Abstract
Sickle cell anemia (SS) is highly phenotypically variable, and early predictors of outcome could guide clinical care. To determine whether early vaso-occlusive complications predicted subsequent adverse outcomes in the Dallas Newborn Cohort, we studied all members with SS or sickle-β0-thalassemia who presented in their first year of life and had 5 years or more of follow-up. We defined 3 potential early predictors: hospitalizations in the first 3 years of life for (1) painful events other than dactylitis, (2) dactylitis, and (3) acute chest syndrome (ACS). We studied the associations of these predictors with the following late adverse outcomes (occurring after the third birthday): death, first overt stroke, use of disease-modifying therapy, and hospitalizations for pain events and ACS. None of the early events predicted death or stroke. Early pain and ACS both predicted a modest, temporary increase in the number of later painful episodes, but early ACS strongly increased the odds of more frequent ACS throughout childhood. Dactylitis had limited utility as a predictor. Although we still lack a useful prognostic framework for young children with SS, those who experience early ACS might be candidates for higher risk interventions to mitigate or cure their disease.
Introduction
Homozygous sickle cell anemia (SS) is the most severe form of sickle cell disease (SCD), but it is phenotypically variable, and the course of disease is difficult to predict.1 Some individuals with SS have frequent vaso-occlusive complications and die prematurely,2,3 whereas others have few problems and a normal life span.4,5 Many genes both within and outside the β-globin locus modify the phenotype of SS, but we have only a limited understanding of a few such genotype-phenotype interactions.6,7
The prediction in very early childhood of later severe disease would permit risk-based counseling for families and could justify the early use of disease-modifying or curative interventions, such as hydroxyurea (HU), chronic transfusions (CTs), or stem-cell transplantation (SCT). The hazards of these treatments vary greatly, especially in comparison with preventive and supportive management alone.8 Accurate and reliable early predictors would provide the opportunity to better balance the risks of these interventions with risks of the disease itself.
Vaso-occlusive complications have been studied as predictors of SCD severity. Platt and colleagues reported for the Cooperative Study of Sickle Cell Disease (CSSCD) that the rate of pain—itself a measure of clinical disease severity—correlated with early mortality in adults.3 Likewise, Miller and colleagues for the CSSCD showed that children who, in the first year of life, experienced dactylitis, a painful vaso-occlusive complication of SCD, were more likely to have adverse outcomes in later childhood, including death, stroke, frequent pain, and recurrent acute chest syndrome (ACS).9 This finding from the multicenter CSSCD infant cohort has not been confirmed in other cohorts. The prognostic significance of ACS and painful episodes other than dactylitis in early childhood is not known.
We sought to test the assumption of many clinicians that children who experienced vaso-occlusive complications in very early life, not limited to dactylitis, were destined to have severe SCD in later childhood. To this end, we studied the Dallas Newborn Cohort10 to determine if hospitalizations for painful events, dactylitis, or ACS during the first 3 years of life were associated with death, stroke, or other measures of severe disease between 3 and 20 years of age. We hypothesized that hospitalizations for early vaso-occlusive complications did not clearly predict later adverse outcomes.
Patients, materials, and methods
Cohort members
This is a retrospective analysis of the Dallas Newborn Cohort,10 whose members are tracked prospectively in our center's comprehensive sickle cell disease database. This newborn inception cohort includes the 4 common SCD genotypes: SS, sickle-hemoglobin C disease (SC), sickle-β0-thalassemia (Sβ0), and sickle-β+-thalassemia (Sβ+). It is unique among SCD cohorts because Texas newborn screening for hemoglobinopathies identified all members, and all subjects with SS or Sβ0 were prescribed prophylactic penicillin until at least 5 years of age. Accrual began in 1983 and is ongoing. The cohort now includes 826 subjects and provides 7275 patient-years of observation. This analysis includes follow-up through February 9, 2005.
We studied every member of the cohort who was younger than 20 years of age, who had a diagnosis of either SS or Sβ0, whose first visit to our center was in the first year of life, who were at least 5 years of age at the time of their last clinical encounter in our center, and who had complete records available for review. The percentage of subjects who were lost to follow-up in the entire cohort was 3.5%.10 We studied individuals with SS and Sβ0 as a single group because of the known clinical similarity of the diseases, the very small number in the Sβ0 subgroup, and the difficulty differentiating the 2 diseases using standard clinical and laboratory assessments. The Institutional Review Board of the University of Texas Southwestern Medical Center approved the use of the clinical database for this project, and waived the requirement for written, informed consent.
Early predictors
We defined “early” vaso-occlusive complications to be those that occurred during the first 3 years of life (before the third birthday). We defined this cut-off before the data were collected and analyzed, and we chose it because pain events other than dactylitis and ACS are uncommon in the first year of life. Further, 2 additional years of “early” follow-up would provide a larger number of early vaso-occlusive complications to study as predictors. This definition is also clinically meaningful because it permitted us to study early disease manifestations before most irreversible organ damage, other than splenic dysfunction, was likely to have occurred in most children.
We defined 3 potential “early” vaso-occlusive predictors: (1) hospitalization for a painful episode (excluding dactylitis), (2) hospitalization for dactylitis, and (3) hospitalization for ACS. These predictors were chosen because they are common, clinically overt manifestations of SCD whose occurrence currently informs clinical decision-making. We studied only inpatient episodes because of the design of our clinical database; we systematically track the discharge diagnoses for all subjects' hospitalizations but not outpatient painful events. Early hospitalizations were identified by query of the database and review of selected medical records, and they were categorized by the subject's age at the time of occurrence. Reasons for hospitalization (discharge diagnoses) had been recorded at the time of each event. If the reason for any single hospitalization was recorded in the database as both painful event and ACS (eg, painful event complicated by ACS), the hospitalization was counted as an episode of ACS only (because ACS was considered to be the more severe complication). In our clinical practice, we have defined ACS as an acute pulmonary illness in a person who has SCD that is characterized by a new radiographic pulmonary infiltrate and some combination of fever, hypoxemia, thoracic pain, and signs and symptoms of respiratory illness.
Late outcomes
We studied the associations of these early predictors with the following “late” outcomes: (1) a death of any cause; (2) a clinically overt first stroke; (3) the use of HU, CTs, or SCT; (4) the numbers and rates of hospitalization for painful episodes; and (5) the numbers and rates of hospitalization for ACS. By definition, all “late” outcomes occurred on or after the third birthday. These outcomes were identified by query of the database and review of selected medical records, and they were categorized by the subject's age at the time of occurrence. If the reason for any single hospitalization was recorded as both painful event and ACS, the hospitalization was counted as an episode of ACS only. Events up to age 20 years were included.
To quantify late pain and ACS, we recorded all subjects' hospitalizations for late pain and ACS that occurred on or after the third birthday through the last clinical encounter or the start date of a disease-modifying therapy (HU, CT, or SCT), whichever occurred first. Only episodes of pain or ACS that occurred before the start of any disease-modifying therapy (HU, CT, or SCT) were included because such treatments are expected to alter the natural frequency of these events. The use of a disease-modifying therapy was also studied as a surrogate measure of disease severity.
For purposes of this study, we defined CT to be a program of regularly scheduled red blood cell transfusions of at least 6 months' duration given for the prevention of complications of SCD. In our center, CT is used to prevent recurrent stroke and, in select patients, to prevent frequent pain or recurrent ACS. At the time of this analysis, we had not commonly used CT for primary stroke prevention. We defined HU use as any administration of HU without regard to duration of treatment or a subject's adherence. HU is prescribed on an individual basis for patients who have frequent pain or recurrent ACS, or when CT is not preferred by the patient and family. To date, we have performed SCT only for prevention of recurrent stroke.
Statistical analysis
Bivariate analysis.
We compared the frequencies of the late categoric outcomes (death, stroke, and use of disease-modifying therapy) between the early predictor-positive and predictor-negative groups by 2-sided Fisher exact tests. For these bivariate analyses (predictor-outcome groupings), we studied the occurrence of early vaso-occlusive complications as binary variables (namely, the occurrence or not of at least one complication). The predictive utility of multiple early hospitalizations was tested in the multivariate analysis (see “Multivariate analysis”). For statistically significant associations, we calculated odds ratios (ORs) with 95% confidence intervals (95% CIs).
We assessed differences in the occurrence of late pain and ACS between the early predictor-positive and predictor-negative groups using means, mean differences, and 2-sided t tests. To control for subjects' different lengths of follow-up, we classified each episode of pain and ACS by the subject's age at the time of its occurrence. Episodes were then categorized into non-overlapping 2-year intervals defined by age of occurrence (3-4.99, 5-6.99, 7-8.99, 9-10.99, 11-12.99, 13-14.99, 15-16.99, and 17-18.99 years of age). To additionally provide a meaningful number of events for analysis these intervals were combined cumulatively (3-6.99, 3-8.99, 3-10.99, 3-12.99, 3-14.99, 3-16.99, and 3-18.99 years of age), and the mean number of episodes of pain or ACS was calculated in each interval. The pain and ACS episodes experienced by an individual were included in one or more of these cumulative intervals only if he or she was alive and evaluable in that interval. For the bivariate analyses, P < .01 was considered statistically significant to partially control for multiple comparisons.
Multivariate analysis.
We used binary logistic regression with backward model building to predict late outcomes from early vaso-occlusive events. Here we studied the number of early vaso-occlusive complications as a continuous variable (to assess the contributions of multiple early events), unlike in bivariate analysis, wherein we considered only their occurrence or not. We included these variables as predictors: early pain (excluding dactylitis), early dactylitis, early ACS, sex, and genotype (SS or Sβ0). Logistic regression models were constructed for the following outcomes: (1) death or stroke, (2) occurrence of late painful episodes, (3) occurrence of late ACS, and (4) the composite occurrence of late pain and ACS.
For each subject, we calculated rates of late pain and ACS that included all events in the interval between the third birthday and the last clinical encounter, or the start date of a disease-modifying therapy (HU, CT, or SCT), whichever occurred first. Because the rates of late pain and ACS were not normally distributed, and could not be normalized by transformation, multiple linear regression was not tenable. Therefore, the rates of late pain and ACS were made dichotomous for binary logistic regression in 2 different ways: (1) dichotomous at the median rate for the whole sample (rate ≤ median versus rate > median), and [2] dichotomous at one episode per year (rate < 1 episode/y versus ≥ 1 episode/y). The cut-off of one episode per year was chosen in addition to the median because some clinicians consider it to be a clinically meaningful threshold that denotes disease severity. We calculated ORs and a 95% CIs for statistically significant predictors in the model. We did not attempt to predict the late use of HU, CT, or SCT, because bivariate analysis showed that CT and SCT were not associated with early vaso-occlusive complications, and we believed that HU use was largely a surrogate marker for late pain and ACS.
All data were analyzed using SPSS 13.0 statistical software (SPSS, Chicago, IL). Figures were generated using GraphPad Prism 4.0c (GraphPad Software, San Diego, CA).
Results
There were 264 subjects (55% male, 97% SS) in the analysis. The mean age at first visit was 4.1 months (SD, 2.3 months), and the mean length of follow-up was 12.1 years (SD, 4.3 years). Twenty of 264 (7.6%) were classified as lost to follow-up.
We found no statistically significant differences between males and females or between SS and Sβ0 genotypes for any of the early or late events (data not shown). All early and late events are reported in Table 1. Two subjects died of pneumococcal sepsis (5 and 6 years of age), and one subject each died of ACS (5 years), complications of multiple strokes (7 years), and Down syndrome with multiple congenital anomalies (5 years).
Table 1.
Early predictors and late outcomes
| Early and late events | No. of events | Children who experienced events |
|
|---|---|---|---|
| No. | Percent* | ||
| Early predictors | |||
| Painful events† | 89 | 54 | 20.5 |
| Dactylitis | 18 | 16 | 6.1 |
| ACS | 151 | 86 | 32.6 |
| Late outcomes | |||
| Death of any cause | 5 | 5 | 1.9 |
| First clinically overt stroke | 30 | 30 | 11.4 |
| Use of HU, CT, or SCT | 78 | 66 | 25 |
| Painful events | 642 | 130‡ | 49.2‡ |
| ACS | 410 | 150‡ | 56.8‡ |
Total of 264 subjects.
Excluding dactylitis.
Subjects who experienced one or more events.
Bivariate analysis
Associations of early events with death and stroke.
We found that hospitalization for pain, dactylitis, or ACS in the first 3 years of life did not predict later death or clinically overt stroke (Table 2). Notably, none of the subjects who had dactylitis later died or had a stroke. This superficially “protective” effect of dactylitis was not statistically significant, so a true protective effect should not be inferred. Because death and stroke were infrequent outcomes, we also studied these 2 events as a single, composite outcome and found, similarly, no associations with any of the early vaso-occlusive complications.
Table 2.
Associations between early predictors and late death, stroke, or the use of HU, CT, or SCT
| Frequency of late outcomes | Occurrence of early predictors |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Painful episode |
Dactylitis |
ACS |
|||||||
| Yes | No | P | Yes | No | P | Yes | No | P | |
| Death | 1.9 | 1.9 | >.999 | 0 | 2.2 | >.999 | 2.4 | 1.7 | .666 |
| Stroke, % | 13.2 | 10.2 | .619 | 0 | 11.6 | .231 | 14.1 | 9.8 | .303 |
| Death or stroke, % | 13.2 | 12.1 | .817 | 0 | 13.2 | .233 | 15.3 | 11.6 | .43 |
| HU, % | 26.4 | 9.2 | .002 | 12.5 | 12.4 | >.999 | 25.9 | 5.8 | <.001 |
| CT, % | 17 | 14.1 | .663 | 6.3 | 15.3 | .48 | 20 | 12.7 | .141 |
| SCT, % | 0 | 0.5 | >.999 | 0 | 0.4 | >.999 | 0 | 0.6 | >.999 |
The percentage of subjects who experienced each outcome is shown based on the occurrence or not of each early predictor. P values are calculated for each comparison of percentages (yes versus no) using the Fisher exact test.
Associations of early events with use of HU, CT, or SCT.
We found that early vaso-occlusive complications predicted only the later prescription of HU (Table 2). The occurrence of early painful episodes gave an OR for later HU use of 3.53 (95% CI, 1.63-7.64). Early ACS gave an OR for HU use of 5.69 (95% CI, 2.55-12.69). However, children who were hospitalized only for early dactylitis, and not for pain or ACS, were not more likely to be treated with HU (P > .999; Table 2). Early vaso-occlusive complications were not associated with later initiation of CT therapy. The single subject who underwent SCT to prevent recurrent stroke was not hospitalized for pain, dactylitis, or ACS in the first 3 years of life.
Associations of early events with late pain.
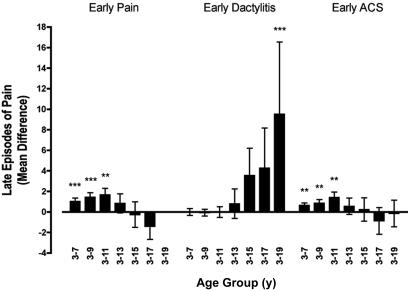
As shown in Figure 1, we found that subjects who were hospitalized for early painful episodes, compared to those who were not, had a small increase in the mean number of late painful episodes through approximately 11 years of age, but this effect was not seen in longer periods of follow-up. Similarly, subjects who were hospitalized for early ACS had a slightly higher mean number of late painful episodes through approximately 11 years of age, but not beyond. Dactylitis was not associated with more frequent late pain considering periods of follow-up that were less than 18 years. However, for the few subjects who had follow-up of 18 years or longer (n = 38), dactylitis was associated with a statistically significantly increased number of late painful episodes over the entire period of follow-up (Figure 1).
Figure 1.
Associations between early predictors and late episodes of pain. Depicted here are the mean differences in the number of late painful episodes for groups defined by the occurrence or not of each of the 3 early predictors (whiskers are SEs). Mean difference = (mean number of painful episodes in predictor-positive group) − (mean number of painful episodes in predictor-negative group). The mean differences are shown for cumulative, overlapping intervals of follow-up beginning with the third birthday. **P < .01; ***P < .001.
Associations of early events with late ACS.
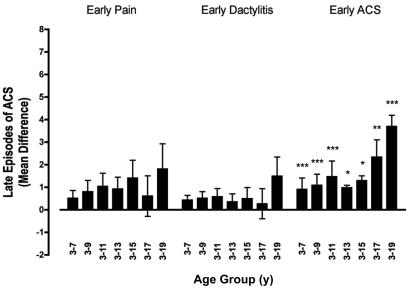
There were no associations between early pain or dactylitis and the mean number of late episodes of ACS (Figure 2). However, children who were hospitalized for early ACS had an increased mean number of late ACS episodes throughout all childhood. The mean difference was as high as 3.7 episodes of late ACS when comparing subjects positive or negative for early ACS (Figure 2).
Figure 2.
Associations between early predictors and late episodes of ACS. Depicted here are the mean differences in the number of late episodes of ACS for groups defined by the occurrence or not of each of the 3 early predictors (whiskers are SEs). Mean difference = (mean number of ACS episodes in predictor positive group) − (mean number of ACS episodes in predictor negative group). The mean differences are shown for cumulative, overlapping intervals of follow-up beginning with the third birthday *P < .05; **P < .01; ***P < .001.
Multivariate analysis
Death and stroke.
Neither alone nor in any combination did early pain, early dactylitis, early ACS, sex, and genotype predict death and stroke. This was consistent with the bivariate analyses.
Late pain.
Early vaso-occlusive complications predicted the rate of late hospitalizations for pain if the cut-off was set at the median rate for all subjects (≤ .071 versus > 0.071 episodes of pain/y), but only 6% of the variability in late pain was explained by this model (Table 3). If this cut-off was set instead at one episode per year, then an early vaso-occlusive complication was not predictive, but early ACS was; however, this model explained only 5% of the variability in late pain (Table 3). Early dactylitis, sex, and genotype were not predictive of the occurrence of late pain. Thus, these models poorly predicted late pain.
Table 3.
Summary of predictive variables in the multivariate models
| Early predictive variables | Late rates dichotomous at median no. of events/y |
Late rates dichotomous at 1 event/y |
||||||
|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | R2 | P | OR | 95% CI | R2 | |
| For outcome: late pain | ||||||||
| Early pain, nondactylitis | .002 | 1.93 | 1.26-2.95 | 0.060 | — | — | — | |
| Early ACS | — | — | — | .009 | 1.57 | 1.12-2.21 | 0.053 | |
| For outcome: late ACS | ||||||||
| Early ACS | <.001 | 2.23 | 1.52-3.29 | 0.149 | <.001 | 2.51 | 1.67-3.86 | 0.236 |
| For outcome: late pain and ACS | ||||||||
| Early ACS | .001 | 1.92 | 1.36-2.70 | 0.152 | <.001 | 1.93 | 1.42-2.62 | 0.123 |
ORs are calculated by binary logistic regression analysis, which adjusts for sex, genotype, early nondactylitis pain, early dactylitis, and early ACS episodes as appropriate. ORs correspond to a single unit increase in the predictive variable (an episode of ACS or pain, where appropriate). Nagelkerke R2 values are reported as coefficients of determination and indicate the proportion of variability in the outcomes explained by the models.
— indicates not predictive.
Late ACS.
The only statistically significant predictor of the occurrence of late ACS was early ACS itself, whether considering the cut-off rate at the median for all subjects (≤ 0.1125 versus > 0.1125 episodes of ACS/y) or one episode per year (Table 3). These models explained 15% and 24%, respectively, of the variability in late ACS (Table 3). Early pain, early dactylitis, sex, and genotype did not predict the occurrence of late ACS.
Late pain and ACS.
The only statistically significant predictor of the occurrence of late pain and ACS (the composite outcome) was early ACS, whether considering the cut-off rate at the median (≤ 0.25 versus > 0.25 episodes of pain and ACS/y) or one episode per year (Table 3). These models explained 15% and 12%, respectively, of the variability in late pain and ACS (Table 3). Early pain, early dactylitis, sex, and genotype did not predict the occurrence of late pain and ACS.
Discussion
It is difficult to predict severe SCD during childhood, at least in part, because it is difficult to define severe disease.1 Two objective indicators of disease severity are death and stroke.3,11 Fortunately, both are becoming rare in children, given the diminishing mortality from invasive bacterial infection and splenic sequestration, as well as the prevention of first stroke using transcranial Doppler ultrasonography (TCD) to direct the initiation of chronic red blood cell transfusions. Beyond these 2 objective outcomes, investigators and clinicians have relied on the enumeration of hospitalizations for vaso-occlusive complications as an index of disease severity, however imperfect. For example, the inclusion criteria for therapeutic clinical trials typically require frequent painful episodes or recurrent ACS,12,13 but the definitions of these events and the particular criteria used to denote severity are not uniform. In the absence of good alternatives, we also studied these usual measures of disease severity here, but we did not arbitrarily define a rate of pain or ACS that was “severe.”
We showed that hospitalizations for vaso-occlusive complica tions during the first 3 years of life did not predict the most objective markers of severe SCD—death and stroke. The low incidence of death in the Dallas Newborn Cohort reflects universal newborn screening and the uniform use of prophylactic penicillin. The CSSCD showed no association between overall pain rate and stroke,11 and we found no increased risk of stroke from pain events that occur in very early childhood. The incidence of stroke in the cohort at the time of this analysis, however, does not reflect the impact of primary stroke prevention, because our center only recently instituted a TCD screening program. Fortunately, death and stroke are becoming rare in children with SCD, so the sickle cell community is now challenged to use other measures of severe disease.
We studied the use of a disease-modifying therapy as a proxy for severe disease and found that early vaso-occlusive complications predicted neither the later use of CT nor SCT. This is not surprising because stroke is the most common indication for both CT and SCT. As such, neither stroke nor its proxies, CT and SCT, was predicted by early vaso-occlusive complications. On the contrary, early vaso-occlusive complications were associated with more frequent later use of HU. Although this association is neither surprising nor wholly unexpected, it should also be interpreted with caution because the decision to prescribe HU to older children was likely biased by the knowledge that they had experienced early complications. The imputation of disease-modifying therapy as a surrogate marker of disease severity is also influenced by the varying prescribing practices of physicians. Therefore, we also studied the frequency of late pain and ACS as more direct, less biased indicators of disease severity.
We found that hospitalizations for pain or ACS in the first 3 years of life predicted only a small and temporary increase in later painful events. Moreover, the multivariate models could explain only a very small fraction of the variability in late pain. It is unclear if such a modest increase in pain is clinically significant. Neither early dactylitis nor pain was associated with late ACS, but subjects who had early ACS were more likely to have recurrent ACS throughout childhood. Although factors other than early ACS, which are unknown, unmeasured, or both, accounted for much of the variability in late ACS (Table 3), we infer a distinct susceptibility to recurrent ACS. Perhaps ACS-related pulmonary injury predisposes to subsequent ACS. An early study by Powars et al suggested that recurrent ACS caused chronic sickle cell lung disease.14 However, ACS in young children tends to be mild,15 and recent studies indicate that neither pulmonary hypertension16 nor steady-state oxyhemoglobin desaturation17 is associated with prior ACS. Another interpretation is that the observed effect is due to asthma and not ACS itself, but it is difficult to separate these 2 disease processes given the definition of ACS as an acute pulmonary illness in an individual with SCD, and that bronchospasm and hypoventilation can precipitate ACS. So, asthma could be the cause or consequence of ACS, or the 2 could share a common pathophysiology. Indeed, Boyd and colleagues reported an association between asthma and ACS,18 and Morris et al found similar alterations of arginine metabolism in both asthma and ACS.19,20
In the CSSCD infant cohort, the occurrence of dactylitis in the first year of life conferred a relative risk of 2.6 for severe disease.9 Although our investigation was not designed to refute or validate this particular CSSCD finding, which included both inpatient and outpatient episodes of dactylitis, we found in contrast that hospitalization for dactylitis in the first 3 years of life had limited prognostic utility in the Dallas Newborn Cohort. Hospitalization for dactylitis did not predict death, overt stroke, the use of a disease-modifying therapy, or later ACS. Early dactylitis was also not associated with late pain, except in one correlation with the 38 subjects who had 18 years or more of follow-up. This is an interesting observation that differs from the predictive pattern of early pain and ACS for later pain events (Figure 1). Perhaps early dactylitis is associated with an increase in late pain over a long period of follow-up, but the size of this subgroup is small and the variability of this particular estimate of mean difference is too high to justify strong inferences.
This retrospective analysis has a number of limitations. First, we studied only hospitalizations for pain, dactylitis, and ACS. Many episodes of pain and dactylitis are managed only at home and not in a hospital or an outpatient facility, and we did not systematically record these outpatient events. Although many factors unrelated to SCD, such as family situation and coping skills, determine whether a patient is admitted to a hospital or not, generally, the more severe episodes are managed by hospitalization. Thus, we have studied the associations among the relatively more severe of the early and late manifestations of SCD. Second, the missing data of patients who were lost to follow-up and censored at their last clinical encounter may have biased the outcomes in either direction; however, there were not many lost subjects. Third, there are numerous clinical, laboratory, and psychosocial factors that could influence outcomes in SCD that we did not consider here. We could not reasonably analyze so many predictors in a cohort of this size, however large. Instead, we chose a priori a small number of predictors and outcomes to test a simple clinical question, and we realize that there may be confounding variables that we have neither measured nor controlled for. Fourth, it is possible that therapeutic improvements over the period of observation in this study could have decreased the predictive nature of the early events, although such an effect would probably be small given the mean follow-up of 12 years. Fifth, the use of disease-modifying interventions in older individuals, which would decrease the frequency of late pain events, might partly account for the lack of association between early events and late events after age 11 years. The overall rate of pain in the study population increased after age 11 years and remained higher until 17 years (data not shown), so such confounding might be modest, but it cannot be dismissed. Moreover, such a censoring effect is not apparent in dactylitis data. Lastly, even though we nominally controlled for multiple comparisons, some of the statistically significant associations we found may still have been type I errors.
In summary, we have shown that ACS during the first 3 years of life predicts recurrent ACS throughout childhood. A potential approach to reduce this risk may be to screen for and aggressively manage any asthma-like disease in children who have early ACS, and this strategy should be the focus of further research. The prognostic significance of other early vaso-occlusive complications is quite limited. Notably, none of the early events predicted the most objective manifestations of severe SCD—death and stroke. Although a multitude of predictors of outcome have been studied,1 we still lack a useful prognostic framework for infants and young children with SCD. Until we are better at prognostication, we should not endorse the use of a disease-modifying therapy in very young children based solely on the occurrence of a few episodes of early pain or dactylitis. Children who experience ACS in the first 3 years of life, however, might be candidates for early, higher risk interventions to mitigate or cure SCD.
Acknowledgment
This work was supported partly by a grant from the National Institutes of Health (U54 HL 70588).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Quinn CT, Miller ST. Risk factors and prediction of outcomes in children and adolescents who have sickle cell anemia. Hematol Oncol Clin North Am. 2004;18:1339–1354. doi: 10.1016/j.hoc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 4.Serjeant GR, Richards R, Barbor PR, Milner PF. Relatively benign sickle-cell anaemia in 60 patients aged over 30 in the West Indies. Br Med J. 1968;3:86–91. doi: 10.1136/bmj.3.5610.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg MH, Dreiling BJ, Morrison FS, Necheles TF. Mild sickle cell disease; clinical and laboratory studies. JAMA. 1973;224:317–321. [PubMed] [Google Scholar]
- 6.Steinberg MH, Rodgers GP. Pathophysiology of sickle cell disease: role of cellular and genetic modifiers. Semin Hematol. 2001;38:299–306. doi: 10.1016/s0037-1963(01)90023-x. [DOI] [PubMed] [Google Scholar]
- 7.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 8.Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526–535. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 9.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 10.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103:4023–4027. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 12.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia: investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 13.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 14.Powars D, Weidman JA, Odom-Maryon T, Niland JC, Johnson C. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine. 1988;67:66–76. [PubMed] [Google Scholar]
- 15.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 16.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 17.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131:129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 19.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22:515–520. doi: 10.1097/00043426-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]