Abstract
Cellular turnover is associated with exposure of surface phosphatidylserine (PS) in apoptotic cells, leading to their phagocytic recognition and removal. But recent studies indicate that surface PS exposure is not always associated with apoptosis. Here we show that several members of the human galectin family of glycan binding proteins (galectins-1, -2, and -4) induce PS exposure in a carbohydrate-dependent fashion in activated, but not resting, human neutrophils and in several leukocyte cell lines. PS exposure is not associated with apoptosis in activated neutrophils. The exposure of PS in cell lines treated with these galectins is sustained and does not affect cell viability. Unexpectedly, these galectins bind well to activated T lymphocytes, but do not induce either PS exposure or apoptosis, indicating that galectin's effects are cell specific. These results suggest novel immunoregulatory contribution of galectins in regulating leukocyte turnover independently of apoptosis.
Introduction
The physiologic causes of leukocyte turnover in homeostasis and during disease conditions are not well understood. It is believed that leukocytes are partly eliminated by programmed cell death or apoptosis1-4 through phagocytosis by macrophages, dendritic cells, or neighboring cells.5,6 The efficient removal of dying cells is important in homeostasis, since it limits accumulation of cellular debris that could be potentially immunogenic or toxic.7-9 However, the role of apoptosis in removing large numbers of cells in inflammation and during the resolution phase remains uncertain. Although human neutrophils undergo apoptosis spontaneously when cultured in vitro, the role of apoptosis in regulating neutrophil turnover in vivo is unclear.6,10-12 Apoptosis impairs cellular functions and might impair proinflammatory functions of neutrophils.13 However, excessive neutrophil influx with loss of membrane integrity during late apoptotic events could contribute to neutrophil-mediated injury of surrounding viable parenchymal tissue.14 Exuberant apoptosis may therefore be proinflammatory instead of anti-inflammatory,15-17 presumably due to the release of cellular contents prior to phagocytic removal. In this regard, nonapoptotic neutrophils can be cleared by phagocytosis in vivo.18 Finally, factors known to induce or to block apoptosis, such as ligation of Fas/FasL and expression of bcl-2, respectively, do not alter neutrophil turnover in mouse models.19-22 These studies suggest that unidentified factors may be involved in the phagocytic removal of viable, rather than apoptotic, cells.
The removal of apoptotic cells occurs partly through tethering to phagocytic cells due to receptor-ligand interactions involving recognition of phosphatidylserine (PS) exposed on the surfaces of apoptotic cells.23 Surface PS is recognized by a defined PS-receptor in macrophages24 and by other receptors.25-27 However, PS exposure in leukocytes can occur independently of apoptosis.28,29 Thus, factors that induce PS exposure independently of apoptosis may be involved in leukocyte turnover.
Recently, we showed that galectin-1 (Gal-1), a prototypical homodimeric member (subunit ∼ 14.5 kDa) of the galectin family, which has immunomodulatory functions,30-34 can induce PS exposure in activated, but not resting, neutrophils, independently of cell death, while concomitantly rendering them sensitive to phagocytic recognition and removal.35 We also found that the signaling pathway of Gal-1 in activated neutrophils is unique and involves elevations of cytosolic Ca2+ and mobilization of PS through the actions of Src kinases and phospholipase Cγ.36
However, there are many conflicting reports about galectin's effects on leukocytes. Several groups have reported that Gal-1 induces apoptosis along with PS exposure in vitro of activated T lymphocytes and several T-leukemic cell lines.37-39 It has also been reported that human Gal-2, a protein structurally related to Gal-1, induces T-cell apoptosis in vitro.40 By contrast, Gal-4, another member of the galectin family with 2 tandemly-repeated carbohydrate-recognition domains, stimulates IL-6 production in CD4+ T cells without accompanying apoptosis.41 Thus, it is important to reinvestigate the activities of these galectins on activated human neutrophils and human T cells.
Here, we report that human galectins-1, -2, and -4 induce PS exposure in activated, but not resting, human neutrophils in a carbohydrate-dependent fashion independently of apoptosis. By contrast, Gal-1 does not induce apoptotic cell death or PS exposure in activated T cells, although it does induce PS exposure in T-leukemic MOLT-4 and CEM cell lines. Unexpectedly, we found that Gal-1 could induce PS exposure in T cells only when the cells were pretreated with dithiothreitol (DTT), a common reducing agent used in many studies assessing the proapoptotic potential of Gal-1 toward T cells. These studies indicate that activated neutrophils, but not activated T lymphocytes, possess unique receptors and signaling pathways for some galectin family members, which trigger PS exposure independently of apoptosis and may be important in regulating neutrophil turnover.
Materials and methods
Preparation of the recombinant forms of human Gal-1, C2S-Gal-1, Gal-2, and Gal-4
The recombinant forms of human galectins-1, -2, and -4 were prepared by established procedures.35,42,43 The C2S-Gal-1 mutant was generated from human Gal-1 by site-directed mutagenesis as described.44,45 Expression and isolation of each of the recombinant proteins were accomplished as described.35,46 To ensure that galectin samples were endotoxin free, Detoxi-Gel Endotoxin removing gel (Pierce Biotechnology, Rockford, IL) was used. Endotoxin levels measured using the Limulus Amebocyte Lysate endotoxin detection kit (Cambrex, Rutherford, NJ) demonstrated that the final preparations of galectins used in experiments contained less than 0.04 ng LPS/mL. Derivatives of each galectin with ALEXA Fluor 488 C5-maleimide (Invitrogen, Carlsbad, CA) were prepared as described.47
Cell lines, isolation of human cells, and flow cytometry
Cell culture, isolation of human cells, cell-growth kinetics, and treatment with apoptosis-inducing agents were according to published procedures.35,36 Incubation of cells with galectins and the use of DTT were generally performed as described.35,39,48,49 Specifically, for T-cell isolation and activation, fresh heparinized blood isolated from healthy donors was diluted equal volume with PBS (Hyclone, Logan, UT) followed by density gradient centrifugation using Ficoll-Hypaque. Following centrifugation, the upper layer of plasma and platelets was carefully removed, followed by washing cells 3 times in HBSS. The cells were then resuspended at 1 × 106 in complete RPMI (RPMI 1640, 10% fetal bovine serum, glutamine [2 mM], penicillin [100 mU/mL], and streptomycin [100 μg/mL]). T-cell activation was accomplished treating cells with 8 μg/mL PHA for approximately 4 days at 37°C in complete RPMI. Flow cytometric analyses were performed on cells dissociated by treatment with hapten sugars,35 with the exception that thiodigalactoside (TDG) rather than lactose was required to dissociate cells incubated with Gal-4. Cell fragmentation following treatments was determined by analyzing changes in the forward-scatter and side-scatter properties of cells using a flow cytometer.49 DNA fragmentation was assessed by the TUNEL assay or by analysis of hypodiploid DNA content.49,50 All samples were analyzed using Cell Quest software (Becton Dickinson, Mountain View, CA) with a minimum of 10 000 counts/sample. Each data set is representative of at least 3 independent experiments.
Results
Gal-2 and Gal-4 induce PS exposure in MOLT-4 and HL60 cells
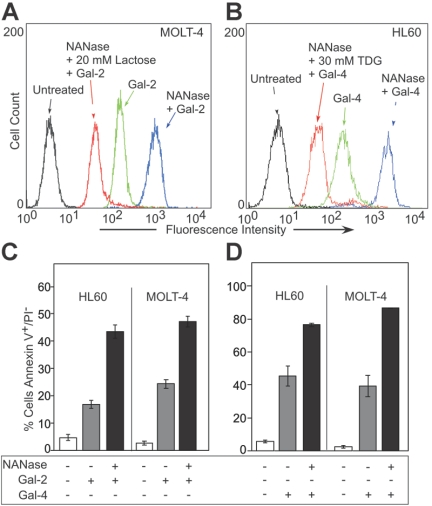
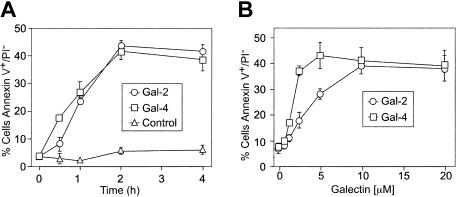
MOLT-4 cells and HL60 cells were treated with either Gal-2 or Gal-4, followed by analysis for PS exposure by staining with FITC–annexin-V using flow cytometric analysis. Annexin-V is the commonly used reagent to detect surface PS, since it specifically binds PS with high affinity51,52 and can interfere in vivo with recognition and uptake of PS-expressing apoptotic cells.53 Both Gal-2 and Gal-4 induced surface PS exposure in both HL60 and MOLT-4 cells (Table 1) The inclusion of 20 mM lactose with Gal-2 or 30 mM TDG with Gal-4 abrogated the induction of PS exposure, whereas sucrose had no effect (Table 1). Of importance, the binding of Gal-2 (Figure 1A) and Gal-4 (Figure 1B) to leukocytes was also dependent on carbohydrate recognition. Pretreatment of either cell line with neuraminidase significantly enhanced both the binding of Gal-2 (Figure 1A) and Gal-4 (Figure 1B) and the ability of Gal-2 (Figure 1C) and Gal-4 (Figure 1D) to redistribute PS. PS exposure was maximal within approximately 2 hours in HL60 cells treated with 10 μM Gal-2 or 3 μM Gal-4 (Figure 2A). Galectin signaling in HL60 cells was dose dependent; Gal-4 was slightly more potent, inducing maximal effects around 2.5 μM, whereas at least 10 μM Gal-2 was required to obtain maximal effects (Figure 2B). These results demonstrate that binding of Gal-2 and Gal-4 to these cells induces PS exposure in a carbohydrate- and dose-dependent fashion.
Table 1.
Gal-2 and Gal-4 induce PS exposure in MOLT-4 and HL60 cells in a carbohydrate-dependent fashion
| Treatment | Annexin-V+/PI−, %* | |
|---|---|---|
| MOLT-4 | HL60 | |
| Control | 2 | 4 |
| Gal-2 | 47 | 49 |
| Gal-2 + lactose† | 6 | 8 |
| Gal-2 + sucrose | 47 | 49 |
| Gal-4 | 37 | 68 |
| Gal-4 + TDG‡ | 4 | 10 |
| Gal-4 + sucrose | 39 | 63 |
MOLT-4 or HL60 cells were treated with 10 mM Gal-2 or 3 mM Gal-4 for 4 hours followed by detection for PS exposure. The data shown represent averages of duplicate determinations where the SE was less than 10%.
Gal-2 was coincubated with either 20 mM lactose or 20 mM sucrose as indicated.
Gal-4 was coincubated with either 30 mM TDG or 30 mM sucrose as indicated.
Figure 1.
Gal-2 and Gal-4 induced PS exposure in MOLT-4 and HL60 cells. (A) MOLT-4 cells were incubated with ALEXA 488–Gal-2, and (B) HL60 cells were incubated with ALEXA 488–Gal-4, followed by flow cytometric analysis. NANase indicates prior treatment of cells with A urefaciens neuraminidase (100 mU) for 1 hour at 37°C. (C-D) HL60 cells or MOLT-4 cells were incubated with either 10 μM Gal-2 (C) or 3 μM Gal-4 (D) for 4 hours followed by flow cytometric analysis for PS externalization. NANase indicates prior treatment of cells with A urefaciens neuraminidase (100 mU) for 1 hour at 37°C. Error bars represent the standard deviation of duplicate samples.
Figure 2.
Kinetics and concentration dependence of Gal-2– and Gal-4–induced PS exposure. (A) Desialylated HL60 cells (dsHL60) cells were incubated with 3 μM Gal-4 or 10 μM Gal-2 for the indicated times followed by detection of PS externalization. Control indicates the inclusion of PBS alone. (B) dsHL60 cells were incubated with Gal-2 or Gal-4 at each indicated concentration for 4 hours followed by detection for PS externalization. Results shown are the averages of duplicate analyses and are representative of at least 2 separate experiments. Error bars indicate the standard deviation of duplicate samples.
Leukocytes induced to expose PS by Gal-2 and Gal-4 fail to display other known features of programmed cell death
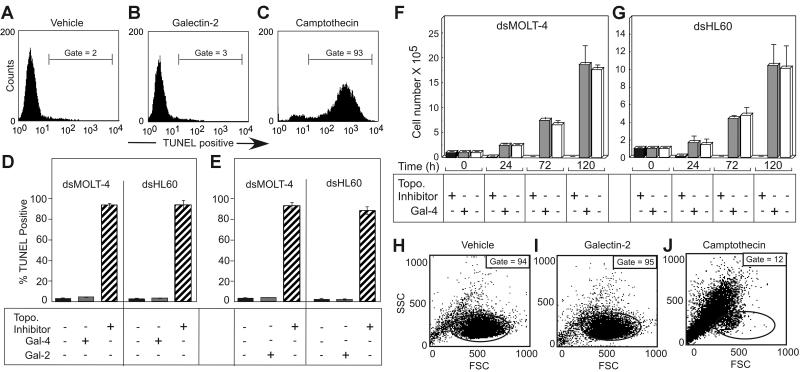
We next examined whether the induction of PS exposure by these galectins was associated with loss of cell viability or apoptosis. MOLT-4 or HL60 cells were treated for 18 hours with either PBS (Figure 3A,D-E), 5 μM Gal-4 (Figure 3D), or 10 μM Gal-2 (Figure 3B,E), followed by the TUNEL assay to detect DNA fragmentation. Known inducers of apoptosis, camptothecin and etoposide in HL60 and MOLT-4 cells, respectively, produced extensive DNA fragmentation over this time period (Figure 3C-E). By contrast, no significant DNA fragmentation was observed in galectin-treated cells (Figure 3B,D-E). Potential DNA fragmentation induced by galectins was also assessed using hypodiploid DNA content, as determined by PI staining. No increases in hypodiploid DNA content were observed following treatment of cells with either Gal-2 or Gal-4, although camptothecin- and etoposide-treated cells displayed extensive hypodiploid DNA content when treated over this same time period (data not shown).
Figure 3.
Galectins induce PS exposure in cell lines without accompanying apoptosis. Representative histograms of dsHL60 cells treated with PBS (A), 10 μM Gal-2 (B), or 20 μM camptothecin (C) for 18 hours followed by flow cytometric analysis for DNA degradation (TUNEL assay). Error bars represent the standard deviation of duplicate samples. The dsMOLT-4 or dsHL60 cells were treated with 5 μM Gal-4 (D), 10 μM Gal-2 (E), or 20 μM topoisomerase inhibitors (etoposide for MOLT-4 cells or camptothecin for HL60 cells) for 18 hours followed by flow cytometric analysis for DNA degradation (TUNEL assay). The dsMOLT-4 cells (F) or dsHL60 cells (G) were treated with either 3 μM Gal-4 or 20 μM topoisomerase inhibitors (etoposide for MOLT-4 cells or camptothecin for HL60) for the indicated times followed by the determination of viable cell number by trypan blue exclusion using a hemocytometer. Results shown are the averages of duplicate analyses and are representative of at least 2 separate experiments. Error bars represent the standard deviation of duplicate samples. Representative dot plots for dsHL60 cells treated with PBS (H), 10 μM Gal-2 (I), or 20 μM camptothecin (J) for 18 hours followed by flow cytometric analysis for changes in the light-scattering properties of the cells. Gate values of cells experiencing no changes in forward- and side-scatter profile are shown.
To further determine whether cell viability might be affected following galectin treatment, MOLT-4 cells (Figure 3F) and HL60 cells (Figure 3G) were treated with 5 μM Gal-4 followed by analysis for the number of viable cells excluding trypan blue using a hemocytometer. Treatment of cells with Gal-4 caused no significant alterations in growth rate, whereas treatment of cells with either camptothecin or etoposide caused loss of viable cells within 24 hours (Figure 3F-G). Treatment of HL60 cells with Gal-2 produced similar results (data not shown). Another cellular feature associated with apoptosis is cell blebbing (zeosis) and cell shrinkage,49 which can be measured by changes in light-scattering properties of the cell in flow cytometric analysis.49 HL60 cells (Figure 3H-J) were treated for 18 hours with Gal-2, and although the cells continued to express surface PS (data not shown), there was no significant change in the light-scatter properties of the cells (Figure 3H-I). Treatment of both MOLT-4 cells and HL60 cells in the same manner with Gal-4 or MOLT-4 cells with Gal-2 produced similar results (data not shown). By contrast, treatments of cells with either camptothecin or etoposide caused significant changes in light-scatter profiles, as shown for HL60 cells treated with camptothecin (Figure 3J). These results demonstrate that both Gal-2 and Gal-4 induce PS exposure in these cell lines independently of cell death.
Activated neutrophils, but not activated T cells, are sensitive to galectin-induced PS exposure
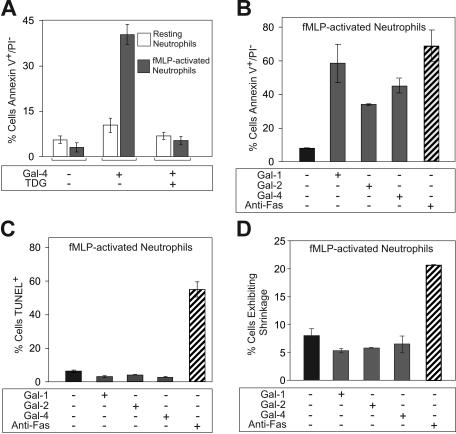
To further explore the specificity of Gal-2– and Gal-4–induced PS exposure in blood leukocytes, we first examined responses of human neutrophils. Gal-4 induced significant PS exposure in fMLP-activated neutrophils, but had only a modest effect on resting neutrophils (Figure 4A) Treatment of cells with Gal-2 and Gal-1 also induced PS exposure in fMLP-activated human neutrophils (Figure 4B), but not in resting neutrophils (data not shown). As a control, cells were treated with anti-Fas, a known inducer of apoptosis,22 which also induced PS exposure (Figure 4B). However, treatment of fMLP-activated neutrophils with either Gal-1, Gal-2, or Gal-4 did not induce apoptosis, as measured by the TUNEL assay, whereas anti-Fas treatment induced apoptosis (Figure 4C). In addition, galectins did not induce cellular shrinkage of fMLP-activated neutrophils, whereas anti-Fas induced cell shrinkage (Figure 4D). These results demonstrate that Gal-1, Gal-2, and Gal-4 induce PS exposure in fMLP-activated, but not resting, neutrophils, independently of cell death.
Figure 4.
Galectins induce PS exposure in fMLP-activated human neutrophils. (A) fMLP-activated or resting neutrophils were treated with either PBS, 3 μM Gal-4, or 3 μM Gal-4 plus 30 mM TDG for 4 hours, as indicated, followed by analysis of PS exposure using annexin-V. (B) fMLP-activated neutrophils were incubated with either 10 μM Gal-1, 10 μM Gal-2, 3 μM Gal-4, or 100 ng/mL anti-Fas for 8 hours followed by detection for PS exposure. (C) fMLP-activated neutrophils were incubated with either 10 μM Gal-1, 10 μM Gal-2, 3 μM Gal-4, or 100 ng/mL anti-Fas for 8 hours followed by detection for DNA degradation (TUNEL assay). (D) fMLP-activated neutrophils were incubated with either 10 μM Gal-1, 10 μM Gal-2, 3 μM Gal-4, or 100 ng/mL anti-Fas for 8 hours followed by flow cytometric analysis for the percentage of cells exhibiting shrinkage. Results shown are the averages of duplicate analyses and are representative of at least 2 separate experiments. Error bars represent the standard deviation of duplicate samples.
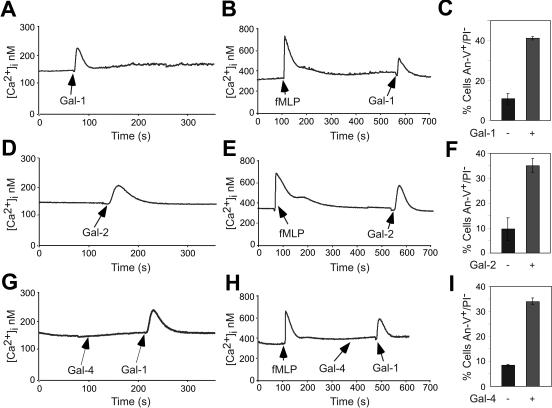
Since both Gal-2 and Gal-4 induced PS exposure independent of cell death in activated neutrophils, we next sought to determine whether Gal-2 and Gal-4 mobilize Ca2+ as previously observed during Gal-1–induced PS exposure.36 Resting and fMLP-activated neutrophils were treated with Gal-1, Gal-2, or Gal-4 followed by detection for changes in intracellular Ca2+. Similar to previous observations for Gal-136 (Figure 5A-B), Gal-2 induced a rapid, yet transient, Ca2+ flux in both resting and fMLP-activated neutrophils (Figure 5D-E). However, both resting and activated neutrophils showed no changes in intracellular Ca2+ levels following Gal-4 treatment (Figure 5G-H). In addition, both resting and activated neutrophils were responsive to Gal-1 following Gal-4 administration, indicating that the cells were sensitive to Gal-1–induced Ca2+ mobilization (Figure 5G-H). In all cases, treatments of cells with either Gal-1, Gal-2, or Gal-4 induced PS exposure (Figure 5C, F, and I, respectively). These results demonstrate that Gal-4 induces PS exposure through separate proximal signaling pathways distinguishable from those for Gal-1 and Gal-2.
Figure 5.
Gal-1 and Gal-2 but not Gal-4 induce Ca2+ flux in both resting and activated neutrophils. Resting neutrophils were treated with 10 μM Gal-1 (A), 10 μM Gal-2 (D), or 5 μM Gal-4 followed by 10 μM Gal-1 (G), followed by detection for changes in intracellular Ca2+ levels. Resting neutrophils were activated with fMLP followed by treatment with either 10 μM Gal-1 (B), 10 μM Gal-2 (E), or 5 μM Gal-4 followed by 10 μM Gal-1 (H), followed by detection for changes in intracellular Ca2+ levels. fMLP-activated neutrophils in parallel assays were treated with 10 μM Gal-1 (C), 10 μM Gal-2 (F), or 5 μM Gal-4 (I) for 4 hours followed by detection of PS exposure. Error bars represent the standard deviation of duplicate samples.
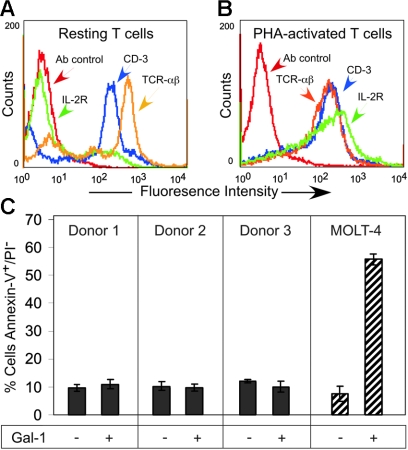
Previous reports indicated that Gal-1 induces PS exposure in activated, but not resting, T cells, with accompanying apoptosis,39 which is clearly different from the responses of activated neutrophils to galectins. To further explore the basis for this apparent difference, resting and PHA-activated T cells (Figure 6A-B) were incubated with Gal-1 for 9 hours. Cell activation was confirmed by incubating cells with anti–IL-2R, anti–CD-3, anti–TCR-αβ, or an isotype control followed by flow cytometric analysis. Unexpectedly, Gal-1 treatment of activated T cells from 3 different donors had no effect on PS redistribution (Figure 6C), although in parallel control assays Gal-1 induced PS exposure in MOLT-4 cells (Figure 6C). Thus, contrary to previous reports, Gal-1 is unable to signal PS exposure or apoptosis in activated T cells.
Figure 6.
Gal-1 does not induce PS exposure in activated human T cells. (A-B) Resting T cells (A) or PHA-activated T cells (B) were incubated with anti–IL-2R, anti–CD-3, anti–TCR-αβ, or isotype control followed by flow cytometric analysis. (C) PHA-activated T cells isolated from 3 separate donors as indicated or MOLT-4 cells were treated with 10 μM Gal-1 followed by detection for PS exposure. Error bars represent the standard deviation of duplicate samples.
In the presence of DTT, galectin-1 induces PS exposure of activated T cells
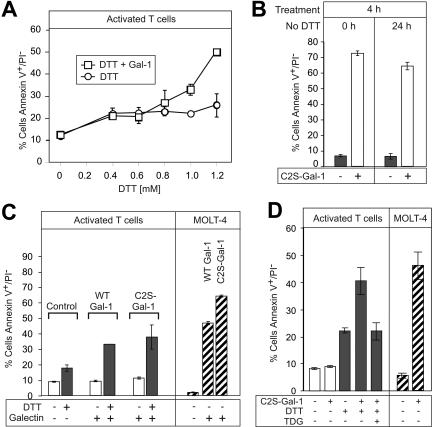
In exploring the basis for these apparently conflicting results in comparison with other published studies, we noted that most previous studies included the reducing agent DTT in Gal-1 preparations used to treat cells, presumably to maintain Gal-1 activity.39,48 Thus, we titrated increasing concentrations of DTT into activated T cells treated with a uniform amount of Gal-1. Consistent with previous results,39,48 Gal-1 induced PS exposure in activated T cells, but only when DTT was included in a concentration-dependent manner (Figure 7A) Thus, while Gal-1 is able to induce PS exposure in fMLP-activated neutrophils, but not activated T cells, it induces robust PS exposure in activated T cells only after inclusion of DTT.
Figure 7.
DTT primes activated human T cells for Gal-1–induced PS exposure. (A) PHA-activated T cells were incubated with RPMI alone, 10 μM Gal-1 alone, or with the respective concentration of DTT with or without 10 μM Gal-1 for 9 hours as indicated, followed by the detection for PS exposure. (B) HL60 cells were treated with either freshly prepared C2S-Gal-1 or C2S-Gal-1 incubated for 24 hours in the absence of DTT. Following C2S-Gal-1 addition, cells were incubated for 4 hours followed by analysis for PS exposure. (C) PHA-activated T cells or MOLT-4 cells were incubated with 10 μM Gal-1 or 10 μM C2S-Gal-1 with or without 1.2 mM DTT for 9 hours, followed by detection for PS exposure. (D) PHA-activated T cells or MOLT-4 cells were incubated with 10 μM C2S-Gal-1 with or without 30 mM TDG as indicated for 9 hours followed by detection for PS exposure. Error bars represent the standard deviation of duplicate samples.
To address the possibility that Gal-1 might be losing activity during these treatments in the absence of DTT, thus affecting its potential activity toward activated T cells, we generated a mutant form of Gal-1, termed C2S-Gal-1, in which a key Cys residue at position 2 is changed to Ser. The C2S mutant form of Gal-1 retains lectin activity for days in the absence of reducing conditions or hapten sugars such as lactose.44,45 When incubated at 37°C in the absence of DTT over 24 hours, the C2S-Gal-1 showed no appreciable loss in activity compared with freshly prepared C2S-Gal-1 (Figure 7B). The C2S-Gal-1 was comparable with wt Gal-1 in inducing PS exposure in MOLT-4 cells (Figure 7C). Additionally, the C2S-Gal-1 retained all carbohydrate-binding properties of the wild-type lectin toward a wide variety of glycan ligands (data not shown).
When activated T cells were treated with either wt Gal-1 or C2S-Gal-1, there was no increase in surface PS exposure (Figure 7C). However, when activated T cells were treated with wt Gal-1 or C2S-Gal-1 in the presence of DTT, they became responsive and PS exposure was apparent (Figure 7C). Furthermore, inclusion of 20 mM TDG abrogated the Gal-1 effect (Figure 7D). These results demonstrate that Gal-1 induces PS exposure in activated T cells only in the presence of DTT.
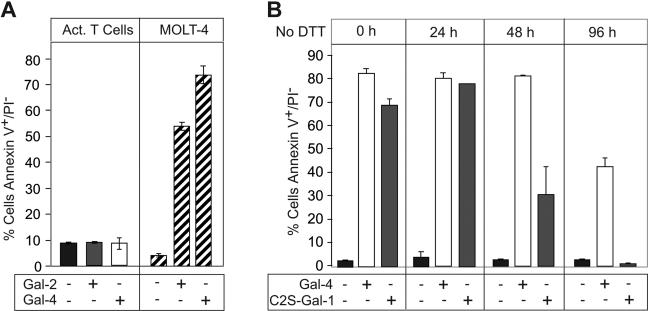
We next addressed whether Gal-2 or Gal-4 could induce PS exposure in T cells in the absence of DTT. Neither Gal-2 nor Gal-4 induced PS exposure in activated T cells (Figure 8A), although upon inclusion of DTT Gal-2 induced PS exposure (data not shown), consistent with previous observations.40 Gal-2 behaved similarly to Gal-1, requiring reducing conditions to maintain complete activity over a 24-hour period (data not shown). However, Gal-4 was much less unstable and in fact was more stable than C2S-Gal-1, as evidenced by its ability to induce PS exposure in HL60 cells following prolonged incubation in the absence of DTT (Figure 8B). These results demonstrate that neither C2S-Gal-1, wt Gal-1, Gal-2, nor Gal-4 induce PS exposure in activated T cells unless DTT is included in the treatment.
Figure 8.
Gal-2 and Gal-4 do not induce PS exposure in activated T cells. (A) PHA-activated T cells or MOLT-4 cells were incubated with 10 μM Gal-2 or 3 μM Gal-4 for 9 hours in the absence of DTT, followed by detection for PS exposure. (B) Gal-4 or C2S-Gal-1 was incubated without DTT for the indicated time followed by treatment of HL60 cells for 4 hours and detection for PS exposure. Error bars represent the standard deviation of duplicate samples.
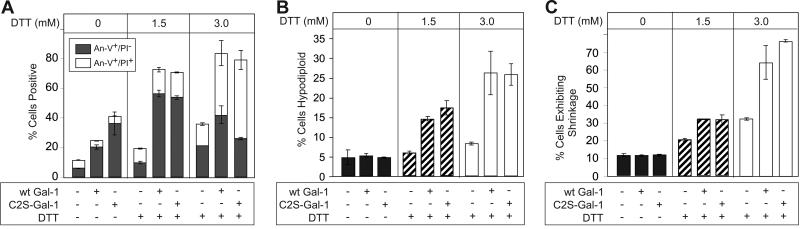
We next tested whether Gal-1 can induce apoptosis in cell lines when coincubated with DTT, as reported by others.39 We incubated MOLT-4 cells with wt or C2S-Gal-1 with or without DTT, and measured PS exposure and hypodiploid content. wt Gal-1 and C2S-Gal-1, when incubated in the absence of DTT, induced PS exposure in MOLT-4 cells (Figure 9A) independently of DNA fragmentation (Figure 9B), cell shrinkage (Figure 9C), or increases in late apoptosis as measured by PI staining (Figure 9A). However, when DTT was included with either wt Gal-1 or C2S-Gal-1, MOLT-4 cells experienced significant increases in PI staining (Figure 9A), DNA fragmentation (Figure 9B), and cell shrinkage (Figure 9C) in a dose-dependent manner. To determine whether this DTT-dependent effect could also be observed in a different T-leukemic cell line, we used T-leukemic CEM cells. CEM cells responded in the same fashion as MOLT-4 cells, becoming sensitive to Gal-1–induced apoptosis only when coincubated with DTT, as measured by both hypodiploid stain, cell shrinkage, and PI staining (data not shown). These results demonstrate that Gal-1 induces PS exposure in activated T cells only when DTT is present and induces apoptosis in MOLT-4 and CEM cells only when primed by DTT treatment. Thus, it is likely that the deleterious effects of DTT on activated T cells make them sensitive to Gal-1 signaling, whereas Gal-1 induces signals in activated neutrophils independently of DTT.
Figure 9.
DTT primes MOLT-4 cells for Gal-1–induced apoptosis. (A) MOLT-4 cells were incubated with either 10 μM wt Gal-1 or 10 μM C2S-Gal-1 with or without DTT at the concentrations indicated for 8 hours, followed by analysis for PS exposure (annexin-V+/PI−) and membrane integrity loss (annexin-V+/PI+). (B) MOLT-4 cells were incubated with either 10 μM wt Gal-1 or 10 μM C2S-Gal-1 with or without DTT at the concentrations indicated for 8 hours, followed by analysis for DNA degradation by assaying for hypodiploid DNA content by flow cytometry. (C) MOLT-4 cells were incubated with either 10 μM wt Gal-1 or 10 μM C2S-Gal-1 with or without DTT at the concentrations indicated for 8 hours, followed by analysis for cellular shrinkage by flow cytometry. Error bars represent the standard deviation of duplicate samples.
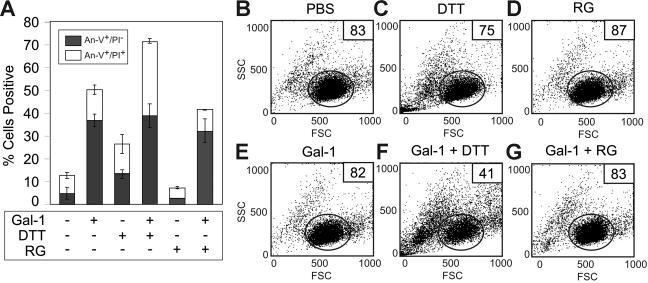
We next sought to determine whether the sensitizing effects of DTT on cells resulted from reduction and therefore potential alterations in cells' surface glycoproteins or receptors or whether DTT might exert its deleterious effects once it accumulates inside the cells. We treated cells with reduced glutathione (RG), a reducing agent that is impermeable to the plasma membrane.54 As previously observed, cells treated with DTT became sensitive to Gal-1–induced cell death, experiencing increases in late apoptosis as evidenced by increased PI staining (Figure 10A) and cellular shrinkage (Figure 10C,F), when compared with controls (Figure 10A-B,E). However, cells treated with RG displayed no increase in sensitivity toward Gal-1–induced cell death (Figure 10A,D,G). These results demonstrate that DTT sensitizes cells toward Gal-1–induced apoptosis through alterations in intracellular processes.
Figure 10.
DTT, but not reduced glutathione, primes MOLT-4 cells for Gal-1–induced apoptosis. (A) MOLT-4 cells were treated with 3 mM DTT or 3 mM reduced glutathione (RG) as indicated with or without 10 μM Gal-1 for 9 hours followed by detection for PS exposure (annexin-V+/PI−) and membrane integrity loss (annexin-V+/PI+). Error bars represent the standard deviation of duplicate samples. MOLT-4 cells were treated with PBS (B), 3 mM DTT (C), 3 mM RG (D), 10 μM Gal-1 (E), 10 μM Gal-1 + 3 mM DTT (F), or 10 μM Gal-1 + 3 mM RG (G) for 9 hours followed by detection for changes in the light-scattering properties of the cell. Gate values of cells experiencing no changes in forward- and side-scatter profile are shown.
Discussion
Our results demonstrate that Gal-1, Gal-2, and Gal-4 induce PS redistribution in activated, but not resting, human neutrophils, and in leukemic MOLT-4 and HL60 cell lines, and that these changes do not accompany cell death. Of importance, activated T cells are not induced to expose PS in response to Gal-1, Gal-2, and Gal-4. These results demonstrate a novel and specific regulatory effect of galectins on leukocytes and further support the possible role of these galectins in the physiologic turnover of neutrophils.
A key membrane event in regulating neutrophil turnover is the externalization of PS, which commonly accompanies apoptosis and serves as a key recognition marker for the phagocytic recognition and removal of apoptotic cells.55 However, the cellular processes and factors that cause PS exposure are not well understood. Our previous studies showed that human Gal-1 induces PS exposure in activated neutrophils independently of apoptosis, which is accompanied in vitro by their efficient recognition and phagocytosis by macrophages.35 Our new studies show that Gal-2 and Gal-4 also induce PS exposure in activated neutrophils but not in activated T cells. Of importance, Gal-4 induced PS exposure in the absence of Ca2+ mobilization, although Gal-1–induced PS exposure requires changes in intracellular Ca2+,36 thus suggesting that there are at least 2 separate proximal pathways whereby PS can be externalized in an apoptosis-independent manner. The increased potency of Gal-4 over Gal-1 and Gal-2 is likely due to the tandem repeat nature of the protein, since engineered tandem repeat forms of Gal-1 demonstrate significant increases in biologic potency.56,57
The ability of galectin family members to signal apoptosis independently of PS exposure corroborates growing evidence for apoptosis-independent mechanisms of leukocyte phagocytosis.55 The ability of galectin family members to induce PS exposure may therefore provide an explanation for the ability of macrophages to recognize and remove neutrophils through nonapoptotic mechanisms.18 Furthermore, the ability of galectins to induce changes enabling phagocytic removal without inducing concomitant apoptosis allows neutrophils to maintain membrane integrity, thus limiting the potential for the unregulated release of deleterious enzymes prior to phagocytic removal.15-17 Consistent with this hypothesis, Gal-1 administration attenuates acute inflammation in vivo and protects parenchyma architecture during inflammatory episodes.58
There is growing evidence that nonapoptotic processes contribute to regulating neutrophil homeostasis. For example, mice that overexpress bcl-2 in myeloid cells have impaired neutrophil apoptosis, yet they have normal numbers of neutrophils that are sensitive to phagocytic removal.59 It appears that mechanisms regulating neutrophil turnover are partially distinct from those regulating lymphocyte turnover. For example, patients in whom bcl-2 is overexpressed, as occurs in follicular lymphoma, accumulate lymphocytes.60 Similarly, patients with autoimmune lymphoproliferative syndrome due to inherited defects in Fas, a proapoptotic signaling receptor, exhibit lymphocytosis.61,62 However, these same patients often have normal or reduced neutrophil counts.61,62 Similarly, mice deficient in Fas or FasL display normal levels of circulating neutrophils and show no change in acute inflammatory responses,21 further suggesting that the turnover of neutrophils and lymphocytes may be regulated by alternative pathways. This is also consistent with evidence that Kupffer cells phagocytose nonapoptotic neutrophils in vivo during inflammatory resolution.18 Potential differences observed between lymphocyte and neutrophil turnover in vivo may partially explain why only activated neutrophils, but not activated T lymphocytes, are sensitive to galectin-induced effects.
Our finding that Gal-1, -2, and -4 induce PS exposure in T-leukemic MOLT-4 cells, but not in activated T cells except when DTT is included, is novel and potentially resolves some long-standing controversies. Many studies have reported the effects of Gal-1 on cells in the presence of DTT49; under these conditions, Gal-1 was reported to induce apoptosis of activated T cells, MOLT-4 cells, and CEM cells.39,49,63,64 The present study demonstrates that Gal-1–induced apoptosis in the presence of DTT results from alterations in cellular physiology and not a requirement to maintain Gal-1 activity. Thus, C2S-Gal-1, which is resistant to oxidative inactivation, does not induce apoptosis in the absence of DTT but only in its presence. It should be noted that the extracellular milieu is normally an oxidizing environment,65 suggesting that reduction of normally oxidized cell-surface proteins may result in the increased sensitivity of cells to Gal-1–induced death. However, the inability of reduced glutathione, a cell-impermeable reducing agent,54 to sensitize cells to Gal-1–induced apoptosis demonstrates that the deleterious effects of DTT result from nonphysiologic changes induced by DTT inside cells. Although very low concentrations of DTT may be protective to cell stress,66 higher concentrations of DTT, similar to those used in this and previous studies,39,49,63,64 have been used to induce the unfolded-protein response, cause changes in gene expression, inhibit protein synthesis, and generate free radicals, causing generalized cell stress,67-70 which often results in apoptotic cell death.70-72 Thus, the DTT priming effect on cells is likely to be complex and could involve numerous pathways. Although we have shown that Gal-1, Gal-2, and Gal-4 do not induce apoptosis in T cells, we have not excluded the possibility that other galectin family members may affect T-cell viability. For example, Gal-3 has been shown to induce apoptosis in activated T cells in the conspicuous absence of DTT.73 Future studies should examine the roles of other galectin family members in leukocyte viability in the absence of reducing agents.
The sensitivity of MOLT-4 and CEM cells, but not T cells, to Gal-1, -2, and -4 may arise from changes in the cell lines that accompanied neoplastic transformation. It is important to note that although both HL60 cells and neutrophils respond to galectin treatment, neutrophils respond only once they are activated, whereas HL60 cells are sensitive without a prior priming event. The ability of these galectins to signal through unique pathways in transformed cells may reflect potential roles for galectin family members in neoplastic disease. Recent studies suggests that PS exposure on tumor cells may serve as a decoy to phagocytic cells and trigger their release of anti-inflammatory cytokines as a mechanism of establishing an immune-privileged environment.74,75 This could be especially important in light of evidence implicating various galectin family members in neoplastic disease.76 Future studies are needed to address the roles of galectin family members in the regulation of innate immunity and in neoplastic-cell survival in vivo.
Acknowledgments
We thank Mrs Chanda Hill for technical assistance.
This work was supported by National Institutes of Health grants HL085607 (R.D.C.) and HL34363 and RR15577 (R.P.M.).
The abbreviations used are as follows: Gal-1 indicates human galectin-1; Gal-2, human galectin-2; Gal-4, human galectin-4; C2S-Gal-1, human galectin-1 with replacement of cysteine-2 by serine-2; fMLP, N-formyl-Met-Leu-Pro; PHA, Phaseolus vulgaris phytohemagglutinin-L; HBSS, Hanks balanced salt solution; HSA, human serum albumin; PI, propidium iodide; PS, phosphatidylserine; TUNEL, terminal deoxynucleotidyl transferase (TdT)–mediated dUTP nick end labeling; DTT, dithiothreitol; RG, reduced glutathione; TDG, thiodigalactoside (β-D-galactopyranosyl 1-thio-β-D-galactopyranoside); 2-ME, 2-mercaptoethanol; dsMOLT-4, desialylated MOLT-4 cells; and dsHL60, desialylated HL60 cells.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- 1.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 3.Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. doi: 10.1038/sj.cdd.4400451. [DOI] [PubMed] [Google Scholar]
- 4.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 5.Simon HU. Targeting apoptosis in the control of inflammation. Eur Respir J Suppl. 2003;44:20s–21s. doi: 10.1183/09031936.03.00000603b. [DOI] [PubMed] [Google Scholar]
- 6.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 7.Adayev T, Estephan R, Meserole S, Mazza B, Yurkow EJ, Banerjee P. Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J Neurochem. 1998;71:1854–1864. doi: 10.1046/j.1471-4159.1998.71051854.x. [DOI] [PubMed] [Google Scholar]
- 8.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 9.Hengartner MO. Apoptosis: corralling the corpses. Cell. 2001;104:325–328. doi: 10.1016/s0092-8674(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 10.Ayub K, Hallett MB. Signalling shutdown strategies in aging immune cells. Aging Cell. 2004;3:145–149. doi: 10.1111/j.1474-9728.2004.00100.x. [DOI] [PubMed] [Google Scholar]
- 11.Ayub K, Hallett MB. Ca2+ influx shutdown during neutrophil apoptosis: importance and possible mechanism. Immunology. 2004;111:8–12. doi: 10.1111/j.1365-2567.2003.01766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheel-Toellner D, Wang KQ, Webb PR, et al. Early events in spontaneous neutrophil apoptosis. Biochem Soc Trans. 2004;32:461–464. doi: 10.1042/BST0320461. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 14.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 15.Misawa R, Kawagishi C, Watanabe N, Kobayashi Y. Infiltration of neutrophils following injection of apoptotic cells into the peritoneal cavity. Apoptosis. 2001;6:411–417. doi: 10.1023/a:1012406121993. [DOI] [PubMed] [Google Scholar]
- 16.Hagimoto N, Kuwano K, Miyazaki H, et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 17.Kuwano K, Hagimoto N, Kawasaki M, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 19.Dibbert B, Weber M, Nikolaizik WH, et al. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci U S A. 1999;96:13330–13335. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fecho K, Bentley SA, Cohen PL. Mice deficient in fas ligand (gld) or fas (lpr) show few alterations in granulopoiesis. Cell Immunol. 1998;188:19–32. doi: 10.1006/cimm.1998.1339. [DOI] [PubMed] [Google Scholar]
- 21.Fecho K, Cohen PL. Fas ligand (gld)- and Fas (lpr)-deficient mice do not show alterations in the extravasation or apoptosis of inflammatory neutrophils. J Leukoc Biol. 1998;64:373–383. doi: 10.1002/jlb.64.3.373. [DOI] [PubMed] [Google Scholar]
- 22.Iwai K, Miyawaki T, Takizawa T, et al. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 23.Hoffmann PR, deCathelineau AM, Ogden CA, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 25.Oka K, Sawamura T, Kikuta K, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki Y, Nakagawa A, Nagaosa K, Shiratsuchi A, Nakanishi Y. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular sertoli cells. J Biol Chem. 2002;277:27559–27566. doi: 10.1074/jbc.M202879200. [DOI] [PubMed] [Google Scholar]
- 27.Zullig S, Hengartner MO. Cell biology. Tickling macrophages, a serious business. Science. 2004;304:1123–1124. doi: 10.1126/science.1099161. [DOI] [PubMed] [Google Scholar]
- 28.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 29.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 30.Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004;19:575–581. doi: 10.1023/B:GLYC.0000014088.21242.e0. [DOI] [PubMed] [Google Scholar]
- 31.Gabius HJ. Probing the cons and pros of lectin-induced immunomodulation: case studies for the mistletoe lectin and galectin-1. Biochimie. 2001;83:659–666. doi: 10.1016/s0300-9084(01)01311-6. [DOI] [PubMed] [Google Scholar]
- 32.Leffler H. Galectins structure and function: a synopsis. Results Probl Cell Differ. 2001;33:57–83. doi: 10.1007/978-3-540-46410-5_4. [DOI] [PubMed] [Google Scholar]
- 33.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- 35.Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- 36.Karmakar S, Cummings RD, McEver RP. Contributions of Ca2+ to galectin-1-induced exposure of phosphatidylserine on activated neutrophils. J Biol Chem. 2005;280:28623–28631. doi: 10.1074/jbc.M414140200. [DOI] [PubMed] [Google Scholar]
- 37.Vas V, Fajka-Boja R, Ion G, Dudics V, Monostori E, Uher F. Biphasic effect of recombinant galectin-1 on the growth and death of early hematopoietic cells. Stem Cells. 2005;23:279–287. doi: 10.1634/stemcells.2004-0084. [DOI] [PubMed] [Google Scholar]
- 38.He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004;279:4705–4712. doi: 10.1074/jbc.M311183200. [DOI] [PubMed] [Google Scholar]
- 39.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 40.Sturm A, Lensch M, Andre S, et al. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173:3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 41.Hokama A, Mizoguchi E, Sugimoto K, et al. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20:681–693. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Ideo H, Seko A, Ohkura T, Matta KL, Yamashita K. High-affinity binding of recombinant human galectin-4 to SO(3)(-)→3Galbeta1→3GalNAc pyranoside. Glycobiology. 2002;12:199–208. doi: 10.1093/glycob/12.3.199. [DOI] [PubMed] [Google Scholar]
- 43.Gitt MA, Massa SM, Leffler H, Barondes SH. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J Biol Chem. 1992;267:10601–10606. [PubMed] [Google Scholar]
- 44.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells, I: physical and chemical characterization. J Biol Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 45.Hirabayashi J, Kasai K. Effect of amino acid substitution by site-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991;266:23648–23653. [PubMed] [Google Scholar]
- 46.Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14:157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- 47.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric Galectin-1 binds with high affinity to {alpha}2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem. 2005;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 48.Carlow DA, Williams MJ, Ziltener HJ. Modulation of O-glycans and N-glycans on murine CD8 T cells fails to alter annexin V ligand induction by galectin 1. J Immunol. 2003;171:5100–5106. doi: 10.4049/jimmunol.171.10.5100. [DOI] [PubMed] [Google Scholar]
- 49.Pace KE, Hahn HP, Baum LG. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- 50.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 51.Tait JF, Gibson D. Measurement of membrane phospholipid asymmetry in normal and sickle-cell erythrocytes by means of annexin V binding. J Lab Clin Med. 1994;123:741–748. [PubMed] [Google Scholar]
- 52.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 53.Stach CM, Turnay X, Voll RE, et al. Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ. 2000;7:911–915. doi: 10.1038/sj.cdd.4400715. [DOI] [PubMed] [Google Scholar]
- 54.Soltysiak-Pawluczuk D, Naciazek-Wieniawska A, Danysz A, Czarnomska A. Effects of thiol compounds on methotrexate uptake by murine lymphocytes from thymus and thymic lymphosarcoma. Cancer Lett. 1992;65:251–257. doi: 10.1016/0304-3835(92)90240-v. [DOI] [PubMed] [Google Scholar]
- 55.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 56.van der Leij J, van den Berg A, Blokzijl T, et al. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204:511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- 57.Battig P, Saudan P, Gunde T, Bachmann MF. Enhanced apoptotic activity of a structurally optimized form of galectin-1. Mol Immunol. 2004;41:9–18. doi: 10.1016/j.molimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. Eur J Immunol. 2000;30:1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 59.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aster JC, Longtine JA. Detection of BCL2 rearrangements in follicular lymphoma. Am J Pathol. 2002;160:759–763. doi: 10.1016/S0002-9440(10)64897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon SW, Procter J, Dale JK, Straus SE, Stroncek DF. Neutrophil and platelet antibodies in autoimmune lymphoproliferative syndrome. Vox Sang. 2003;85:307–312. doi: 10.1111/j.0042-9007.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 62.Jackson CE, Fischer RE, Hsu AP, et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64:1002–1014. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 64.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 66.Liu L, Trimarchi JR, Keefe DL. Thiol oxidation-induced embryonic cell death in mice is prevented by the antioxidant dithiothreitol. Biol Reprod. 1999;61:1162–1169. doi: 10.1095/biolreprod61.4.1162. [DOI] [PubMed] [Google Scholar]
- 67.Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cronin SR, Rao R, Hampton RY. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol. 2002;157:1017–1028. doi: 10.1083/jcb.200203052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell. 1998;1:161–170. doi: 10.1016/s1097-2765(00)80017-9. [DOI] [PubMed] [Google Scholar]
- 70.Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tartier L, McCarey YL, Biaglow JE, Kochevar IE, Held KD. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 2000;7:1002–1010. doi: 10.1038/sj.cdd.4400726. [DOI] [PubMed] [Google Scholar]
- 72.Held KD, Sylvester FC, Hopcia KL, Biaglow JE. Role of Fenton chemistry in thiol-induced toxicity and apoptosis. Radiat Res. 1996;145:542–553. [PubMed] [Google Scholar]
- 73.Fukumori T, Takenaka Y, Yoshii T, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- 74.Matsuno R, Aramaki Y, Tsuchiya S. Involvement of TGF-beta in inhibitory effects of negatively charged liposomes on nitric oxide production by macrophages stimulated with lps. Biochem Biophys Res Commun. 2001;281:614–620. doi: 10.1006/bbrc.2001.4419. [DOI] [PubMed] [Google Scholar]
- 75.Aramaki Y. Liposomes as immunomodulator: inhibitory effect of liposomes on NO production from macrophages. Biol Pharm Bull. 2000;23:1267–1274. doi: 10.1248/bpb.23.1267. [DOI] [PubMed] [Google Scholar]
- 76.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]