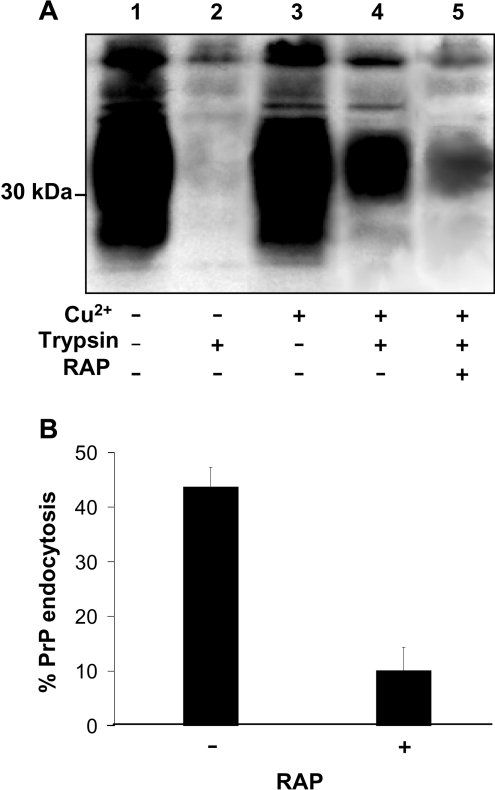
To initially test the hypothesis that members of the LDL family of endocytic receptors may act as transmembrane adaptors in the Cu2+-mediated endocytosis of PrPC, potential PrPC–LDL family interactions were inhibited using RAP. RAP is a specialized chaperone that binds tightly to LDL family members in the early secretory pathway to assist correct folding and disulfide bond formation, and to prevent premature association of family members with their ligands [17]. Exogenously applied RAP has been used to universally antagonize binding of ligands to LDL family members [17]. Incubation of SH-SY5Y cells expressing murine PrPC with the 3F4 epitope tag with 100 μM Cu2+ resulted in the rapid endocytosis of cell surface PrPC, as shown using surface biotinylation and subsequent trypsin digestion of biotinylated material remaining at the cell surface after 20 min at 37 °C [7,11]. However, in the presence of 20 μg/ml recombinant human RAP, a concentration used in previous studies [18], the endocytosis of PrPC was reduced significantly (Figure 1A). Densitometric analysis of multiple blots from three separate experiments revealed that 44±8% of biotin-labelled PrPC was endocytosed in response to 100 μM Cu2+. However, in cells incubated with RAP, only 10±6% of PrPC was endocytosed in response to 100 μM Cu2+ (Figure 1B), indicating a role for LDL family members in the Cu2+-mediated endocytosis of PrPC.
Figure 1. Cu2+-stimulated endocytosis of PrPC is inhibited by RAP.
(A) SH-SY5Y cells expressing PrPC were surface biotinylated and then either untreated or treated with 20 μg/ml recombinant RAP in the presence or absence of 100 μM Cu2+ for 20 min at 37 °C. Prior to lysis, the cells were, where indicated, incubated with trypsin to digest cell-surface PrPC. Cells were then lysed and total PrPC immunoprecipitated from the sample using antibody 3F4 and then subjected to Western blot analysis. The biotin-labelled PrPC fraction was detected with HRP-conjugated streptavidin. (B) Densitometric analysis (means±S.E.M.) for multiple blots (lanes 4 and 5 in A) from three separate experiments.
Knockdown of LRP1, but not LRP1B, by siRNA inhibits the endocytosis of PrPC
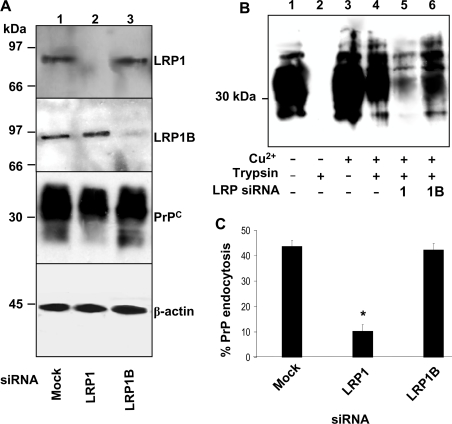
Owing to its ability to bind to a wide variety of ligands and its neuronal expression, LRP1 was selected as a likely candidate of the LDL family to be involved in the endocytosis of PrPC, along with the more recently discovered LRP1 splice-variant, LRP1B, which is suggested to share a number of ligands with LRP1, but to internalize them at a lower rate [19]. To determine which is involved in the Cu2+-mediated endocytosis of PrPC, SH-SY5Y cells expressing PrPC were incubated with siRNAs directed against either LRP1 or LRP1B for 30 h. The cells were then surface biotinylated, incubated with 100 μM Cu2+ followed by trypsin digestion. LRP1 protein expression was absent in cells incubated with the siRNA against LRP1, whereas the siRNA against LRP1B had no effect on LRP1 expression (Figure 2A). Similarly, the siRNA against LRP1B reduced LRP1B expression significantly, but had no effect on the expression of LRP1 (Figure 2A). The amount of PrPC in the cell lysates was not altered by the knockdown of either LRP1 or LRP1B (Figure 2A). Although prior incubation with siRNA directed against LRP1B had no effect on the Cu2+-mediated endocytosis of PrPC, knockdown of LRP1 reduced the Cu2+-mediated endocytosis of PrPC significantly (Figure 2B). Densitometric analysis of multiple blots from three separate experiments revealed that only 10±3% of cell-surface PrPC was endocytosed in the cells incubated with the siRNA against LRP1 compared with 44±4% in the untreated cells (Figure 2C). There was no significant difference in the amount of PrPC endocytosed in the cells incubated with siRNA directed against LRP1B as compared with the untreated cells (Figure 2C).
Figure 2. Endocytosis of PrPC is blocked by siRNA directed against LRP-1, but not LRP-1B.
SH-SY5Y cells expressing PrPC were incubated with a 2 μM solution of the indicated LRP siRNA prepared with DharmaFECT-1 transfection reagent in OptiMEM for 30 h. (A) Cell lysates were immunoblotted for LRP1 expression using Ab 5A6 and LRP1B expression using a goat polyclonal antibody, along with PrPC and β-actin. (B) Cells were surface biotinylated and treated with or without 100 μM Cu2+ for 20 min at 37 °C. Prior to lysis, the cells were, where indicated, incubated with trypsin to digest cell-surface PrPC. Cells were then lysed and total PrPC immunoprecipitated from the sample using antibody 3F4. Samples were then subjected to Western blot analysis. The biotin-labelled PrPC fraction was detected with HRP-conjugated streptavidin. (C) Densitometric analysis (means±S.E.M.) for multiple blots (lanes 5 and 6 in B) from three separate experiments. *, Students t-test with values of P<0.05 were taken as statistically significant.
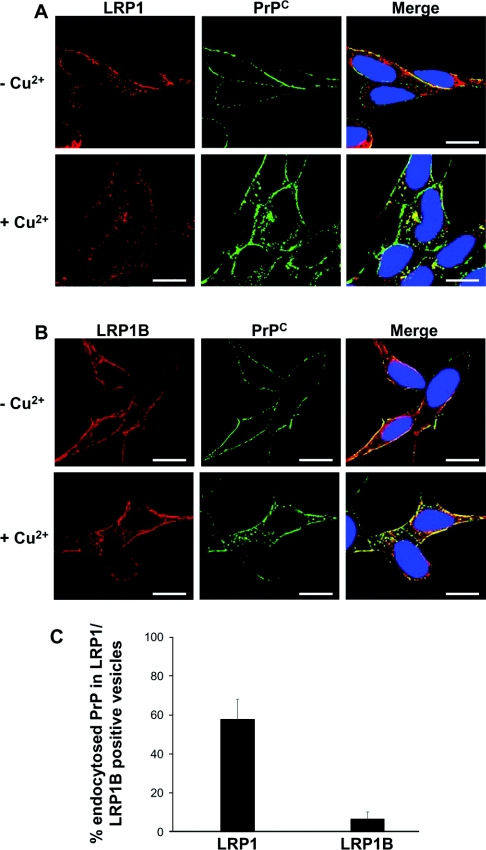
The subcellular localization of LRP1 and LRP1B relative to that of PrPC was also examined. In order to visualize the fate of cell-surface PrPC only, cells were pre-labelled with antibody 3F4 prior to incubation in the presence or absence of Cu2+. The cells were then fixed and permeabilized before incubation with antibodies against either LRP1 or LRP1B. Both LRP1 and LRP1B were localized at the cell surface, and in intracellular compartments in both Cu2+ and non-Cu2+-treated cells (Figures 3A and 3B). In cells incubated in the absence of Cu2+, 3F4-labelled PrPC was located exclusively at the cell surface, with little co-localization with either LRP1 or LRP1B. After Cu2+ treatment, a portion of the 3F4-labelled PrPC was endocytosed into intracellular compartments. In these Cu2+-treated cells, co-localization of PrPC with both LRP1 and LRP1B was seen to increase at the cell surface. However, only LRP1, but not LRP1B, co-localized with intracellular PrPC in Cu2+-treated cells (Figure 3C), consistent with a role for LRP1 in the Cu2+-mediated endocytosis of PrPC.
Figure 3. LRP1 but not LRP1B co-localizes with PrPC after its Cu2+-mediated endocytosis.
SH-SY5Y cells expressing PrPC were seeded on to glass coverslips and grown to 50% confluence. Cells were then pre-incubated with antibody 3F4 at a dilution of 1:1000 in PBS for 30 min at 4 °C, washed three times in PBS and then incubated for 20 min at 37 °C in OptiMEM in either the absence or the presence of 100 μM Cu2+. Cells were fixed, permeabilized and then incubated with either (A) a goat polyclonal antibody against LRP1 or (B) a rabbit polyclonal antibody against LRP1B overnight at 4 °C. Cells were then incubated with AlexaFluor® 488 conjugated rabbit anti-mouse antibody and then with either AlexaFluor® 594 conjugated rabbit anti-goat (for LRP1) or AlexaFluor® 594 conjugated goat anti-rabbit (for LRP1B). Nuclei were stained using DAPI (4′,6-diamidino-2-phenylindole). Cells were viewed using a DeltaVision Optical Restoration Microscopy system. Images are representative of three individual experiments. Scale bar=10 μm. (C) The co-localization of intracellular PrPC, internalized in response to Cu2+, with LRP1 or LRP1B was quantified using Imaris 4. The data represent the percentage of intracellular PrPC-containing vesicles that also stained positive for LRP1 or LRP1B (±S.E.M.); n≥10 cells.
Overexpression or knockdown of APP has no effect on the endocytosis of PrPC
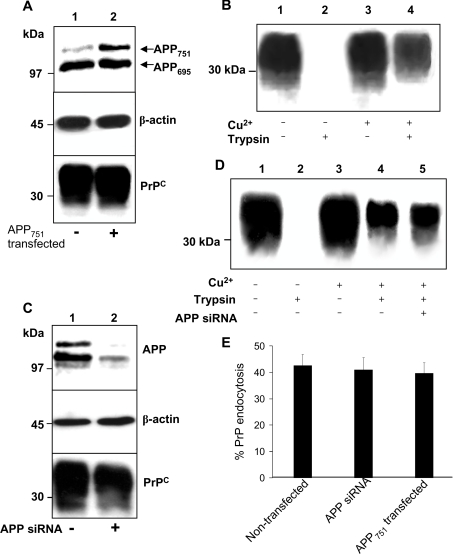
As the neuronal protein APP is also endocytosed by LRP1 [20], we examined whether APP might modulate the endocytosis of PrPC. SH-SY5Y cells expressing PrPC were also stably transfected with a pIREShyg vector containing the cDNA encoding APP751. Immunoblotting of cell lysates revealed that there was an approx. 2-fold increase in APP751 expression in these cells above endogenous levels (Figure 4A). When lysate samples from mock- and APP751-transfected cells were immunoblotted for PrPC the levels of PrPC expression were identical (Figure 4A). The Cu2+-mediated endocytosis of PrPC in the APP751 transfected cells was then examined. There was no difference in the amount of cell-surface PrPC endocytosed in the APP751 transfected cells as compared with cells transfected with the empty pIREShyg vector (Figures 4B and 4E). Similarly, there was no change in the amount of PrPC endocytosed when the non-KPI-containing isoform of APP, APP695, was overexpressed in the SH-SY5Y cells (results not shown).
Figure 4. Cu2+-stimulated endocytosis of PrPC is unaltered either by overexpression or knockdown of APP.
(A) SH-SY5Y cells expressing PrPC were stably transfected with a pIREShyg vector containing the cDNA encoding APP751. Mock transfectants were created by transfection of an empty pIREShyg vector. Lysates were immunoblotted for APP, PrPC and β-actin. (B) Cells stably expressing APP751 were surface biotinylated and incubated with or without 100 μM Cu2+ for 20 min at 37 °C. Prior to lysis, the cells were incubated with trypsin to digest cell-surface PrPC. Cells were then lysed and total PrPC immunoprecipitated from the sample using antibody 3F4 and then subjected to Western blot analysis. The biotin-labelled PrPC fraction was detected with peroxidase-conjugated streptavidin. (C) SH-SY5Y cells expressing PrPC were incubated for 48 h with a 2 μM solution of the siRNA against APP, prepared with DharmaFECT-1 transfection reagent in OptiMEM. Mock-transfectants were incubated in the presence of DharmaFECT-1 only. Lysates were immunoblotted for APP, PrPC and β-actin. (D) Endocytosis experiment as described in (B) using the cells incubated in the presence or absence of APP siRNA. (E) Densitometric analysis (means±S.E.M.) for multiple blots from three separate experiments performed in (B) and (D).
To confirm that APP does not interfere with the endocytosis of PrPC, the expression of APP in the SH-SY5Y cells was knocked down using siRNA. A significant reduction in APP expression (87±6%) was observed in the cells incubated with the siRNA against APP, with no effect on total PrPC levels (Figure 4C). Cells were then surface biotinylated and incubated in the presence or absence of 100 μM Cu2+ for 20 min followed by trypsin digestion. There was no significant difference in the amount of cell-surface PrPC endocytosed in the absence of APP siRNA as compared with the presence of the APP siRNA (43±4% and 41±5% of cell surface PrPC was endocytosed respectively; Figures 4D and 4E). Together these data indicate that APP does not modulate the endocytosis of PrPC by LRP1.
DISCUSSION
To date, PrPC has been reported to bind to several proteins present at the cell surface, including stress-inducible protein 1 [21], neural cell-adhesion molecules [22,23] and the 37 kDa/67 kDa laminin receptor [24]. Of these proteins, only the 37 kDa/67 kDa laminin receptor has been directly implicated in the internalization of PrPC [25]. However, the 37 kDa/67 kDa laminin receptor was responsible for the internalization of only 25–50% of membrane-bound recombinant PrPC [25] and the binding of PrPC to all these proteins involves regions located C-terminally to residue 90, making them unlikely candidates to act as transmembrane adaptors in the Cu2+-mediated endocytosis of PrPC, which is dependent on the polybasic region at the extreme N-terminus of the protein [11]. Consistent with the existence of other transmembrane receptors for PrPC, we show in the present study, for the first time, that LRP1 is required for the Cu2+-mediated endocytosis of PrPC. By exploiting the fact that ligand binding to LDL-family members can be universally antagonized by soluble RAP [17], we have shown that soluble RAP reduces significantly the Cu2+-mediated endocytosis of PrPC in SH-SY5Y cells. More selective targeting of individual LDL-family members with siRNAs revealed that LRP1, but not LRP1B, was responsible for facilitating the Cu2+-mediated endocytosis of PrPC in SH-SY5Y cells. We cannot rule out the involvement of other LDL-family members or other, as yet unidentified, proteins in the endocytosis of PrPC in other cell types.
The involvement of LRP1 in PrPC endocytosis may not be entirely surprising, as over 40 ligands have been reported to bind to this scavenger receptor [14]. For example, LRP1 is an endocytic receptor for another GPI-anchored protein, uPAR (urokinase-type plasminogen activator receptor). LRP1 internalizes uPAR after uPAR has bound to a complex of uPA and PAI-1 (plasminogen activator inhibitor-1) [26,27]. Like PrPC, uPAR has been localized to lipid rafts [28] and its endocytosis is inhibited by both RAP- and LRP1-specific antibodies [13]. Although Cu2+ causes PrPC to dissociate from detergent-insoluble lipid rafts [11], it is not known where PrPC initially interacts with LRP1. A recent study demonstrated that LRP1 is capable of associating transiently with lipid rafts [29], thus the initial interaction between PrPC and LRP1 may occur within lipid rafts after the octapeptide repeats become fully loaded with Cu2+, and a conformational change in the N-terminus of PrPC [30] brings the polybasic region, critical for the clathrin-dependent endocytosis of PrPC, [10,11] into an orientation that favours interaction either directly or indirectly with the ligand-binding domains of LRP1. A recent study suggested that clusters of basic residues are also critical in mediating the interaction between the uPA–PAI-1 complex and LRP1 [31]. It has been postulated that endogenous HSPGs (heparan sulfate proteoglycans) may be involved in the clathrin-dependent endocytosis of PrPC [10]. Interestingly, HSPGs have been implicated in facilitating the binding of many ligands to LRP1 [13], and thus it is possible that cell-surface HSPGs may be involved in the LRP1-dependent endocytosis of PrPC, perhaps through the formation of large multimeric protein complexes.
LRP1 mediates the endocytosis of another protein involved in neurodegenerative diseases, the Alzheimer's APP, from which the neurotoxic amyloid-β peptide is proteolytically cleaved [32]. LRP1 has been shown to associate directly with the ectodomain of APP isoforms containing the KPI-domain [33]. When cells were cultured in the presence of RAP, the level of cell-surface APP was shown to increase, and blocking the interaction of APP with LRP1 reduced the production of the amyloid-β peptide [18]. The interaction of both the KPI-containing and non-KPI-containing isoforms of APP with LRP1 can also occur via the cytoplasmic adaptor protein Fe65, which bridges the cytoplasmic domains of APP and LRP1 [34,35]. Neither overexpression of KPI-containing or non-KPI-containing APP isoforms nor knockdown of APP by siRNA affected the Cu2+-mediated endocytosis of PrPC. This may be due to PrPC and APP binding to different regions in the ectodomain of LRP1 and/or that, as a scavenger receptor, LRP1 is not limiting in the cell.
In conclusion, we have shown for the first time that LRP1 mediates the endocytosis of PrPC in neuronal cells. Further studies will be required to elucidate whether interaction with LRP1 has a role to play in the conversion of PrPC into PrPSc.
Acknowledgments
This work was supported by grants from the Medical Research Council (MRC) of Great Britain (G9824728) and the Wellcome Trust (080229 and the Bioimaging Facility, University of Leeds). D. R. T. was in receipt of a studentship from the MRC.
References
- 1.Aguzzi A., Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 2.Campana V., Sarnataro D., Zurzolo C. The highways and byways of prion protein trafficking. Trends Cell Biol. 2005;15:102–111. doi: 10.1016/j.tcb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Taylor D. R., Hooper N. M. The prion protein and lipid rafts. Mol. Membr. Biol. 2006;23:89–99. doi: 10.1080/09687860500449994. [DOI] [PubMed] [Google Scholar]
- 4.Moir R. D., Atwood C. S., Huang X., Tanzi R. E., Bush A. I. Mounting evidence for the involvement of zinc and copper in Alzheimer's disease. Eur. J. Clin. Invest. 1999;29:569–570. doi: 10.1046/j.1365-2362.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 5.Stockel J., Safar J., Wallacen A. C., Cohen F. E., Prusiner S. B. Prion protein selectively binds copper(II) ions. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- 6.Pauly P. C., Harris D. A. Copper stimulates endocytosis of the prion protein. J. Biol. Chem. 1998;273:33107–33110. doi: 10.1074/jbc.273.50.33107. [DOI] [PubMed] [Google Scholar]
- 7.Perera W. S. S., Hooper N. M. Ablation of the metal ion-induced endocytosis of the prion protein by disease-associated mutation of the octarepeat region. Curr. Biol. 2001;11:519–523. doi: 10.1016/s0960-9822(01)00147-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee K. S., Magalhaes A. C., Zanata S. M., Brentani R. R., Martins V. R., Prado M. A. Internalization of mammalian fluorescent cellular prion protein and N-terminal deletion mutants in living cells. J. Neurochem. 2001;79:79–87. doi: 10.1046/j.1471-4159.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- 9.Shyng S. L., Heuser J. E., Harris D. A. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J. Cell Biol. 1994;125:1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunyach C., Jen A., Deng J., Fitzgerald K. T., Frobert Y., Grassi J., McCaffrey M. W., Morris R. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J. 2003;22:3591–3601. doi: 10.1093/emboj/cdg344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor D. R., Watt N. T., Perera W. S., Hooper N. M. Assigning functions to distinct regions of the N-terminus of the prion protein that are involved in its copper-stimulated, clathrin-dependent endocytosis. J. Cell Sci. 2005;118:5141–5153. doi: 10.1242/jcs.02627. [DOI] [PubMed] [Google Scholar]
- 12.Willnow T. E. The low-density lipoprotein receptor gene family: multiple roles in lipid metabolism. J. Mol. Med. 1999;77:306–315. doi: 10.1007/s001090050356. [DOI] [PubMed] [Google Scholar]
- 13.Nykjaer A., Willnow T. E. The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol. 2002;12:273–280. doi: 10.1016/s0962-8924(02)02282-1. [DOI] [PubMed] [Google Scholar]
- 14.Strickland D. K., Gonias S. L., Argraves W. S. Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 2002;13:66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- 15.Hyman B. T., Strickland D., Rebeck G. W. Role of the low-density lipoprotein receptor-related protein in β-amyloid metabolism and Alzheimer disease. Arch. Neurol. 2000;57:646–650. doi: 10.1001/archneur.57.5.646. [DOI] [PubMed] [Google Scholar]
- 16.Pietrzik C. U., Busse T., Merriam D. E., Weggen S., Koo E. H. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–5700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr. Opin. Lipidol. 1998;9:149–155. doi: 10.1097/00041433-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Ulery P. G., Beers J., Mikhailenko I., Tanzi R. E., Rebeck G. W., Hyman B. T., Strickland D. K. Modulation of β-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J. Biol. Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Knisely J. M., Lu W., McCormick L. M., Wang J., Henkin J., Schwartz A. L., Bu G. Low density lipoprotein (LDL) receptor-related protein 1B impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J. Biol. Chem. 2002;277:42366–42371. doi: 10.1074/jbc.M207705200. [DOI] [PubMed] [Google Scholar]
- 20.Knauer M. F., Orlando R. A., Glabe C. G. Cell surface APP751 forms complexes with protease nexin 2 ligands and is internalized via the low density lipoprotein receptor-related protein (LRP) Brain Res. 1996;740:6–14. doi: 10.1016/s0006-8993(96)00711-1. [DOI] [PubMed] [Google Scholar]
- 21.Zanata S. M., Lopes M. H., Mercadante A. F., Hajj G. N., Chiarini L. B., Nomizo R., Freitas A. R., Cabral A. L., Lee K. S., Juliano M. A., et al. Stress-inducible protein 1 is a cell surface ligand for cellular prion that triggers neuroprotection. EMBO J. 2002;21:3307–3316. doi: 10.1093/emboj/cdf325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt-Ulms G., Legname G., Baldwin M. A., Ball H. L., Bradon N., Bosque P. J., Crossin K. L., Edelman G. M., DeArmond S. J., Cohen F. E., Prusiner S. B. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J. Mol. Biol. 2001;314:1209–1225. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- 23.Santuccione A., Sytnyk V., Leshchyns'ka I., Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 2005;169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hundt C., Peyrin J. M., Haik S., Gauczynski S., Leucht C., Rieger R., Riley M. L., Deslys J. P., Dormont D., Lasmezas C. I., Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001;20:5876–5886. doi: 10.1093/emboj/20.21.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauczynski S., Peyrin J. M., Haik S., Leucht C., Hundt C., Rieger R., Krasemann S., Deslys J. P., Dormont D., Lasmezas C. I., Weiss S. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001;20:5863–5875. doi: 10.1093/emboj/20.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cubellis M. V., Wun T. C., Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 1990;9:1079–1085. doi: 10.1002/j.1460-2075.1990.tb08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nykjaer A., Petersen C. M., Moller B., Jensen P. H., Moestrup S. K., Holtet T. L., Etzerodt M., Thogersen H. C., Munch M., Andreasen P. A., et al. Purified α2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the α2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J. Biol. Chem. 1992;267:14543–14546. [PubMed] [Google Scholar]
- 28.Stahl A., Mueller B. M. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J. Cell Biol. 1995;129:335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L., Gonias S. L. The low-density lipoprotein receptor-related protein-1 associates transiently with lipid rafts. J. Cell. Biochem. 2005;96:1021–1033. doi: 10.1002/jcb.20596. [DOI] [PubMed] [Google Scholar]
- 30.Morante S., Gonzalez-Iglesias R., Potrich C., Meneghini C., Meyer-Klaucke W., Menestrina G., Gasset M. Inter- and intra-octarepeat Cu(II) site geometries in the prion protein: implications in Cu(II) binding cooperativity and Cu(II)-mediated assemblies. J. Biol. Chem. 2004;279:11753–11759. doi: 10.1074/jbc.M312860200. [DOI] [PubMed] [Google Scholar]
- 31.Skeldal S., Larsen J. V., Pedersen K. E., Petersen H. H., Egelund R., Christensen A., Jensen J. K., Gliemann J., Andreasen P. A. Binding areas of urokinase-type plasminogen activator-plasminogen activator inhibitor-1 complex for endocytosis receptors of the low-density lipoprotein receptor family, determined by site-directed mutagenesis. FEBS J. 2006;273:5143–5159. doi: 10.1111/j.1742-4658.2006.05511.x. [DOI] [PubMed] [Google Scholar]
- 32.Vardy E. R. L. C., Catto A. J., Hooper N. M. Proteolytic mechanisms in amyloid-β metabolism: therapeutic implications for Alzheimer's disease. Trends Mol. Med. 2005;11:464–472. doi: 10.1016/j.molmed.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kounnas M. Z., Moir R. D., Rebeck G. W., Bush A. I., Argraves W. S., Tanzi R. E., Hyman B. T., Strickland D. K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted β-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita A., Whelan C. M., Smith C. J., Mikhailenko I., Rebeck G. W., Strickland D. K., Hyman B. T. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 2001;21:8354–8361. doi: 10.1523/JNEUROSCI.21-21-08354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietrzik C. U., Yoon I. S., Jaeger S., Busse T., Weggen S., Koo E. H. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]