Summary
Arc (also termed Arg 3.1) is an immediate early gene whose mRNA is rapidly transcribed and targeted to dendrites of neurons as they engage in information processing and storage. Moreover, Arc is known to be required for durable forms of synaptic plasticity and learning. Despite these intriguing links to plasticity, Arc’s molecular function remains enigmatic. Here, we demonstrate that Arc protein interacts with dynamin and specific isoforms of endophilin to enhance receptor endocytosis. Arc selectively modulates trafficking of AMPA-type glutamate receptors (AMPARs) in neurons by accelerating endocytosis and reducing surface expression. The Arc-endocytosis pathway appears to regulate basal AMPAR levels since Arc KO neurons exhibit markedly reduced endocytosis and increased steady-state surface levels. These findings reveal a novel molecular pathway that is regulated by Arc and likely contributes to late-phase synaptic plasticity and memory consolidation.
Introduction
Long-term forms of learning and memory are dependent on rapid, de novo RNA and protein synthesis (Davis and Squire, 1984). Similar macromolecular synthesis is essential for long-term forms of synaptic plasticity including long-term potentiation (LTP) and depression (LTD) (Steward and Worley, 2002). Efforts to identify molecules that underlie transcription-dependent plasticity have revealed a set of immediate early genes (IEGs) that target to excitatory synapses, including Homer (Brakeman et al., 1997), Narp (Tsui et al., 1996), Arc (Link et al., 1995; Lyford et al., 1995), and CPG-2 (Cottrell et al., 2004). Arc is especially notable because it has been strongly linked to behavioral plasticity. Antisense knockdown of Arc results in selective deficits of long-term, but not short-term synaptic potentiation and spatial learning (Guzowski et al., 1999). Similar phenotypes are evident in Arc/Arg3.1 knock-out mice that show gross deficits in long-term memory consolidation but not task acquisition or short-term memory (Plath et al., 2006). Arc is a single copy gene that is highly conserved in vertebrates and is induced in divergent behavioral paradigms in many species (Bock et al., 2005; Matsuoka et al., 2003; Velho et al., 2005). Indeed, Arc mRNA and protein induction during behavioral learning is so robust and reproducible that cellular imaging of Arc induction provides a powerful methodology to detect neural networks that underlie information processing and memory (Burke et al., 2005; Guzowski et al., 1999; Ramirez-Amaya et al., 2005; Tagawa et al., 2005; Zou and Buck, 2006). Arc is further notable in that its mRNA traffics to dendrites and specifically accumulates at sites of synaptic activity (Steward et al., 1998). Arc protein also accumulates in dendrites and becomes enriched at the site of local synaptic activity suggesting that Arc protein is locally synthesized (Steward et al., 1998).
Despite these advances, the molecular function of Arc protein remains unknown. Here, we demonstrate that Arc plays a regulatory role in AMPAR trafficking via its interaction with components of the endocytic machinery, endophilin and dynamin. In a cellular model that monitors the effects of acute protein up-regulation, Arc down-regulates AMPARs by increasing the basal rate of endocytosis. Conversely, Arc KO neurons display increased levels of surface AMPARs and decreased rates of endocytosis. These studies reveal a novel mechanism for protein synthesis-dependent synaptic plasticity and AMPAR trafficking.
Results
Arc Interacts with Endophilin and Dynamin
To gain insight into Arc function, we performed a yeast 2-hybrid screen. Two clones were confirmed to interact in yeast and correspond to the C-terminal half of dynamin 2 [dynamin 2(503-871)] and endophilin 3 [endophilin3 (172-347)] (Figure 1A). Dynamin is a large GTPase essential for intracellular membrane trafficking, including clathrin-mediated synaptic vesicle recycling (Kosaka and Ikeda, 1983), and receptor-mediated endocytosis (Vieira et al., 1996). Dynamin possesses a plextrin homology (PH) domain that is implicated in targeting to phospholipid membranes (Zheng et al., 1996), and a proline rich domain (PRD) that includes a SH3 ligand. Three dynamin genes are present in vertebrates, and are all expressed in brain (Praefcke and McMahon, 2004). When co-expressed in HEK293 cells, Arc co-IPs with dynamin 2(503-871) while dynamin 2(612-871), which lacks the PH domain, does not (Figure 1B). To confirm that Arc and dynamin interact in vivo, detergent lysates of a rat forebrain P2 fraction were precipitated with Arc Ab. Dynamin co-IPs with Arc (Figure 1C).
Figure 1.
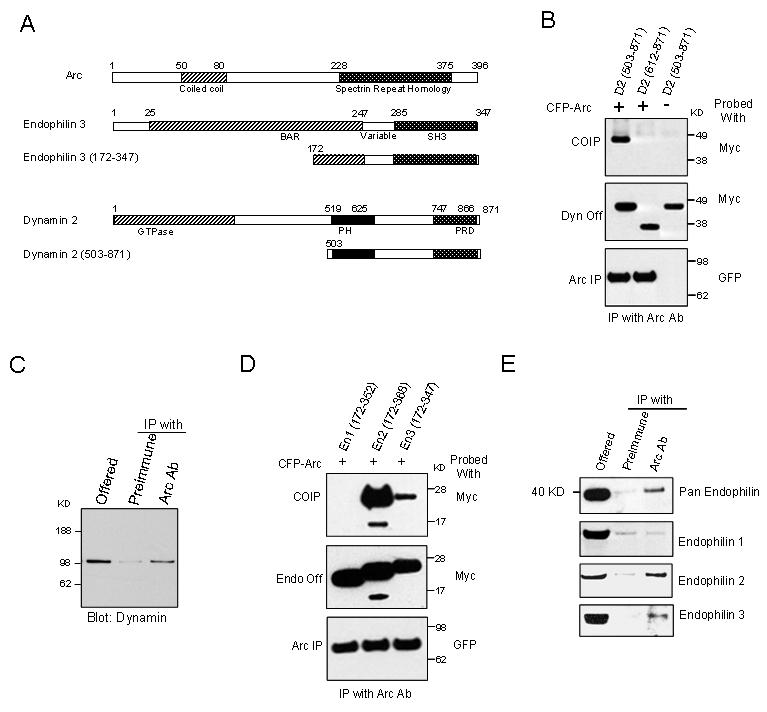
Arc Interacts with Endophilin and Dynamin(A) Schematic diagram showing the domain structure of Arc, endophilin 3, dynamin 2, and the yeast two hybrid fragments of each.
(B) When co-expressed in HEK293 cells Arc co-IPs dynamin 2(503-871) but not dynamin 2(612-871) (lacks the PH domain). Control blots show expressed dynamin (dynamin Off) and immunoprecipitated Arc (Arc IP).
(C) Dynamin co-IPs with Arc from a rat forebrain synaptosomal fraction (P2). 10% of lysate used for IP was routinely loaded in offered lane.
(D) Arc co-IPs endophilin 2(172-368) and endophilin 3(172-347), but not endophilin 1(172-352) from HEK293 cells. (E) Pan endophilin Ab detects co-IP with Arc from rat forebrain P2 fraction. Samples were reblotted with Abs selective for individual endophilin A proteins. Arc IP fractions show enrichment for endophilin 2 and 3, but not endophilin 1.
Endophilin proteins play a role in vesicle formation and function (Ringstad et al., 1997; Schuske et al., 2003; Verstreken et al., 2003). There are five endophilin genes in the mammalian genome (endophilin A1-3 and B1-2) that share the domain structure of an N-terminal BAR (Bin/amphiphysin/Rvs) and a C-terminal SH3 domain. The three endophilin A proteins are highly conserved. In mammals, endophilin 1 and 3 are brain-specific. Endophilin 1 is predominantly present in presynaptic terminals and plays a role in endocytosis of clathrin-coated vesicles (Ringstad et al., 1999). Endophilin 2 is expressed in many tissues, and in neurons is reported to bind voltage gated calcium channels (Chen et al., 2003). The Y2H fragment of endophilin 3 includes the C-terminal 76 aa of the BAR domain, the variable region, and the SH3 domain (Figure 1A). When co-expressed with Arc in HEK293 cells, endophilin 3(172-347) co-IPs with Arc (Figure 1D). We also tested equivalent endophilin 1 and 2 constructs. Endophilin 1(172-352) does not co-IP with Arc while endophilin 2(172-368) does. To determine whether Arc and endophilin interact in vivo, we probed Arc IPs with an antibody detects endophilin 1, 2 and 3 (Ringstand et al., 1997). Endophilin proteins were enriched ∼5 fold in the Arc-IP fraction compared to control IPs (Figure 1E). When these same fractions were probed with commercial Abs with confirmed selectivity for individual endophilins (Supplemental Figure 1), endophilin 1, which is abundant in forebrain, was not enriched in Arc fractions. By contrast, endophilin 2 and 3 were enriched in the Arc IP (>2 fold in three independent experiments). Thus, three independent antibodies support the assertion that Arc interacts with endophilin in vivo.
Arc and Endophilin 2 and 3 are Enriched in Postsynaptic Structures
We examined the subcellular localization of Arc and endophilins in adult hippocampus to determine where these proteins may functionally interact. Consistent with previous reports (Gad et al., 2000; Ringstad et al., 1999), endophilin 1 immunogold EM shows prominent localization to presynaptic vesicles (Figure 2A). By contrast, endophilin 2 gold particles were enriched in both the pre- and postsynaptic compartments, and were prominent over the PSD and in the lateral margins of dendritic spines. Endophilin 3 gold particles were relatively enriched in the postsynaptic compartment compared with endophilin 1. These localizations were confirmed by quantitative counts of gold particles (see Figure 2 legend). Vesicular structures containing endophilin 2 and 3 gold particles were present in both the spine and along microtubules in dendrites. Arc immuno-EM gold particles were present throughout the spine but were enriched over the postsynaptic density (PSD) where they appeared at, or very near, the plasma membrane (Figure 2B). In some cases Arc was also localized to the lateral margin of the PSD. EM localization of Arc to vesicular-like structures in dendritic spines has been previously reported (Moga et al., 2004). Consistent with EM localizations, Arc protein localized in DIV 21 hippocampal primary neurons to dendrites with puncta in spines, and colocalized with both the AMPAR subunit GluR1 and endophilin 3 (Figures 2C).
Figure 2.
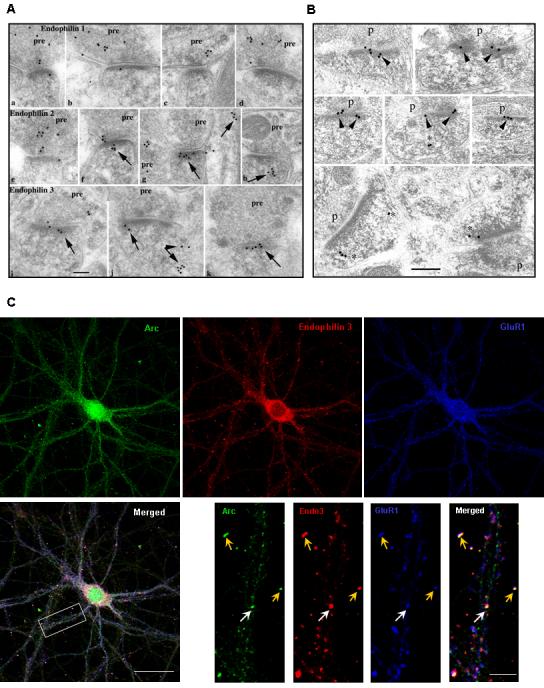
Endophilin 2, 3 and Arc Localize to Post-synaptic Sites with GluR1 in Hippocampal Neurons (A)Immunogold localization of endophilin 1 (a-d), 2 (e-h) and 3 (i-m) in the hippocampus CA1 stratum radiatum (a,b, e,f, i,j,l) and molecular layer of the dentate gyrus (c,d,g,h,k,m). Arrows indicate clusters of gold in the postsynaptic spine (f-k) or dendrite shaft (g-right, l,m). This labeling often is associated with vesicular or tubulovesicular structures (arrows in f-h,j,l,m). Labeling for endophilin 1 is common mainly in presynaptic terminals (pre). Endophilin 2 labeling also is common in presynaptic terminals as well as in postsynaptic spines. Endophilin 3 labeling is seen less commonly in presynaptic terminals (compared to endophilin 1 and 2) but is prominent in postsynaptic spines (similar to endophilin 2). Scale bar is 100 nm.
Results from the counting of 180 synapses (from random sections) of the hippocampus CA1 stratum radiatum are as follows. For endophilin 1, there were 0.483 gold/postsynaptic spine and 1.933 gold/presynaptic terminal, so that there were 4x as many gold particles in the pre as in the post-synapse. The difference is highly significant (p≪0.001). For endophilin 2, there were 1.283 gold/postsynaptic spine and 2.056 gold/presynaptic terminal, so that there were 1.6x as many gold particles in the pre as in the post-synapse. The difference is highly significant (p<0.001). For endophilin 3, there were 0.367 gold/postsynaptic spine and 0.422 gold/presynaptic terminal, so that there were 1.2x (essentially 1x) as many gold particles in the pre as in the post-synapse.
(B) Immuno EM images of CA1 region of adult rat brain showing Arc localization in the postsynaptic density (PSD). Bottom panel shows Arc immunoreactivity at the lateral margin of the PSD.
(C) Immunostaining of 4 week old hippocampal cultured neurons shows endogenous Arc and endophilin 3 localized at dendrites, and in synapses marked by GluR1 (yellow arrows). Some Arc/endophilin 3/GluR1 puncta colocalize together at non-synaptic sites (white arrows) in the dendritic shaft. (Scale dendrite)
Arc Cooperates with Endophilin to Associate with Early Endosomes
To assess the function of their interactions, Arc, dynamin 2 and endophilin transgenes were examined in HeLa cells (Figure 3A). When expressed individually, Arc localized throughout the cytoplasm with enrichment in the nucleus and the plasma membrane. Endophilin 3(172-347) localized diffusely throughout the cytoplasm in a reticular pattern. Dynamin 2(503-871) localized to vesicular structures abundant near the plasma membrane (Figure 3A). When co-expressed, Arc and endophilin 3(172-347) proteins was strikingly redistributed to vesicular structures. Arc also co-localized to vesicles when co-expressed with dynamin 2(503-871) or dynamin 2 GFP (not shown). When Arc, endophilin 3 and dynamin 2(503-871) were co-expressed, all three proteins co-localized in vesicles. Arc and endophilin 3 full length protein did not co-localize on vesicles when co-expressed alone, but did when co-expressed with dynamin (not shown).
Figure 3.

Arc is Recruited with Endophilin and Dynamin to Vesicles in HeLa Cells(A) Arc expressed alone has a cytoplasmic and nuclear distribution in HeLa cells, with some enrichment at the plasma membrane. En3-CT [Endophilin 3(172-347)] alone has a cytoplasmic distribution with enrichment at the plasma membrane. Dyn2-CT [Dynamin 2(503-871)] alone localizes to vesicular structures near the plasma membrane. When Arc and En3-CT are expressed together, vesicular structures form that contain both proteins. Arc and Dyn2-CT co-expression results in a redistribution of Arc to dynamin 2 containing vesicular structures. When Arc, Dyn2-CT, and En3-FL (endophilin 3 full length) are co-expressed, all three proteins colocalize in vesicular structures.
(B) Arc and full-length endophilin 3 have no effect on Alexa-555 conjugated transferrin uptake when expressed alone (a and c). Arc and myc antibodies were used to stain Arc and endophilins respectively. (b) En3-CT inhibits transferrin uptake. (d) Arc and En3-CT co-expression results in endosomes that internalize transferrin. (Scale bars represent 30 μm)
To assess the nature of these vesicular structures, we stained HeLa cells with markers for known organelles. Arc-endophilin vesicles co-localized with endogenous transferrin receptors, a marker for early endosomes, but not LAMP1, a lysosomal marker (Supplemental Figure S2A). A subset of vesicles also co-localized with Rab11, a marker for recycling endosomes. Similar associations with vesicular markers were evident for dynamin 2 alone, Arc-dynamin 2(503-871) and Arc-endophilin-dynamin 2 vesicles (data not shown). These data suggest that Arc-endophilin-dynamin vesicles are predominantly early endosomes in HeLa cells.
To confirm that vesicles are endosomes, we examined uptake of Alexa555 labeled transferrin into living cells. When expressed individually, neither Arc nor endophilin 3 altered transferrin uptake or its intracellular distribution (Figure 3Ba). Endophilin 3(172-347) alone did not increase transferrin uptake, but rather appeared to reduce transferrin uptake (Figure 3Bb). This is consistent with a previous report that C-terminal fragments of endophilin are dominant negatives for endocytosis (Petrelli et al., 2002). When Arc was co-expressed with endophilin 3(172-347), endocytosed transferrin accumulated in Arc-endophilin vesicles (Figure 3Bd). TIRF microscopy showed that Arc-endophilin endosomes are dynamic near the plasma membrane (Supplemental Figure S2B and C). These observations confirm that Arc-endophilin vesicles are endosomes and are able to take up cargo.
Distinct Regions of Arc are Required for Interactions with Endophilin and Dynamin, and Both Interactions are Required for Association with Vesicles
We examined binding interactions that mediate Arc’s ability to associate with endosomes. Arc(1-154) co-IPs endophilin 3(172-347), while Arc(155-396) does not (Figure 4A). Similarly, the internal deletion mutant Arc(Δ50-80) co-IPs with endophilin3 (172-347), while Arc(Δ81-90), Arc(Δ91-100) and Arc(Δ101-130) show reduced co-IP (Figure 4B). Longer exposures of the co-IP blot revealed that deletion of Arc(91-100) most effectively disrupts binding (not shown). In support of a direct role of this region in binding, Arc peptide 89-100 selectively blocked binding of Arc to GST endophilin 3(172-347) (Figure 4C). Moreover, the same peptide linked to CNBr activated beads selectively bound endophilin 3 (Supplemental Figure 3A). This analysis indicates that Arc 89-100 is necessary and sufficient to bind endophilin.
Figure 4.
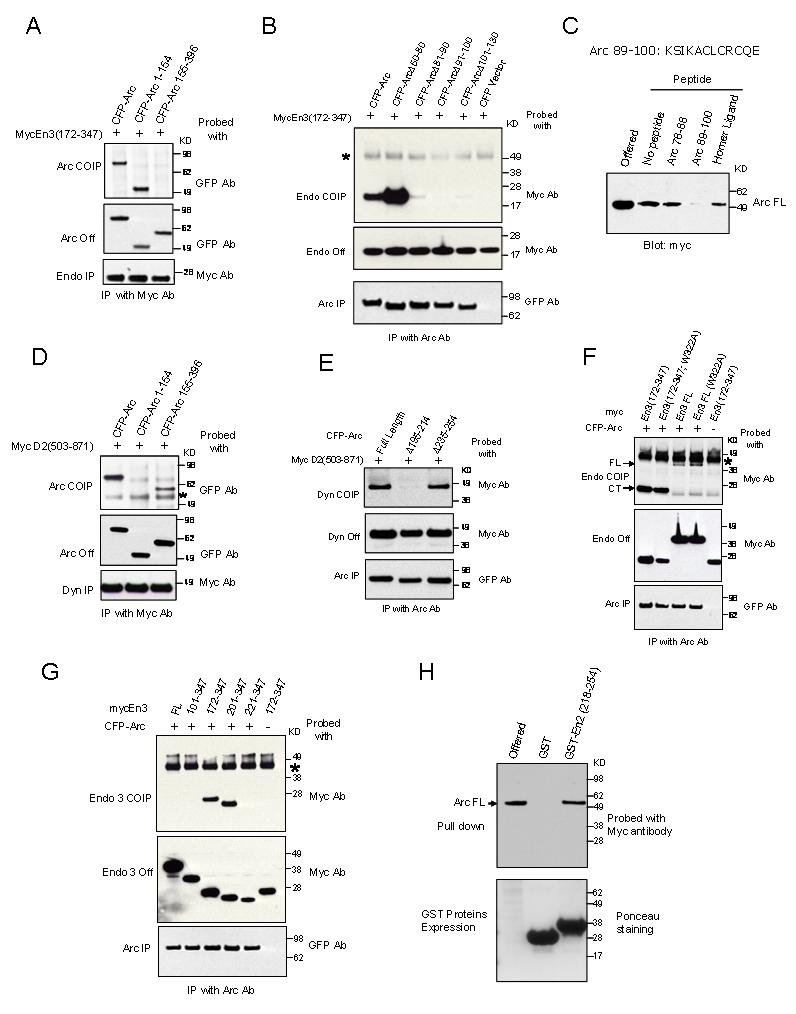
Distinct Regions of Arc Bind to Endophilin and Dynamin(A) Arc or Arc (1-154) co-IP endophilin 3(172-347), while Arc (155-396) does not.
(B) The region of Arc that binds endophilin maps to aa 81-130. Internal deletion of 50-80 does not reduce binding to endophilin 3(172-347), while deletions in the region 81-130 do. Non-specific bands are indicated with *.
(C) GST-Endophilin 3(172-347) binds Arc expressed in HEK293 cells, and the interaction is blocked by peptide Arc 89-100, but not by Arc 76-88 or an unrelated peptide (Homer ligand). Final concentration of peptides is 100μM.
(D)Arc (155-396) co-IPs with dynamin 2(503-871), while Arc (1-154) does not.
(E) Internal deletions of 20 aa that span the region Arc (195-214) disrupt co-IP with Dynamin 2(503-871) co-expressed in HEK293 cells. No change in binding with the deletion of region Arc(235-254).
(F) Arc co-IPs endophilin 3(172-347) more effectively than endophilin 3 (En3-FL) and is not altered by SH3 mutation [W322A].
(G)Deletions of BAR domain modify co-IP with Arc. (H)GST-Endophilin 2 (218-254) binds Arc.
The region of Arc required to bind dynamin 2 was also examined. Arc(155-396) co-IPs with dynamin 2(503-871), while Arc(1-154) does not (Figure 4D). Internal deletion mutants of Arc indicated a requirement for aa 195-214 but not aa 235-254 in co-IP experiment.(Figure 4E). We conclude that regions essential for interaction with endophilin and dynamin are distinct and do not correspond to previously described domains.
Arc mutants were assayed for their ability to associate with vesicles when co-expressed with endophilin 3(172-347). Only Arc constructs that retain the ability to bind both endophilin and dynamin associate with vesicles (Supplemental Table T1A).
Arc Interacts with the C-terminus of the BAR Domain of Endophilin 2 and 3, andBinding Is Allosterically Regulated
Arc binds endophilin 3 (172-347) more strongly than endophilin 3 suggesting inhibition by the N-terminus of the BAR domain (Figures 1A and 4F). Domains present in endophilin 3 (172-347) include the C-terminus of the BAR domain, the variable region and the SH3 domain (Figure 1A). Point mutation of the SH3 that destroys binding to SH3 ligands (W322A) does not alter binding to Arc (Figure 4F). The BAR domain in endophilin consists of three helices that span aa 30-104, 110-173, 180-247, respectively (Weissenhorn, 2005). Deletion of the first helix does not increase binding while deletion of the second helix does (Figure 4G). Partial deletions of the third helix [endophilin 3(221-347)] abolish binding to Arc. These data suggest that the N-terminus of the BAR domain inhibits binding to Arc and the third helix of the BAR domain is required. To determine whether this region is directly involved in binding we generated a bacterial GST fusion protein that includes the C-terminus of the third helix of the BAR domain [GST-E2(218-254)]. This peptide selectively binds Arc (Figure 4H). Chimeras between endophilin 2/3 and endophilin 1 (does not bind Arc) confirm the requirement of the C-terminus of the BAR domain (Supplemental Figure S3B and C).
Analysis of vesicle association of endophilin mutants with Arc confirms the requirement for physical interaction between Arc and endophilin (Supplemental Table T1B). In contrast to binding data, endophilin mutants that lack a functional SH3 domain do not associate with vesicles when expressed alone or in combination with Arc. Since the SH3 is required for interaction with dynamin, these observations support a model of cooperative interaction in vesicle association.
Arc Interacts with Endophilin and Dynamin and Associates with Endosomes in Neurons
Primary neuronal cultures were transfected with Arc and full length endophilin 3, either individually or together. When Arc was expressed individually, the transgene accumulates in dendrites with a uniform, dispersed distribution, and shows enrichment in dendritic spines (Supplemental Figure S4A). Similarly, endophilin 3 transgene is present in dendrites (Supplemental Figure S4A). Some endophilin puncta co-localized with surface GluR1 (used to mark synapses), while other puncta localized to the dendritic shaft. When co-expressed, Arc and endophilin 3 co-localized in puncta along the length of dendrites (Figure 5A). Thus, Arc redistributes to endophilin puncta in neurons. In addition, internalized GluR1 co-localized with endophilin 3 puncta (Figure 5B), suggesting that these puncta are receptor trafficking vesicles. Arc-endophilin vesicles also co-localized with endocytosed transferrin (Figure 5C), further suggesting that these are trafficking endosomes. The structure-function properties of the Arc-endophilin interaction in neurons is similar to HeLa cells (see Supplemental Figures S5 and S6). Endophilin mutants that cannot bind to dynamin do not form endosomes with Arc, and an Arc mutant that cannot bind to endophilin does not co-localize with endophilin puncta. Arc also redistributes to endosomes when co-expressed with dynamin2(503-871) (Figure 7A) in neurons. Dynamin2(503-871), when expressed alone, co-localized with AMPARs in a similar manner to endophilin (Supplemental Figure S7B).
Figure 5.
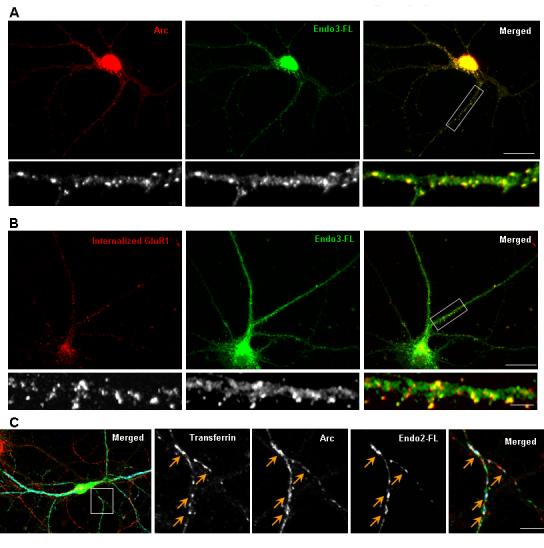
Arc and Endophilin Associate with Endosomes in Neurons(A)Arc and endophilin 3 transgenes co-localize in large puncta in the dendritic shaft and in spines.
(B) Neurons were incubated with a GluR1 N-terminal antibody at 10°C, and were subsequently allowed to undergo basal endocytosis for 30 min at 37°C. Any remaining surface GluR1 were stripped with an acid wash. Internalized GluR1 puncta co-localize with many of the endophilin-Arc transgene vesicular puncta.
(C) Neurons were incubated with Alexa555-transferrin for one hour at 37°C, labeling both early and recycling endosomal pools. A number of Arc-endophilin transgene puncta co-localized with transferrin in dendrites (yellow arrows).
Figure 7.

The Arc-Dynamin Interaction is Required for the Arc-dependent Decrease in Surface AMPARs. (A) Arc and dynamin2-CT (aa503-871) transgenes co-localize in large puncta in the dendritic shaft and in spines.
(B) Representative images of Arc transgene expression, showing a loss of surface GluR1 puncta/intensity, whereas Arc-214 Δ195 expression had no effect on the number of GluR1 puncta/intensity. Quantition shows that Arc expression causes a significant decrease in the number and total intensity of surface GluR1 puncta (n = 63/21). In contrast,-214, Arc had no significant Δ195effect on GluR1 puncta or total intensity (n = 57/10). (*p < 0.001).
Arc Induces Selective Down Regulation of AMPA Receptors and Accelerates Endocytosis
Since Arc, endophilin and dynamin co-localize at synapses that contain AMPARs (Supplementary Figure S7), we asked whether they function in their trafficking. To model the condition of transient up regulation of Arc we examined neurons ∼16 hrs after transfection. Arc transgene expression resulted in a marked reduction of surface GluR1 (Figure 6A and B). This action was dependent on Arc’s ability to bind endophilin since Arc (Δ91-100) had no effect on surface GluR1 (Figure 6A and B). A deletion mutant of Arc (Δ195-214) that does not bind dynamin also had no effect on surface GluR1 (Figure 7B), suggesting that both the endophilin and dynamin binding domains are necessary for Arc’s modulation of AMPAR trafficking. Neither endophilin 3, nor endophilin 3(172-347) downregulated surface GluR1 (Figure 6B), suggesting Arc is necessary to induce AMPAR downregulation.
Figure 6.
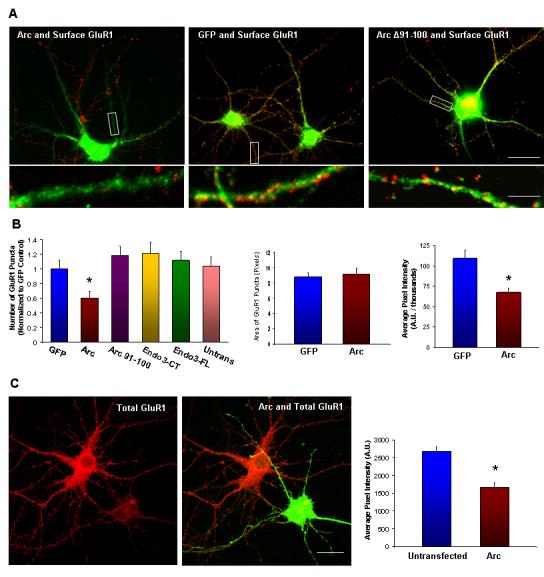
Arc Expression Decreases Surface and Total AMPARs(A) Representative images of Arc transgene expression (green), showing a loss of surface GluR1 puncta (assessed using an N-terminal antibody, (red) as compared to neighboring untransfected cells, 16 hours post-transfection. GFP transfected neurons had normal numbers of-100 expression GluR1 had no puncta. effect on the number of GluR1 puncta.
(B) Arc expression causes a significant decrease in the number of surface GluR1 puncta (n = 48 dendrites from16 cells, *p < 0.01) compared with neighboring untransfected cells. GFP transfection had no effect. In contrast,-100, endophilin Arc 3(172-347) or endophilin 3 had no significant effect on GluR1 puncta. Arc expression did not change GluR1 puncta size or average pixel intensity, but total intensity was significantly decreased *p < 0.001).
(C) Arc transgene reduced total GluR1 (assessed using a C-terminal antibody), as compared to untransfected neurons 16 hours post-transfection (n = 60 regions from 20 cells, *p < 0.001).
Arc expression also resulted in a reduction of total GluR1, assessed using a C-terminal antibody (Figure 6C). This effect is not as dramatic as the reduction of surface GluR1 (∼30% decrease vs. ∼50% respectively), but suggests that high Arc expression leads to downregulation of receptors. In contrast to GluR1, Arc expression resulted in a small increase in total NMDA receptor subunit NR1 puncta (Supplemental Figure S8). Due to limitations of the antibody, we could not determine if this corresponds to increased surface NR1. Nevertheless, the result indicates that Arc does not generally down-regulate all glutamate receptors
To assess the hypothesis that Arc down regulates surface GluR1 by increasing basal endocytosis, we assayed the amount of GluR1 internalized during a 30 minute epoch at 37°C. Despite markedly reduced surface GluR1, the amount of internalized GluR1 was similar to that seen in control neurons (Figure 8A, C and S9). Accordingly, the percent of surface GluR1 internalized was increased in neurons that express Arc. We conclude that Arc increases the rate of GluR1 endocytosis.
Figure 8.
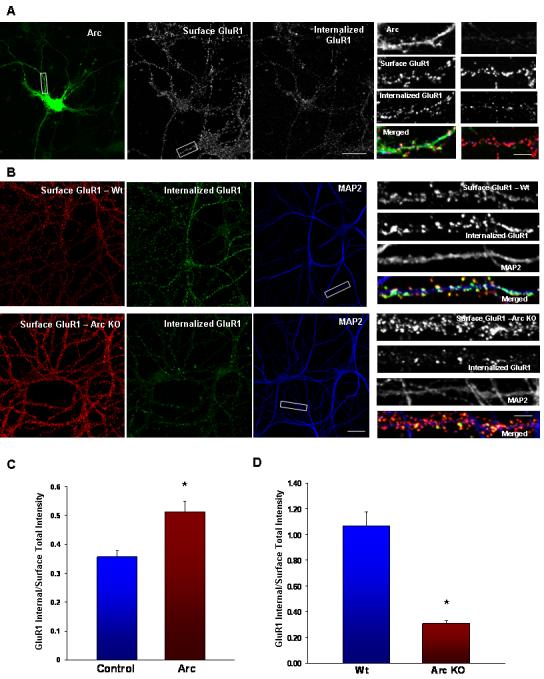
Arc Increases AMPAR Endocytosis while Arc KO Neurons Exhibit Increased Surface AMPARs and a Deficit in Endocytosis (A)Arc transfected neuron with both surface and internalized GluR1 images using different secondary antibodies after 30 min basal endocytosis. Magnified dendrites show an Arc transfected dendrite and an untransfected dendrite.
(B) Representative images of surface and internalized GluR1 after 30 min of basal endocytosis in Wt and KO hippocampal neurons. MAP2 staining is shown to highlight individual dendrites (Scale bar, 30μm)
(C) Quantification of surface and internalized GluR1. Arc expression results in a decrease in the surface to internalized GluR1 ratio (n = 30/10, p < 0.02), indicating that more endocytosis of receptors occurred in Arc transfected neurons. Similar results are reported in Figure 3 of Shepherd et al (this issue), although the results reported reflect two different experiments. (*p < 0.05).
(D)Total intensity of surface and internalized GluR1 puncta was quantified to assess the amount of endocytosis during 30 min. Arc KO neurons (n = 48/16) have a significantly higher surface to internalized ratio than Wt neurons (n = 51/17). (*p ≪ 0.001).
Loss of Arc Protein Results in Increased Surface AMPARs and a Reduction in AMPAR Endocytosis
To assess the effect of loss of Arc function on AMPAR trafficking, we utilized a mouse knock-out of Arc/Arg3.1. These mice are viable, and do not exhibit gross changes in brain architecture (Plath et al., 2006). DIV 21-28 low-density hippocampal neurons from Arc KO mice exhibited a ∼2 fold increase in the steady state level of surface GluR1 as compared with Wt control neurons (Figures 7B, D and S9). Despite these high surface levels, the amount of AMPAR internalized in 30 minutes was dramatically less than Wt (Supplemental Figure S9). Since surface expressed AMPARs represent the pool available for internalization, this result indicates a marked reduction in the rate of endocytosis of GluR1 in Arc KO neurons (Figure 8D). Although Arc appears to be expressed late in development, we wanted to rule out any non-specific developmental effects caused by the loss of Arc. We performed “rescue” experiments by introducing Arc protein into KO neurons and assessed surface GluR1 levels. To facilitate this we expressed Arc by Sindbis virus in DIV 21-28 KO neurons and measured surface GluR1 levels one day after transfection. Arc expressing neurons significantly downregulated surface GluR1 levels compared to surrounding untransfected neurons (Figure 9). Importantly GFP Sindbis virus expression in KO neurons had no affect on surface levels, indicating that the virus itself does not affect surface GluR1 levels. Surface GluR1 levels in Arc expressing KO neurons were comparable to Wt levels (Figure 9) indicating that addition of Arc protein to Arc KO neurons rescued the KO phenotype. To test whether Arc’s rescue of surface GluR1 levels was due to its ability-100)into to KO neurons. This mutant was unable to rescue surface GluR1 levels, indicating that the KO phenotype is due to Arc’s modulation of the endocytic machinery.
Figure 9.
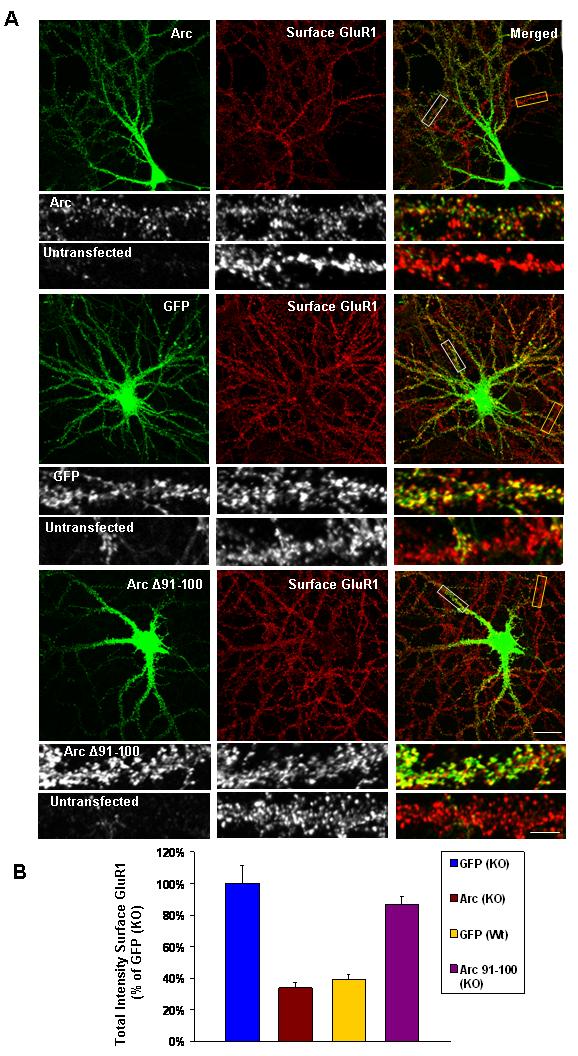
Arc Expression in Arc KO Neurons Rescues Surface GluR1 Levels (A)Representative images of Arc Sindbis virus expression in Arc KO low-density hippocampal neurons, showing a reduction of surface GluR1 as compared to neighboring untransfected cells. GFP Sindbis virus expression has no affect on surface GluR1. In contrast to-100 Arc Sindbis virus expression does not affect surface GluR1 levels. (White boxes show magnified transfected dendrites and yellow boxes highlight untransfected dendrites. Scale bars, 30μm and 8 μm in magnified dendrites)
(B)Quantification of surface GluR1 experiments. Arc expression in Arc KO neurons (n = 30/10) significantly reduces total intensity of surface GluR1 (34 ± 3% of GFP transfected KO neurons, n = 30/10, p < 0.001) and is comparable to GluR1 levels in-100 expression in Arc KO neurons does not rescue GluR1 levels (87 ± 5%, n = 30/10, p = 0.3)
Discussion
The present study identifies a molecular function for the immediate early gene Arc. Our data support a model in which Arc binds endophilin and dynamin in a cooperative assembly that modifies postsynaptic endosome function and AMPAR trafficking (See Figure 10). This pathway plays a role in the regulation of steady state AMPAR expression, since genetic deletion of Arc results in a substantial up-regulation of surface AMPAR. Arc promises to provide insight into forms of synaptic plasticity that are dependent on postsynaptic endocytosis and receptor trafficking, including processes involved in late-phase plasticity and memory.
Figure 10.
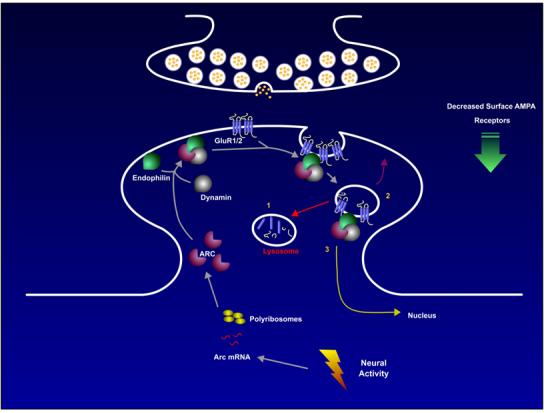
Proposed Model for Arc Modulation of AMPAR TraffickingArc mRNA is induced after neuronal activity and is transported to dendrites. Upregulation of Arc protein occurs in a synapse-specific manner via local translation. Arc protein recruits endophilin and dynamin, which is anchored to the PSD via its interaction with VSCC (Chen et al., 2003) and Shank (Okamoto et al., 2001), respectively. The complex of Arc, endophilin and dynamin modulates endosome formation, and selectively recruits AMPA receptors. These endosomes may then 1. Recycle back to the membrane. 2. Traffic to the lysosomal compartment where protein is degraded or sent to the proteosome 3. Traffic to the nucleus as a signaling endosome carrying other cargo. It is interesting to note that Arc protein is present in the nucleus of select populations of brain neurons where it is coincidently induced with Arc protein in dendrites. Changes in the kinetics of these vesicular pathways are anticipated to underlie changes in steady state levels of AMPAR at synapses.
Arc-Endophilin-Dynamin and Endocytosis
Clathrin-mediated endocytosis is a general mechanism of membrane protein regulation and is conserved in most species and cell types (Mousavi et al., 2004). Endophilin and dynamin are two core endocytic proteins. Dynamin, a large GTPase, is involved in pinching off and fissioning of membrane, which releases endosomes from the plasma membrane surface (Praefcke and McMahon, 2004; Roux et al., 2006). In addition to its enzymatic action on lipids, dynamin also recruits many other proteins to regulate endosome formation. The proline-rich domain (PRD) at the C terminus contains numerous binding motifs for SH3 domains, which recruits proteins such as endophilin (Ringstad et al., 1997), amphiphysin (Grabs et al., 1997) and syndapin (Qualmann et al., 1999). These proteins are thought to regulate kinetics, spatial distribution and induction of endocytosis (Le Roy and Wrana, 2005). Many of these proteins also sub serve specific roles in specialized endocytic pathways such synaptic vesicle recycling in the presynaptic nerve terminal. The classic dynamin mutation, shibire, causes paralysis due to a severe defect in synaptic vesicle recycling at the neuromuscular junction (Chen et al., 1991; Poodry et al., 1973). Mutations in endophilin also perturb synaptic vesicle recycling in Drosophila and in C. elegans (Schuske et al., 2003; Verstreken et al., 2002). Endophilin binds synaptojanin, a polyphosphoinositide phosphatase involved in uncoating clathrin from endosomes (Micheva et al., 1997). Synaptojanin mutations in Drosophila and C. elegans produce similar endocytic phenotypes as endophilin mutants (Schuske et al., 2003; Verstreken et al., 2003). Precisely how these proteins act in concert during endocytosis remains unclear.
Postsynaptic endocytosis of receptors is thought to be mediated by a similar repertoire of proteins, although perhaps via specific protein isoforms. Dynamin 2 and 3 have been shown to be mostly post-synaptic and are localized to the PSD via their interaction with the post-synaptic scaffolding proteins Shank and Homer, respectively (Gray et al., 2003; Okamoto et al., 2001). We show that distinct isoforms of endophilins (2 and 3) are localized to postsynaptic membranes, whereas endophilin 1 is predominantly presynaptically localized. Strikingly, Arc only interacts with endophilins 2 and 3 but not 1, suggesting that isoform specificity can indeed confer particular properties to specific endocytic pathways. Specific endocytic zones, segregated from the postsynaptic density (PSD), can be found in the lateral margins of excitatory synapses (Blanpied et al., 2002). These sites appear to be sites of glutamate receptor internalization (Racz et al., 2004). We find that immunogold EM labeling of endophilins 2/3 and Arc occurs at the lateral margins of spines and near the PSD, precisely where AMPARs diffuse out of the PSD and are endocytosed.
Distinct regions of Arc interact with dynamin’s PH domain and endophilin’s BAR domain. Both interactions appear novel and merit further structural studies. The region of endophilin that is necessary and sufficient to bind Arc corresponds to the last leucine turn of helix 3 of the BAR domain. When associated with vesicles, the BAR domain is predicted to form a dimer of antiparallel triple helices that form a concave surface that contacts phospholipids in vesicles and thus has a role in sensing or inducing membrane curvature (Peter et al., 2004). The Arc-interacting region is at the vertex of the convex surface facing away from the lipid vesicle. Formation of the BAR dimer, which is stabilized by lipid vesicles (Peter et al., 2004), could be important for Arc binding. Arc-BAR binding could position Arc to simultaneously bind the PH domain of dynamin and cooperate with endophilin’s SH3 domain to bind dynamin’s PRD, or other proteins with SH3 ligands such as synaptojanin (McPherson et al., 1996). Endophilin is a nexus of several functions for membrane receptor trafficking, in addition to those involved in lipid biophysics. The SH3 domain of endophilin interacts with CIN85, which binds the ubiquitin ligase cbl in complex with the EGF receptor, resulting in internalization and down-regulation of EGF receptors (Soubeyran et al., 2002). By this mechanism, recruitment of endophilin to membrane receptors regulates the duration and intensity of EGF and c-Met protooncogene signaling (Petrelli et al., 2002; Soubeyran et al., 2002). Endophilin’s SH3 domain is also reported to interact with the cytosolic tail of membrane metalloproteases, and recruits these enzymes to endosomes (Howard et al., 1999).Further work on these protein interactions should shed light on how Arc is able to specifically modify postsynaptic endocytosis. It is clear that Arc is not essential for endocytosis to occur, but acts as an adaptor molecule to modify the kinetics of AMPA receptor endocytosis, and perhaps subsequent trafficking.
Arc and AMPA Receptor Trafficking
AMPARs undergo clathrin-dependent endocytosis (Carroll et al., 1999), and AMPAR-mediated synaptic transmission and synaptic plasticity are regulated by both endocytosis and exocytosis (Man et al., 2000; Wang and Linden, 2000). Expression of dominant negative dynamin 2 inhibits AMPAR endocytosis (Carroll et al., 1999) and interfering dynamin peptides block cerebellar LTD (Wang and Linden, 2000). Modulation of a recycling pool of AMPARs is critical for LTP (Park et al., 2004). Small GTPases such as Ras, Rap and the Rab family of proteins have been shown to be important for AMPAR trafficking (Brown et al., 2005; Park et al., 2004; Zhu et al., 2002) and modulation of GTPase activity by GAPs such as SynGAP have also been implicated in endosomal trafficking of AMPARs (Rumbaugh et al., 2006). These recent studies elucidate the specific protein machinery involved in AMPAR endocytosis, but the signals that regulate them still remain relatively unclear. The most widely accepted model proposes that the GluR1 and GluR2 subunits play key roles in the differential regulation of AMPAR trafficking at rest or during activity (Lee et al., 2004; Malinow, 2003). The long rather than the short cytoplasmic tails of GluR1/GluR2 heteromers are thought to be crucial for activity-dependent insertion and LTP. However, it is thought that constitutive cycling of GluR2/GluR3 subunits replaces synaptic GluR1/GluR2-containing AMPARs (Malinow, 2003). Activity-dependent removal of AMPARs leads to LTD (Carroll et al., 2001) but the role of subunit composition during this regulated removal is still unclear. Phosphorylation of the AMPAR tails has emerged as an important mechanism for regulating trafficking (Boehm and Malinow, 2005). For example, the phosphorylation of the GluR2 tail by protein kinase C at Ser880 has been shown to regulate GluR2 trafficking by modulating the binding of GRIP/ABP and PICK1 via a PDZ domain binding motif (Dong et al., 1997; Xia et al., 1999). This pathway is critical for in vivo regulation of cerebellar LTD (Chung et al., 2003; Steinberg et al., 2006).
The molecular mechanism underlying Arc’s selective endocytosis of AMPA receptors remains unknown. We do not detect direct interactions of AMPA receptors with Arc or endophilin (unpublished observations, S.C and P.F.W). It is notable that while endophilin alone associates with vesicles in neurons that include GluR1/2, it is only when Arc is expressed that we detect a reduction in surface AMPA receptors. High levels of Arc accelerate the internalization of AMPARs, whereas deletion of Arc slows endocytosis. Steady-state surface AMPAR levels are consequently downregulated with sustained Arc expression and increased when Arc is deleted for long periods of time. This suggests that Arc acts as an adaptor to specifically localize or concentrate endocytic proteins at sites where AMPAR endocytosis can occur. Arc may also increase diffusion of AMPARs out of the PSD.
Arc expression in pyramidal neurons of hippocampal slice cultures produces a selective down-regulation of AMPARs that requires Arc’s ability to bind endophilin, and is blocked by agents that inhibit NMDA receptor dependent LTD, including the calcineurin inhibitor FK506 and peptides that mimic the C-terminus of GluR2 (Verde et al., 2006). In our studies of Arc KO neurons, Arc effects appear to be preferential for GluR1, as GluR2 endocytosis is not altered. However, when Arc is expressed in Wt neurons, we see down regulation of both GluR1 and GluR2 (Shepherd et al., 2006; Figure 2C). These results confirm that Arc can endocytose GluR2 in an acute manner. As a synthesis of these findings, we suggest that Arc’s apparent GluR1 selectivity in the Arc KO is a consequence of long term depletion of Arc, which shifts sub-unit composition of AMPAR pools towards more GluR2-lacking AMPARs. Further studies should clarify Arc’s role in regulating AMPAR subunit composition.
Arc and Synaptic Plasticity
Long-term maintenance of synaptic plasticity requires new protein synthesis (Huber et al., 2000; Nguyen and Kandel, 1996; Otani and Abraham, 1989). Gene transcription is upregulated after plasticity-inducing stimuli, resulting in an upregulation of a set of activity-dependent proteins (Lanahan and Worley, 1998). Arc is one of the few proteins upregulated that has been found to specifically regulate AMPAR trafficking directly. AMPARs have recently been shown to modulate the transcription of Arc message, suggesting a possible feedback loop (Rao, et al., 2006). Another gene induced by activity, CPG-2, also regulates endocytosis of receptors, but seems to sub serve a more general function as an endocytic protein regulating internalization of postsynaptic proteins (Cottrell et al., 2004). CPG-2 seems important for both activity-induced AMPAR and constitutive AMPAR and NMDAR internalization. Further studies should clarify whether the Arc-endophilin-dynamin endocytic pathway involves CPG-2.
In support of a role for the Arc-endophilin-dynamin endocytosis pathway in synaptic plasticity, Arc transgene expression in CA1 neurons of cultured hippocampal slices results in a selective down-regulation of AMPA receptors that mimics LTD (Verde et al, 2006). This action is not produced by Arc (Δ91-100), suggesting a role for Arc-endophilin endocytosis. In addition, this mutant cannot rescue the Arc-dependent increases in surface AMPARs observed in Arc KO neurons.
Arc is also required for maintenance of LTP (Guzowski et al., 2000; Plath et al., 2006). However, the precise mechanism by which Arc maintains LTP remains to be determined. The physiological consequence of Arc expression can be anticipated to depend on its spatial extent and temporal dynamics, which are highly regulated. If the steady state level of Arc protein were up regulated in response to an increase in neuronal activity, the Arc-endophilin endocytosis mechanism might evoke cell wide down regulation of AMPA receptors. In support of this notion, homeostatic scaling of AMPAR is absent in Arc KO neurons (Shepherd et al., 2006). This suggests that Arc facilitates plasticity by controlling optimal surface AMPAR levels and neuronal excitability. If Arc’s action were restricted to subsets of synapses within a neuron, for example by mRNA trafficking, it is possible that the Arc-endophilin pathway could contribute to other forms of protein synthesis-dependent plasticity such as heterosynaptic LTD or competitive capture (Fonseca et al., 2004).
Arc is very highly conserved from chickens to humans (∼88% conservation at the amino acid level), and has been implicated in many different learning behaviors such as bird song learning and rodent spatial mapping. This suggests that its modulation of post-synaptic endocytosis in the brain is a well conserved process important for synaptic plasticity and memory consolidation.
Methods
Yeast Two-hybrid Screening
Full-length Arc open reading frame was used to screen a yeast expression library, as described previously (Brakeman et al., 1997)
Expression Constructs
All the expression constructs were made by PCR. Internal deletion and point mutant were made either using QuikChange Site-Directed Mutagenesis Kit (Stratagene) or by megaprimer method (Barik, 2002). Sequence to generate each mutant will be supplied upon request. PCR products were cloned to expression vectors ECFPC1 (Clontech), pGEX 4T2 (Pharmacia), pRK5 (Genentech) with myc or HA-tags. All constructs were verified by sequencing.
Antibodies
All antibodies were previously described or were acquired commercially: PSD-95 (mAb, Affinity), GluR1-N (pAb, JH1816;), GluR1-C (JH1710); GluR2-C (pAb, JH1707); NR1 (pAb, JH2590), Arc (pAb), Arc (mAb, Santa Cruz), Endophilin goat pAbs from Santa Cruz; 1 (sc-10874), 2 (sc-10876), 3 (sc-10879), Pan endophilin (pAb), Myc (9E10 mAb, Santa Cruz), Transferrin receptor (mAB, Zymed), LAMP1 (mAb, Signal Transduction Labs), Rab11 (mAb, Signal Transduction Labs), EGF (pAb, Calbiochem), GFP mAb (Covence), Dynamin (mAb Hudy1,Upstate).
Cell Culture and Neuronal Transfection.
Low-density hippocampal neurons were prepared as described previously (Banker and Cowan, 1977). Neuronal transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in DIV 12-14 neurons and were analyzed 16-24 hr after initial incubation.
Preparation of Crude Synaptosomal Fraction from Brain
Rat brains were homogenized in 10 volumes of buffered sucrose (0.32 M sucrose, 4 mM HEPES/NaOH, pH 7.4, 1mM EDTA, 1mM EGTA and protease inhibitor cocktail) with a glass-Teflon homogenizer, centrifuged at 800g for 15 min. The supernatant was again centrifuged at 9000g for 15 min and the pellet collected as crude synaptosomal fraction, P2 (Huttner et al., 1983).
Coimmunoprecipitation and In Vitro Binding
P2 was sonicated in PBS with 0.2% deoxycholete detergent and protease inhibitors. The homogenate was centrifuged at 100,000 X g for 20 min at 4°C and supernatants were incubated with 2.5 μl of rabbit-immune serum. After 1.5 hr of mixing at 4°C Protein A agarose slurry was added and incubated for another hour. The beads were washed with PBS+1%Triton X-100 three times and eluted with SDS loading buffer. HEK293 cells were transfected with Arc, endophilin or dynamin constructs and cell lysates were prepared in PBS with 1% Triton X-100 and protease inhibitors (Roche). Arc antibody or myc monoclonal antibody (Roche) were incubated with cell lysate for 2 hour. Protein A agarose (for Arc Ab) or Protein G (for myc Ab) sepharose slurry was added and incubated with mild agitation at 4 °C for another hour. The beads were washed twice with PBS+0.5-1% Triton X-100 and once with PBS. Proteins were eluted with SDS loading buffer and samples were boiled for 3 min. GST Protein expression and binding was done according to the protocol described in Tu et al. (Tu et al., 1998) except for the blocking experiment where HEK293 cells lysates were preincubated with 100μM peptides.
CNBr-activated Sepharose 4B Coupled Peptide Pull Down
Arc 89-100 peptide coupled to CNBr-activated Sepharose 4B (Amersham) according to the manufacturers protocol. Cell lysates were prepared from mutants of endophilin 3 transfected HEK293 cell in PBS with 1% Triton X-100 and protease inhibitors. Equal volume of bead was added to the lysates and incubated in the cold room for 2 hours. The beads were washed 3 times with binding buffer and bound proteins were eluted with SDS loading buffer and analyzed by western.
Immuno-electron Microscopy
Immuno-electron microscopy was performed by post-fixation immunogold labeling as described in (Kim et al., 2003) and (Darstein et al., 2003), using goat endophilin antibodies (described in antibodies section above) at a dilution of 1:100.
Immunocytochemistry, Microscopy, and Data Analysis
Cells were fixed in 4% paraformaldehyde, 4% sucrose PBS solution for 20 min at 4°C and were subsequently permeabilized with 0.2% Triton X-100 in PBS for 10 min. Cells were then blocked for 1 hr in 10% normal donkey/goat serum (NGS). Primary antibodies were diluted in 10% NGS and incubated with neurons for 1 hr at room temperature or overnight at 4°C. Alexa secondary antibodies (1:500; Molecular Probes, Eugene, OR) to the appropriate species were diluted in 10% NGS and incubated at room temperature for 1 hr. Coverslips were mounted on precleaned slides with PermaFluor and DABCO.
Immunofluorescence was viewed with either a Zeiss LSM 510 confocal laser scanning microscope or a Nikon TE800 epifluorescence microscope fitted with a CoolSnap ES CCD digital camera (Roeper Scientific).
Receptor Trafficking Experiments
For transferrin uptake experiments HeLa cells on coverslips were serum starved for 6 hours and were incubated with 15 ug/ml transferrin conjugated to Alexa555 (Molecular Probes) for 15 min at 37°C and subsequently fixed. Transferrin uptake in neurons was carried out by serum starving neurons for an hour, incubating neurons with 100ug/ml Alexa555-transferrin for 1 hr at 37°C and subsequently fixed. To label surface GluR1-containing AMPA receptors, -N JH1816 pAb was 2.5 added to μg neuronal of growth media and incubated at 10°C for 20 min. The unbound excess antibody was quickly washed with fresh warmed growth medium and then fixed and mounted according to the methods described above. For internalization assays neurons were put back at 37°C for various time points. Surface GluR1 were removed by incubating neurons in stripping buffer (0.5M NaCl and 0.2M ascetic acid) live for 5 min and were then fixed. For endocytosis assays that labeled surface and internalized GluR1, Alexa 555 secondary was added in excess live at 10°C. Neurons were fixed, permeabilized and subsequently exposed to Alexa 488 secondary to stain internalized receptors (background in the non-permeabilized control was negligible).
Quantification of surface GluR1 puncta was carried out essentially as described (Rumbaugh et al., 2003), using Metamorph imaging software (Universal Imaging, Downingtown, PA). Images were acquired as multi-channel TIFF files with a dynamic range of 4096 gray levels (12-bit binary; MultiTrack acquisition for confocal). To measure punctate structures neurons were thresholded by gray value at a level close to 50% of the dynamic range. Background noise from these images was negligible. After a dendrite segment was selected, all puncta were treated as individual objects and the characteristics of each, such as pixel area, average fluorescence, and total fluorescence, were logged to a spreadsheet. In addition, each dendrite length was logged in order to calculate puncta density. Transfected cells were compared with neighboring untransfected cells in individual coverslips. To calculate total GluR1 expression, we measured three 20x20 regions (400 pixels) in the perinuclear region of the soma from GluR1-C labeled neurons. No threshold was used as the soma is clearly delineated from background (Signal to noise > 10). The average single pixel intensity from each region was calculated and averages from all regions were derived. Significance was determined by a paired student’s T test. Scale bars represent 30μm and 8μm in magnified dendrites.
Supplementary Material
Acknowledgments
This work was supported by grants from NIMH (P.F.W), NIMH Conte Center (R.L.H. P.I.), the Howard Hughes Medical Institute (R.L.H) and the NIH/NIDCD Intramural Program (R.S.P). H.O. was supported by a fellowship from the Human Frontier Science Program Organization. We would like to thank Oswald Steward for helpful discussions and Jennifer Henderson for preliminary studies of Arc-endophilin interaction. Thanks to Jing Wu for providing the Arc Sindbis virus. We also thank Dr. Ya-Xian Wang for help with the immunogold labeling. Endophilin 1, 2 and Dynamin 2 expression constructs were a generous gift from Dr. De Camilli (Yale University).
References
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Barik S. Megaprimer PCR. Methods Mol Biol. 2002;192:189–196. doi: 10.1385/1-59259-177-9:189. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bock J, Thode C, Hannemann O, Braun K, Darlison MG. Early socio-emotional experience induces expression of the immediate-early gene Arc/arg3.1 (activity-regulated cytoskeleton-associated protein/activity-regulated gene) in learning-relevant brain regions of the newborn chick. Neuroscience. 2005;133:625–633. doi: 10.1016/j.neuroscience.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem Soc Trans. 2005;33:1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Burke SN, Chawla MK, Penner MR, Crowell BE, Worley PF, Barnes CA, McNaughton BL. Differential encoding of behavior and spatial context in deep and superficial layers of the neocortex. Neuron. 2005;45:667–674. doi: 10.1016/j.neuron.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Beattie EC, Xia H, Luscher C, Altschuler Y, Nicoll RA, Malenka RC, von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Deng L, Maeno-Hikichi Y, Lai M, Chang S, Chen G, Zhang JF. Formation of an endophilin-Ca2+ channel complex is critical for clathrin-mediated synaptic vesicle endocytosis. Cell. 2003;115:37–48. doi: 10.1016/s0092-8674(03)00726-8. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Cottrell JR, Borok E, Horvath TL, Nedivi E. CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors. Neuron. 2004;44:677–690. doi: 10.1016/j.neuron.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J Neurosci. 2003;23:8013–8019. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron. 2004;44:1011–1020. doi: 10.1016/j.neuron.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, Hemar A, McNiven MA. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Howard L, Nelson KK, Maciewicz RA, Blobel CP. Interaction of the metalloprotease disintegrins MDC9 and MDC15 with two SH3 domain-containing proteins, endophilin I and SH3PX1. J Biol Chem. 1999;274:31693–31699. doi: 10.1074/jbc.274.44.31693. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Worley P. Immediate-early genes and synaptic function. Neurobiol Learn Mem. 1998;70:37–43. doi: 10.1006/nlme.1998.3836. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Yoshida-Matsuoka J, Yamagata K, Sugiura H, Ichikawa M, Norita M. Rapid induction of Arc is observed in the granule cell dendrites in the accessory olfactory bulb after mating. Brain Res. 2003;975:189–195. doi: 10.1016/s0006-8993(03)02634-9. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J Biol Chem. 1997;272:27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Mousavi SA, Malerod L, Berg T, Kjeken R. Clathrin-dependent endocytosis. Biochem J. 2004;377:1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto PM, Gamby C, Wells D, Fallon J, Vallee RB. Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton. J Biol Chem. 2001;276:48458–48465. doi: 10.1074/jbc.M104927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Abraham WC. Inhibition of protein synthesis in the dentate gyrus, but not the entorhinal cortex, blocks maintenance of long-term potentiation in rats. Neurosci Lett. 1989;106:175–180. doi: 10.1016/0304-3940(89)90222-x. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–190. doi: 10.1038/416187a. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammerman B, Errington ML, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, et al. Arg3.1/Arc is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006 doi: 10.1016/j.neuron.2006.08.024. In Press. [DOI] [PubMed] [Google Scholar]
- Poodry CA, Hall L, Suzuki DT. Developmental properties of Shibire: a pleiotropic mutation affecting larval and adult locomotion and development. Dev Biol. 1973;32:373–386. doi: 10.1016/0012-1606(73)90248-0. [DOI] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol Biol Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci U S A. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci U S A. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske KR, Richmond JE, Matthies DS, Davis WS, Runz S, Rube DA, van der Bliek AM, Jorgensen EM. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc mediates synaptic scaling of AMPA receptors. Neuron. 2006 doi: 10.1016/j.neuron.2006.08.034. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J`C, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Velho TA, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- Verde ER, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased Expression of the Immediate-early Gene Arc Reduces AMPA Receptor-mediated Synaptic Transmission. Neuron. 2006 doi: 10.1016/j.neuron.2006.09.031. This Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W. Crystal structure of the endophilin-A1 BAR domain. J Mol Biol. 2005;351:653–661. doi: 10.1016/j.jmb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zou Z, Buck LB. Combinatorial effects of odorant mixes in olfactory cortex. Science. 2006;311:1477–1481. doi: 10.1126/science.1124755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


